Abstract
Chronic lower extremity wounds (diabetic foot ulcers) are a serious and prevalent complication of diabetes. These wounds exhibit low healing rates and present a high risk of amputation. Current diagnostic options for foot ulcers are limited to macroscopic wound analysis such as wound depth, implicated tissues, and infection. Molecular diagnostics promises to improve foot ulcer diagnosis, staging, and assessment of the treatment response. In this perspective, we report recent progress in understanding the pathophysiology of diabetic wound healing and point to recently emerged novel molecular targets for wound diagnostics. We discuss selected diagnostic wound dressings under preclinical development that detect one or several inflammatory markers, bacterial secretions, hyperglycemia, and mechanical stress. We also highlight key translational challenges of investigational diagnostic bandages for diabetic foot ulcers.
Keywords: diagnostics, diabetic foot ulcer, wound healing, wound dressing, infection, diabetes
Introduction
Chronic lower extremity wounds (diabetic foot ulcers, DFU) are a serious complication of diabetes.1,992 With a lifetime incidence of 15–25% among patients with diabetes, a large fraction of these patients will be affected by DFU.2 The prevalence of DFU was 0.7% in all Medicare patients and 6.0% in Medicare patients with diabetes, and particularly affects patients with type 2 diabetes.3 With standard wound care, about half of DFUs heal after seven months, but the recurrence rate is at about 60% after three years.4,5 Amputations are necessary in approximately 20% of patients with moderate or severe DFU.4 According to the Centers for Disease Control in the United States, DFUs are the leading cause for nontraumatic lower limb amputations.6 Moreover, the financial burden of DFU on the health care system is considerable. In 2014, the Medicare costs for DFU wound care (including cost of infections) were 6.2 billion USD.3 A systematic review found mean health care costs of 44,200 USD per year per DFU patient in the United States in 2015.7
In the clinical pathogenesis of diabetic foot ulceration, peripheral neuropathy and peripheral arterial disease play a key role.2 Neuropathy in lower extremities leads to a loss of protective sensations and foot deformations and results in abnormal mechanical loading, formation of thickened skin (callus), and subcutaneous hemorrhage and skin ulceration.2 Minor trauma (e.g., due to mechanical injury) can also result in wounding, especially in case of loss of protective sensations due to neuropathy.2 Continued biomechanical loading on the insensitive wounded foot impairs healing and promotes chronic wound formation.2 As explained in recent reviews on diabetic wound pathogenesis, typical properties of difficult-to-heal diabetic wounds are chronic inflammation, impaired angiogenesis, and delayed granulation tissue formation.2,8
Clinical practice guidelines on DFU diagnostics recommend macroscopic evaluations to stage DFU and assess the need for antibiotic therapy and revascularization.9 Ulcer depth and the implication of other tissues (tendon, muscle, bone) guide DFU classification.9,10 Evaluating the presence of infections of the diabetic wound is an important part of the diagnostic workup, as they present a serious threat to the affected foot and limb and can result in sepsis if left untreated. In most cases, Staphylococcus aureus is the predominant pathogen.9 Chronic and more severe infections are often polymicrobial and characterized by aerobic Gram-negative rods and anaerobes accompanying the Gram-positive cocci.9 As most diabetic wounds are colonized by potential pathogens, diagnostic evaluation of infection includes at least two signs of inflammation (redness, warmth, induration, and pain/tenderness) or purulent secretions.9,11 In the case of clinically relevant infection, deep tissue specimens should be taken for culture to determine the causative bacterial strain(s) by gram staining, colony morphology, and microscopic analysis of bacteria.9 Culturomics approaches using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry have also been successfully used in DFU infections.12 Current practice guidelines recommend against using superficial swabs because they are less reliable for guidance of antimicrobial therapy than deep tissue specimens.13 Moreover, the presence of peripheral arterial disease is determined by examining the arterial pedal wave forms and measuring the ankle pressure and ankle brachial index using a Doppler instrument.
While currently recommended wound diagnostics are predominantly macroscopic evaluations, recent new insights into the pathophysiology of diabetic wound healing revealed previously unidentified diagnostic targets and led to the development of novel diagnostic wound dressings. In this perspective, we present selected diagnostic targets and critically discuss diagnostic bandages under preclinical investigation. We also evaluate the translational potential of these diagnostic wound dressings.
Recently Emerged Diagnostic Targets
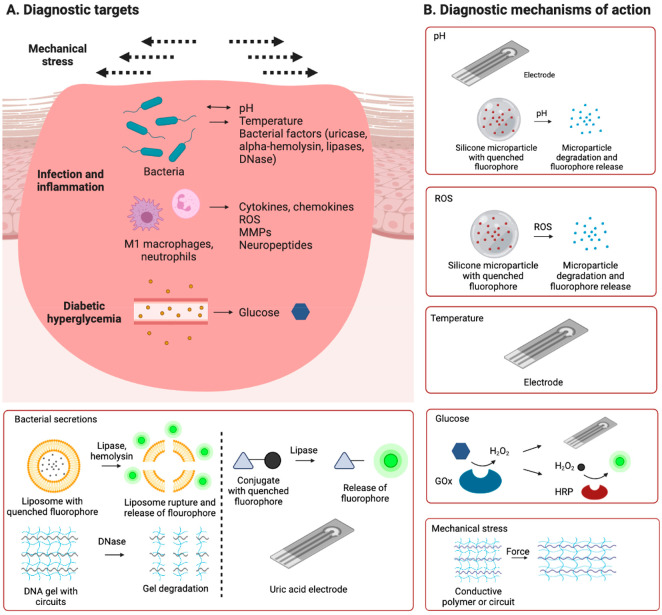
While clinical practice guidelines recommend macroscopic diagnostic workup of diabetic wounds, our understanding of the molecular pathophysiology of diabetic wound healing has been strongly improved in the past two decades. In this section, we present selected molecular targets that recently emerged for diagnostic wound dressings (Figure 1A, Table 1).
Figure 1.
Targets and mechanisms of action of investigational diagnostic wound dressings. A range of molecular targets associated with bacterial infection, inflammation, and hyperglycemia, and mechanical stress on the wound were proposed as diagnostic targets (A). Diagnostic bandages under preclinical investigation detected these targets by various molecular mechanisms of action (B).
Table 1. Potential Molecular Targets for Diagnostic Bandages in Diabetic Wound Healing.
| Category | Molecular targets |
|---|---|
| Inflammation | Cytokines (e.g., MCP-1, IL-1β) |
| ROS (e.g., hydroxyl radical, hydrogen peroxide) | |
| Neuropeptides (e.g., substance P) | |
| MMPs (e.g., MMP-9) | |
| pH | |
| Temperature | |
| Bacterial components | Uricase |
| alpha-Hemolysin | |
| Lipases | |
| Wound regeneration | Growth factors (e.g., VEGF) |
The physiological wound healing response involves four phases (Figure 2). In the hemostasis phase, tissue injury activates platelets and initiates the coagulation cascade, which results in the formation of a thrombus.2 Hemostasis is followed by the inflammation phase, where M1 macrophages and neutrophils enter the wound site, precipitate an acute inflammatory response, and remove bacteria and damaged tissue.2 When macrophages differentiate to the anti-inflammatory M2 phenotype, the wound proceeds to the proliferation phase where M2 macrophages secrete pro-healing factors to induce stromal cell proliferation, angiogenesis, and the formation of granulation tissue.2 Eventually, the wound enters the remodeling phase where the collagen matrix is reorganized into a dense, cross-linked network and the density of blood vessels decreases, leading to the formation of mature tissue.2
Figure 2.
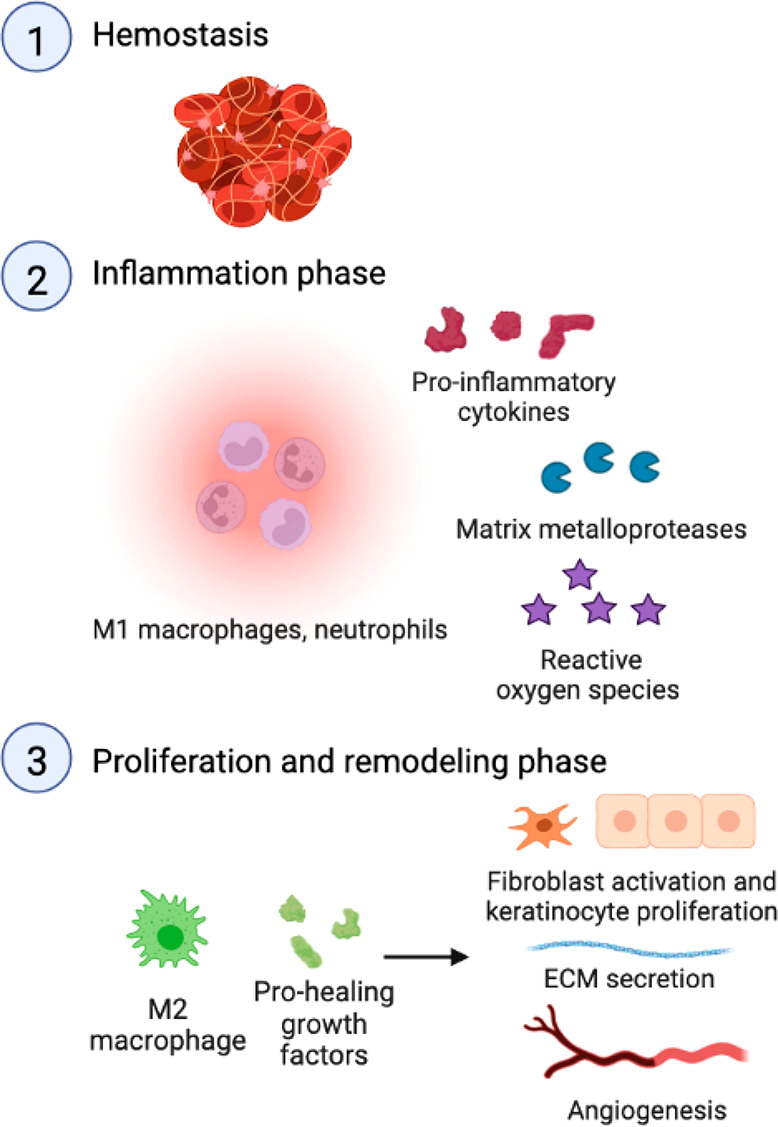
Physiological wound healing cascade includes the hemostasis, inflammation, proliferation, and remodeling phase.
The notion that the inflammatory phase of diabetic wound healing is likely the most dysregulated phase has gained broad acceptance in the last two decades.14 Even prior to injury, a pro-inflammatory baseline state was identified in diabetic patients and various animal models of diabetes. Elevations of pro-inflammatory cytokines were observed both systemically and in intact diabetic skin. In noninjured diabetic skin, there are more immune cells in the dermis and around blood vessels and an excess of pro- (M1) over anti-inflammatory (M2) macrophages. Moreover, skin-resident mast cells were found to be degranulated in intact diabetic skin and thus less able to mount an acute inflammatory response to injury.15 Neuropathy also impairs the inflammation process, as the expression and release of pro-inflammatory neuropeptides such as substance P are decreased in intact diabetic skin and after wounding, respectively.16 These pathophysiologic changes in intact diabetic skin reduce the acute inflammatory response in response to injury.2 Therefore, the identification of these chronic inflammatory markers in intact diabetic skin could be indicative of the risk of the diabetes patient to develop chronic wounds and trigger clinical decisions for preventive action.
The chronic inflammation in intact diabetic skin impairs the skin’s ability to mount an acute inflammatory response when the tissue is injured. In the absence of an acute inflammatory response, diabetic wounds do not progress into the proliferative phase and become chronically inflamed. In wounds of patients with diabetes and rodent models of diabetes, a chronic state of low-grade inflammation characterized by an excess of pro-inflammatory M1 over anti-inflammatory M2 macrophages was reported.2,17 In chronic diabetic wounds, M1 macrophages secrete pro-inflammatory chemo- and cytokines (e.g., monocyte chemoattractant protein-1, interleukin-1β) and continually recruit macrophages to the wound site where they are polarized to the pro-inflammatory M1 phenotype in a positive feedback loop. The polarization of macrophages to the pro-healing M2 macrophage phenotype is therefore impaired in diabetic wounds. M2 macrophages and their secreted pro-healing growth factors such as vascular endothelial growth factor (VEGF) are reduced in diabetic wounds.8,17 As a result, a typical signature of diabetic wounds is an increased ratio of M1 to M2 macrophages, increased levels of pro-inflammatory chemo- and cytokines, and lower levels of pro-healing growth factors. Diabetic wound pH is impacted by these pathophysiological particularities. In contrast to the acidic pH in acute wounds of about 5.5, diabetic wound pH reaches values in the neutral and alkaline range of up to pH 8.5 in patients with diabetes and animal models of diabetes.18−25 The high wound pH is likely related to low-grade chronic inflammation and leakage of physiological fluids, which are at slightly alkaline pH, into the chronic wounds.26,27
The highly abundant wound immune cells (neutrophils, M1 macrophages) secrete high levels of pro-inflammatory chemo- and cytokines, reactive oxygen species (ROS), and proteases such as matrix metalloprotease-9 (MMP-9). While ROS and MMP-9 have important roles in physiological wound healing, their overexpression in diabetic wounds negatively impacts key processes of tissue repair and thus prevents the progression into the proliferation phase.2 Increased ROS concentrations were identified in wounds of patients with diabetes and animal models of diabetes and associated with impaired angiogenesis, extracellular matrix modifications, and a negative impact on fibroblast and keratinocyte proliferation and migration.2,17,28 ROS covalently modified a coactivator of hypoxia inducible factor 1α (HIF-1α) and impaired HIF-1 signaling and reduced angiogenesis in diabetic mouse wounds.29 Increases in expression and activity of p66Shc, a hydrogen peroxide-producing enzyme in diabetic mouse wounds, decreased granulation tissue thickness and angiogenesis.30 Immune cells further secrete high amounts of MMPs, zinc-dependent endopeptidases that degrade and remodel ECM components. In wounds of diabetic mice and patients with diabetes, MMP-9 upregulation impaired angiogenesis and re-epithelialization.31 Furthermore, hyperglycemia impaired mast cells’ capacity to release VEGF, which promotes angiogenesis independent of degranulation.15 Therefore, increased levels of ROS (hydroxyl radical, hydrogen peroxide), MMP-9, reduced VEGF, and hyperglycemia are molecular features of diabetic wounds.
The assessment of bacterial infection is a central component of the diagnostic workup in DFU, but current methods are limited to macroscopic methods as explained above. In diabetic foot ulcers, Gram-positive Staphylococcus aureus and Streptococcus and Gram-negative Pseudomonas aeruginosa were prevalent, and wound infection with S. aureus and/or P. aeruginosa was associated with poor diabetic wound healing.32−35 Wound fluid of wounds infected with S. aureus and/or P. aeruginosa contains high amounts of indicative bacterial secretions such as lipases, hemolysins, and uric acid oxidase (uricase). Bacterial infection elicits an inflammatory response and leads to the upregulation of pro-inflammatory cytokines, ROS, and products of glycolysis such as lactate.36 Wound pH is also associated with wound healing, as the basic and slightly alkaline pH found in diabetic wounds promotes the growth of pathogenic bacteria.21−23
As our understanding of how various immune cell types and their secreted factors negatively impact the pathophysiology of diabetic wound healing, a variety of potential novel diagnostic targets have emerged and promise to generate new molecular diagnostics.
Diagnostic Wound Dressings under Preclinical Investigation
A variety of new diagnostic wound dressings that mainly focus on monitoring the inflammatory status of the wound and on detecting bacterial secretions (Figure 1B, Table 2) have been developed and tested in vivo. While preclinical proof-of-concept for the bandages presented in this section has been established, definitive data demonstrating their diagnostic utility benefit in DFU patients have not been published yet. Nonetheless, the diversity of these strategies raises hopes that novel diagnostic options will soon be available for DFU patients.
Table 2. Selected Investigational Diagnostic Wound Dressings for Diabetic Wounds.
| Materials | Analytes | Detection method |
|---|---|---|
| Carbon electrode; poly(imide) substrate; polydimethylsiloxane encapsulation; poly(butadiene) derivative; poly(aniline); poly(ethylenimine); gold nanoparticles; commercial temperature sensor19 | pH, temperature, uric acid | Potentiometry |
| Carbon electrode; poly(imide) substrate; polydimethylsiloxane encapsulation; poly(ethylenimine); poly(aniline); glucose oxidase and Prussian blue electrode coating20 | pH, temperature, glucose | Potentiometry |
| Poly(ethylene glycol) diacrylate; commercial temperature sensor37 | Temperature | Potentiometry |
| Polyurethane base layer, ciprofloxacin-loaded silicon particles, cross-linked chitosan38 | pH, ROS | Optical |
| Zwitterionic poly(carboxybetaine); phenol red dye; glucose oxidase, HRP, optical HRP substrate24 | pH, glucose | Optical |
| Methylarylated gelatin hydrogel, carboxyfluorescein-loaded liposomes39 | Bacterial secretions (phospholipase and alpha-hemolysin) | Optical |
| Agarose gel, carboxyfluorescein-loaded vesicles40 | Bacterial secretions (cytotoxins) | Optical |
| Polyurethane, lipase-sensitive pro-drug of hemicyanine41 | Bacterial secretions (lipase) | Optical |
| Poly(ethylene glycol) diglycidyl ether-cross-linked DNA gel with graphene oxide dopant; copper electrodes with SU-8 coating; poly(imide)42 | Bacterial secretions (DNase) | Potentiometry |
| Carbon electrode, cationic derivative of poly(vinyl alcohol), uricase, ferrocene43 | Bacterial secretions (uricase) | Potentiometry |
| Silk fibroin, graphene, poly(dimethylsiloxane)44 | Mechanical stress | Potentiometry |
| Poly(3-aminophenylboronic acid), poly(aniline), poly(vinyl alcohol)45 | Mechanical stress | Potentiometry |
| Cross-linked gelatin; poly(3,4-ethylenedioxythiophene) and poly(styrenesulfonate) and multiwalled carbon nanotubes46 | Mechanical stress | Potentiometry |
An increase of wound pH in diabetic wounds into the neutral to alkaline range up to pH 8.5 favors wound infection, as explained above. Antibiotic therapy treats infection, improves wound healing, and thus lowers wound pH. Wound pH has therefore been investigated as a biomarker for wound infection and antibiotic treatment response. Two systems were recently developed to measure wound pH by potentiometry which both rely on a carbon/polyaniline working electrode.19,20 Deprotonation of polyaniline leads to an accumulation of charge and a voltage output, which is measured by a potentiometer for the determination of pH. These pH sensors showed linearity in acidic and alkaline conditions in vitro. One sensor was embedded in an elastomer made of a poly(butadiene) derivative and contained the antiseptic agent cetyltrimethylammonium bromide and was tested in S. aureus-infected rat wounds in vivo where it detected pH-lowering effects of antibiotic treatment in a range of 6.5 to 8.19 A similar sensor embedded in a stretchable dressing made of a poly(imide) substrate and polydimethylsiloxane was tested in S. aureus-infected mice and detected higher pH values in nontreated than cefazolin-treated mice in the range of 6.8 to 8.4.20
As potentiometric strategies face several challenges such loss of function and/or release of toxic substances in wound fluid or due to mechanical stress on the skin, measurements limited to one point of the wound, and the need for a power source, optical pH sensing wound dressings were developed. The quenching effect of red luminescent porous silicon particles on the blue luminescence of a loaded drug, ciprofloxacin, was used for an optical pH sensor.38 The ciprofloxacin-loaded silicon particles were sandwiched between a polyurethane base layer and a cross-linked chitosan wound-exposed protection layer. As alkaline species such as hydroxide and phosphate oxidize the silicon surface to silicon oxide, the quenching effect becomes weaker, and the blue signal becomes stronger. This system discriminated pH 7.0 and 7.5 in vitro and differentiated infected and noninfected mouse wounds in vivo based on excitation using a UV light and fluorescence detection. However, as this system also responded to ROS, the contribution of pH effects to the fluorescence change was difficult to quantify in the in vivo study. Wound pH is also a marker of diabetic wounds irrespective of infection. A zwitterionic poly(carboxybetaine) hydrogel loaded with the pH-sensitive dye phenol red detected higher wound pH in streptozotocin-induced diabetic mouse wounds than in nondiabetic control wounds.24
ROS are secreted by immune cells and thus an important marker of inflammation. The pH-sensing fluorescent bandage described above is also sensitive to ROS, as ROS oxidize the red silica layer and thus reduce its quenching effect on the blue ciprofloxacin.38 Single molecular oxygen (1O2) prepared by mixing hydrogen peroxide and hypochlorite decreased the ratio of the red-to-blue fluorescence ratio in vitro.38 While this bandage also changed color in vivo, the contribution of pH and ROS cannot be determined, as the bandage is sensitive to both factors.38 In general, new ROS sensing systems would be highly useful in wound diagnostics, as recently developed ROS-reducing therapeutic bandages are highlighting the central role of ROS in diabetic wound healing.29,47
Increased wound temperature is an integral part of the inflammatory response. Real-time wound temperature monitoring therefore promises to yield information about bacterial infections. A potentiometric graphene oxide electrode with a polymeric poly(ethylenimine) layer showed a linear change in resistance between 24 and 44 °C in vitro and sensed a temperature increase of 1–2 °C in a S. aureus-infected mouse wound model.20 Other wound temperature-sensing wound dressings embedded a commercial temperature sensor chip in the wound dressing. A bandage containing a temperature sensor in a poly(imide) substrate determined a decrease in wound temperature in S. aureus-infected rat wounds upon treatment with the antibiotic cefazolin, while the temperature stayed constant in the absence of antibiosis.19 In another bandage, a commercial temperature sensor was embedded in a poly(ethylene glycol) diacrylate hydrogel and determined a decrease in wound temperature after antibiotic treatment with gentamicin in an S. aureus-infected porcine wound model.37
As wound infection has deleterious effects on wound healing, molecular diagnostics are being developed to provide real-time wound infection monitoring. These strategies are based on quantifying factors secreted by bacteria. A methacrylated gelatin hydrogel was loaded with carboxyfluorescein-containing liposomes to sense membrane-degrading factors secreted by bacteria.39 The fluorescence of this dye is quenched when the liposomes are intact. Upon destabilization of liposomes by bacterial phospholipase and alpha-hemolysin, carboxyfluorescein is released and becomes fluorescent. The liposomal hydrogel showed a strong increase in fluorescence in the presence of pathogenic wound bacteria Escherichia coli and P. aeruginosa in vitro and on mouse wounds in vivo. Another bacteria-sensing bandage was based on a vesicle-containing agarose hydrogel.40 These vesicles were made of synthetic lipids and contained quenched carboxyfluorescein, which became fluorescent after lysis by cytotoxins secreted by biofilm-forming pathogenic bacteria S. aureus, P. aeruginosa, and Enterococcus faecalis in an ex vivo porcine burn wound model.40 While these systems were shown to work well, vesicles loaded with hydrophilic cargo are often leaky in solution and may break during drying procedures; this issue often limits shelf life.48−53 A polyurethane dressing loaded with a lipase-sensitive pro-drug sensed bacterial lipase activity.41 The chromogenic probe contained a lipase-sensitive ester bond which releases the dye hemicyanine after enzymatic cleavage and turns the dressing from yellow to green to red. High lipase-secreting bacteria P. aeruginosa showed a stronger chromogenic response than low lipase-secreting bacteria E. coli in vitro. In an ex vivo experiment, a burn wound on pig skin was inoculated with P. aeruginosa and covered with the wound dressing, and a color change was observed. Bacterial deoxyribonucleases (DNases), enzymes secreted by bacteria to evade neutrophil extracellular traps, were detected by a potentiometric DNA gel.42 This poly(ethylene glycol) diglycidyl ether-cross-linked DNA gel contained a graphene oxide dopant and copper electrodes on a poly(imide) substrate. In the presence of bacterial DNases, the DNA gel is degraded. The loss of the integrity of the dopant-containing hydrogel changes the dielectric permittivity of the region above the electrodes and results in a detectable change in capacitance. The DNase sensor showed a stronger signal change in mouse wounds infected with S. aureus than in noninfected control wounds.
Uric acid is another important biomarker associated with bacterial infection.19 The uric acid concentration of wound fluid is decreased if the wound is colonized by uricase-producing bacteria.19 An enzyme-free design for uric acid sensing was developed where a carbon working electrode was modified with reductive graphene oxide and gold nanoparticles, and uric acid was detected by differential pulse voltammetry.19 Uric acid was sensed with high linearity in vitro. In S. aureus-infected rat wounds, uric acid values of about 350 μM were detected and rose to about 500 μM with antibiotic treatment.19 Several approaches have used uricase-mediated oxidation of uric acid to 5-hydroxyisourate and hydrogen peroxide and electrochemical detection of hydrogen peroxide, but validation studies in animal models are missing.43,54 Uricase was embedded in a cationic derivative of poly(vinyl alcohol) which contained ferrocene as an electron conductor for potentiometric detection.43 This system responded to uric acid in wound fluid extracted from wound dressings worn by patients with chronic wounds.43
Wound hyperglycemia has negative effects on various stromal and immune cells implied in wound healing.8 Glucose is generally sensed by glucose oxidase-mediated catalytic oxidation of glucose to glucuronic acid and hydrogen peroxide followed by electrochemical hydrogen peroxide quantification. A glucose oxidase/Prussian blue-coated carbon working electrode linearly sensed glucose between 0 and 2 mM in vitro and quantified glucose in mouse wounds in vivo.20 Electroactive substances such as uric acid and ascorbic acid did not interfere with the assay.20 Prussian blue is an electrocatalyst and electron donor in the reduction of hydrogen peroxide to hydroxide and acts as a transducer.55 An optical glucose sensor was developed based on a zwitterionic poly(carboxybetaine) hydrogel containing glucose oxidase, horseradish peroxidase (HRP), and an optical HRP substrate.24 HRP used hydrogen peroxide generated by glucose oxidation in the chromogenic and fluorogenic oxidation of its substrate. The signal was detected by a conventional smartphone camera. The wound dressing showed higher wound glucose levels in streptozotocin-induced diabetic mouse wounds than in nondiabetic control wounds.
Monitoring body motions that put mechanical stress on the wound may be predictive of wound healing, as repeated tension and compression negatively affect wound healing. A motion-detecting bandage was developed based on microelectronic circuits in a silk fibroin microneedle-structured dressing.44 When applied on joints of human volunteers, bending movements and bending angle were detected by changes in resistance. Conductive hydrogels are also capable of sensing motion as stretching modifies electric current. A hydrogel dressing made of conductive polymers poly(3-aminophenylboronic acid) and poly(aniline) and the biocompatible polymer poly(vinyl alcohol) sensed bending of finger, elbow, and knee joints of human volunteers and movements of ankle joints of rats with streptozotocin-induced diabetes.45 Another motion-sensing hydrogel was composed of a cross-linked gelatin scaffold with a mixture of the conductive polymers poly(3,4-ethylenedioxythiophene) and poly(styrenesulfonate) and multiwalled carbon nanotubes.46 This hydrogel was applied on joints of human volunteers, and a change in resistance was detected upon bending and flexing.46
Conclusions, Challenges, and Future Developments
A number of diagnostic bandages with novel mechanisms of action are in preclinical development for diabetic wounds. Their sensor systems and target analytes are highly diverse and mainly address the inflammatory phase and bacterial infection. The diversity of these strategies builds confidence that clinicians will soon have molecular diagnostics at their disposal to assess wound healing rate, the need for therapeutic intervention, and evaluate treatment efficacy.
Despite the promising advances in diagnostic bandages, considerable challenges remain in the investigation of diabetic wound pathophysiology and diagnostic target validation. Consensus regarding which animal model best represents human DFU pathophysiology is lacking,56 which may impair clinical translation. Of note is that the majority of diagnostic wound dressings presented here were not tested in diabetic animals. This is problematic as it remains unclear for certain systems if they are sensitive to pathophysiologically relevant analyte concentrations (e.g., ROS-sensing dressings) and effective in diabetic wound fluid.
Diagnostic bandage formulation development has also presented challenges to the field. Sensor materials and the wound dressing matrix need to exhibit low toxicity and low immunogenicity. Release of toxic compounds needs to be avoided in the harsh environment of the wound fluid (high protease activity, acidic or basic pH) to reduce local toxicity and systemic effects after absorption. In addition, diagnostic dressings that remain on the wound over prolonged periods of time need to exert functions of traditional wound dressings, such as exudate uptake to avoid wound maceration. Scaling up manufacturing, high stability for appropriate shelf life, and cost considerations are also important factors in formulation development.8,49,50
Diagnostic bandages aiming at predicting wound healing rate will likely be combinations of different measures including inflammation (pH, temperature, ROS), bacterial infection, and mechanical stress. Ideally, these diagnostic bandages provide information on the entire wound (including wound margins). To select the optimal antibiotic and to reduce the risk of bacterial resistance, it is essential to find biomarkers that are specific to certain bacteria. For treatments that specifically address a single molecular target, companion diagnostics will be needed to select patients with high target expression and to assess treatment response in these patients. For these systems, the selection of meaningful cut-offs will be highly important for patient selection.
We predict that advances in diagnostic bandages will provide new diagnostic options for foot ulcers in the coming decade. Molecular wound diagnostics will pave the way for personalized medicine in diabetic wound healing. Theranostic wound dressings monitoring several wound parameters at once and releasing drugs on demand have the potential to revolutionize DFU diagnostics and treatment and promise to decrease the human and financial burden of DFU if they are cost-effective.
Author Contributions
CRediT: Tracy Fu writing-original draft (equal), writing-review & editing (equal); Polina Stupnitskaia writing-review & editing (supporting); Simon Matoori conceptualization (lead), supervision (lead), writing-original draft (lead), writing-review & editing (lead).
The authors declare no competing financial interest.
Notes
The Figures and Table of Content were created using biorender.
References
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Matoori S. Diabetes and its Complications. ACS Pharmacology & Translational Science 2022, 10.1021/acsptsci.2c00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzis D.; Eleftheriadou I.; Veves A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31 (8), 817–836. 10.1007/s12325-014-0140-x. [DOI] [PubMed] [Google Scholar]
- Nussbaum S. R.; Carter M. J.; Fife C. E.; DaVanzo J.; Haught R.; Nusgart M.; Cartwright D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Heal. 2018, 21 (1), 27–32. 10.1016/j.jval.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Armstrong D. G.; Boulton A. J. M.; Bus S. A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376 (24), 2367–2375. 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- Tchanque-Fossuo C. N.; Dahle S. E.; Lev-Tov H.; West K. I. M.; Li C.; Rocke D. M.; Isseroff R. R. Cellular versus Acellular Matrix Devices in the Treatment of Diabetic Foot Ulcers: Interim Results of a Comparative Efficacy Randomized Controlled Trial. J. Tissue Eng. Regen. Med. 2019, 13 (8), 1430–1437. 10.1002/term.2884. [DOI] [PubMed] [Google Scholar]
- Imam B.; Miller W. C.; Finlayson H. C.; Eng J. J.; Jarus T. Incidence of Lower Limb Amputation in Canada. Can. J. Public Heal. 2017, 108 (4), e374–e380. 10.17269/cjph.108.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B.; Cadarette S.; Wodchis W.; Wong J.; Mittmann N.; Krahn M. Cost-of-Illness Studies in Chronic Ulcers: A Systematic Review. J. Wound Care 2017, 26, S4–S14. 10.12968/jowc.2017.26.Sup4.S4. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Veves A.; Mooney D. J. Advanced Bandages for Diabetic Wound Healing. Sci. Transl. Med. 2021, 13 (585), eabe4839 10.1126/scitranslmed.abe4839. [DOI] [PubMed] [Google Scholar]
- Schaper N. C.; Netten J. J.; Apelqvist J.; Bus S. A.; Hinchliffe R. J.; Lipsky B. A. Practical Guidelines on the Prevention and Management of Diabetic Foot Disease (IWGDF 2019 Update). Diabetes. Metab. Res. Rev. 2020, 36 (S1), e3266 10.1002/dmrr.3266. [DOI] [PubMed] [Google Scholar]
- Santema T. B.; Lenselink E. A.; Balm R.; Ubbink D. T. Comparing the Meggitt-Wagner and the University of Texas Wound Classification Systems for Diabetic Foot Ulcers: Inter-Observer Analyses. Int. Wound J. 2016, 13 (6), 1137–1141. 10.1111/iwj.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A.; Wright-Hughes A.; Backhouse M. R.; Lipsky B. A.; Nixon J.; Bhogal M. S.; Reynolds C.; Brown S. CODIFI (Concordance in Diabetic Foot Ulcer Infection): A Cross-Sectional Study of Wound Swab versus Tissue Sampling in Infected Diabetic Foot Ulcers in England. BMJ. Open 2018, 8 (1), e019437. 10.1136/bmjopen-2017-019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Złoch M.; Maślak E.; Kupczyk W.; Jackowski M.; Pomastowski P.; Buszewski B. Culturomics Approach to Identify Diabetic Foot Infection Bacteria. Int. J. Mol. Sci. 2021, 22 (17), 9574. 10.3390/ijms22179574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutluoglu M.; Uzun G.; Turhan V.; Gorenek L.; Ay H.; Lipsky B. A. How Reliable Are Cultures of Specimens from Superficial Swabs Compared with Those of Deep Tissue in Patients with Diabetic Foot Ulcers?. J. Diabetes Complications 2012, 26 (3), 225–229. 10.1016/j.jdiacomp.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Eming S. A.; Martin P.; Tomic-Canic M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Trans. Med. 2014, 6, 265sr6. 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellechea A.; Leal E. C.; Kafanas A.; Auster M. E.; Kuchibhotla S.; Ostrovsky Y.; Tecilazich F.; Baltzis D.; Zheng Y.; Carvalho E.; Zabolotny J. M.; Weng Z.; Petra A.; Patel A.; Panagiotidou S.; Pradhan-Nabzdyk L.; Theoharides T. C.; Veves A. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65 (7), 2006–2019. 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharidis G.; Veves A. Autonomic Nerve Dysfunction and Impaired Diabetic Wound Healing: The Role of Neuropeptides. Autonomic Neuroscience 2020, 223, 102610. 10.1016/j.autneu.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. M.; Kimball A.; Boniakowski A.; Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diab. Rep. 2018, 18, 1–8. 10.1007/s11892-018-0970-z. [DOI] [PubMed] [Google Scholar]
- Mai H.; Wang Y.; Li S.; Jia R.; Li S.; Peng Q.; Xie Y.; Hu X.; Wu S. A PH-Sensitive near-Infrared Fluorescent Probe with Alkaline p: K a for Chronic Wound Monitoring in Diabetic Mice. Chem. Commun. 2019, 55 (51), 7374–7377. 10.1039/C9CC02289A. [DOI] [PubMed] [Google Scholar]
- Xu G.; Lu Y.; Cheng C.; Li X.; Xu J.; Liu Z.; Liu J.; Liu G.; Shi Z.; Chen Z.; Zhang F.; Jia Y.; Xu D.; Yuan W.; Cui Z.; Low S. S.; Liu Q. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 2021, 31 (26), 2100852. 10.1002/adfm.202100852. [DOI] [Google Scholar]
- Tang N.; Zhang R.; Zheng Y.; Wang J.; Khatib M.; Jiang X.; Zhou C.; Omar R.; Saliba W.; Wu W.; Yuan M.; Cui D.; Haick H. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022, 34 (3), 2106842. 10.1002/adma.202106842. [DOI] [PubMed] [Google Scholar]
- McArdle C.; Lagan K. M.; McDowell D. A. The PH of Wound Fluid in Diabetic Foot Ulcers - the Way Forward in Detecting Clinical Infection?. Curr. Diabetes Rev. 2014, 10 (3), 177–181. 10.2174/1573399810666140609143217. [DOI] [PubMed] [Google Scholar]
- McArdle C.; Lagan K.; Spence S.; McDowell D. Diabetic Foot Ulcer Wound Fluid: The Effects of PH on DFU Bacteria and Infection. J. Foot Ankle Res. 2015, 8 (S1), A8. 10.1186/1757-1146-8-S1-A8. [DOI] [Google Scholar]
- McArdle C. D.; Lagan K. M.; McDowell D. A. Effects of PH on the Antibiotic Resistance of Bacteria Recovered from Diabetic Foot Ulcer Fluid An In Vitro Study. J. Am. Podiatr. Med. Assoc. 2018, 108 (1), 6–11. 10.7547/16-033. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Zhang J.; Song J.; Yang J.; Du Z.; Zhao W.; Guo H.; Wen C.; Li Q.; Sui X.; Zhang L. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of PH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2020, 30 (6), 1905493. 10.1002/adfm.201905493. [DOI] [Google Scholar]
- Gethin G. The Significance of Surface PH in Chronic Wounds. Wounds UK 2007, 3 (3), 52. [Google Scholar]
- Rippke F.; Berardesca E.; Weber T. M. PH and Microbial Infections. Curr. Probl. Dermatol. 2018, 54, 87–94. 10.1159/000489522. [DOI] [PubMed] [Google Scholar]
- Power G.; Moore Z.; O’Connor T. Measurement of PH, Exudate Composition and Temperature in Wound Healing: A Systematic Review. J. Wound Care 2017, 26 (7), 381–397. 10.12968/jowc.2017.26.7.381. [DOI] [PubMed] [Google Scholar]
- Kunkemoeller B.; Kyriakides T. R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27 (12), 823–838. 10.1089/ars.2017.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher D.; Neofytou E.; Wong V. W.; Maan Z. N.; Rennert R. C.; Inayathullah M.; Januszyk M.; Rodrigues M.; Malkovskiy A. V.; Whitmore A. J.; Walmsley G. G.; Galvez M. G.; Whittam A. J.; Brownlee M.; Rajadas J.; Gurtner G. C. Transdermal Deferoxamine Prevents Pressure-Induced Diabetic Ulcers. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (1), 94–99. 10.1073/pnas.1413445112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G. P.; Albiero M.; Menegazzo L.; Boscaro E.; Pagnin E.; Iori E.; Cosma C.; Lapolla A.; Pengo V.; Stendardo M.; Agostini C.; Pelicci P. G.; Giorgio M.; Avogaro A. The Redox Enzyme P66Shc Contributes to Diabetes and Ischemia-Induced Delay in Cutaneous Wound Healing. Diabetes 2010, 59 (9), 2306–2314. 10.2337/db09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. I.; Nguyen T. T.; Peng Z.; Chang M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals 2019, 12 (2), 79. 10.3390/ph12020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A.; Wright-Hughes A.; Backhouse M. R.; Lipsky B. A.; Nixon J.; Bhogal M. S.; Reynolds C.; Brown S. CODIFI (Concordance in Diabetic Foot Ulcer Infection): A Cross-Sectional Study of Wound Swab versus Tissue Sampling in Infected Diabetic Foot Ulcers in England. BMJ. Open 2018, 8 (1), e019437 10.1136/bmjopen-2017-019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I.; Nusbaum A. G.; Gil J.; Patel S. B.; Chen J.; Valdes J.; Stojadinovic O.; Plano L. R.; Tomic-Canic M.; Davis S. C. Interactions of Methicillin Resistant Staphylococcus Aureus USA300 and Pseudomonas Aeruginosa in Polymicrobial Wound Infection. PLoS One 2013, 8 (2), e56846 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldufsky J.; Wood S. J.; Jayaraman V.; Majdobeh O.; Chen L.; Qin S.; Zhang C.; DiPietro L. A.; Shafikhani S. H. Pseudomonas Aeruginosa Uses T3SS to Inhibit Diabetic Wound Healing. Wound Repair Regen. 2015, 23 (4), 557–564. 10.1111/wrr.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters C.; Deleon K.; Trivedi U.; Griswold J. A.; Lyte M.; Hampel K. J.; Wargo M. J.; Rumbaugh K. P. Pseudomonas Aeruginosa Biofilms Perturb Wound Resolution and Antibiotic Tolerance in Diabetic Mice. Med. Microbiol. Immunol. 2013, 202 (2), 131–141. 10.1007/s00430-012-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler M.; Zieker D.; Weinreich J.; Löb S.; Königsrainer I.; Symons S.; Bühler S.; Königsrainer A.; Northoff H.; Beckert S. Wound Fluid Lactate Concentration: A Helpful Marker for Diagnosing Soft-Tissue Infection in Diabetic Foot Ulcers? Preliminary Findings. Diabet. Med. 2011, 28 (2), 175–178. 10.1111/j.1464-5491.2010.03123.x. [DOI] [PubMed] [Google Scholar]
- Pang Q.; Lou D.; Li S.; Wang G.; Qiao B.; Dong S.; Ma L.; Gao C.; Wu Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7 (6), 1902673. 10.1002/advs.201902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Wo F.; Jin Y.; Tan J.; Lai Y.; Wu J. Drug-Porous Silicon Dual Luminescent System for Monitoring and Inhibition of Wound Infection. ACS Nano 2017, 11 (8), 7938–7949. 10.1021/acsnano.7b02471. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Yao D.; Qian Z.; Hou S.; Li L.; Jenkins A. T. A.; Fan Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous In Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. 10.1016/j.biomaterials.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Thet N. T.; Alves D. R.; Bean J. E.; Booth S.; Nzakizwanayo J.; Young A. E. R.; Jones B. V.; Jenkins A. T. A. Prototype Development of the Intelligent Hydrogel Wound Dressing and Its Efficacy in the Detection of Model Pathogenic Wound Biofilms. ACS Appl. Mater. Interfaces 2016, 8 (24), 14909–14919. 10.1021/acsami.5b07372. [DOI] [PubMed] [Google Scholar]
- Singh H.; Li W.; Kazemian M. R.; Yang R.; Yang C.; Logsetty S.; Liu S. Lipase-Responsive Electrospun Theranostic Wound Dressing for Simultaneous Recognition and Treatment of Wound Infection. ACS Appl. Bio Mater. 2019, 2 (5), 2028–2036. 10.1021/acsabm.9b00076. [DOI] [PubMed] [Google Scholar]
- Xiong Z.; Achavananthadith S.; Lian S.; Madden L. E.; Ong Z. X.; Chua W.; Kalidasan V.; Li Z.; Liu Z.; Singh P.; Yang H.; Heussler S. P.; Kalaiselvi S. M. P.; Breese M. B. H.; Yao H.; Gao Y.; Sanmugam K.; Tee B. C. K.; Chen P. Y.; Loke W.; Lim C. T.; Chiang G. S. H.; Tan B. Y.; Li H.; Becker D. L.; Ho J. S. A Wireless and Battery-Free Wound Infection Sensor Based on DNA Hydrogel. Sci. Adv. 2021, 7 (47), 1617. 10.1126/sciadv.abj1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoyChoudhury S.; Umasankar Y.; Jaller J.; Herskovitz I.; Mervis J.; Darwin E.; Hirt P. A.; Borda L. J.; Lev-Tov H. A.; Kirsner R.; Bhansali S. Continuous Monitoring of Wound Healing Using a Wearable Enzymatic Uric Acid Biosensor. J. Electrochem. Soc. 2018, 165 (8), B3168–B3175. 10.1149/2.0231808jes. [DOI] [Google Scholar]
- Gao B.; Guo M.; Lyu K.; Chu T.; He B. Intelligent Silk Fibroin Based Microneedle Dressing (i-SMD). Adv. Funct. Mater. 2021, 31 (3), 2006839. 10.1002/adfm.202006839. [DOI] [Google Scholar]
- Zhao Y.; Li Z.; Song S.; Yang K.; Liu H.; Yang Z.; Wang J.; Yang B.; Lin Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29 (31), 1901474. 10.1002/adfm.201901474. [DOI] [Google Scholar]
- Zheng M.; Wang X.; Yue O.; Hou M.; Zhang H.; Beyer S.; Blocki A. M.; Wang Q.; Gong G.; Liu X.; Guo J. Skin-Inspired Gelatin-Based Flexible Bio-Electronic Hydrogel for Wound Healing Promotion and Motion Sensing. Biomaterials 2021, 276, 121026. 10.1016/j.biomaterials.2021.121026. [DOI] [PubMed] [Google Scholar]
- Duscher D.; Trotsyuk A. A.; Maan Z. N.; Kwon S. H.; Rodrigues M.; Engel K.; Stern-Buchbinder Z. A.; Bonham C. A.; Barrera J.; Whittam A. J.; Hu M. S.; Inayathullah M.; Rajadas J.; Gurtner G. C. Optimization of Transdermal Deferoxamine Leads to Enhanced Efficacy in Healing Skin Wounds. J. Controlled Release 2019, 308, 232–239. 10.1016/j.jconrel.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Bao Y.; Schmidt A.; Fischer E. J.; Ochoa-Sanchez R.; Tremblay M.; Oliveira M. M.; Rose C. F.; Leroux J.-C. An Investigation of PS-b-PEO Polymersomes for the Oral Treatment and Diagnosis of Hyperammonemia. Small 2019, 15 (50), e1902347 10.1002/smll.201902347. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Mooney D. J. Development of a Liposomal Near-Infrared Fluorescence Lactate Assay for Human Blood. Biomaterials 2022, 283, 121475. 10.1016/j.biomaterials.2022.121475. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Leroux J.-C. Twenty-Five Years of Polymersomes: Lost in Translation?. Mater. Horizons 2020, 7 (5), 1297–1309. 10.1039/C9MH01669D. [DOI] [Google Scholar]
- Matoori S.; Mooney D. J. Near-Infrared Fluorescence Hydrogen Peroxide Assay for Versatile Metabolite Biosensing in Whole Blood. Small 2020, 16 (20), 2000369. 10.1002/smll.202000369. [DOI] [PubMed] [Google Scholar]
- Giacalone G.; Matoori S.; Agostoni V.; Forster V.; Kabbaj M.; Eggenschwiler S.; Lussi M.; De Gottardi A.; Zamboni N.; Leroux J.-C. Liposome-Supported Peritoneal Dialysis in the Treatment of Severe Hyperammonemia: An Investigation on Potential Interactions. J. Controlled Release 2018, 278, 57–65. 10.1016/j.jconrel.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Forster V.; Agostoni V.; Bettschart-Wolfensberger R.; Bektas R. N.; Thöny B.; Häberle J.; Leroux J. C.; Kabbaj M. Preclinical Evaluation of Liposome-Supported Peritoneal Dialysis for the Treatment of Hyperammonemic Crises. J. Controlled Release 2020, 328, 503–513. 10.1016/j.jconrel.2020.08.040. [DOI] [PubMed] [Google Scholar]
- Kassal P.; Kim J.; Kumar R.; De Araujo W. R.; Steinberg I. M.; Steinberg M. D.; Wang J. Smart Bandage with Wireless Connectivity for Uric Acid Biosensing as an Indicator of Wound Status. Electrochem. commun. 2015, 56, 6–10. 10.1016/j.elecom.2015.03.018. [DOI] [Google Scholar]
- Karyakin A. A. Prussian Blue and Its Analogues: Electrochemistry and Analytical Applications. Electroanalysis 2001, 13, 813–819. . [DOI] [Google Scholar]
- Tellechea A.; Pradhan-Nabzdyk L.; LoGerfo F. W.; Veves A. Neuropeptides, Inflammation, and Diabetic Wound Healing: Lessons from Experimental Models and Human Subjects. Diabetic Foot 2018, 1, 131–154. 10.1007/978-3-319-89869-8_8. [DOI] [Google Scholar]