Abstract
Bordetella dermonecrotic toxin (DNT) catalyzes the transglutamination of glutamine-63/61 of Rho GTPases, thereby constitutively activating Rho proteins. Here we identified second substrates for transglutamination of RhoA by DNT. The enzymatically active fragment of DNT (residues 1136 to 1451, ΔDNT) induced the incorporation of l-[14C]lysine in RhoA in a concentration-dependent manner. Also, Rac and Cdc42, but not Ras, were transglutaminated with lysine by ΔDNT. Transglutamination of the GTPase with l-lysine inhibited intrinsic and Rho-GAP-stimulated GTP hydrolysis of RhoA. In contrast to lysine, treatment of RhoA with alanine, arginine, and glutamine were not able to substitute for lysine in the transglutamination reaction. DNT increased the incorporation of l-[14C]lysine into embryonic bovine lung cells. Microinjection of GST-RhoA together with the enzymatically active DNT fragment into Xenopus oocytes, subsequent affinity purification of modified GST-RhoA, and mass spectrometry identified attachment of putrescine or spermidine at glutamine-63 of RhoA. A comparison of putrescine, spermidine, and lysine as substrates for DNT-induced transglutamination of RhoA revealed that lysine is a preferred second substrate at least in vitro.
Rho GTPases, including the Rho, Rac, and Cdc42 isoforms, are major regulators of the actin cytoskeleton and act as molecular switches in a large array of signaling events. They are involved in migration and morphogenesis and in secretion and phagocytosis processes, and they play essential roles in cell cycle progression, gene expression, and transformation (7). The GTPases also serve as eukaryotic substrates for various bacterial protein toxins (2, 14, 19). Large clostridial cytotoxins (e.g., Clostridium difficile toxins A and B) inhibit Rho, Rac, and Cdc42 by monoglucosylation at threonine-37 and threonine-35, respectively (10, 11). C31-like exoenzymes (e.g., Clostridium botulinum exoenzyme C3) inhibit the biological functions of the GTPases RhoA, -B, and -C by ADP-ribosylation at asparagine-41 (3, 4, 21). Rho GTPases are not only inhibited but also activated by bacterial toxins. Cytotoxic necrotizing factor 1 (CNF1) and CNF2 from Escherichia coli cause constitutive activation of Rho GTPases by deamidation at glutamine-63 of Rho (glutamine-61 of Rac and Cdc42). Glutamine-63 of Rho is essentially involved in the catalysis of GTP hydrolysis by Rho proteins. Deamidation of this glutamine residue inhibits the GTPase activity of Rho and renders Rho proteins constitutively active.
Another Rho GTPase-activating toxin is dermonecrotic toxin (DNT), which is produced by various Bordetella species (8, 17). DNT induces stress fiber formation, focal adhesion assembly, and tyrosine phosphorylation of focal adhesion kinase and paxillin (8, 9, 13). Recent studies showed that DNT also causes deamidation of Rho GTPases at glutamine-63/61 (16, 20). However, the biological actions (such as cytopathic effects) of DNT differ from those of CNFs (5, 9, 15, 17). Accordingly, it was reported that DNT not only deamidates Rho proteins but also catalyzes the specific transglutamination of GTPases (20). We attempted to identify here cellular substrates for transglutamination of Rho and report that polyamines and lysine are second substrates for transglutamination by DNT.
MATERIALS AND METHODS
Materials.
Small GTPases and p50RhoGAP were prepared from fusion proteins. Because of better expression and stability, the RhoA mutant F25N-RhoA was used with identical results as wild-type protein. Amino acids, spermidine, and putrescine were purchased from Sigma; l-[14C]lysine was from Hartmann Analytics (Braunschweig, Germany), and [14C]ethylenediamine was from Biotrend (Cologne, Germany).
Purification of ΔDNT fragment.
The active DNT fragment (ΔDNT) consisting of amino acid residues 1136 through 1451 was purified as a glutathione S-transferase (GST) fusion protein. Expression of the protein in E. coli BL21 cells, growing at 37°C, was induced by adding 0.2 mM (final concentration) IPTG (isopropyl-β-d-thiogalactopyranoside) at an optical density (OD) of 0.5. At 6 h after induction, cells were collected, lysed by sonication in lysis buffer (20 mM Tris-HCl, pH 7.4; 10 mM NaCl; 5 mM MgCl2 1% Triton X-100), and purified by affinity chromatography with glutathione-Sepharose (Pharmacia). Loaded beads were washed twice in washing buffer A (20 mM Tris-HCl, pH 7.4; 10 mM NaCl; 5 mM MgCl2) and washing buffer B (150 mM NaCl; 50 mM Tris-HCl [pH 7.5]) at 4°C. ΔDNT was eluted from the beads as a GST fusion protein with glutathione (10 mM glutathione, 50 mM Tris-HCl [pH 7.5]) for 10 min at room temperature.
Partial purification of DNT.
Bordetella bronchiseptica strain GS8BB11 obtained from Roy Gross (Wurzburg, Germany) was grown in Steiner-Scholte medium at 37°C. Cells were collected after overnight growth by centrifugation and then lysed by sonication in lysis buffer (20 mM Tris-HCl, pH 7.4; 10 mM NaCl; 5 mM MgCl2; 1% Triton X-100). DNT was partially purified from smaller proteins by centrifugation of the cell lysate through 100-kDa membranes (Microcon; Amicon) and washing with lysis buffer.
Modification of GTPases with GST-ΔDNT.
Small GTPases (1 μg/lane) were incubated with GST-ΔDNT and [14C]lysine (40 μM and 258 mCi/mmol or as indicated) in transglutamination buffer (20 mM Tris-HCl, pH 7.5; 5 mM MgCl2; 8 mM CaCl2; 1mM dithiothreitol; 1 mM EDTA) for 10 min at 29°C. As a control, RhoA was incubated without the toxin but in the presence of cosubstrate. The molar ratio of toxin to GTPases was 1:20. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), radioactively labeled protein bands were detected with a PhosphorImager (Molecular Dynamics).
Competition assay.
Small GTPases were incubated with GST-ΔDNT in the presence of [14C]ethylenediamine (10 μM, 56 mCi/mmol) and increasing concentrations of lysine, putrescine, and spermidine, as indicated, in transglutamination buffer for 10 min at 29°C. The molar ratio of toxin to GTPases was 1:20. Proteins from 20-μl samples were isolated from free ethylenediamine by the addition of 50 μl of bovine serum albumin (10 mg/ml) and subsequent precipitation with 15 volumes of trichloroacetic acid (TCA; 300 mg/ml) for 30 min on ice. Precipitates were filtered through nitrocellulose membranes and then washed once, and incorporated [14C]ethylenediamine was detected by β-counting (shown as the incorporated radioactivity and presented as the mean ± the standard deviation [SD] of three independent experiments). The significance of the data was determined by using the Student's t test.
GTPase assay.
Recombinant Rho proteins were modified by GST-ΔDNT in the presence or absence of primary amines and amino acids. The reaction was stopped after 10 min by freezing in liquid nitrogen. After thawing, the proteins were loaded with [γ-32P]GTP for 5 min at 37°C in loading buffer (50 mM Tris-HCl, pH 7.5; 10 mM EDTA; 2 mM dithiothreitol). MgCl2 (12 mM, final concentration) and unlabeled GTP (2 mM, final concentration) were added. For stimulation of GTPase activity by Rho-GAP, 50 nM p50Rho-GAP was added to 1 μM Rho, and the mixtures were incubated for 4 min at 37°C. The GTPase activity was analyzed by a filter binding assay as described above.
In vivo labeling of Rho proteins.
Subconfluent embryonic bovine lung (EBL) cells were washed with phosphate-buffered saline and cultivated for 24 h in minimal essential Eagle medium without l-lysine and for 1 h with 500 mM 14C-labeled (258 mCi/mmol) l-lysine in the absence or presence of 10 μM cycloheximide. The cells were then treated with partially purified DNT from Bordetella lysates for 1 h and lysed. Proteins were isolated from free l-lysine by filtration through nitrocellulose membranes. Transglutamination was analyzed by measuring the bound radioactivity (shown as the mean of incorporated radioactivity ± SD of two independent experiments. The significance of the data was determined by using the Student's t test).
Purification of GST-RhoA from Xenopus oocytes injected with GST-RhoA and ΔDNT.
Follicle cell-free oocytes of oogenesis stages V or VI were obtained as described earlier (18) and injected with 50-nl aliquots of either GST-RhoA alone, ΔDNT alone, or a mixture of both GST-RhoA and ΔDNT. Oocytes were kept at 19°C in sterile oocyte Ringer solution (ORi; 90 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES [pH 7.4]) supplemented with 50 mg of gentamicin per liter. After overnight incubation, oocytes were washed in Ca2+-free ORi, homogenized in 20 μl per oocyte of buffer A (0.1 M sodium phosphate buffer, pH 8; 0.3 mM MgCl2) supplemented with 0.5% digitonin (Sigma), 10 μM Pefabloc, 5 μM pepstatin, 5 μM antipain, and 10 μM leupeptin. Digitonin extracts were cleared twice by centrifugation (10 min at 10,000 × g and at 4°C), diluted 1.5-fold with buffer A, and supplemented with glutathione-Sepharose 4B beads (Pharmacia). After 1 h of end-over-end mixing at ambient temperature, beads were washed five times with ice-cold buffer A. Proteins were released from the glutathione-Sepharose beads by two rounds of continuous gentle shaking, each round lasting 10 min at 37°C, and with 50 μl of SDS-PAGE sample buffer. Eluted proteins were resolved on linear SDS-polyacrylamide slab gels (8% acrylamide) in parallel with molecular mass markers (Rainbow; Amersham). After staining with Bio-Safe Coomassie Stain (Bio-Rad) and drying, the desired protein bands were excised.
ESI-MS.
Precipitated Rho-proteins separated by one-dimensional SDS-PAGE and stained with Coomassie blue were reduced, alkylated, and digested with trypsin (sequencing grade) as described elsewhere (22). Peptides were extracted and subjected to a single desalting and concentration step as described previously (23). Peptides were eluted twice with 0.5 μl of 60% methanol–5% formic acid into a nano-electrospray needle that was mounted on a Q-TOF1 instrument (Micromass, Manchester, United Kingdom). Tryptic peptide maps of the GST-RhoA treated with toxin and of the untreated control were compared in order to detect modified peptides, which were then sequenced by tandem MS (MS/MS).
Proteolytic digestion of recombinant RhoA in the gel matrix.
RhoA was separated from the toxin by SDS-PAGE. The excised gel plugs of RhoA were destained for 1 h in 40% acetonitrile–60% hydrogen carbonate (50 mM, pH 7.8) in order to remove the Coomassie blue, gel buffer, SDS, and salts. The plug was subsequently dried in a vacuum centrifuge for 15 min. Thereafter, 30 μl of digestion buffer with trypsin was added, and digestion was carried out for 12 h at 37°C. The proteolytic peptide mixture was extracted into 100 μl of 60% acetonitrile overnight at room temperature. Finally, the gel plug was removed and the peptide solution was dried for subsequent matrix-assisted laser desorption ionization (MALDI)-MS analysis.
MALDI-MS.
A saturated matrix solution of recrystallized 4-hydroxy-α-cyanocinnamic acid (Aldrich) in trifluoroacetic acid was freshly prepared, and marker peptides (5 μM ACTH 18–39 clip human and 5 μM angiotensin II human) were added for internal calibration. Matrix and peptide solution were mixed in equal amounts. By using the dried-drop method of matrix crystallization, 1 μl of the sample matrix solution was placed on the MALDI stainless steel target and allowed to air dry for several minutes at room temperature, resulting in a thin layer of fine granular matrix crystals. MALDI–time-of-flight (TOF)–MS was performed on a Bruker Biflex mass spectrometer equipped with a nitrogen laser (λ = 337) to desorb and ionize the samples. Mass spectra were recorded in the reflector positive mode in combination with delayed extraction.
RESULTS
Lysine serves as a second substrate for DNT.
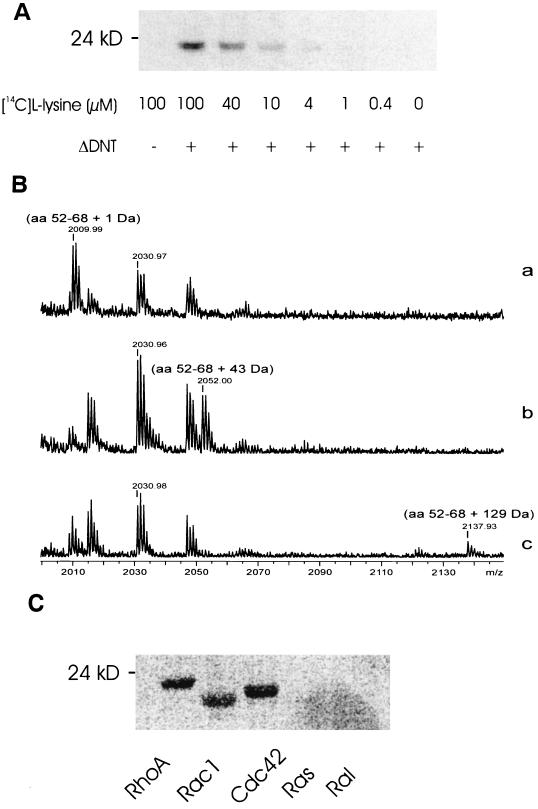
Eukaryotic transglutaminases catalyze cross-linking of glutamine to lysine residues of peptides. The cellular concentration of the amino acid lysine is reportedly in the millimolar range (12). Therefore, we studied whether l-lysine is a second substrate for DNT. To this end, recombinant RhoA (2 μM) was treated with ΔDNT (100 nM) at increasing concentrations of l[14C]lysine. Thereafter, the proteins were separated by SDS-PAGE and analyzed by phosphorimaging. Whereas no labeling was observed without ΔDNT, in the presence of the active toxin fragment the incorporation of label was detected at 1 to 4 μM l-[14C]lysine (Fig. 1A). To analyze the modification of lysine-labeled RhoA in more detail, we applied MALDI-TOF-MS. As a control, we used ethylenediamine which serves as an in vitro substrate for ΔDNT (20). The mass analysis recovered the tryptic RhoA peptides glutamine-52 through arginine-68. After treatment of RhoA with ΔDNT in the absence or presence of ethylenediamine and lysine, the RhoA peptide exhibited increases in mass of 1, 43, and 129 Da, respectively, indicating deamidation, transglutamination with ethylenediamine, or cross-linking with lysine, respectively (Fig. 1B). Thus, l-lysine serves as a second substrate for DNT-catalyzed transglutamination of RhoA, which occurs at glutamine-63. We next studied whether, besides RhoA, other small GTPases are modified by ΔDNT and l-[14C]lysine. As shown in Fig. 1C, Rac and Cdc42, in addition to RhoA, but not GTPases of the Ras family, were cross-linked with l-lysine by ΔDNT.
FIG. 1.
Cross-linking of small GTPases with l-lysine by DNT. (A) Recombinant RhoA (2 μM) was treated with ΔDNT (100 nM) at increasing concentrations of l-[14C]lysine. After incubation, the proteins were separated from free lysine by SDS-PAGE, and the modification was analyzed by phosphorimaging. (B) MALDI-MS spectra in gel digestion of modified RhoA. Gel plugs of RhoA modified in the absence (a) or presence (b) of ethylenediamine or in the presence of lysine (c), were excised and destained for 1 h in 40% acetonitrile–60% hydrogencarbonate (50 mM, pH 7.8). The plugs were subsequently dried in a vacuum centrifuge for 15 min. Thereafter, trypsin digestion was carried out for 12 h at 37°C. (a) Deamidation of glutamine-63 of RhoA by GST-ΔDNT results in a mass shift of the peptide of 1 Da. (b) Transglutamination of glutamine-63 of RhoA by GST-ΔDNT in the presence of ethylenediamine results in a mass shift of the peptide of 43 Da. (c) Transglutamination of glutamine-63 of RhoA by GST-ΔDNT in the presence of lysine results in a mass shift of 129 Da. (Note that the mass is given as mass + H+). (C) The small GTPases RhoA, Rac1, Cdc42, Ras, and Ral were incubated with GST-ΔDNT in the presence of 40 μM l-[14C]lysine. After incubation, the proteins were separated from free lysine by SDS-PAGE, and the modification was analyzed by phosphorimaging (shown).
Cross-linking of RhoA with l-lysine leads to inhibition of GTPase activity.
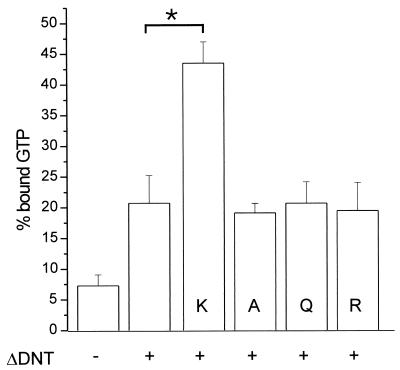
To analyze the functional consequences of the ΔDNT-induced cross-linking of RhoA with different amines, we studied the GTPase activity of RhoA after modification. After incubation of RhoA with DNT and lysine, arginine, glutamine, and alanine for 10 min, the reaction was stopped by freezing the samples in liquid nitrogen. Unlike ΔDNT, RhoA remains fully active after liquid nitrogen treatment, allowing determination of the GTPase activity of RhoA in a filter binding assay. Figure 2 shows the p50GAP-stimulated GTPase activity of modified RhoA. GTP hydrolysis by RhoA, which was incubated with the toxin plus alanine, glutamine, and arginine or with ΔDNT alone was only partially inhibited, indicating deamidation of a small percentage of RhoA molecules. In contrast, the GTPase activity of RhoA treated with the toxin plus l-lysine was inhibited to a large extent. The data indicate that l-lysine serves as substrate for DNT, whereas other amino acids tested are not accepted by the toxin.
FIG. 2.
GTPase activity of modified RhoA. Recombinant Rho proteins were modified by GST-ΔDNT in the presence or absence of amino acids (K, l-lysine; A, alanine; Q, glutamic acid; R, arginine [20 mM each]). The reaction was stopped, and proteins were loaded with [γ-32P]GTP. Thereafter, the GTPase activity was stimulated by adding p50GAP. The hydrolysis of GTP was determined after 4 min by a filter binding assay (shown is the mean of the remaining bound radioactivity as a percentage of loaded radioactivity plus the SD of three independent experiments). Data with l-lysine are significantly different (∗, P < 0.001) from controls.
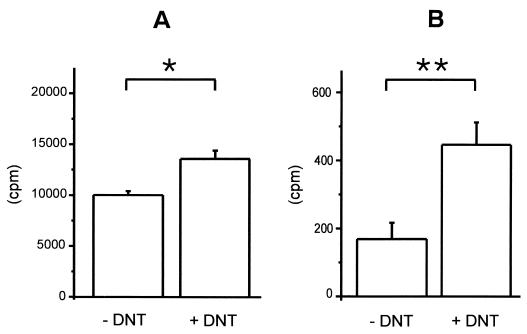
To analyze whether l-lysine modification of RhoA is relevant in mammalian cells, we incubated bovine EBL cells in lysine-free medium. The cells were then incubated with l-[14C]lysine in the absence (Fig. 3A) or presence (Fig. 3B) of cycloheximide as an inhibitor of protein synthesis to avoid unspecific incorporation of l-[14C]lysine into proteins. Afterward, the cells were treated for 1 h with partially purified full-length DNT from Bordetella lysates. Proteins were isolated from lysed cells and free lysine by filtration through nitrocellulose membranes, and the transglutamination was analyzed by measuring the bound radioactivity. When the incubation was performed with cells grown in medium supplemented with unlabeled l-lysine, only a very slight increase in incorporation of l-[14C]lysine was detected after DNT intoxication (not shown). In contrast, in medium without unlabeled l-lysine a significant increase in l-[14C]lysine within the protein fraction was detected after DNT treatment but not without DNT, indicating that an increased incorporation of l-[14C]lysine was induced by DNT in mammalian cells (Fig. 3).
FIG. 3.
Incorporation of l-[14C]lysine by DNT in mammalian cells. EBL cells cultivated for 24 h in medium without l-lysine and for 1 h with l-[14C]lysine in the absence (A) or presence (B) of 10 μM cycloheximide were treated with partially purified DNT from Bordetella lysates for 1 h and lysed. Proteins were isolated from free l-lysine by filtration through nitrocellulose membranes. Transglutamination was analyzed by measuring the bound radioactivity (shown is the incorporated radioactivity as the mean plus the SD of two independent experiments). The P values between DNT-treated samples and the control were determined by using the Student's t test (∗, P < 0.1; ∗∗, P < 0.01).
Analysis of second substrates of DNT in Xenopus oocytes.
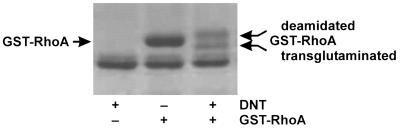
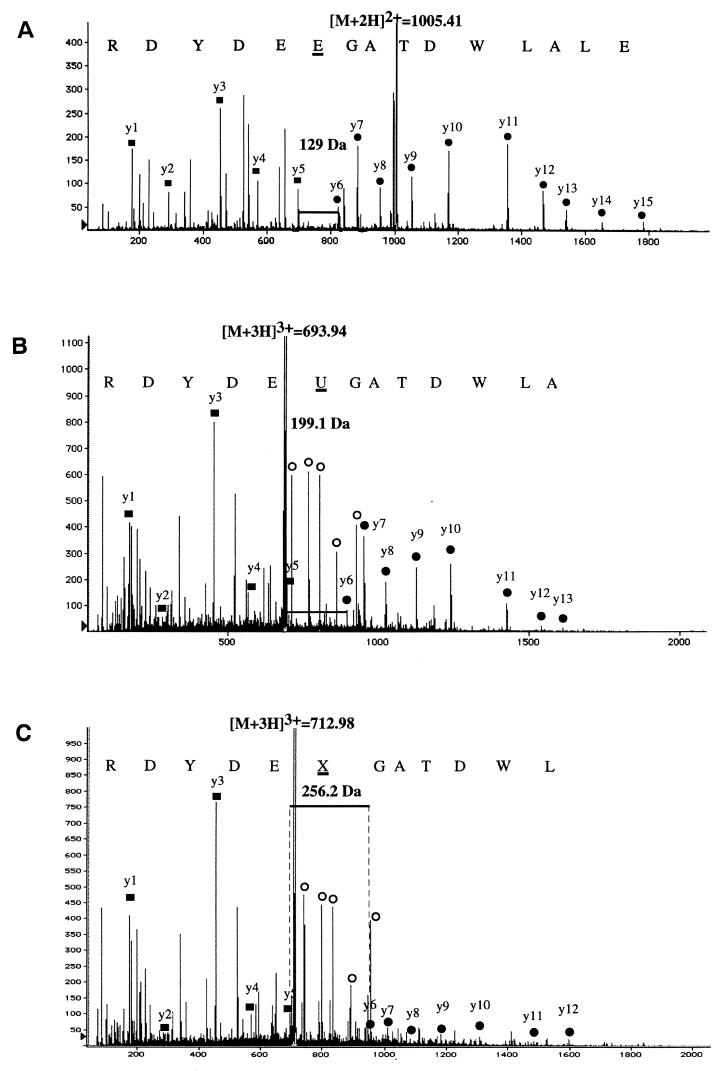
To study the second substrate(s) of DNT, which are cross-linked by transglutamination with Rho GTPase, we injected ΔDNT and recombinant GST-RhoA together or individually into Xenopus oocytes. After overnight incubation, the oocytes were lysed, and GST-RhoA was affinity purified and then analyzed by SDS-gel electrophoresis. As shown in Fig. 4, GST-RhoA from oocytes coinjected with ΔDNT split into two bands, migrating either faster or slower than control GST-RhoA. Both protein bands were cut from the gel and digested with trypsin. The, tryptic peptides of the ΔDNT treated RhoA were then analyzed by MS and peptide sequencing. As shown in Fig. 5A, mass analysis of the tryptic digest of the “upper” band recovered the RhoA peptide glutamine-52–arginine-68 with an increase in mass of 1 Da in comparison with the control, indicating the deamidation of glutamine-63 of RhoA. Deamidation was confirmed by peptide sequencing. The same peptide was identified in the tryptic digest of the “lower” band, thereby exhibiting additional mass increases. Besides the unmodified peptide, a peptide was found to exhibit an increase in mass of 71 Da, indicating the addition of putrescine. Sequencing of the peptide identified a modified glutamine-63 with a mass of 199.1 Da that could correspond to transglutamination with putrescine (128.0 + 88.1 − 17 = 199.1; Fig. 5B). A further peptide was identified and sequenced, exhibiting a mass increase of about 128 Da, indicating transglutamination with spermidine (128.0 + 145.2 − 17 = 128.2; Fig. 5C). In Xenopus oocytes, we could identify putrescine and spermidine as possible second substrates for transglutamination of RhoA by DNT.
FIG. 4.
Deamidation and transglutamination of RhoA by ΔDNT in Xenopus oocytes. ΔDNT and recombinant GST-RhoA were injected together or individually into Xenopus oocytes. After overnight incubation, oocytes were lysed, and GST-RhoA was affinity purified and analyzed by SDS-gel electrophoresis. GST-RhoA from oocytes coinjected with ΔDNT split into two bands, which migrated either faster or slower than control GST-RhoA, indicating transglutamination and deamidation, respectively.
FIG. 5.
Mass analysis and sequencing of the tryptic digests of proteins shown in Fig. 4. (A) Analysis of the “upper” band recovered RhoA peptide glutamine-52–arginine-68 with an increase in mass of 1 Da (2,008.8 Da) compared to the control (2,007.8 Da), indicating deamidation of glutamine-63 of RhoA. (B) Identification of a modified glutamine-63 of 199.1 Da (“U”) from the RhoA peptide glutamine-52–arginine-68 obtained from the “lower” band of modified RhoA which corresponds to transglutamination of glutamine-63 with putrescine (128.0 + 88.1 − 17 = 199.1). (C) Identification of a modified glutamine-63 of 256.2 Da (“X”) from the RhoA peptide glutamine-52–arginine-68 obtained from the “lower” band of modified RhoA which could correspond to transglutamination of glutamine-63 with spermidine (128.0 + 145.2 − 17 = 256.2).
l-Lysine is a preferential substrate of DNT in vitro.
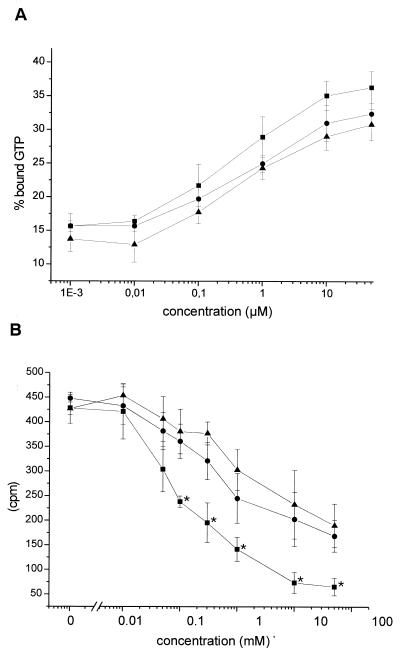
We next compared the transglutamination catalyzed by ΔDNT with the various amines in a time course by measuring inhibition of GTP hydrolysis. As shown in Fig. 6A, the DNT-induced modification of RhoA, as measured by inhibition of GTP hydrolysis, increased with higher lysine, putrescine, and spermidine concentrations and was slightly more elevated in the presence of l-lysine than with spermidine or putrescine, suggesting a preference for l-lysine as substrate of DNT. In the GTPase assay both deamidation and transglutamination were analyzed, because both modifications are catalyzed by DNT and block GTP hydrolysis of the GTPase. Thus, the difference between the substrates may be masked by deamidated RhoA. To further compare the substrate properties of lysine and polyamines, we performed competition experiments with [14C]ethylenediamine as the labeled substrate with increasing concentrations of lysine, putrescine, and spermidine. Figure 6B shows that with 0.1 mM l-lysine, the incorporation of [14C]ethylenediamine is reduced by about 50% compared to cross-linking in the absence of any competitor. In contrast, between 1 and 5 mM putrescine and spermidine, respectively, were needed for the same extent of competition of labeling by [14C]ethylenediamine (Fig. 6B). From all of the substrates studied, l-lysine was the strongest competitor of ethylenediamine.
FIG. 6.
Substrate preference of ΔDNT. (A) GTPase activity. Small GTPases were incubated with GST-ΔDNT in the presence of increasing concentrations of lysine (■), putrescine (●), or spermidine (▴) as indicated. After incubation, the reaction was stopped, and the proteins were loaded with [γ-32P]GTP. GTPase activity was stimulated by adding p50GAP. The hydrolysis of GTP was determined by a filter binding assay (shown is the remaining bound radioactivity as a percentage of the loaded radioactivity as the mean ± the SD of three independent experiments). (B) Competition of l-lysine, putrescine, and spermidine with [14C]ethylenediamine as the substrate for DNT. Small GTPases were incubated with GST-ΔDNT in the presence of [14C]ethylenediamine and increasing concentrations of lysine (■), putrescine (●), or spermidine (▴) as indicated. Proteins were isolated from free ethylenediamine by precipitation with TCA and filtration. Incorporation of [14C]ethylenediamine was detected by counting (shown is the incorporated radioactivity as the mean ± the SD of three independent experiments). The data in the presence of l-lysine were significantly different (∗, P < 0.1) from those in the presence of putrescine (or spermidine).
DISCUSSION
Transglutaminases are widely distributed in mammalian cells and tissues. In general, they are cross-linking enzymes catalyzing the exchange of an amide group of γ-carboxamide of glutamine residues for primary amines (e.g., peptide-bound lysine residues or polyamines) to form ɛ-(γ-glutamyl)lysine or (γ-glutamyl)polyamine bonds (1, 6). Recently, it was shown that the Bordetella DNT acts preferentially as a transglutaminase to modify specifically Rho GTPases (20). In contrast to mammalian transglutaminases, no cross-linking of Rho molecules to each other occurs and no typical transglutaminase substrates such as casein or fibronectin are modified by the toxin (20).
Since transglutaminases cross-link glutamine and lysine residues of polypeptides, we sought to determine whether free lysine might be a second substrate for transglutamination by DNT. This question is especially relevant because the cellular concentration of the potential second substrate lysine is reportedly in the millimolar range (12). Using radioactively labeled l-lysine and MALDI-TOF-MS analysis, we were able to detect cross-linking of RhoA with l-lysine by ΔDNT in vitro.
To analyze the second substrates of DNT which are cross-linked with RhoA in eukaryotic cells, we injected the catalytic domain (ΔDNT), together with recombinant GST-RhoA, into Xenopus oocytes and subsequently isolated GST-RhoA by affinity purification. Besides deamidation, MS analysis of the protein led to the identification of putrescine and spermidine as possible polyamine substrates for transglutamination of glutamine-63 by DNT but not for transglutamination of lysine. These data corroborate recent studies by Horiguchi and coworkers (16). The reason we were not able to identify lysine as a second substrate in the Xenopus system is not clear. It may be due to a lower lysine concentration in Xenopus oocytes compared to mammalian cells or to easier detection of polyamine-modified peptides by MS (Fig. 1B).
To compare the substrate properties of putrescine, spermidine, and lysine, we measured the inhibition of the GTP hydrolytic activity of RhoA with increasing concentrations of these compounds. In this assay, lysine was slightly more efficient as a substrate of DNT than was spermidine or putrescine (not shown). However, differences between the substrates may be masked, because in the GTPase assay deamidation, as well as transglutamination, is analyzed, and both modifications are catalyzed by DNT and lead to the inhibition of the GTP hydrolytic activity (16, 20). To further compare the substrate properties of lysine and polyamines, competition experiments with [14C]ethylenediamine were performed. We observed that l-lysine is about 10 times more effective in preventing [14C]ethylenediamine incorporation than putrescine and spermidine, indicating that lysine is the preferred substrate for transglutamination by DNT compared to the polyamines putrescine and spermidine. In addition, our studies with radiolabeled lysine indicate that this amino acid is also a substrate for transglutamination by DNT in mammalian cells.
Detection of different second substrates for transglutamination of RhoA by DNT generates the question of the preferential substrate in intact cells. Most likely, the cellular concentrations of these agents define their substrate properties. Polyamines, such as putrescine and spermidine, are present in many cells in submillimolar concentrations. The cellular concentration of lysine appears to be the same or greater than that of polyamines and is reportedly in the millimolar range (12). For example, in granulocytes the concentration of lysine is 5- and 10-fold higher than that of putrescine and spermidine, respectively (Wissenschaftliche Tabellen Geigy; CIBA-Geigy, Basel, Switzerland). Furthermore, the concentration of unbound substrate is probably critical for transglutamination. The free concentration of polyamines is much lower than the total concentration due to the binding of RNA and DNA. Therefore, we assume that lysine is a preferred substrate for the modification of Rho GTPases by DNT in many cells.
It is well established that the deamidation of glutamine-63 of RhoA inhibits GTP hydrolysis and transfers the GTPase into a persistently active state. Thus far, the functional consequences of transglutamination in vivo are not that clear. Like deamidation, transglutamination of RhoA with polyamines (16, 20) and lysine (shown here) inhibits the intrinsic and GTPase-activating-protein-stimulated GTP hydrolysis. However, the interaction of transglutaminated RhoA with effectors might be more complex. Matsuda et al. (16) reported that polyaminated RhoA interacts with the Rho-binding domain of the effector Rho kinase (Rock) even in the GDP-bound form. However, no increase in kinase activity by modified RhoA was observed, and no attempt was made to separate deamidated and polyaminated Rho. It has been shown that DNT-treated cells have an initial downward shift of Rho in SDS-PAGE, which is followed by an upward shift after several hours (16). Findings described recently (9, 16, 20) and those shown here indicate that the downward shift of RhoA is caused by transglutamination and that the upward shift is caused by deamidation. Therefore, both types of Rho modifications induced by DNT are governed by different time dependencies. In this respect it is noteworthy that the morphological changes typical for Rho activation by DNT and CNF, such as stress fiber formation, flattening of cells, increase in cell size, and multinucleation, appear to occur later with DNT than with CNF (5, 9, 15, 17). Moreover, the morphological changes induced by DNT and CNF are different in some cell types, e.g., in EBL cells, DNT causes an initial rounding up of cells (17), whereas CNF induces cell flattening (G. Schmidt, unpublished observation). It remains to be studied whether the two types of Rho modification (deamidation and transglutamination) are the underlying mechanisms for the different morphological changes and functional consequences.
ACKNOWLEDGMENTS
We thank Bradley Stiles for critically reading a previous version of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 388).
REFERENCES
- 1.Aeschlimann D, Paulsson M. Transglutaminases: protein cross-linking enzymes in tissue and body fluids. Thromb Haemost. 1994;71:402–415. [PubMed] [Google Scholar]
- 2.Aktories K. Bacterial toxins that target Rho proteins. J Clin Investig. 1997;99:827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktories K, Rosener S, Blaschke U, Chatwal G S. Botulinum ADP-ribosyltransferase C3. Purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988;172:445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- 4.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein rho C is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilament in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanié L, Oswald E, Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 6.Folk J E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 8.Horiguchi Y, Inoue N, Masuda M, Kashimoto T, Katahira J, Sugimoto N, Matsuda M. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc Natl Acad Sci USA. 1997;94:11623–11626. doi: 10.1073/pnas.94.21.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiguchi Y, Senda T, Sugimoto N, Katahira J, Matsuda M. Bordetella bronchiseptica dermonecrotizing toxin stimulates assembly of actin stress fibers and focal adhesions by modifying the small GTP-binding protein Rho. J Cell Sci. 1995;108:3243–3251. doi: 10.1242/jcs.108.10.3243. [DOI] [PubMed] [Google Scholar]
- 10.Just I, Selzer J, Von Eichel-Streiber C, Aktories K. The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J Clin Investig. 1995;95:1026–1031. doi: 10.1172/JCI117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 12.Kabus P, Koch G. Quantitiative determination of amino acids in tissue culture cells by high performance liquid chromatography. Biochem Biophys Res Commun. 1982;108:783–790. doi: 10.1016/0006-291x(82)90897-x. [DOI] [PubMed] [Google Scholar]
- 13.Lacerda H M, Pullinger G D, Lax A J, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21rho-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in swiss 3T3 cells. J Biol Chem. 1997;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- 14.Lerm M, Schmidt G, Aktories K. Bacterial protein toxins targeting Rho GTPases. FEMS Microbiol Lett. 2000;188:1–6. doi: 10.1111/j.1574-6968.2000.tb09159.x. [DOI] [PubMed] [Google Scholar]
- 15.Lerm M, Selzer J, Hoffmeyer A, Rapp U R, Aktories K, Schmidt G. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1 (CNF1): activation of c-Jun-N-terminal kinase in HeLa cells. Infect Immun. 1998;67:496–503. doi: 10.1128/iai.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda M, Betancourt L, Matsuzawa T, Kashimoto T, Takao T, Shimonishi Y, Horiguchi Y. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 2000;19:521–530. doi: 10.1093/emboj/19.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullinger G D, Adams T E, Mullan P B, Garrod T I, Lax A J. Cloning, expression, and molecular characterization of the dermonecrotic toxin gene of Bordetella spp. Infect Immun. 1996;64:4163–4171. doi: 10.1128/iai.64.10.4163-4171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmalzing G, Gloor S, Omay H, Kröner S, Applehans H, Schwarz W. Upregulation of sodium pump activity in Xenopus laevis oocytes by expression of heterologous β1 subunits of the sodium pump. Biochem J. 1991;279:329–336. doi: 10.1042/bj2790329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt G, Aktories K. Bacterial cytotoxins target Rho GTPases. Naturwissenschaften. 1998;85:253–261. doi: 10.1007/s001140050495. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt G, Goehring U-M, Schirmer J, Lerm M, Aktories K. Identification of the C-terminal part of Bordetella dermonecrotic toxin as a transglutaminase for Rho GTPases. J Biol Chem. 1999;274:31875–31881. doi: 10.1074/jbc.274.45.31875. [DOI] [PubMed] [Google Scholar]
- 21.Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- 22.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 23.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]