Abstract
Previous studies in prosimian galagos (Otolemur garnetti) have demonstrated that posterior parietal cortex (PPC) is subdivided into several functionally distinct domains, each of which mediates a specific type of complex movements (e.g., reaching, grasping, hand-to-mouth) and has a different pattern of cortical connections. Here we identified a medially located domain in PPC where combined forelimb and hindlimb movements, as if climbing or running, were evoked by long-train intracortical microstimulation (LT-ICMS). We injected anatomical tracers in this climbing/running domain of PPC to reveal its cortical connections. Our results showed the PPC climbing domain had dense intrinsic connections within rostral PPC and reciprocal connections with forelimb and hindlimb region in primary motor cortex (M1) of the ipsilateral hemisphere. Fewer connections were with dorsal premotor cortex (PMd), supplementary motor (SMA) and cingulate motor (CMA) areas, as well as somatosensory cortex including areas 3a, 3b and 1–2, secondary somatosensory (S2), parietal ventral (PV) and retroinsular (Ri) areas. The rostral portion of the climbing domain had more connections with primary somatosensory cortex than the caudal portion. Cortical projections were found in functionally matched domains in M1 and premotor cortex (PMC). Similar patterns of connections with fewer labeled neurons and terminals were seen in the contralateral hemisphere. These connection patterns are consistent with the proposed role of the climbing/running domain as part of a parietal-frontal network for combined use of the limbs in locomotion as in climbing and running. The cortical connections identify this action-specific domain in PPC as a more somatosensory driven domain.
Keywords: prosimian, primate, posterior parietal cortex, long-train stimulation, cortical connection, parietal-frontal network, AB_2187552, AB_1502299
Graphical abstract
A domain for combined forelimb and hindlimb movements in posterior parietal cortex (PPC) of galagos was identified. This domain had dense intrinsic connections within PPC and reciprocal connections with frontal and parietal sensorimotor areas of the ipsilateral cortex. It also had connections with the homotopic region of the contralateral cortex.

Introduction
The present study is a part of an ongoing effort to characterize the major features of functionally distinct networks for specific actions that involve small regions (domains) in posterior parietal cortex (PPC) of primates. These domains interact with functionally matched domains in motor and premotor cortex. Our research effort was initially motivated by the discovery by Graziano and coworkers that primary motor cortex (M1) of macaques is divided into a number of small regions that produce different specific actions when electrically stimulated (Graziano et al., 2002, 2005). Previously, the functional organization of M1 was mapped by electrically stimulating many sites in M1 with short bursts of electrical pulses delivered with microelectrodes (e.g., Strick and Preston, 1982; Gould et al., 1986; Nudo et al., 1992, 1996). Such maps revealed an overall crude somatotopic representation of the body parts that were involved in the brief movements, but not the more extensive complex movements that Graziano and coworkers described for sites in motor cortex as a result of longer 500 ms trains of electrical pulses. These more complex movements suggested that motor cortex is subdivided into a number of small regions or domains that directly mediate ethologically relevant behaviors such as bringing the hand to the mouth, reaching to grasp, defending the head from a blow and “climbing”. The region where stimulation produced movements resembling “climbing” was unusual, in that the electrical stimulation evoked bilateral movements of forelimbs and hindlimbs (Graziano et al., 2005; Graziano and Aflalo, 2007). In addition, those sites that evoked climbing movements appeared to be in the supplementary motor area (SMA), while other sites that evoked complex movements of the face or forelimbs were in M1 or premotor cortex (PMC). Our interest here was in further exploring the cortical circuits involved in complex movements of the combination of forelimbs and hindlimbs. In our experiments, the movements could be characterized as “climbing”, “jumping” or “running”, as it was difficult to classify these movements as completely natural as they were evoked in anesthetized primates with the head fixed in a head holder, and the limbs were limited somewhat in what movements were possible.
Our previous studies of action-specific networks in cortex have involved parts of PPC, M1 and PMC in macaques, owl monkeys, squirrel monkeys and prosimian galagos (Gharbawie et al., 2011a, 2011b; Stepniewska et al., 2005, 2009a, 2009b, 2011, 2014). The results suggested that these primates have as many as eight or more functionally distinct cortical networks for evoking different behaviors. These networks have been most extensively investigated in galagos for the reason that they have relatively smooth cortex without a central fissure, so that more of the relevant cortex is exposed on the surface of the brain. Our studies revealed an array of action-specific domains in PPC in a roughly somatotopic pattern. The most medial stimulation sites evoked hindlimb movements, with progressively more lateral sites evoking combined movements of the forelimb and hindlimb, reaching, defensive movements of the forelimb, hand to body, grasping, and most laterally, defensive or aggressive movements of the face as well as eye movements were evoked (Figure 1). Domains for most classes of movements were found in roughly the same mediolateral order in M1 and PMC. Domains in PPC were found to selectively project to functionally matched domains in M1 and PMC (Stepniewska et al., 2009a). In addition, optical imaging experiments showed that stimulation of PPC domains only activated functionally matched PMC and M1 domains (Stepniewska et al., 2011), and muscimol inactivation results showed that inactivation of PMC and M1 domains suppressed functionally matched PPC domains while activities of unmatched PPC domains were preserved (Stepniewska et al., 2014). These observations support the proposed existence of parallel parietal-frontal networks for specific actions in primates (Kaas and Stepniewska, 2016).
Figure 1.
A dorsolateral view of the left cerebral hemisphere of a galago showing the location of specific movement domains identified by long-train microstimulation in areas M1, PMC, and PPC. Corresponding domains in these areas are marked with the same color. HL/FL, climbing; D, defensive; R, reaching; HB, hand to body; G, grasping; F, face movements; E, eye movements. V1, primary visual area; V2, secondary visual area; DM, dorsomedial visual area; DL, dorsolateral visual area; FEF, frontal eye field; 3b, primary somatosensory area; 3a, somatosensory area 3a; 1–2, somatosensory areas 1 and 2; A, auditory cortex; IT, inferiortemporal cortex; MT, middle temporal cortex; PMD, dorsal premotor area; PMV, ventral premotor area; PMC, premotor cortex; PPCr, rostral posterior parietal cortex; PPCc, caudal posterior parietal cortex; SMA, supplementary motor area; M1, primary motor cortex. FSa, anterior frontal sulcus; FSp, posterior frontal sulcus; LS, lateral sulcus; STS, superior temporal sulcus; IPS, intraparietal sulcus. Adapted from Figure 5A in Kaas and Stepniewska (2016).
The goals of the present study were to define and characterize the regions of parietal and frontal cortex where coordinated movements of forelimbs and hindlimbs can be evoked, and determine the connections of the forelimb/hindlimb region of PPC with other cortical areas. Features of the forelimb/hindlimb domain have been only briefly described (Stepniewska et al., 2005, 2009b), and the connections of this domain have not been explored.
Materials and Methods
Four adult galagos (Otolemur garnetti) were examined in this study. We used long-train intracortical microstimulation (LT-ICMS) to evoke movements of the limbs from PPC. In order to reveal cortical connections, anatomical tracers were injected into locations of PPC where combined forelimb and hindlimb movements were evoked. After the tracers being transported to neurons across cortex, the parietofrontal region was mapped more extensively to reveal parts of PMC and M1 where movements of forelimbs and hindlimbs together could be evoked. All experimental procedures followed the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health and were approved by the Animal Care and Use Committee of Vanderbilt University.
Surgery, ICMS mapping and tracer injection
Surgical procedures were carried out under aseptic conditions with the animal’s vital signs including respiration rate, blood pressure, expiration CO2, arterial O2 saturation and body temperature being monitored throughout the procedure. Each galago was anesthetized with an initial intramuscular injection of ketamine hydrochloride (20–40 mg/kg) and maintained with isoflurane (1–2%) delivered through a tracheal tube during the surgical procedure. After the animal was fixed on the stereotaxic apparatus, craniotomy and durotomy were performed in one hemisphere to expose the region of PPC around the intraparietal sulcus (IPS). The opening extended rostrally to the posterior frontal sulcus (FSp) of motor cortex and dorsally to the midline. The cortical surface was photographed and printed to allow documentation of the sites of microelectrode placement and injections of tracers.
When proceeding to the ICMS stage during the survival surgery, the anesthesia was switched to the intravenous administration of ketamine (20–40 mg/kg/hour) supplemented with intramuscular injections of xylazine (0.2–0.4 mg/kg) to maintain the galago at a stable surgical level. A short 40–60 minutes session of LT-ICMS was then used to delineate the domain in PPC where combined forelimb and hindlimb movements contralateral to the simulation sites were evoked. Stimuli were generated by a Master 8 stimulator (A.M.P.I, Jerusalem, Israel) with a biphasic stimulus isolator (Bak Electronics Inc., Umatilla, FL), characterizing as 300Hz, 500 ms trains of 0.4 ms biphasic electrical pulses. A low-impedance (1 MΩ) tungsten microelectrode tilted at a 10° angle from the vertical was used to penetrate the cortex perpendicularly to deliver the current pulses at a depth of 1.8–2.2 mm below the pial surface where the cortical layer V is located.
Anatomical tracers (cholera toxin subunit B, CTB used for retrograde tracing; fluoror-uby, FR and biotinylated dextran amines, BDA used for bidirectional tracing) were then injected into the electrophysiologically defined forelimb/hindlimb domain in PPC. 0.5 μL of CTB (1% in distilled water, Sigma-Aldrich, St. Louis, MO) was injected in each of three cases (17–17 LH, 18–03 LH, 18–06 LH), and 0.4 μL of CTB was injected in case 18–19 LH. In addition, 0.5 μL of FR (10% in distilled water, MW 3k and 10k, Invitrogen, Carlsbad, CA) and a total volume of 1 μL of BDA (10% in distilled water, MW 3k and 10k, Invitrogen) were injected in case 17–17 LH and case 18–19 LH respectively. All injections were made with Hamilton syringes at two depths, 1.6 mm and 1 mm, below the pial surface to include the whole cortical thickness in the injection site. The exposed cortex was then covered with gelfilm and the opening in the skull was covered with a thin piece of hard dental cement that was fixed to the skull before suturing the skin. The galagos were given an injection of antibiotics and closely monitored as they recovered from the anesthesia. After full recovery, they were returned to home cages.
After a two-week period for the transportation of the tracer to neurons in cortex, a terminal ICMS session was performed to obtain extensive functional maps of PPC, M1 and PMC regions where combined forelimb and hindlimb movements could be evoked with the electrode placement at an interval of 400–800 μm. The applied current amplitude was initially determined based on previous studies (Stepniewska et al., 2005, 2009b), and was adjusted accordingly to elicit visible movements or measure the current threshold. Amplitudes up to 300 μA were applied, beyond which the stimulation site was considered to be non-excitable if no movement was evoked. Two or three experimenters that were blind to the location of microelectrode placement were present to observe and document the movements. To mark the functional boundaries of cortical areas, microlesions were made at selected locations by passing 10 μA direct current via the lesion making device (Ugo Basile SRL., Gemonio, Italy) during the electrode withdrawal from the depth of 2 mm below the pial surface. All penetration sites and electrolytic lesions were marked on photographs of the operated cortex.
Perfusion and tissue processing
At the end of the terminal experiments, galagos were administered a lethal dose of sodium pentobarbital (120 mg/kg), and then perfused intracardially with 0.9% phosphate-buffered saline (PBS), followed by 2% paraformaldehyde in phosphate buffer (fixative) and 10% sucrose in fixative. Brains were removed from the skull. Left and right cortices were then separated from subcortical structures and manually flattened to unfold sulci before being squeezed between two glass slides. After being post fixed in the same fixative for two hours, the flattened tissues were transferred into 30% sucrose solution for cryoprotection and stored at 4°C overnight for microtomy.
Cortices were sectioned in parallel to the pial surface at a thickness of 40 μm and saved in four series. One or two series were stained for cytochrome oxidase (CO; Wong-Riley, 1979), myelinated fibers (Gallyas, 1979), or vesicular glutamate transporter 2 (VGluT2; Balaram et al., 2013) to reveal cortical architectures, particularly the primary visual (V1), somatosensory (area 3b) and auditory (A1) areas. Other series were mounted, air-dried and coverslipped directly for fluorescence microscopy, or were processed to reveal CTB (Angelucci et al., 1996; Liao et al., 2013) or BDA (Veenman et al., 1992) labeled profiles. In cases for BDA-CTB double staining, sections were first processed for BDA followed by CTB to visualize labeling of both tracers in different colors in the same section. In order to visualize FR labeling profiles under the brightfield microscope, one series of the sections was immunoreacted as described in Chang (1993) with modifications. In brief, the sections were first rinsed in 0.5% Triton containing 0.01 M PBS (pH 7.4, washing solution) and immersed in a blocking solution (0.5% Triton and 5% normal goat serum in 0.01 M PBS) for 1 hour, followed by being incubated in a 1:1000 dilution of the primary antibody (rabbit anti-tetramethylrhodamine, Invitrogen) in the blocking solution at 4°C for two nights. Sections were repeatedly rinsed in washing solution, and then immersed in a secondary antibody solution of biotinylated goat anti-rabbit IgG (BA-1000, Vector Laboratories Inc, Burlingame, CA) that was diluted at 1:300 in the same blocking solution for 1.5 hours at room temperature. After three washes, the sections were processed with a standard avidin-biotin complex (ABC) reaction. Sections were incubated for 1.5 hours in the ABC solution (PK-4005, Vector Laboratories) that was made at a ratio of 1 drop of A and 1 drop of B for every 7 mL 0.01 M PBS, and then washed and incubated for 10 minutes in a 3,3’-diaminbenzidine tetrahydrochloride (DAB) solution (50 mg DAB in 100 mL 0.01 M PBS) containing 0.03% nickel ammonium sulfate. Finally, 30% hydrogen peroxide solution was added into the DAB solution at a ratio of 1:10000 to visualize the FR labeling, which were shown in brown color.
Antibody characterization
The primary antibodies used in the present study were mouse anti-VGluT2 (AB_2187552; monoclonal; cat. no. MAB5504; Millipore, Burlington, MA) in a concentration of 1:5000 for VGluT2, and rabbit anti-tetramethylrhodamine (AB_1502299; polyclonal; cat. no. A6397; Invitrogen, Carlsbad, CA) in a concentration of 1:1000 for FR. The VGluT2 antibody was tested against galago brain tissue using standard western blot techniques and showed a single band at 56 kDa (Baldwin et al., 2013).
Data analysis and anatomical reconstruction
A Zeiss Axio Imager 2 microscope (Carl Zeiss AG, Oberkochen, Germany) coupled to a Dell Precision T3610 workstation (Dell Inc., Round Rock, TX) running Stereo Investigator version 2018 software (MBF Bioscience, Williston, VT) was used to plot the injection sites and labeling. For each case, four or five sections with the fixed interval across the superficial, middle, and deep cortical layers were selected for plotting. Retrogradely labeled neurons and anterogradely labeled axonal terminals were marked and outlined at the 10x power and subsequently verified at the 20x power. Sections of architectural staining were scanned with a HP Photosmart printer C4780 (HP Inc., Palo Alto, CA) and adjusted only for contrast and brightness with Adobe Photoshop CS5 (Adobe Inc., San Jose, CA). Plots were then imported into Adobe Illustrator CS5 (Adobe Inc., San Jose, CA) to be compiled and aligned with the architectural images based on blood vessels and electrolytic lesions. Identification of architectonic borders of cortical regions followed those of previous studies (Preuss and Goldman-Rakic, 1991; Wu and Kaas, 2003; Wong and Kaas, 2010). In cases where these areas were architechtonically indistinct, areal borders were estimated based on measurements from other cases and outlined with dashed lines. Heatmaps of the cortical distributions of labeled neurons were created with a Python script (https://github.com/kaaslabqw/neuron-densityanalysis) that was designed to calculate and visualize the density of distribution of labeled neurons.
Results
The results are presented in two parts. First, we briefly describe the microstimulation results, including the general characteristics of the evoked movements of forelimb and hindlimb combination, and profile of the cortical domains in PPC, PMC and M1 where stimulation sites evoking such movements formed clusters. Second, we show the patterns of intracortical and interhemispheric connections of the PPC domain that was devoted to combined forelimb and hindlimb movements as revealed by tracer injections in detail.
Climbing movements and the climbing domains
Combined forelimb and hindlimb movements evoked by LT-ICMS were recognized in experiments as complex movements that involved multiple body parts and were coordinated across multiple joints during most or all of the stimulation period. These movements were characterized as some combinations of flexion or extension of the elbow, shoulder, hip or knee causing retraction or protraction of the forelimb and hindlimb contralateral to the stimulated side. In 2 stimulation sites, bilateral limb movements were observed. However, as we focused on monitoring contralateral limb movements, it is likely that ipsilateral and bilateral movements might have occurred at more sites. Although some movement components (e.g., forelimb downward pushing or hindlimb kicking) might be missed due to the restraint of the animal in the stereotaxic apparatus, the combined limb movements that were observed in the experiments resembled those of an animal trying to climb, jump or run. The key feature of these movements was the involvement of both the forelimb and hindlimb, different from previously reported or observed forelimb reaching, defensive movements, grasping or hindlimb movements. We therefore viewed them as a distinct class of ethologically relevant actions and termed them “climbing movements” attempting to be consistent with early classifications of Graziano (2009).
Climbing movements were observed from 203 out of 599 stimulation sites (33.9%) in PPC, M1 and PMC across four animals, with no systematic differences in movement components or sequences observed among those movements elicited from different cortical areas. In 12 sites, climbing movements were also accompanied by rotation of the contralateral wrist or ankle, flexion or extension of the contralateral digits or toes, or twitch of the trunk. Threshold values for consistently evoking climbing movements were usually much higher in PPC (104 sites across 4 individuals, median = 120 μA, mean = 121.54 μA) than those in M1 (56 sites across 3 individuals, median = 30 μA, mean = 36.25 μA). Variations of threshold values in the same cortical area were possibly resulted from differences in anesthesia levels.
The stimulation sites from which climbing movements were evoked tended to cluster to form a domain in PPC, identified here as the PPC “climbing domain”. The PPC climbing domain was situated 2.2–2.8 mm above the IPS, extending 3–5 mm rostrocaudally and 1–2 mm dorsoventrally in general (larger extension in cases 17–17 LH and 18–19 LH than cases 18–03 LH and 18–06 LH). It was bordered ventrally by forelimb-movement-alone domains (defense, reaching or hand-to-body; see Stepniewska et al., 2005) and bordered dorsally by hindlimb-movement-alone domains (hindlimb flexion or extension or foot rotation; Figure 2). This arrangement was recognized not only in PPC, but similarly in M1 and PMC of all examined cases. A matching climbing domain was delineated above the anterior frontal sulcus (FSa), sandwiched between hindlimb-movement-alone domains and forelimb-movement-alone domains, as shown in cases 17–17 LH and 18–06 LH (Figures 2a-b, e-f). Our mapping results conform to those of Stepniewska et al. (2009b) in that the rostral portion of PPC (PPCr) in galagos is functionally organized in a roughly somatotopic fashion, with domains involved in hindlimb movements located more medially and domains involved in forelimb movements more laterally.
Figure 2.

The location of specific movement domains as defined by long-train microstimulation in the left hemisphere (LH) of frontal (a) and parietal cortex (b) of galago 17–17, parietal cortex of galagos 18–03 (c) and 18–19 (d), and frontal (e) and parietal cortex (f) of galago 18–06. Stimulation sites are color-coded according to the type of evoked movements. Sites where more than one type of movement was observed during the same stimulation train are marked with multi-color concentric symbols. Clusters of the same symbol are shaded in the corresponding color, roughly depicting the specific movement domains. Cortical areas that represent limb and trunk movements are arranged in a crude somatotopic fashion in PPC, M1 and PMC. Representations of combined forelimb and hindlimb movements are sandwiched between more medially located representations of hindlimb-only movements and more laterally located representations of forelimb-only movements. Injection cores of anatomical tracers are outlined. Dashed lines mark contours of sulci and estimated areal boundaries. For abbreviations see list. Scale bars = 1 mm.
Overall, as a class of behaviorally distinct movements, climbing movements, characterized by combined retraction or protraction of both forelimb and hindlimb of the contralateral side, were evoked by LT-ICMS in PPC, M1 and PMC. The cortical representations of the climbing movements clustered into domains that fit somatotopically in the motor maps of parietal and frontal cortex.
Connection patterns of the PPC climbing domain
Tracers were injected into the defined climbing domain in PPC to reveal intracortical and interhemispheric connections. The locations of injection sites in relation to the electrophysiologically identified movement domains in each case are shown in Figure 2. Examples of the injection sites, architectonic boundaries as well as labeled neurons and axonal terminals are shown in Figure 3. Overall distributions of labeled neurons and axonal terminals in eight cortical hemispheres are presented in surface views of the manually flattened cortices in Figures 4–14.
Figure 3.
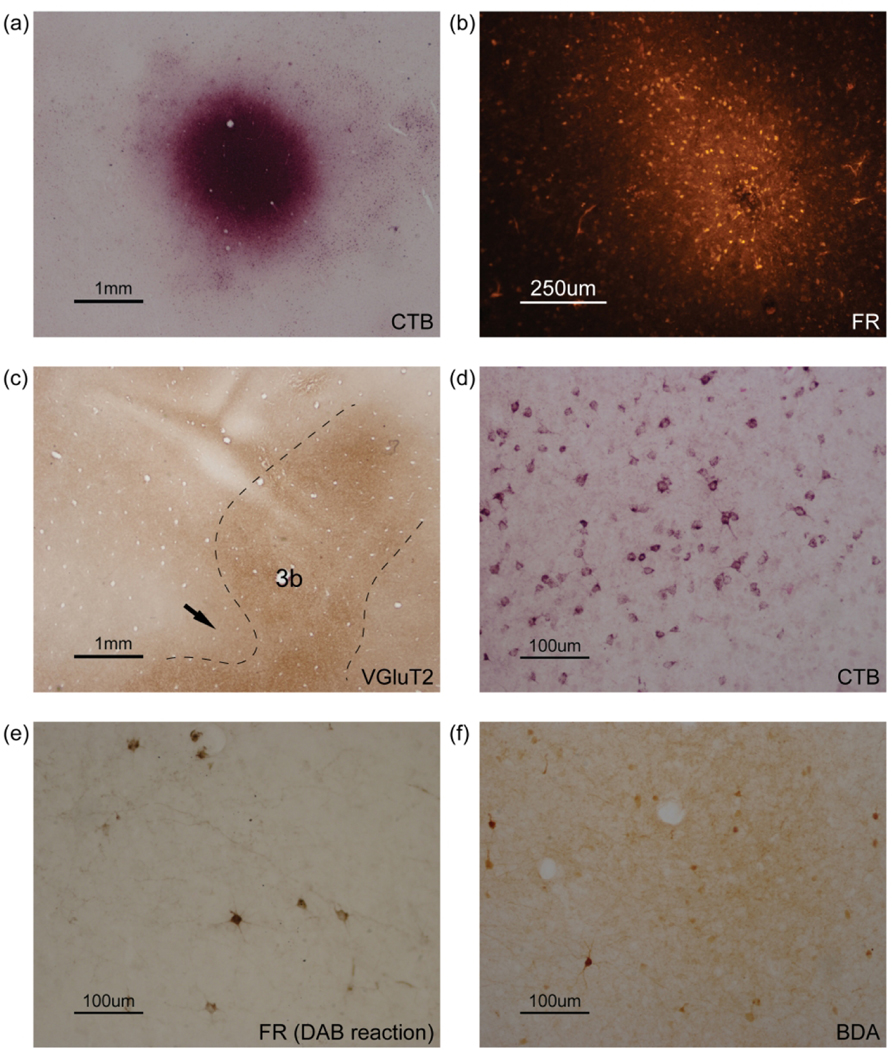
Photomicrographs of flattened cortical sections showing (a) CTB and (b) FR injection sites of case 17–17 LH. (c) Photomicrograph of a flattened cortical section immunoreacted for vesicular glutamate transporter 2 (VGluT2) showing area 3b of case 18–03. Dashed lines mark estimated boundaries of area 3b. The arrow indicates the presumptive hand-face border. Photomicrographs of flattened cortical sections showing (d) CTB-labeled neurons of case 18–06 LH, (e) FR-labeled neurons and sparse terminals of case 17–17 LH and (f) BDA-labeled neurons and dense terminals of case 18–19 LH.
Figure 4.
Distribution of labeled neurons (a) and a heat map of labeled neuron density (b) after a CTB injection in the PPC climbing domain of case 18–06 LH, shown in flattened cortex. (a) The injection site is outlined with black and filled with blue. Each blue dot indicates the location of a labeled neuron. Estimated areal boundaries drawn from histological results are indicated by thin lines, while those drawn from physiological results and measurements are indicated by dotted lines. Thick gray dashed lines mark the dorsal conjunction with the medial wall. Region of torn tissue marked in black. (b) In the heat map, the lighter color indicates higher density of labeled neurons. The injection core is marked in white. CTB-labeled neurons are extensively distributed in frontal and parietal cortex, with the highest density adjacent to the injection site, and in dorsal frontal motor areas and caudal somatosensory areas of the LS. M, medial; R, rostral. For abbreviations see list.
Figure 14.
Distributions of BDA-labeled axonal terminals of case 18–19 RH (a) and FR-labeled axonal terminals of case 17–17 RH (b), shown in flattened cortex. In both cases labeled terminals are focused in regions homotopic to the injection sites of LH. Other conventions as in Figure 4.
Determination of the areal borders
Areal borders of the cortex were defined by physiological and architectonic criteria. Physiologically, values of the threshold current were lowest for M1 (typically below 40 μA) while highest for PPC (up to 300 μA). Architectonically, primary sensory areas were easier to distinguish than other areas in the flattened sections stained for CO, myelinated fibers and VGluT2. In all of these preparations, the primary somatosensory area 3b stood out as a dark stripe extending mediolaterally and curving rostrally (Figure 3c), as previously reported (Wu and Kaas, 2003; Wong and Kaas, 2010). Anteriorly adjoining area 3a and posteriorly adjoining area 1–2 were usually lighter in color. The IPS, FSa and FSp were apparent in the flattened sections as depressions in some cases, which were used as landmarks for PPC and M1 respectively. Among other areas, V1 was easily distinguished by blobs in CO and VGluT2 preparations (Rockoff et al., 2014). A1 and the middle temporal visual area (MT) in some cases were recognized by dark patches outstanding from surrounding areas (Lyon and Kaas, 2002; Kaskan and Kaas, 2007; Wong and Kaas, 2010).
Cortical connections of the ipsilateral hemisphere
Eight tracer injections used for retrograde and anterograde labeling were made in different locations of the PPC climbing domain of four galagos.
Case 18–06 LH
A CTB injection was placed in the center of the delineated climbing domain in PPC (Figure 2f). The injection core involved most of the climbing domain while extending somewhat laterally into the forelimb defense domain and medially into the hindlimb movement domain. The plot details the locations of labeled neurons (Figure 4a), and the heat map reflects the density of distributions of labeled neurons (Figure 4b).
Dense distributions of labeled neurons were found around the injection site, suggesting strong intrinsic connections within PPC. Other dense patches of neurons were found along the upper bank of the lateral sulcus (LS), where higher-order somatosensory areas including secondary somatosensory (S2), parietal ventral (PV) and presumptive retroinsular (Ri) areas were located (Wu and Kaas, 2003). Compared to areas 1–2 where numerous neurons were labeled, areas 3b and 3a contained much fewer labeled neurons, most of which were distributed in the dorsomedial portion of the region. A great number of neurons were also labeled in the dorsal and medial frontal cortex, most likely the limbs and trunk representations of M1, dorsal premotor (PMd) and supplementary motor areas (SMA). Several foci of labeled neurons were present in the caudal portion of cingulate cortex (CgC) and cingulate motor areas (CMA), as well as in ventral M1 and ventral premotor area (PMv) below the level of FSa-FSp. Other labeled neurons were in prefrontal cortex (PFC). Sparse distributions of labeled neurons were observed in the caudal portion of PPC (PPCc), dorsal third visual (V3d), dorsomedial visual (DM) areas and retrosplenial (RS) cortex (Wong and Kaas, 2010). A few neurons were scattered in MT, inferior temporal cortex (IT), A1 and surrounding regions. V1, on the other hand, was free of label.
Distributions of CTB-labeled neurons in M1, PMC and PPC in relation to movement domains that defined by LT-ICMS are also illustrated in this case (Figure 5). Labeled neurons spread into hindlimb movement, climbing, defensive and hand-to-body domains of PPC (Figure 5c). In the frontal cortex, a great number of labeled neurons fell into the matching climbing domain, as well as hindlimb movement and hand-to-body domains. Less but still numerous labeled neurons were seen in the defensive domain of PMC and M1 (Figure 5b).
Figure 5.
Reconstruction of the movement map and the label distribution of case 18–06 LH shown in flattened cortex, with photomicrographs of electrophysiologically mapped regions superimposed (a). The alignment of delineated movement domains and identified CTB-labeled neurons in frontal sensorimotor areas (b) and PPC (c) are illustrated. The core of the CTB injection is outlined in panel (a) and filled in panel (c).
Case 18–19 LH
CTB and BDA were injected 4 mm apart rostrocaudally in the climbing domain of PPC (Figures 2d, 6a). The connection pattern revealed by the CTB injection placed in the caudal part of the climbing domain was similar to that of case 18–06 LH (Figures 6a, 6c, 4). Most CTB-labeled neurons were found in the PPC region surrounding the injection site with a higher density in the dorsomedial portion than the ventrolateral portion, suggesting stronger intrinsic connections with the representations of limb and trunk movements than those of face movements. Considerable numbers of labeled neurons concentrated in the frontal motor areas (the dorsal region of M1, PMd and SMA), as well in CMA and the caudal portion of CgC. PFC and PMv also contained some small patches of labeled neurons. Numerous neurons were labeled in the higher-order somatosensory areas of the LS including S2, PV, VS, the parietal rostral area (PR) and Ri, however, very few neurons were labeled in the somatosensory areas 3a, 3b and 1–2. In this case, we found a cluster of CTB-labeled neurons in the sections of deep layers of the rostroventral part of the frontal cortex, likely corresponding to the orbital frontal (OF) and claustral (CLI) areas of Wong and Kaas (2010). In addition, sparse cells were labeled in the dorsomedial extrastriate visual areas including the dorsal second area (V2d) and V3d, RS and IT.
Figure 6.
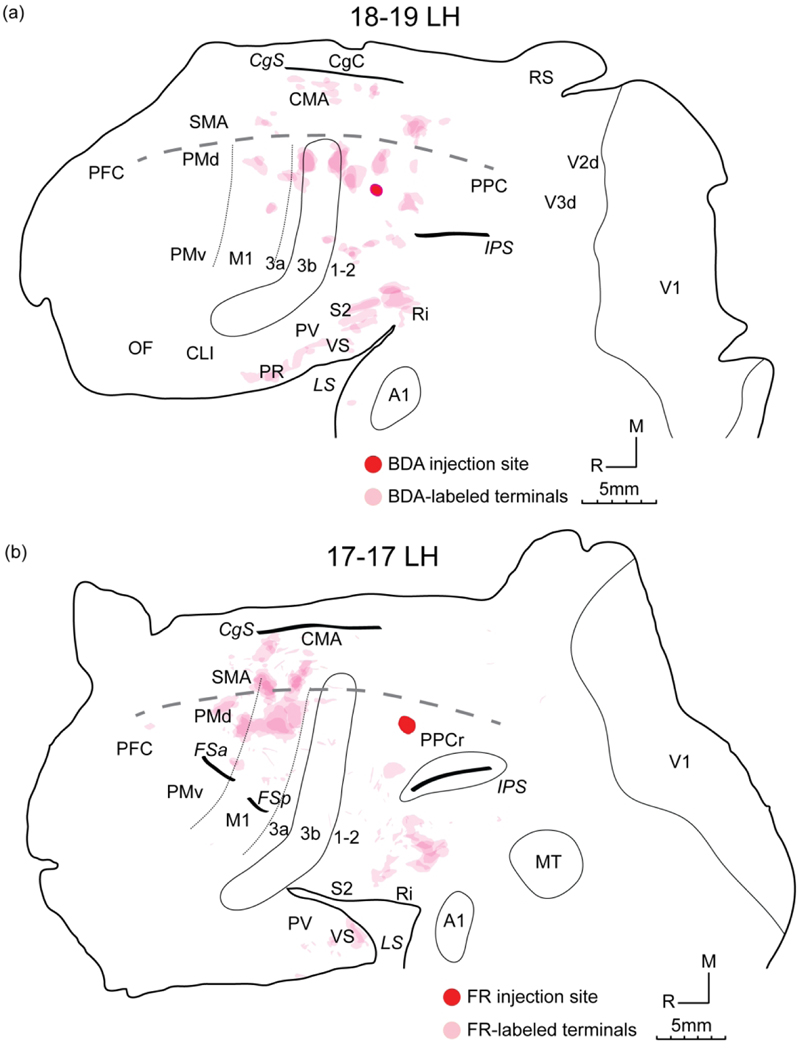
Distributions of CTB and BDA labeling (a), and heat maps of BDA- (b) and CTB-labeled neuron densities (c) after more rostral BDA injections and a more caudal CTB injection in the PPC climbing domain of case 18–19 LH, shown in flattened cortex. (a) The BDA injection core is outlined with white and filled with red. Identified BDA-labeled axonal terminals are shaded in light pink (see Figure 7a for only labeled terminal foci). BDA-labeled neurons are most densely distributed near the injection site. Many CTB-labeled neurons are distributed in frontal and parietal cortex with a higher density around the CTB injection site. Other conventions as in Figure 4.
Three focal BDA injections close to each other were placed in the most rostral part of the climbing domain (Figures 2d, 6a), forming an injection core at the approximate border of PPCr and area 1–2. The BDA injection produced a more restricted distribution of labeled neurons compared to that of the CTB injection in the same case (Figure 6b-c). BDA-labeled neurons were primarily concentrated around the injection site, while extending rostrally to the dorsal regions of areas 1–2, 3b and 3a. A relatively dense cluster of neurons were labeled in the medial portion of PPCr. Sparsely scattered neurons were also labeled in the dorsal portion of M1, SMA, CMA and CgC. More ventrally, some patches of labeled neurons were observed in areas 1–2 and 3b at the approximate level of the IPS, supposedly above the hand-face border (Kaas et al., 2006; Wong and Kaas, 2010). Many neurons were also labeled in S2, PV, VS and Ri. This injection may have extended slightly into a climbing domain in area 1–2 (see Gharbawie et al., 2011b for a grasping domain in area 2).
Anterograde labeling from the BDA injection was also acquired from this case (Figures 6a, 7a). Major patches of dense labeled terminals were seen in the dorsal portion of areas 3a, 3b, 1–2 and PPCr. Several patches were present in the ventral portion of areas 3b and 1–2 as well. BDA-labeled terminal fields were also observed in CMA and somatosensory areas of the LS (S2, PV, VS and Ri). In addition, a few small patches of terminals were labeled in the dorsal region of M1. The overlapping distribution of labeled cells and axonal terminals revealed reciprocal connections between the PPC climbing domain and multiple motor and somatosensory areas, including the climbing domain in frontal cortex.
Figure 7.
(a) Distribution of BDA-labeled axonal terminals after BDA injections in the rostral portion of the PPC climbing domain of case 18–19 LH, shown in flattened cortex. Labeled terminals are primarily throughout dorsal parieto-frontal areas, along the CgS and the upper bank of the LS. (b) Distribution of FRlabeled axonal terminals after a FR injection in the caudal portion of the PPC climbing domain of case 17–17 LH, shown in flattened cortex. Labeled terminals are primarily in dorsal and medial frontal motor areas and caudal somatosensory areas of the LS. Other conventions as in Figure 4.
Case 17–17 LH
Two different tracers were injected into the delineated climbing domain, with CTB placed rostrally and FR placed caudally (Figures 2b, 8a). The core of CTB injection involved the PPC climbing domain and the caudal portion of area 1–2. A mass of CTB-labeled neurons extended rostrocaudally throughout PPCr, areas 1–2, 3b, 3a, M1 and the caudal part of PMd. A wide and dense band of labeled neurons occupied the dorsal portion of aforementioned regions, likely corresponding to the representations of hindlimbs and trunk, while a narrower band was identified at the approximate level of the IPS-FSa, likely to be the representation of forelimbs (Sur et al., 1980). Numerous labeled-neurons were distributed on the medial wall of the parietal-frontal sensorimotor cortex including the medial region of PPCr, CMA and SMA, the caudal portion of the CgC, and the upper bank of the LS including S2, PV, VS and Ri. Few small foci were also present in PMv, ventral M1 and PFC. Occasionally, labeled neurons were found in the caudal portion of PPC, the extrastriate visual area, MT and IT (Figure 8b).
Figure 8.
Distributions of CTB and FR labeling (a), and heat maps of CTB- (b) and FR-labeled neuron densities (c) after a more rostral CTB injection and a more caudal FR injection in the PPC climbing domain of case 17–17 LH, shown in flattened cortex. (a) The FR injection core is outlined with white and filled with red. Identified FR-labeled axonal terminals are shaded in light pink (see Figure 7b for only labeled terminal foci). FR-labeled neurons are densely distributed in the dorsal portion of frontal and parietal cortex, as well as in the upper bank of the LS. Regions of high density of CTB-labeled neurons largely overlap those of FR-labeled neurons. Other conventions as in Figure 4.
The FR injection was located 2 mm caudal to the CTB injection with a smaller core, yet the distribution of FR-labeled neurons largely overlapped that of CTB-labeled neurons, only fewer in number (Figures 8a, 8c). FR-labeled terminals were concentrated in the dorsal and medial regions of M1, PMd, SMA, CMA where numerous labeled cells were also observed. The dorsal region of areas 3a, 3b, 1–2, PPC, and presumptive VS and Ri regions contained labeled terminals as well (Figure 7b).
In relation to LT-ICMS mapping results (Figure 9), more CTB- and FR-labeled neurons fell into the frontal climbing domain compared to those found in frontal hindlimb movement and defensive domains (Figure 9b). Plenty of FR-labeled terminals were identified in the frontal climbing and hindlimb movement domains, while only few were found in the frontal defensive domain (Figure 9c).
Figure 9.
Reconstruction of the movement map and the label distribution of case 17–17 LH shown in flattened cortex, with photomicrographs of electrophysiologically mapped regions superimposed (a). The alignment of delineated movement domains and identified CTB- and FR-labeled neurons (b) and FR-labeled terminals (c) in frontal sensorimotor areas, as well as all labels in PPC (d) are illustrated, respectively. The cores of the CTB and FR injections are outlined in panel (a) and filled in panel (d).
Case 18–03 LH
The CTB injection of this case involved the climbing domain and parts of the hindlimb movements and forelimb defense domains in PPC (Figures 2c, 10a). A dense, rostrocaudally expanded stripe of labeled neurons was observed across the dorsal region of PPCr, areas 1–2, 3b, 3a and M1 where the representations of hindlimbs, trunk and forelimbs were presumptively located. Some dense patches were also found in the medial parts of PPCr, areas 3b, 3a, SMA and CMA. Labeled neurons with a slightly lower packing density were present in PV, S2, VS and Ri along the upper bank of the LS. Scattered neurons were labeled in PMd, PMv, CgC, as well as in putative OF, CLI and IT areas. Density analysis of the labeled neurons provided further evidence for strong intrinsic connections within PPC, as well as connections between PPCr and parietal-frontal sensorimotor areas (Figure 10b). Overall, the results from this case were similar to those from the other three cases.
Figure 10.
Distribution of CTB-labeled neurons (a) and a heat map of labeled neuron density (b) after a CTB injection in the PPC climbing domain of case 18–03 LH, shown in flattened cortex. CTB-labeled neurons are concentrated in dorsal and medial portions of frontal and parietal cortex and the upper bank of the LS. Other conventions as in Figure 4.
In summary, the PPC climbing domain received ipsilateral cortical connections mainly from the adjacent PPC regions, the dorsal portion of frontal motor areas and higher-order somatosensory areas of the LS. We noticed that the rostral portion of the climbing domain had more connections with the primary somatosensory cortex than the caudal part. The PPC climbing domain also projected to dorsal parietal-frontal sensorimotor areas and somatosensory areas of the LS, which demonstrated the existence of reciprocal connections between the PPC climbing domain and these cortical regions.
Callosal connections of the contralateral hemisphere
We identified greatly reduced numbers and distributions of labeled neurons and axonal terminals contralateral to the injected hemisphere, which demonstrated a restricted pattern of callosal connections for the climbing domain in PPCr.
Case 18–03 RH had a CTB injection primarily in the PPC climbing domain of the left hemisphere. In the right hemisphere, a prominent patch of labeled neurons was located above the rostral portion of the IPS, occupying the dorsal part of area 1–2 and PPCr, homotopic to the contralateral injection site (Figure 11a-b). Some labeled neurons were identified in CgC and CMA, as well as in Ri, S2 and PV of the LS. Labeled neurons were also scattered in the dorsal portion of M1, 3a and 3b, with slight invasion into dorsal PMC. Case 18–06 RH showed a similar labeling pattern in the hemisphere contralateral to the CTB injection site, with more neurons labeled in the dorsomedial portion of PPCr (Figure 11c-d).
Figure 11.
Distributions of CTB-labeled neurons (a, c) and heat maps of labeled neuron density (b, d) of cases 18–03 RH and 18–06 RH. Both are obtained from the right hemispheres (RH) contralateral to injected hemispheres, shown in flattened cortex. Corresponding injection sites of each case are shown in the insets respectively. In both cases, high densities of CTB-labeled neurons are in the regions homotopic to the injection sites of LH, as well as in the dorsal portion of frontal and parietal cortex and the caudal portion of the LS. Other conventions as in Figure 4.
In case 17–17 RH, the distribution of CTB-labeled neurons in the uninjected hemisphere was comparable but sparse to that of the injected hemisphere (Figures 8a-b, 12a, 12c). A major focus of CTB-labeled neurons was located in the dorsal region of area 1–2, nearly matching the location of the injection core in the opposite hemisphere. Some neurons were labeled across the expected representations of forelimbs and hindlimbs of areas 3b, 3a and M1. Additional foci of CTB-labeled neurons were found rostral to the IPS, and in Ri and S2/PV of the LS, with few neurons spreading to more ventral areas. FR-labeled neurons were fewer and less densely packed than CTB-labeled neurons in the same case, while following the same restricted pattern (Figure 12b).
Figure 12.
Distributions of CTB and FR labeling (a), and heat maps of CTB- (b) and FR-labeled neuron densities (c) of case 17–17 RH, shown in flattened cortex. Corresponding injection sites are shown in the inset. See Figure 14b for illustration of only FR-labeled terminals. Other conventions as in Figure 4.
In case 18–19 RH (Figure 13), several small foci of BDA-labeled neurons were found in the dorsal portion of PPCr that matched the BDA injection sites in the other hemisphere. A few neurons were also labeled in the dorsal portion of area 3b, the upper bank of the LS and CgC. CTB-labeled neurons were present primarily in the dorsal and medial aspects of PPCr, corresponding to the CTB injection site in the opposite hemisphere. Other cortical areas such as Ri, S2, PV, CLI, PMC, CgC and the presumable auditory belt area (Wong and Kaas, 2010) contained a few CTB-labeled neurons.
Figure 13.
Distributions of CTB and BDA labeling (a) and a heat map of CTB-labeled neuron density (b) of case 18–19 RH, shown in flattened cortex. Corresponding injection sites are in shown in the inset. See Figure 14a for illustration of only BDA-labeled terminals. Other conventions as in Figure 4.
Distributions of labeled axonal terminals in the uninjected hemispheres are also illustrated in cases 18–19 RH and 17–17 RH (Figure 14), which are similar in overall pattern. The PPC climbing domain projected mostly to the matching regions of contralateral PPCr and area 1–2, as well to CgC, Ri, S2 and PV in both cases. Sparse projections were identified in areas 3a, M1, CMA and PMC of case 17–17 RH (Figure 14b).
In summary, most of the callosal connections of the PPC climbing domain were with the homotopic region of the contralateral hemisphere. Other callosal connections were identified in the dorsal and medial aspects of the parietal-frontal regions, higher-order somatosensory areas of the LS and CgC. Distributions of labeled neurons and axonal terminals largely overlapped, indicating the connections of PPC across the corpus callosum are reciprocal.
Discussion
In previous studies, we have defined a number of small regions in PPC of monkeys and prosimian galagos where 500 ms trains of electrical pulses evoke specific types of complex behaviors that often appear to be ethologically relevant (Gharbawie et al., 2011a, 2011b; Stepniewska et al., 2005, 2009a, 2009b). These small regions or domains form a sequence that roughly relates to body parts, especially in galagos where they have been most investigated. Domains where stimulation produces face movements are located most laterally in PPC, with domains producing hand and arm movements more medial, followed most medially by a region where coordinated forelimb and hindlimb movements are electrically evoked (Figure 1). Such action-specific domains were first described in M1 and adjoining PMC of macaque monkeys by Graziano et al. (2002, 2005) where they formed a lateromedial sequence from face to hand and forelimb, to forelimb and hindlimb actions. The complex actions evoked from the most medial domains were classified as resembling climbing or leaping. For convenience, the domain identified in PPC of galagos is called the “climbing domain” here, while recognizing that it is difficult to functionally classify the complex movements in an anesthetized primate with the head fixed. In addition, there are clear differences between the locomotive behavior of galagos and macaques, as macaques spend more time on the ground than galagos which are known as great leapers (Schmitt, 2010).
The focus of the present study was to determine the cortical connections of the PPC climbing domain, as such connections reveal the locations of other cortical regions that are directly involved in the actions produced by electrically stimulating neurons within the domain. We also used LT-ICMS to evoke movements from M1 and PMC, as well as from PPC, with the expectation that some of the connections of the PPC climbing domain would be with functionally matched domains in M1 and PMC, as previously found for other domains. Thus, the first part of the discussion will be on these microstimulation results, and how they relate to previous findings.
Injections of tracers into the PPC climbing domain revealed widespread connections with sensorimotor areas of frontal, parietal, cingulate and insular cortex, as well as connections within PPC. These connections were mainly ipsilateral, but connections with contralateral cortex were also revealed. Remarkably few connections involved higher-order visual areas. Therefore, the second part of the discussion relates these anatomical findings to previous results on other domains, and considers what the connections might mean in terms of defining cortical networks for specific actions, and finally identifies the cortical areas involved in influencing the functionality of the PPC climbing domain.
Movements evoked from the climbing domain of parietal-frontal cortex in galagos and other primates
In the present paper, cortical sites were considered to be in the climbing domain if combined forelimb and hindlimb movements were evoked by LT-ICMS. Movements of the contralateral limbs were always present, while sites with movements of all four limbs were occasional. The PPC region in galagos where hindlimb movements combined with forelimb movements that resemble those used to “support the body, stand up, climb or jump” was previously described by Stepniewska et al. (2005, 2009b). A similar domain for combined movements of the limbs was noted in medial M1, but not described. Here we stimulated medial parts of M1 and PMC in galagos and identified regions in both areas where LT-ICMS evoked combined forelimb and hindlimb movements (Figure 2).
The region of frontal motor cortex associated with forelimb and hindlimb movements was first identified with long-train stimulation by Graziano et al. (2002). The movements evoked while the monkey was restrained in a primate chair “resembled climbing or leaping postures” (Graziano, 2009). The sites evoking combined forelimb and hindlimb movements were characterized as being in SMA, where electrical stimulation of sites in monkeys and humans had been found to evoke bilateral movements of all four limbs before (for review, see Graziano, 2009). However, the stimulation sites in Graziano et al. (2002) could also have been in both M1 and PMC. In New World owl monkeys and squirrel monkeys, domains for combined forelimb and hindlimb movements have been illustrated in medial parts of PPC and M1 (Kaas and Stepniewska, 2016), but not described. A PPC domain for climbing movements has not been described in macaque monkeys, either. However, the expected location of such a domain is in medial PPC, as in galagos and squirrel monkeys, but skews more caudally because of the occurrence of some rotation of the topographic pattern in macaques (Kaas et al., 2018). A lateral to medial progression of domains in motor and premotor cortex reflects a crude somatotopic pattern from the face to hindlimbs in all stimulated primates, as has long been observed in short-train stimulation studies (for review, see Graziano, 2009). Microstimulation of PPC and motor cortex in non-primate mammals reveal similar results of movement representation from face to limbs in lateral to medial sequences, indicating that those features emerged in mammalian evolution before the emergence of primates (Baldwin et al., 2017; Halley et al., 2020).
The PPC climbing domain does not appear to be uniform in function, as different sites within the domain produced different types and combinations of limb movements. Thus, the domain may have column-like subregions in aid of climbing and running. Other domains appear to map variations of a movement pattern, such as where to look for the eye movement domain in LIP of macaques (Thier and Andersen, 1996, 1998) and squirrel monkeys (ongoing research), or where to reach for the reaching domain in PPC (Stepniewska et al., 2020).
Cortical connections of the climbing domain in PPC
Although the existence of a climbing domain in parietal-frontal networks has been known for some time, after being first reported in M1 and PMC of macaques (Graziano et al., 2002; Graziano and Aflalo, 2007), subsequently in PPC, M1 and PMC of galagos (Stepniewska et al., 2005) and New World owl monkeys and squirrel monkeys (Kaas and Stepniewska, 2016), the connections of such a domain has not been described before. In order to reveal the cortical connections of the PPC climbing domain in galagos, anatomical tracers were injected into cortical sites in PPC where LT-ICMS evoked climbing movements. The retrograde transport of these tracers labeled a dense array of neurons around the injection core, many neurons elsewhere in PPC, and neurons in other regions of cortex of both cerebral hemispheres. Terminal axonal arbors of neurons that project from the injection core were also labeled with the anterograde transport of the tracers. Here we consider the regions having connections with the PPC climbing domain in regard to what is known about the connections of other PPC domains, and consider the possible functions of the cortical connections of the PPC climbing domain.
The intrinsic connections of the PPC climbing domain
Injections of tracers into the PPC climbing domain label many neurons within the domain, including those near the injection core, as is commonly observed in studies of cortical connections (e.g., Cusick and Kaas, 1988; Malach et al., 1997; Stepniewska et al., 2016). These connections indicate that a population of adjoining neurons that make up cortical modules or columns have connections within a larger functional unit, the domain (Kaas, 2012). Electrical stimulation of a site in a domain likely activates most of these adjacent neurons, as well as patches of nearby neurons (Stepniewska et al., 2016; Card and Gharbawie, 2020). The nearby projections involve the activation of both excitatory and inhibitory neurons, but a general finding is that within a sensory area of cortex, the longer axon projections more likely terminate in inhibitory neurons, and thereby contribute to the suppressive receptive field surrounds (Reed et al., 2010, 2011). What is clearly apparent from the results (e.g., Figure 3) is that neurons throughout PPCr, the region where movements can be evoked by LT-ICMS, project to the climbing domain. Such widespread connections within PPCr have been reported for other domains (Stepniewska et al., 2009a; Gharbawie et al., 2011a), suggesting these PPC domains are highly interconnected. However, optical imaging of PPC while stimulating neurons in a domain does not reveal widespread activity within PPCr (unpublished observation; Stepniewska et al., 2011). Instead, only a few nearby sites are highly activated. Similar results were obtained when sites in M1 of macaques were stimulated (Card and Gharbawie, 2020). A reasonable interpretation of such results is that neurons that are activated at the injection site activate clusters of excitatory neurons at only a few nearby sites. However, longer projections, especially those to more distant domains, mainly activate inhibitory neurons that suppress overall activities of these domains. This premise is further supported by the results from electrically simulating sites in two different PPC domains at the same time (Stepniewska et al., 2020). The dominant effect was for the two domains to suppress each other in order to prevent competing movements. Thus, mutual suppression could be the dominant function of widespread connections within PPCr. However, some connections that are found in domains with complementary functions may terminate mainly on excitatory neurons, thereby facilitating neural activity and the function of the domain. Therefore, one movement is enhanced or two compatible movements are combined when stimulating two movement domains simultaneously.
Connections of the PPC climbing domain with somatosensory and visual areas of cortex
PPC has long been known to receive sensory information from visual areas of cortex, but also from somatosensory and even auditory cortex. Much of the evidence for such inputs comes from microelectrode recording from neurons in PPC that demonstrate the influences of sensory stimuli on neural activity (for review, see Andersen et al., 1997; Colby and Goldberg, 1999), but less is known about how sensory information gets to specific PPC domains.
Our results indicate that the climbing domain gets many inputs from parietal somatosensory areas, including the region of areas 1 and 2 of macaques, which is identified as area 1–2 in galagos (Sur et al. 1980). Other somatosensory inputs are from S2 and PV. Very few inputs come from areas 3b (S1) and 3a, where labeled neurons are mainly in the hindlimb representation, with some labeled neurons in the forelimb region. The climbing domain also projects to all these somatosensory areas. We expect that most cortical connections are reciprocal, but the labeling of cells and dendrites can mask labeled axonal arbors, so our evidence on anterograde labeling is clearest where the anterograde label is densest.
Injections of tracers into area 3b and S2 of galagos did not label many neurons in PPCr (Wu and Kaas, 2003). Likewise, injections in the hand-to-mouth, forelimb defense and face defense domains of PPC in galagos labeled neurons in areas 1–2, S2 and PV, but only a few neurons in area 3b (Stepniewska et al., 2009a). Another area or region, retroinsular cortex (Ri), likely has somatosensory and auditory functions, and is densely connected with the climbing domain, as well as with other domains in PPCr. Some of the neurons labeled by injections in the PPC climbing domain could also be in areas of the auditory belt (de la Mothe et al., 2006), thereby providing auditory information.
Perhaps surprisingly, visual inputs to the PPC climbing domain are less direct than the somatosensory inputs. The injections in the climbing domain labeled only a few scattered neurons throughout caudal visual cortex. Injections of more lateral domains in galagos involving forelimb or face movements labeled only small patches of neurons in middle superior temporal visual area (MST), fundal superior temporal visual area (FST) and dorsal third visual area (V3d) (Stepniewska et al., 2009a). Instead, injections of the climbing domain, as well as other PPCr domains in galagos, labeled many neurons in PPCc. However, injections of PPCc labeled many neurons in visual areas DM, V3, V2, MST and IT (Stepniewska et al., 2016). Thus, PPC domains likely receive most of their visual information from higher-order visual areas indirectly via a relay through PPCc. This is also likely to be the case in other primates. In macaques, direct inputs from MT, MST and V3 to the lateral intraparietal area (LIP) have been reported (Andersen et al., 1990; Boussaoud et al., 1990; Blatt et al., 1990), but other visual information likely comes from visually dominated areas of PPCc (Beck and Kaas, 1999).
Motor and premotor connections of the PPC climbing domain
The other major cortical connections of the PPC climbing domain are with motor areas, including M1, PMd, PMv, SMA and CMA. The injections labeled neurons in parts of M1, PMd and PMv that have domains for forelimb and hindlimb movements, including the functionally matched climbing domains. Fewer labeled neurons were found in regions where domains for grasping and reaching are located. Neurons in the PPC climbing domain also project to locations in M1 and PMC that have labeled neurons. Projections from other PPCr domains in galagos are to functionally matched domains in M1 and PMC, as optical imaging of frontal cortex during the electrical stimulation of PPC domains revealed increased activity only in functionally matched domains (Stepniewska et al., 2011). In addition, the actions evoked by stimulating PPCr domains are dependent on the functioning of matched M1 domains, as selectively inactivating M1 domains render the stimulation of matching PPCr domains ineffective (Stepniewska et al., 2014; Cooke et al., 2015). Thus, the feedforward projections from PPCr activate matching domains in PMC and M1, and evoked actions from PPC stimulation depend on these connections. The feedback connections appear to come from somewhat broader distributions of neurons, so that feedback from functionally matched domains in frontal cortex likely facilitates activity in PPCr domains, while inputs from functionally mismatched domains of frontal cortex likely have a suppressive role.
The motor areas that occupy the medial wall of cerebral cortex in galagos (Wu et al., 2000) and other primates (e.g., Picard and Strick, 1996) include SMA and CMA. These regions are considered to be higher-order motor areas where movements can be evoked by electrical stimulation (see Wu et al., 2000 for galagos). SMA and CMA are thought to be involved in mediating and planning complex actions related to motivations and ongoing stimuli (Kermadi et al., 1998, 2000; Backus et al., 2001; for review, see Tanji, 1994). The influences these motor areas and the PPC climbing domain have on each other are unclear.
Conclusions
The present study provided further evidence for climbing domains in PPC, PMC and M1 in prosimian galagos. In addition, the connectional patterns of the climbing domain in PPC are described for the first time. Some of these connections likely mediate interactions that are mainly suppressive between functionally different domains in PPC. Other connections provide visual, somatosensory and perhaps auditory information to the climbing domain. Projections from the PPC climbing domain to M1 and PMC facilitate the activation of matching climbing domains in M1 and PMC. While the evoked movements of forelimbs and hindlimbs have been characterized as climbing or running movements, more detailed studies of the evoked movements are needed.
Acknowledgments
We thank Dr. Hui-Xin Qi, Dr. Jamie Reed and Mary Feurtado for help during surgical procedures, Laura Trice and Steve Jeoung for histological assistance, and Yuan Yang for helping with the Python script. This work was supported by National Eye Institute grant (EY02686) to J.H.K.
Abbreviations
Cortical fields
- 1–2
somatosensory areas 1 and 2
- 3a
somatosensory area 3a
- 3b (or S1)
primary somatosensory area
- A1
primary auditory area
- CLI
claustral cortex
- CgC
cingulate cortex
- CMA
cingulate motor area
- DL
dorsolateral visual area
- DM
dorsomedial visual area
- FST
fundal superior temporal visual area
- IT
inferotemporal cortex
- M1
primary motor cortex
- MST
middle superior temporal visual area
- MT
middle temporal visual area
- OF
orbital frontal cortex
- PFC
prefrontal cortex
- PMC
premotor cortex
- PMd
dorsal premotor area
- PMv
ventral premotor area
- PPC
posterior parietal cortex
- PPCc
caudal posterior parietal cortex
- PPCr
rostral posterior parietal cortex
- PR
rostral parietal area
- PV
ventral parietal area
- Ri
retroinsular area
- RS
retrosplenial area
- SMA
supplementary motor area
- S2
secondary somatosensory area
- V1
primary visual area
- VS
ventral somatosensory area
- V2
secondary visual area
- V2d
dorsal secondary visual area
- V3d
dorsal third visual area
Sulci
- CgS
cingulate sulcus
- FSa
anterior frontal sulcus
- FSp
posterior frontal sulcus
- IPS
intraparietal sulcus
- LS
lateral sulcus
Anatomical tracers
- BDA
biotinylated dextran amines
- CTB
cholera toxin subunit B
- FR
fluoro-ruby
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Andersen RA, Asanuma C, Essick G, & Siegel RM (1990). Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. Journal of Comparative Neurology, 296(1), 65–113. 10.1002/cne.902960106 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, & Xing J. (1997). Multimodal Representation of Space in the Posterior Parietal Cortex and Its Use in Planning Movements. Annual Review of Neuroscience, 20(1), 303–330. 10.1146/annurev.neuro.20.1.303 [DOI] [PubMed] [Google Scholar]
- Angelucci A, Clascá F, & Sur M. (1996). Anterograde axonal tracing with the subunit B of cholera toxin: A highly sensitive immunohistochemical protocol for revealing fine axonal morphology in adult and neonatal brains. Journal of Neuroscience Methods, 65(1), 101–112. 10.1016/0165-0270(95)00155-7 [DOI] [PubMed] [Google Scholar]
- Backus DA, Ye S, Russo GS, & Crutcher MD (2001). Neural activity correlated with the preparation and execution of visually guided arm movements in the cingulate motor area of the monkey: Preliminary findings. Experimental Brain Research, 140(2), 182–189. 10.1007/s002210100807 [DOI] [PubMed] [Google Scholar]
- Balaram P, Hackett TA, & Kaas JH (2013). Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. Journal of Chemical Neuroanatomy, 50–51(1), 21–38. 10.1016/j.jchemneu.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Balaram P, & Kaas JH (2013). Projections of the superior colliculus to the pulvinar in prosimian galagos (Otolemur garnettii) and VGLUT2 staining of the visual pulvinar. Journal of Comparative Neurology, 521(7), 1664–1682. 10.1002/cne.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Cooke DF, & Krubitzer L. (2017). Intracortical Microstimulation Maps of Motor, Somatosensory, and Posterior Parietal Cortex in Tree Shrews (Tupaia belangeri) Reveal Complex Movement Representations. Cerebral Cortex (New York, N.Y.: 1991), 27(2), 1439–1456. 10.1093/cercor/bhv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck PD, & Kaas JH (1999). Cortical connections of the dorsomedial visual area in old world macaque monkeys. Journal of Comparative Neurology, 406(4), 487–502. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, & Stoner GR (1990). Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. Journal of Comparative Neurology, 299(4), 421–445. 10.1002/cne.902990404 [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, & Desimone R. (1990). Pathways for motion analysis: Cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. Journal of Comparative Neurology, 296(3), 462–495. 10.1002/cne.902960311 [DOI] [PubMed] [Google Scholar]
- Card NS, & Gharbawie OA (2020). Principles of intrinsic motor cortex connectivity in primates. Journal of Neuroscience, 40(22), 4348–4362. 10.1523/JNEUROSCI.0003-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT (1993). Immunoperoxidase labeling of the anterograde tracer fluoro-ruby (tetramethylrhodamine-dextran amine conjugate). Brain Research Bulletin, 30(1–2), 115–118. 10.1016/0361-9230(93)90046-E [DOI] [PubMed] [Google Scholar]
- Colby CL, & Goldberg ME (1999). Space and Attention in Parietal Cortex. Annual Review of Neuroscience, 22(1), 319–349. 10.1146/annurev.neuro.22.1.319 [DOI] [PubMed] [Google Scholar]
- Cooke DF, Stepniewska I, Miller DJ, Kaas JH, & Krubitzer L. (2015). Reversible deactivation of motor cortex reveals functional connectivity with posterior parietal cortex in the prosimian galago (Otolemur garnettii). Journal of Neuroscience, 35(42), 14406–14422. 10.1523/JNEUROSCI.1468-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG, & Kaas JH (1988). Surface view patterns of intrinsic and extrinsic cortical connections of area 17 in a prosimian primate. Brain Research, 458(2), 383–388. https://doi.or [DOI] [PubMed] [Google Scholar]
- de La Mothe LA, Blumell S, Kajikawa Y, & Hackett TA (2006). Cortical connections of the auditory cortex in marmoset monkeys: Core and medial belt regions. Journal of Comparative Neurology, 496(1), 27–71. 10.1002/cne.20923 [DOI] [PubMed] [Google Scholar]
- Gallyas F. (1979). Silver staining of myelin by means of physical development. Neurological Research, 1(2), 203–209. 10.1080/01616412.1979.11739553 [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, & Kaas JH (2011) a. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in new world monkeys. Cerebral Cortex, 21(9), 1981–2002. 10.1093/cercor/bhq260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H, & Kaas JH (2011) b. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. Journal of Neuroscience, 31(32), 11660–11677. 10.1523/JNEUROSCI.1777-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, Cusick CG, Pons TP, & Kaas JH (1986). The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. Journal of Comparative Neurology, 247(3), 297–325. 10.1002/cne.902470303 [DOI] [PubMed] [Google Scholar]
- Graziano MSA (2009). The Intelligent Movement Machine. Oxford University Press, Inc. 10.1093/acprof:oso/9780195326703.001.0001 [DOI] [Google Scholar]
- Graziano MSA, & Aflalo TN (2007). Mapping behavioral repertoire onto the cortex. Neuron, 56(2), 239–251. 10.1016/j.neuron.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Aflalo TNS, & Cooke DF (2005). Arm movements evoked by electrical stimulation in the motor cortex of monkeys. Journal of Neurophysiology, 94(6), 4209–4223. 10.1152/jn.01303.2004 [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, & Moore T. (2002). Complex movements evoked by microstimulation of precentral cortex. Neuron, 34(5), 841–851. 10.1016/S0896-6273(02)00698-0 [DOI] [PubMed] [Google Scholar]
- Halley AC, Baldwin MKL, Cooke DF, Englund M, & Krubitzer L. (2020). Distributed Motor Control of Limb Movements in Rat Motor and Somatosensory Cortex: The Sensorimotor Amalgam Revisited. Cerebral Cortex, 1, 1–17. 10.1093/cercor/bhaa186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Qi H-X, & Stepniewska I. (2020). Evolution of Parietal-Frontal Networks in Primates. In Kaas Jon H. (Ed.), Evolutionary Neuroscience; (2nd ed., pp. 657–667). 10.1016/b978-0-12-820584-6.00027-1 [DOI] [Google Scholar]
- Kaas JH (2012). Evolution of columns, modules, and domains in the neocortex of primates. Proceedings of the National Academy of Sciences of the United States of America, 109(SUPPL.1), 10655–10660. 10.1073/pnas.1201892109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, & Iyengar S. (2006). Cortical network for representing the teeth and tongue in primates. Anatomical Record - Part A Discoveries in Molecular, Cellular, and Evolutionary Biology, 288(2), 182–190. 10.1002/ar.a.20267 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, & Stepniewska I. (2018). The evolution of parietal cortex in primates. In Vallar G. & Coslett HB (Eds.), Handbook of Clinical Neurology (Vol. 151, pp. 31–52). Elsevier B.V. 10.1016/B978-0-444-63622-5.00002-4 [DOI] [PubMed] [Google Scholar]
- Kaas JH, & Stepniewska I. (2016). Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates. Journal of Comparative Neurology, 524(3), 595–608. 10.1002/cne.23838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan PM, & Kaas JH (2007). Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti). Anatomical Record, 290(3), 349–366. 10.1002/ar.20440 [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, & Rouiller EM (2000). Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosensory and Motor Research, 17(3), 255–271. 10.1080/08990220050117619 [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Calciati E, & Rouiller EM (1998). Neuronal activity in the primate supplementary motor area and the primary motor cortex in relation to spatio-temporal bimanual coordination. Somatosensory and Motor Research, 15(4), 287–308. 10.1080/08990229870709 [DOI] [PubMed] [Google Scholar]
- Liao CC, Gharbawie OA, Qi H, & Kaas JH (2013). Cortical connections to single digit representations in area 3b of somatosensory cortex in squirrel monkeys and prosimian galagos. Journal of Comparative Neurology, 521(16), 3768–3790. 10.1002/cne.23377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, & Kaas JH (2002). Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain, Behavior and Evolution, 59(3), 114–129. 10.1159/000064159 [DOI] [PubMed] [Google Scholar]
- Malach R, Schirman TD, Harel M, Tootell RBH, & Malonek D. (1997). Organization of intrinsic connections in owl monkey area MT. Cerebral Cortex, 7(4), 386–393. 10.1093/cercor/7.4.386 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, & Grenda R. (1992). Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience, 12(8), 2918–2947. 10.1523/jneurosci.12-08-02918.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, & Merzenich MM (1996). Use-Dependent Primary Motor Alterations of Movement Representations Cortex of Adult Squirrel Monkeys in of Physiology and. Journal of Neuroscience, 16(2), 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, & Strick PL (1996). Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex, 6(3), 342–353. 10.1093/cercor/6.3.342 [DOI] [PubMed] [Google Scholar]
- Preuss TM, & Goldman-Rakic PS (1991). Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. Journal of Comparative Neurology, 310(4), 475–506. 10.1002/cne.903100403 [DOI] [PubMed] [Google Scholar]
- Reed JL, Qi HX, & Kaas JH (2011). Spatiotemporal properties of neuron response suppression in owl monkey primary somatosensory cortex when stimuli are presented to both hands. Journal of Neuroscience, 31(10), 3589–3601. 10.1523/JNEUROSCI.4310-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi HX, Zhou Z, Bernard MR, Burish MJ, Bonds AB, & Kaas JH (2010). Response properties of neurons in primary somatosensory cortex of owl monkeys reflect widespread spatiotemporal integration. Journal of Neurophysiology, 103(4), 2139–2157. 10.1152/jn.00709.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockoff EC, Balaram P, & Kaas JH (2014). Patchy distributions of myelin and vesicular glutamate transporter 2 align with cytochrome oxidase blobs and interblobs in the superficial layers of the primary visual cortex. Eye and Brain, 6, 19–27. 10.2147/EB.S59797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimitt D. (2010). Primate locomotor evolution: Biomechanical studies of primate locomotion and their implications for understanding primate neuroethology. In Platt ML, & Ghazanfar AA (Eds.), Primate neuroethology (pp. 31–63). Oxford University Press. 10.1093/acprof:oso/9780195326598.001.0001 [DOI] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PCY, & Kaas JH (2009) a. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. Journal of Comparative Neurology, 517(6), 783–807. 10.1002/cne.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, & Kaas JH (2016). Cortical Connections of the Caudal Portion of Posterior Parietal Cortex in Prosimian Galagos. Cerebral Cortex, 26(6), 2753–2777. 10.1093/cercor/bhv132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PCY, & Kaas JH (2009) b. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. Journal of Comparative Neurology, 517(6), 765–782. 10.1002/cne.22181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, & Kaas JH (2005). Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proceedings of the National Academy of Sciences of the United States of America, 102(13), 4878–4883. 10.1073/pnas.0501048102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, & Kaas JH (2011). Optical imaging in galagos reveals parietal-frontal circuits underlying motor behavior. Proceedings of the National Academy of Sciences of the United States of America, 108(37), 725–732. 10.1073/pnas.1109925108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Miller DJ, & Kaas JH (2020). Interactions within and between parallel parietal-frontal networks involved in complex motor behaviors in prosimian galagos and a squirrel monkey. Journal of Neurophysiology, 123(1), 34–56. 10.1152/jn.00576.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Gharbawie OA, Burish MJ, & Kaas JH (2014). Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation. Journal of Neurophysiology, 111(5), 1100–1119. 10.1152/jn.00491.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, & Preston JB (1982). Two representations of the hand in area 4 of a primate. I. Motor output organization. Journal of Neurophysiology, 48(1), 139–149. 10.1152/jn.1982.48.1.139 [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, & Kaas JH (1980). Representation of the body surface in somatic koniocortex in the prosimian Galago. Journal of Comparative Neurology, 189(2), 381–402. 10.1002/cne.901890211 [DOI] [PubMed] [Google Scholar]
- Tanji J. (1994). The supplementary motor area in the cerebral cortex. Neuroscience Research, 19(3), 251–268. 10.1016/0168-0102(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Thier P, & Andersen RA (1996). Electrical microstimulation suggests two different forms of representation of head-centered space in the intraparietal sulcus of rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America, 93(10), 4962–4967. 10.1073/pnas.93.10.4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier P, & Andersen RA (1998). Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. Journal of Neurophysiology, 80(4), 1713–1735. 10.1152/jn.1998.80.4.1713 [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, & Honig MG (1992). Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. Journal of Neuroscience Methods, 41(3), 239–254. 10.1016/0165-0270(92)90089-V [DOI] [PubMed] [Google Scholar]
- Wong P, & Kaas JH (2010). Architectonic subdivisions of neocortex in the galago (Otolemur garnetti). Anatomical Record, 293(6), 1033–1069. 10.1002/ar.21109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. (1979). Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Research, 171, 11–28. [DOI] [PubMed] [Google Scholar]
- Wu CWH, Bichot NP, & Kaas JH (2000). Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. Journal of Comparative Neurology, 423(1), 140–177. [DOI] [PubMed] [Google Scholar]
- Wu CWH, & Kaas JH (2003). Somatosensory cortex of prosimian galagos: Physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. Journal of Comparative Neurology, 457(3), 263–292. 10.1002/cne.10542 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.