Abstract
A recent study using new holographic optogenetic stimulation technology has provided direct evidence that hippocampal place cell activity is sufficient to drive memory and navigation-related behaviors.
As animals navigate through the world, their survival depends on the ability to maintain a memory for episodes that occurred in different locations. During such memory-guided navigation, sparse sets of neurons in the hippocampus show striking correlations with an animal’s instantaneous location in an environment. These neurons, called ‘place cells’, are maximally active when an animal occupies one or few restricted spatial locations and collectively form a neural map of the external environment1. Moreover, the activity of place cells is hypothesized to provide the neural basis for episodic memory2 and support the ability of animals to navigate both physical and conceptual spaces3. Until recently, direct tests of the causal role of place cells in memory-guided behavior remained experimentally intractable. A new study by Robinson et al.4 used a cutting-edge combination of two-photon calcium imaging and holographic optogenetic technologies to provide the first causal evidence that place cell activity can drive memory-guided navigation (Figure 1).
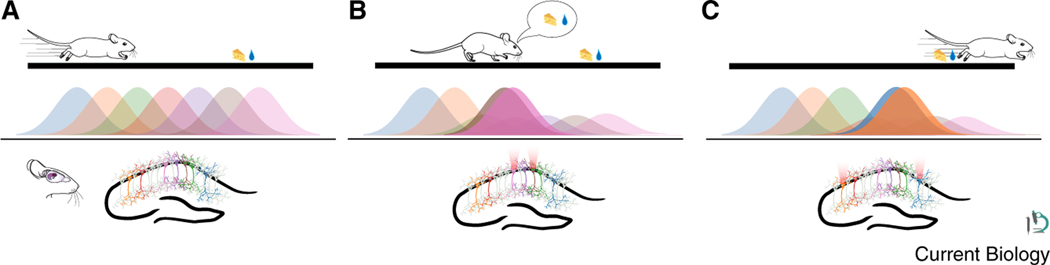
Figure 1. Stimulating place cells drives behaviors associated with the spatial locations the place cells represent.
(A) When mice explore environments, sparse sets of hippocampal place cells form representations for confined spatial locations. (B) Specifically stimulating place cells associated with reward locations drives reward-associated behaviors like slowed running and licking for liquid rewards. (C) Stimulating place cells associated with locations in which the mouse typically runs fast, such as the beginning of a linear track, causes motivated animals to overrun reward locations. Images from scidraw.io (CC 4.0). Mouse images: Ethan Tyler and Lex Kravits 10.5281/zenodo.3925949 and 10.5281/zenodo.3925915. Pyramidal neuron: Federico Claud 10.5281/zenodo.3925905. Sagittal view of brain from Wenbo Tang 10.5281/zenodo.3925923.
The work of Robinson et al.4 builds on decades of research indicating that the hippocampus plays a key role in memory and navigation2,5. The profound memory deficits observed in patient H.M. after bilateral hippocampal resection, combined with subsequent animal and human lesion work, all pointed to the importance of hippocampal processing in memory2. In parallel, the discovery of hippocampal place cells provided a significant leap forward in understanding how hippocampal neural activity supports navigation1. Subsequent work on place cells ranged from the dissection of which sensory cues drive the firing properties of place cells to observations that place cells can encode information regarding non-spatial sensory cues (for review, see6).
A direct demonstration that the firing activity of place cells causally controls behavior, however, remained elusive. This missing pillar of the place cell literature persisted because of the lack of appropriate technology to directly test their behavioral relevance. Place cells are genetically diverse, existing in deep and superficial layers of the hippocampus and across multiple hippocampal sub-regions (for review, see7). They are, in essence, ‘functionally defined’: the firing activity observed as an animal navigates through an environment defines a neuron as a ‘place cell’. Based on this functional definition, nearly any hippocampal pyramidal cell has the potential to be a place cell at any location in space8,9. Moreover, for any given environment, only a sparse set of place cells are active: across environments, place cells turn on, turn off, or move their location of preferred firing, and the place cells active in different environments are scattered throughout the cell layer in a seemingly random manner10. Thus, manipulating place cells requires observing cellular activity with relatively high temporal resolution and the ability to manipulate the spiking activity of functionally (not genetically) defined neurons during behavior.
Despite these challenges, several lines of research in rodents hinted at the importance of place cell activity in behavior. A seminal set of experiments used targeted optogenetic manipulation of hippocampal cells that express immediate early genes (a marker for increased neural activity) in a particular environment to show that reactivation of these cells could drive fear memory recall11. Another influential group of studies discovered that sets of place cells are rapidly activated in temporally compressed sequences during rest and sleep12. These sequences often predict future behavior and disrupting them reduces subsequent memory performance13. One previous study successfully optogenetically stimulated sequences of individual place cells with high temporal specificity; however, the authors could only stimulate a single neuron at a time14. This limitation was likely the reason they did not observe any behavioral effects of their manipulation. Clearly, to fully test the link between place cell activity and behavior, an approach was needed that provided expanded bandwidth for manipulation while maintaining spatial and temporal specificity.
Recently developed two-photon holographic optogenetic stimulation fills this need. In brief, by adding a further light-path with spatial light modulators to a two-photon microscope, this technique creates numerous customizable small beamlets from a larger laser beam. These small beamlets can then be centered over individual experimenter-specified neurons to induce two-photon stimulation of optogenetic proteins and therefore drive action potentials in the cells of interest. By updating the ‘phase masks’ of the spatial light modulators, the experimenter can change which cells are stimulated. Several of the authors of the new Robinson et al.4 paper were central to the development of this approach15. The first evidence that holographic optogenetic stimulation could be used to emulate physiologically relevant neural activity and drive behavior was demonstrated in visual cortex. Stimulating cells in V1 that were active during a particular visual experience could evoke the same neural activity and behavior associated with that experience, even when the visual stimulus was not present16,17.
Robinson et al.4 capitalized on this technological innovation, combining it with several other state-of-the-art techniques to truly test the causal role of place cell activity in behavior. The authors trained thirsty, head-fixed mice to navigate in virtual reality for liquid rewards. Importantly, the animals were trained to withhold their licking until they reached a spatially restricted reward zone on the virtual track. As mice navigated, the authors used two-photon calcium imaging to capture the activity of large populations of pyramidal neurons in the CA1 region of the hippocampus. The CA1 neurons not only expressed calcium indicators but also expressed a light activated ion channel that was sensitive to a different wavelength of light than the imaging laser, allowing the authors to perform simultaneous holographic stimulation of place cells identified through calcium imaging.
With these powerful tools in hand, Robinson et al.4 asked a straightforward but critically unanswered question: is place cell activity associated with a particular location sufficient to drive memory recall and evoke behaviors associated with that location? Strikingly, Robinson et al.4 demonstrated that, if you stimulate place cells that were endogenously active near a reward at an earlier location on the track, the animal will show behavior associated with the anticipation of a reward, for example slowing its running speed and increasing its licking rate. In another set of experiments, the authors found that stimulating place cells active near the beginning of the virtual reality track just before the reward location resulted in the animal overrunning the reward zone without licking — as if it perceived its location as earlier on the track than it really was.
Of note, despite the technological feat of the paper, Robinson et al.4 were able to successfully stimulate only a relatively small number of cells at the same time (∼45 cells on average). Thus, one surprising result from this study is that the hippocampal circuit can be sensitive to a manipulation of a relatively small number of place cells. However, this technological limitation serendipitously sidesteps a frequent criticism of optogenetic manipulation — that the strength of the manipulation results in a pattern of neural activity that would never occur under normal physiological conditions.
As a further indication of the sensitivity of the hippocampal circuit and its plasticity mechanism, the animals in the Robinson et al.4 study seemed to learn from the stimulations, slowing down in anticipation of the stimulation of reward-associated place cells and a lasting decrease in accurate licking behavior. The stimulation also caused lasting reorganization of the place cell code.
This elegant work now fills a conspicuous gap in the hippocampal literature and provides new evidence of the role of hippocampal activity in behavior and circuit plasticity. The work of Robinson et al.4 also shows that these newly developed sophisticated optogenetic stimulation technologies can be extended to deep tissues in a straightforward manner. This paper opens the door for testing a multitude of hypotheses built from many decades of careful characterization of hippocampal physiology and highlights the need to look downstream of the hippocampus to understand how place cell representations are transformed into motor actions. Moreover, recent work suggests that hippocampal place cell sequences, as well as the remapping of these sequences, are general mechanisms for hippocampal learning and are capable of representing non-spatial variables. This suggests that hippocampal place cells are less of a ‘neural GPS’ and more of a representation of the statistical relationship between different behaviorally relevant states of the world18–20. Interesting future applications of this technology could be to test these emerging theories of the computational roles of place cells.
REFERENCES
- 1.O’Keefe J, and Dostrovsky J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. [DOI] [PubMed] [Google Scholar]
- 2.Scoville WB, and Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens TEJ, Muller TH, Whittington JCR, Mark S, Baram AB, Stachenfeld KL, and Kurth-Nelson Z. (2018). What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509. [DOI] [PubMed] [Google Scholar]
- 4.Robinson NTM, Descamps LAL, Russell LE, Buchholz MO, Bicknell BA, Antonov GK, Lau JYN, Nutbrown R, Schmidt-Hieber C, and Häusser M. (2020). Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell 183, 1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris RG, Garrud P, Rawlins JN, and O’Keefe J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. [DOI] [PubMed] [Google Scholar]
- 6.Moser EI, Moser M-B, and McNaughton BL. (2017). Spatial representation in the hippocampal formation: a history. Nat. Neurosci. 20, 1448–1464. [DOI] [PubMed] [Google Scholar]
- 7.Mallory CS, and Giocomo LM. (2018). Heterogeneity in hippocampal place coding. Curr. Opin. Neurobiol. 49, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, and Magee JC. (2015). Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 18, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D, Lin BJ, and Lee AK. (2012). Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853. [DOI] [PubMed] [Google Scholar]
- 10.Colgin LL, Moser EI, and Moser MB. (2008). Understanding memory through hippocampal remapping. Trends Neurosci. 31, 469–477. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Ramirez S, Pang PT, Puryear CB, Govingarajan A, Deisseroth K, and Tonegawa S. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MA, and McNaughton BL. (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. [DOI] [PubMed] [Google Scholar]
- 13.Jadhav SP, Kemere C, German PW, and Frank LM. (2012). Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickgauer JP, Deisseroth K, and Tank DW. (2014). Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker AM, Russell LE, Dalgleish HWP, and Hausser M. (2014). Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods 12, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshel JH, Kim YS, Machado TA, Quirin S, Benson B, Kadmon J, Raja C, Chibukhchyan A, Ramakrishnan C, Inoue M, et al. (2019). Cortical layer-specific critical dynamics triggering perception. Science 365, eaaw5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid-Carrillo L, Han S, Yang W, Akrouh A, and Yuste R. (2019). Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aronov D, Nevers R, and Tank DW. (2017). Mapping a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachenfeld KL, Botvinick MM, and Gershman SJ. (2017). The hippocampus as a predictive map. Nat. Neurosci. 20, 1643–1653. [DOI] [PubMed] [Google Scholar]
- 20.Plitt MH, and Giocomo LM. (2019). Experience dependent contextual codes in the hippocampus. bioRxiv, 10.1101/864090. [DOI] [PMC free article] [PubMed] [Google Scholar]