Abstract
[11C]Carfentanil ([11C]CFN) is a selective radiotracer for in vivo positron emission tomography imaging studies of the μ-opioid system that, in our laboratories, is synthesized by methylation of the corresponding carboxylate precursor with [11C] MeOTf, and purified using a C2 solid-phase extraction cartridge. Changes in the commercial availability of common C2 cartridges have necessitated future proofing the synthesis of [11C]CFN to maintain reliable delivery of the radiotracer for clinical imaging studies. An updated synthesis of [11C]CFN is reported that replaces a now obsolete purification cartridge with a new commercially available version and also substitutes the organic solvents used in traditional production methods with ethanol.
Keywords: C-11, carbon-11, opioid, PET radiochemistry, radiosynthesis
1 ∣. INTRODUCTION
[11C]Carfentanil ([11C]CFN) has been widely used for pre-clinical and clinical positron emission tomography (PET) imaging studies of the μ-opioid system since its introduction at Johns Hopkins University in the early 1980s.1,2 Reliable methods for quantification of [11C]CFN binding are available,3 and recent work suggests that the radiotracer binds preferentially to μ-opioid receptor subtype 1, compared4 to subtype 2. Our physician colleagues have used [11C]CFN to investigate the role of the μ-opioid system in tobacco smoking,5 obesity and weight loss,6 pain regulation as well as sex and genetic influences on these mechanisms,7-11 social distress and reward,12 drug addiction,13,14 and migraine15 and to understand emotion regulation and the pathophysiology and treatment responses in major depression.16-18 We have also recently used [11C]CFN to compare the effects of intranasal and intravenous administration of opioids in rhesus monkeys.19 These studies have shown the displaceability of this radiotracer by competition with endogenous opioid neurotransmitter, enabling its use to measure not only μ-opioid receptor availability in vivo but also the release of endogenous opioids with suitable challenges (eg, pain, transcranial direct current stimulation, and psychological challenges). As a potent and selective μ-opioid receptor agonist, it is imperative that both radioactivity and mass of carfentanil administered to subjects are carefully controlled. In the case of radioactivity, biodistribution studies in rodents have been described,20,21 while human dosimetry of [11C]CFN has been reported by Newberg and colleagues.22 Newberg confirmed that the radiation dosimetry of [11C]CFN was suitable for PET imaging studies. In a typical imaging study, our patients receive 15 to 18 mCi of [11C]CFN, and this dose (in combination with the dosimetry) permits multiple injections for test-retest protocols during 1 hospital visit. The CFN is estimated to be several thousand times more potent than morphine,23 with potential for abuse and/or toxicity, even at low doses. For example, the appearance of CFN in illicit US drug markets recently has lead to an increase in overdoses and overdose-related deaths across the country.24 Reflecting this, we also carefully control injection limits of unlabeled drug associated with radiotracer doses used in clinical PET studies, and our mass limits are ≤0.03 μg/kg, consistent with dose limits used at other institutions using [11C]CFN.22 This corresponds to 2.1 μg or 5.3 nanomoles, administered to an average 70 kg subject. It is therefore necessary that reliable radiosyntheses of high specific activity [11C]CFN are in place to make doses for clinical PET imaging applications, and we have set a specific activity release criteria of 4000 Ci/mmol (see Section 2). In hundreds of administrations of this radiotracer using the protocols of the above manuscripts (ie, 50/50 bolus/continuous infusion and mass dose limits of ≤0.03 μg/kg) across a large range of volunteers, both healthy and with disease processes, we have detected no objective or subjective pharmacological effects associated with [11C]CFN. This data, in conjunction with the reported dosimetry,22 support use of [11C]CFN as a safe imaging agent for μ-opioid receptors.
Radiochemistry production facilities typically synthesize [11C]CFN by methylation of the carboxylate precursor. In early syntheses, this was accomplished using [11C]MeI and the resulting [11C]CFN could be purified using semipreparative HPLC1,21 or solid-phase extraction (SPE).25 More recently, groups often methylate the precursor using [11C]MeOTf instead and purify by SPE20,26-28 according to the method developed by Jewett.29 This approach employs a C2 SPE cartridge for purification. This is critical because, while other cartridges such as C8 and C18 trap [11C]CFN, they also retain significant quantities of desmethyl-carfentanil precursor, which coelutes with [11C]CFN and contaminates the PET drug. The choice of C2 cartridge is also critical, since some of them retain a polar radioactive impurity (likely [11C]MeOH or related impurity) which also coelutes with [11C]CFN and, historically, we have used 3M Empore C2 extraction disks to purify the radiotracer.26 However, in recent years, the 3M Empore C2 cartridges have been discontinued necessitating development of alternative approaches for purifying [11C]CFN. We report an updated synthesis of [11C]CFN that uses Agilent Bond Elut C2 cartridges to accomplish the purification. This new synthesis also replaces the organic solvents used in traditional production methods with ethanol, keeping the production of [11C] CFN in line with our ongoing efforts to simplify radiochemical syntheses and implement pharmaceutical quality by design in our PET drug manufacturing facility.30-32
2 ∣. EXPERIMENTAL
2.1 ∣. General considerations
Desmethyl-carfentanil precursor was custom synthesized by Sigma Aldrich. Unlabeled carfentanil reference standard was obtained from our existing in house inventory or appropriate commercial supplier (eg, Cayman Chemical, Product No. 19410; https://www.caymanchem.com/product/19410). Carfentanil is a schedule II substance that needs to be handled according to local-controlled substance regulations (eg, Drug Enforcement Agency in the United States). Caution should also be used when working with carfentanil in the laboratory, including procedures such as making up standard solutions. Because of the possibility of absorption through the skin, gloves and other personal protective equipment should be used. The standard should be handled in a manner consistent with its material safety data sheet and with the same care and attention as any other hazardous laboratory chemical.
2.2 ∣. Synthesis of [11C]CFN
[11C]CFN was prepared in a TRACERlab FXC-Pro synthesis module configured as previously reported26 and fitted with an Agilent Bond Elut C2 cartridge (conditioned with ethanol [5 mL] and water [10 mL] prior to use). The synthesis module was loaded as follows: reaction vessel: desmethyl carfentanil—tetrabutyl ammonium salt (0.4 mg in ethanol [100 μL]); vial 1:1% ammonium hydroxide (1 mL); vial 3: 20% ethanol (3 mL); vial 4: Milli-Q Water (10 mL); vial 5: ethanol (0.5 mL); vial 6: Sterile Water for Injection (9.5 mL); round-bottomed dilution flask: 1% ammonium hydroxide (5 mL). [11C]CO2 (~3 Ci) was produced using a GE PETTrace cyclotron and converted to [11C]MeOTf (~1 Ci) as previously described.26 [11C]MeOTf was then bubbled through the precursor solution at 15 mL/min at room temperature for 3 minutes. After this time, 1% NH4OH (1 mL) was added to the reaction vessel. This crude reaction mixture was then transferred to the dilution flask where it was further diluted with 1% NH4OH (5 mL). This mixture was passed through the Agilent Bond Elut C2 cartridge to trap [11C]CFN. The cartridge was washed with 20% EtOH (3 mL), followed by Milli-Q water (10 mL), to remove impurities from the cartridge, and dried for 1.0 minute with He gas. [11C]CFN was eluted with EtOH (0.5 mL) and diluted with Sterile Water for Injection, USP (9.5 mL). The formulated product was then passed through a Millipore-GV 0.22-μm filter into a sterile dose vial and submitted for quality control (QC) testing.
2.3 ∣. Quality control testing
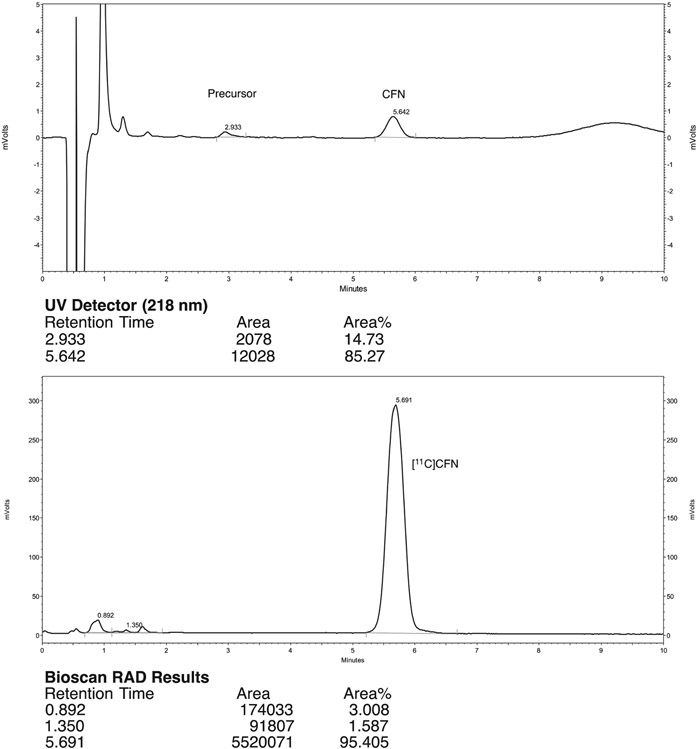
Quality control of [11C]CFN was conducted according to the guidelines outlined in chapter 823 of the US Pharmacopeia and previously reported.26,30 HPLC analysis is described in Section 2.3.1, including a representative analytical HPLC trace (Figure 1). Results for 3 process verification batches using the new synthesis and purification methods are summarized in Table 1. Doses met all acceptance criteria confirming their suitability for clinical use and validating the synthesis method for future production.
FIGURE 1.
Typical analytical HPLC trace for [11C]carfentanil ([11C]CFN) prepared using the new synthesis method
TABLE 1.
QC data for process verification batches of [11C]carfentanil
| QC Test | Release Criteria | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|---|
| Visual inspection | Clear, colorless, free of particulates | Pass | Pass | Pass |
| pH | 4.5-8.0 | 4.5 | 5.0 | 5.0 |
| Radioactivity conc. | >50 mCi/10 mL | 130 | 112 | 147 |
| Mass CFN μg/mL) | N/A | 0.36 | 0.18 | 0.18 |
| Mass precursor μg/mL) | N/A | 0.18 | 0.18 | 0.18 |
| Total mass conc. (CFN + precursor) | ≤ 3.0 μg/mL | 0.54 | 0.36 | 0.36 |
| Specific activity | ≥4000 Ci/mmol | 8918 | 15 057 | 32 218 |
| Radiochemical purity | >90% | 95 | 99 | 96 |
| Radiochemical identity | RRTa = 0.9-1.1 | 1.03 | 1.03 | 1.00 |
| Radionuclidic identity | t1/2 = 18.4-22.4 min | 20.3 | 20.2 | 20.2 |
| Filter integrity | >44 psi | 49 | 49 | 50 |
| Bacterial endotoxins | <17.5 EU/mLb | <2.00 | <2.00 | <2.00 |
| Sterility | Sterile | Sterile | Sterile | Sterile |
Abbreviations: CFN, carfentanil; QC, quality control.
RRT, relative retention time (tR [11C]CFN / tR of [12C]CFN reference standard).
EU, endotoxin units.
2.3.1 ∣. HPLC analysis
Chemical and radiochemical purities were analyzed using a Shimadzu LC2010 HPLC equipped with a Bioscan/Eckert and Ziegler radioactivity detector and an ultraviolet detector. Column: Phenomenex Luna C8(2) 5 μ, 100 x 2.0 mm; mobile phase: 35% MeOH: 65% 20mM NH4OAc; pH 4.5; flow rate: 0.8 mL / min; oven: 40°C; ultraviolet: 218 nm; typical trace: Figure 1 (tR[Precursor] = 3.0 minutes; tR[Carfentanil] = 5.5-6.0 minutes; limit of detection for standard and precursor = 0.05 μg/mL; limit of quantitation for standard and precursor = 0.18 μg/mL).
3 ∣. RESULTS AND DISCUSSION
In the synthesis of [11C]CFN historically used in our laboratory, the desmethyl precursor was dissolved in dimethyl sulfoxide (DMSO) and reacted with [11C]MeOTf, and the SPE cartridges used to collected [11C]CFN were washed with 10% n-propanol.26 This existing method typically produces >200 mCi of [11C]CFN (Table 2, entry 1). We used this method to synthesize a number of batches of [11C]CFN to test out possible purification cartridges with which to replace the discontinued 3 M Empore C2 cartridges. Initially, we tested Waters tC2 and HLB cartridges. The cartridges adequately trapped and released [11C]CFN, and doses met the required radiochemical purity specification of >90%. However, both tC2 (entries 2 and 3) and HLB (Entry 4) cartridges also trapped appreciable amounts of precursor (>150 μg / 10 mL dose), which contaminated final doses. Changing direction, we sourced Bond Elut C2 cartridges loaded with 50 and 100 mg of resin from Agilent. The 50 mg cartridges proved inadequate in trapping [11C]CFN, such that we observed a marked decline in product yield (entry 5). Gratifyingly, the 100-mg cartridges were found to be acceptable substitutes for discontinued 3M Empore C2 cartridges. They trapped and released [11C]CFN as expected but did not retain appreciable amounts of precursor or any polar radiochemical impurities (entry 6).
TABLE 2.
Purification of [11C]CFN using different SPE cartridges
| Cartridge | na | Reaction/Reformulation Solvents | Yield, mCi | Cold mass, μg/mL | Precursor, μg/mL | RCP, % | |
|---|---|---|---|---|---|---|---|
| 1 | 3M Empore C2 (45 mg) | 10 | DMSO/10%n-PrOH | 203 ± 29 | 0.28 ± 0.2 | 0.23 ± 0.2 | 97 ± 1 |
| 2 | Waters tC2 (145 mg) | 1 | DMSO/10%n-PrOH | 210 | < 0.5 | > 15 | 93 |
| 3 | Waters tC2 (100 mg) | 1 | DMSO/10%n-PrOH | 211 | 0.61 | 15.6 | 97 |
| 4 | Waters HLB (10 mg) | 1 | DMSO/10%n-PrOH | 230 | < 0.5 | > 15 | 90 |
| 5 | Agilent C2 (50 mg) | 3 | DMSO/10%n-PrOH | 109 ± 6 | < 0.2 | 0.09 ± 0.009 | 97 ± 1 |
| 6 | Agilent C2 (100 mg) | 3 | DMSO/10%n-PrOH | 175 ± 20 | 0.32 ± 0.2 | 0.12 ± 0.1 | 95 ± 1 |
| 7 | Agilent C2 (100 mg) | 2 | EtOH/20%EtOH | 164 ± 20 | < 0.2 | 0.09 ± 0.0 | 96 ± 1 |
Abbreviations: RCP, radiochemical purity; SPE, solid-phase extraction.
When multiple experiments were conducted, results are reported as mean ± standard deviation.
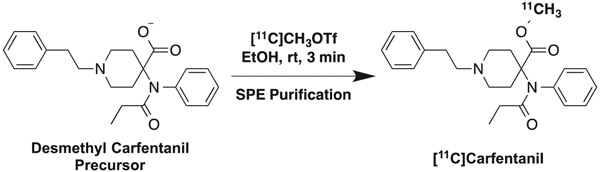
In recent work developing greener approaches to carbon-11 radiochemistry,30,31 we were able to replace the reaction solvent with ethanol and rinse SPE cartridges with 20% ethanol (Scheme 1). Since the synthesis uses ethanol as the only organic solvent, this negates the need to conduct residual solvent analysis (RSA) during QC testing of final doses per updates to chapter 823 of the US Pharmacopeia. Using this method, in conjunction with the Agilent C2 (100 mg) purification cartridge yielded 164 ± 20 mCi of [11C]CFN (Table 2, entry 7). While this is somewhat lower than the corresponding syntheses using either the 3M Empore C2 or Agilent C2 cartridges and DMSO/n-PrOH reaction/reformulation solvents (Table 2, entries 1 and 6), it yields adequate amounts of product for our clinical PET studies and has attractive operationally simplicity stemming from elimination of RSA during QC testing. If higher yields are required, DMSO/n-PrOH be used instead, but RSA testing would be necessary during QC. Finally, to qualify the new synthesis and purification strategy for production of clinical doses, we conducted 3 process verification batches. [11C]MeOTf was bubbled through a solution of desmethyl carfentanil precursor (as the tetrabutyl ammonium salt, which was prepared as previously described30) dissolved in ethanol (0.4 mg in 100 μL ethanol). Following synthesis, the crude reaction mixture was diluted into 6 mL of 1% ammonium hydroxide and passed through an Agilent Bond Elut C2 cartridge, where the [11C]CFN was trapped. The cartridge was washed with 20% ethanol to remove unreacted precursor and polar radiochemical purities. [11C]CFN was then eluted from the cartridge with EtOH (0.5 mL) and diluted with Sterile Water for Injection, USP (9.5 mL). The dose was then passed through a 0.22 μm sterile filter into a sterile glass vial to yield 130 ± 18 mCi of isolated and formulated [11C]CFN at the end of synthesis. The nondecay corrected radiochemical yield based upon 3 Ci of [11C]CO2 = 4 ± 1%, radiochemical purity = 97 ± 2% and specific activity = 18 731 ± 12 077 Ci/mmol, n = 3. Synthesis time was approximately 35 minutes, the same as our previously reported synthesis of [11C]CFN. All doses were submitted for QC testing (see Section 2) and met all acceptance criteria confirming their suitability for clinical use (Table 1). Radiochemical purity and pH of batches were also reanalyzed 2-hour post-end of synthesis, confirming doses were stable and allowing us to assign a 2-hour expiration time to doses of [11C]CFN.
SCHEME 1.
Synthesis of [11C]carfentanil
4 ∣. CONCLUSIONS
In summary, the process for synthesizing [11C]CFN has been updated to eliminate hazardous organic solvents and avoid continued use of SPE cartridges which are no longer commercially available. As [11C]CFN remains the radiotracer of choice for PET imaging of the μ-opioid system, the new method described herein will ensure continued reliable availability of [11C]CFN for future clinical imaging studies.
ACKNOWLEDGEMENT
Financial support of this work by the University of Michigan Department of Radiology is gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors do not report any conflict of interest.
REFERENCES
- 1.Dannals RF, Ravert HT, Frost JJ, Wilson AA, Burns HD, Wagner HN. Radiosynthesis of an opiate receptor binding radiotracer: [11C]carfentanil. Int J Appl Radiat Isot. 1985;36(4):303–306. [DOI] [PubMed] [Google Scholar]
- 2.Frost JJ, Wagner HN Jr, Dannals RF, et al. Imaging opiate receptors in the human brain by positron tomography. J Comp Asst Tomog. 1985;9(2):231–236. [DOI] [PubMed] [Google Scholar]
- 3.Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ. Quantification of brain μ-opioid receptors with [11C]carfentanil: reference tissue methods. Nucl Med Biol. 2003;30:177–186. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson O, Antoni G. [11C]Carfentanil binds preferentially to μ-opioid receptor subtype 1 compared to subtype 2. Mol Imaging. 2015;14(8):476–483. [PubMed] [Google Scholar]
- 5.Domino EF, Hirasawa-Fujita M, Ni L, Guthrie SK, Zubieta JK. Regional brain [11C]carfentanil binding following tobacco smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta JK. Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. J Clin Endocrinol Metab. 2015;100(8):3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DosSantos MF, Martikainen IK, Nascimento TD, et al. Reduced basal ganglia μ-opioid receptor availability in trigeminal neuropathic pain: a pilot study. Mol Pain. 2012;8:Article 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. [DOI] [PubMed] [Google Scholar]
- 9.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met geno-type affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. [DOI] [PubMed] [Google Scholar]
- 10.Zubieta JK, Bueller JA, Jackson LR, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26(21):5777–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu DT, Sanford BJ, Meyers KK, et al. Response of the μ-opioid system to social rejection and acceptance. Mol Psychiatry. 2013;18(11):1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–1229. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11): 2000–2009. [DOI] [PubMed] [Google Scholar]
- 15.DaSilva AF, Nascimento TD, DosSantos MF, Zubieta JK. Migraine and the mu-opioidergic system—can we directly modulate it? Evidence from neuroimaging studies. Curr Pain Headache Rep. 2014;18(7):Article 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubieta JK, Ketter TA, Bueller JA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60(11):1145–1153. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63(11):1199–1208. [DOI] [PubMed] [Google Scholar]
- 18.Peciña M, Bohnert AS, Sikora M, et al. Association between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA Psychiat. 2015;72(11):1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saccone PA, Lindsey AM, Koeppe RA, et al. Intranasal opioid administration in rhesus monkeys: PET imaging and antinociception. J Pharmacol Exp Ther. 2016;359(2):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huichun W, Zhenwei Z, Ping L. Synthesis of opiate receptor radioligand 11C-carfentanil and its biodistribution in rats. Chinese J Nucl Med. 2011;31(1):46–49. [Google Scholar]
- 21.Saji H, Tsutsumi D, Magata Y, Iida Y, Konishi J, Yokoyama A. Preparation and biodistribution in mice of [11C]carfentanil: a radiopharmaceutical for studying brain μ-opioid receptors by positron emission tomography. Ann Nucl Med. 1992;6(1):63–67. [DOI] [PubMed] [Google Scholar]
- 22.Newberg AB, Ray R, Scheuermann J, et al. Dosimetry of 11C-carfentanil, a μ-opioid receptor imaging agent. Nucl Med Commun. 2009;30(4):314–318. [DOI] [PubMed] [Google Scholar]
- 23.Van Daele PG, De Bruyn MF, Boey JM, Sanczuk S, Agten JT, Janssen PA. Synthetic analgesics: N-(1-[2-arylethyl]-4-substituted 4-piperidinyl) N-arylalkanamides. Arzneim-Forsch (Drug Res). 1976;26:1521–1531. [DOI] [PubMed] [Google Scholar]
- 24.MacQuarrie B Opioid epidemic's newest killer is 10,000 times stronger than morphine. Boston Globe, October 16, 2016. (https://www.bostonglobe.com/metro/2016/10/16/region-braces-for-arrival-new-more-powerful-synthetic-opioid/uLdoivGZdopm468poRzU3J/story.html; accessed March 27, 2017). [Google Scholar]
- 25.Studenov AR, Jivan S, Buckley KR, Adam MJ. Efficient in-loop synthesis of high specific radioactivity [11C]carfentanil. J Label Compd Radiopharm. 2003;46(9):837–842. [Google Scholar]
- 26.Shao X, Hoareau R, Runkle AC, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J Label Compd Radiopharm. 2011;54(14):819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao X, Kilbourn MR. A simple modification of GE tracerlab FX C Pro for rapid sequential preparation of [11C]carfentanil and [11C] raclopride. Appl Radiat Isot. 2009;67:602–605. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J-M, Xu Z-H, Zhang X-J, Xiang X-H, Tian J-H. Automated synthesis of 11C-carfetnail with 11C-choline module and micro PET imaging. Tongweisu (J Isot). 2011;24(3):182–187. [Google Scholar]
- 29.Jewett D A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28(6):733–734. [DOI] [PubMed] [Google Scholar]
- 30.Shao X, Fawaz MV, Jang K, Scott PJH. Ethanolic carbon-11 chemistry: The introduction of green radiochemistry. Appl Radiat Isot. 2014;89:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao X, Schnau PL, Fawaz MV, Scott PJH. Enhanced radiosyntheses of [11C]raclopride and [11C]DASB using ethanolic loop chemistry. Nucl Med Biol. 2013;40(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart MN, Hockley BG, Scott PJH. Green approaches to late-stage fluorination: radiosyntheses of 18F-labelled radiopharmaceuticals in ethanol and water. Chem Commun. 2015;51(79):14805–14808. [DOI] [PubMed] [Google Scholar]