Abstract
Introduction
Coronavirus disease-2019 (COVID-19) infection can result in pulmonary complications ranging from mild illness to severe life-threatening disease. There are limited studies correlating the association between the clinical course of COVID-19 and histopathological findings. This study aimed to examine the postmortem histopathological changes in lung tissue of COVID-19-positive patients and to correlate those changes with disease severity.
Materials and methods
This prospective observational study was conducted in adult COVID-19-positive patients. Postmortem core needle biopsy (CNB) of the lung was done using ultrasonography guidance within 1 hour of death. Histopathological analyses were performed by two expert pulmonary pathologists. The demographic and clinical data of the patients were recorded to correlate them with histopathological findings.
Results
In total, 48 patients were assessed for inclusion, and 21 patient relatives consented for the study. The median duration of illness was 21 (range 9–38) days, the predominant histopathological finding was diffuse alveolar damage (DAD) in most patients (19/21), followed by pneumonia (13/21). Exudative, intermediate, and advanced DAD patterns were seen in 9.5%, 52.4%, and 28.6% of cases, respectively. Advanced DAD was associated with a longer duration of disease. The pneumonia findings were associated with positive respiratory and blood cultures. The microvascular thrombus was seen only in one patient.
Conclusion
The predominant pathological findings in our patients were DAD and pneumonia. The DAD type correlated with the duration of illness, and we attributed pneumonia findings to secondary infection. The incidence of microvascular thrombi was low, and it might reflect the effect of treatment with anticoagulation.
How to cite this article
Maddani SS, Rao R, Deepa HC, Noronha AK, Chaudhuri S, Vishwas P. Pathological Lung Patterns of COVID-19 and its Clinical Correlation to Disease Severity. Indian J Crit Care Med 2022;26(12):1285–1292.
Keywords: Coronavirus disease-2019, Diffuse alveolar damage, Histopathology, Lung biopsy, Pneumonia
Highlights
Histopathological lung findings of COVID-19 patients largely were DAD and pneumonia.
The median duration of illness was 21 (range 9–38), and more than 90% of patients had one or more comorbidities.
Diffuse alveolar damage findings correlated to the duration of illness, and pneumonia findings were attributed primarily to secondary bacterial infection.
Only one patient had evidence of microvascular thrombosis on histological examination that might reflect the effect of treatment with anticoagulation.
Introduction
Coronavirus disease-2019 caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is associated with variable clinical features from mild illness to severe disease, and the latter is associated with considerable mortality.1,2 The pathogenesis of COVID-19 pneumonia begins with viral–host cell interaction by spike protein binding to host receptors via the angiotensin-converting enzyme-2 receptor domains. Histopathological changes linked to SARS-CoV-2 are crucial for a better understanding of the COVID-19 disease's pathogenesis. To investigate this disease process and mortality, lung biopsy studies were conducted, and they predominantly showed DAD.3,4 Other findings of organizing fibrosis, pulmonary microthrombosis, alveolar hemorrhage, and superimposed pneumonia were also identified by postmortem biopsy studies.5–7
Most investigations have shown comparable histological characteristics, but there are limited studies correlating clinical course, laboratory data, and therapy with histopathological findings.8 The incidence of vascular microthrombi has also varied from 21 to 100% across studies.9–12 This variable finding needs further evaluation. The cause of mortality also depends on the local epidemiology, and the management of COVID-19 disease keeps evolving with time. Hence, clinical parameters associated with pathological changes need to be done. We aimed to conduct this study to understand the pathological lung pattern of COVID-19 disease and its correlation with disease severity and mortality.
Materials and Methods
Study Design and Subjects Included
After obtaining Institutional Ethics Committee (IEC) clearance, this prospective observational study was conducted at a tertiary care teaching college hospital. The study was registered with the Clinical Trial Registry of India (CTRI/2021/08/035447) and was conducted from August 2021 to April 2022. About 48 adult COVID-19 disease patients diagnosed by reverse transcription-polymerase chain reaction (RT-PCR) or by antigen testing were screened for inclusion in the study after their death. About 21 patients were included in the study as consent was obtained only from these patients. Informed consent for tissue sampling was obtained from the patient's next of kin. The demographic and clinical data of the patients were recorded to correlate them with histopathological findings.
Tissue Biopsy Technique
Postmortem CNB of the lung was done using ultrasonography guidance within 1 hour of death. Samples were collected from four sites on each hemi-thorax (for example, anterior upper, anterior lower, lateral upper, and lateral lower), and a minimum of three samples were collected from each sampling site. Hence, the least possible of 12 samples were collected from each hemithorax. The samples thus obtained were fixed in a 10% neutral buffered formalin solution and were transported to the lab with all standard precautions. The samples were processed and, after paraffin embedding, were stained with hematoxylin and eosin.
Histopathological Processing and Examination
Two expert pulmonary pathologists performed histopathological analysis. The histological findings were looked at under the following points: (1) presence of DAD and its characteristics, (2) presence or absence of bronchopneumonia, (3) presence or absence of alveolar hemorrhage, (4) presence or absence of capillary microthrombi, and (5) other findings. The mean findings of the different sample sites were used to obtain a single estimate for each patient.
Statistical Analysis
Statistical analysis was performed using STATA statistical software, version 16. Descriptive statistics for continuous variables and proportions for categorical variables were calculated. Continuous data were summarized using medians and interquartile ranges (IQR: 25th percentile, 75th percentile) or mean and standard deviation (SD).
Results
Clinicodemographic Characteristics
We screened 48 patients and included 21 patients in our study, among them, 14 were male and 7 were female. The age-group varied from 36 to 89 years, with a median age of 65 (IQR: 51.5–73). About 90% of them had one or more comorbidities, the most common being diabetes mellitus (71.4%), followed by hypertension (67%) and chronic kidney disease (23.8%). Predominant presenting features were fever, cough, and shortness of breath. The median duration of illness was 21 (range 9–38) days, and the median Intensive Care Unit (ICU) length of stay was around 14 (IQR: 8–19) days. The median sequential organ failure assessment (SOFA) score was 6 (IQR: 5.5–7.5).
Intravenous steroids (IV) were used in most patients, except for 2 in whom it was contraindicated. Anticoagulation with 1 mg/kg of enoxaparin twice daily bis in die (BD) was routinely used in severe COVID-19 patients at our hospital. Unfractionated heparin was used in acute kidney injury (AKI) patients, and in 2 patients, anticoagulation was not used because of thrombocytopenia. Antiviral remdesivir was used in all patients. The clinico-demographic characteristics of patients are mentioned in Table 1.
Table 1.
Demographic and clinical characteristics of the patients
| Female/male (n) | 7/21 |
| Age in years, median (IQR) | 65 (51.5–73) |
| Comorbidities, n (%) | |
| Diabetes mellitus (DM) | 15 (71.4%) |
| Hypertension (HTN) | 14 (66.7%) |
| Chronic kidney disease (CKD) | 5 (23.8%) |
| Ischemic heart disease (IHD) | 4 (19.04%) |
| Cerebrovascular accident (CVA) | 2 (9.5%) |
| Chronic obstructive pulmonary disease (COPD) | 1 (4.8%) |
| Number of comorbidities | |
| 0 | 2 (9.5%) |
| 1 | 6 (28%) |
| 2 | 5 (23.8%) |
| 3 | 7 (33.3%) |
| 4 | 1 (4.8%) |
| Symptoms, n (%) | |
| Fever | 13 (61.9%) |
| Cough | 12 (57.1%) |
| Shortness of breath (SOB) | 11 (52.4%) |
| Altered sensorium | 4 (19.1%) |
| Pain in abdomen | 1 (4.8%) |
| COVID-19 diagnosis, n (%) | |
| RT-PCR | 17 (81%) |
| Positive antigen test | 4 (19%) |
| Use of corticosteroids, n (%) | 19 (90.5%) |
| Use of anticoagulants, n (%) | 19 (90.5%) |
| Use of antiviral (remdesivir), n (%) | 21 (100%) |
| SOFA score (at 24 hours) | 6 (5.5–7.5) |
| Laboratory parameters from 24 h before death, median (IQR) | |
| D-dimer (µg/mL) | 5.7 (2.55–8.95) |
| CRP (µg/L) | 151 (124.5–252.5) |
| WBC count (µL) | 24,600 (14,100–29,950) |
| Procalcitonin (µg/L) | 0.9 (0.21–1.95) |
| Blood cultures, n (%) | |
| A. baumanii | 1 (4.8%) |
| MSSA | 1 (4.8%) |
| K. pneumoniae | 3 (14.3%) |
| E. coli | 1 (4.8%) |
| Respiratory cultures, n (%) | |
| A. baumanii | 6 (28.6%) |
| K. pneumoniae | 6 (28.6%) |
| Galactomannan positive test, n (%) | 7 (33.3%) |
| Intensive care unit stay in days, median (IQR) | 14 (8–19) |
COVID-19, coronavirus disease-2019; CRP, C-reactive protein; IQR, interquartile range; RT-PCR, reverse transcription-polymerase chain reaction; SOFA, sequential organ failure assessment; WBC, white blood cell; A. baumanii, Acinetobacter baumanii; E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae; MSSA, methicillin-sensitive Staphylococcus aureus
Reverse transcription-polymerase chain reaction detected COVID-19 disease in most patients, except in 4, where the antigen test was positive. Chest radiographs of patients showed bilateral infiltrates in the majority, in concordance with most viral pneumonia. The median CRP was 151 (IQR: 124.5–252.5)µg/L, and the median D-dimer was 5.7 (IQR: 2.55–8.95)µg/mL. In addition, the median white blood cell (WBC) count in these subjects was 24600 (IQR 14100–29950)µg/L. Most of the patients had leukocytosis further during the course of the disease, and procalcitonin was used to diagnose infection along with appropriate cultures. The median procalcitonin values were 0.9 (IQR: 0.21–1.95)µg/L. The procalcitonin value of less than 0.5µg/L was considered negative, and 4 patients had positive cultures despite the negative procalcitonin value. In 10 patients, multidrug-resistant (MDR) Klebsiella pneumoniae and Acinetobacter baumanii were found in respiratory cultures. Six patients exhibited positive blood cultures, 4 of whom had K. pneumoniae and A. baumanii growth, and one culture each was positive for Escherichia coli, Candida albicans, and methicillin-sensitive Staphylococcus aureus (Table 2). Based on the culture sensitivity report, these patients were treated with higher antibiotics like meropenem, polymyxin B, and tigecycline. Serum galactomannan was positive in 7 patients, and 5 were treated with voriconazole (Flowchart 1).
Table 2.
Infectious parameters
| Sl no. | Base parameters at admission | Parameters at the time of diagnosis of infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CRP (µg/L) | Procalcitonin (µg/L) | Initial cultures | Empirical antibiotics | CRP (µg/L) | Procalcitonin (µg/L) | Cultures (RES/BLD) | Sensitivity | Antibiotics | |
| 1 | 113 | 1.13 | Nil | PIPZ, AZEE | 127 | 1.08 | K. pneumoniae (Res) | XDR | MERM, PLYB, TECO |
| 2 | 8 | 0.25 | Nil | PIPZ | 22 | 0.1 | Nil | – | CEFS |
| 3 | 122 | 0.15 | Nil | PIPZ, AZEE | 246 | 0.2 | Nil | – | PIPZ |
| 4 | 35 | 0.12 | Nil | PIPZ, AZEE | 81 | 0.22 | K. pneumoniae (Res) | XDR | CEFS, PLYB |
| 5 | 13 | 0.23 | Nil | PIPZ | 95 | 148 | K. pneumoniae (Res) | Non-MDR | PIPZ |
| 6 | 132 | 1.8 | Nil | PIPZ, AZEE | 75 | 0.3 | A. baumanii (Res) | XDR | CEFS, PLYB |
| 7 | 117 | 0.2 | MSSA (BLD) | PIPZ | 112 | 0.7 | A. baumanii (Res) | XDR | TIGY, PLYB |
| 8 | 47 | 0.15 | Nil | PIPZ, AZEE | 79 | 0.7 | A. baumanii (Res) | XDR | MERM, PLYB, TECO |
| 9 | 96 | 0.77 | Nil | CTRI, AZEE | 151 | 0.9 | Nil | – | CEFT |
| 10 | 220 | 0.25 | Nil | PIPZ, AZEE | 33 | 0.28 | Nil | – | MERM, LIZD |
| 11 | 148 | 1.6 | Nil | PIPZ | 32 | 0.31 | K. pneumoniae (Bld) | XDR | CEFS, PLYB, TECO |
| 12 | 98 | 0.18 | Nil | CTRI, DOXY | 5 | 0.2 | Nil | – | CEFS |
| 13 | 147 | 2.1 | Nil | PIPZ | 78 | 1.7 | Nil | – | MERM, FLUZ |
| 14 | 259 | 60 | E. coli (Bld) | MERM | 248 | 27 | K. pneumoniae (Res) | XDR | MERM, CLST |
| 15 | 280 | 41 | Nil | PIPZ | 212 | 6.4 | Nil | – | MERM |
| 16 | 113 | 0.24 | Nil | MERM | 131 | 1.8 | A. baumanii | XDR | MERM, PLYB, TIGY |
| 17 | 350 | 13.4 | K. pneumoniae (Bld) | MERM | 350 | 13.4 | K. pneumoniae (Bld) | Non-MDR | MERM |
| 18 | 95 | 1.37 | Nil | PIPZ | 130 | 1.3 | A. baumanii, K. pneumoniae (Res) | XDR | MERM, PLYB, TIGY, TECO |
| 19 | 83 | 0.42 | Nil | PIPZ | 57 | 0.38 | A. baumanii (Res) | XDR | MERM, PLYB |
| 20 | 171 | 0.21 | Nil | CTRI, AZEE | 73 | 0.2 | Nil | – | MERM |
| 21 | 79 | 1 | Nil | PIPZ | 122 | 0.35 | Nil | – | PIPZ |
AZEE, azithromycin; BLD, blood culture; CEFS, cefaperazone + sulbactaum; CRP, C-reactive protein; CTRI, ceftriaxone; LIZD, linezolid; MERM, meropenem; MSSA, methicillin-sensitive Staphylococcus aureus; PIPZ, piperacillin + tazobactam; PLYB, polymyxin B; RES, respiratory culture; TECO, teicoplanin; TIGY, tigecycline; XDR, extensively drug-resistant, A. baumanii, Acinetobacter baumanii; K. pneumoniae, Klebsiella pneumoniae
Flowchart 1.
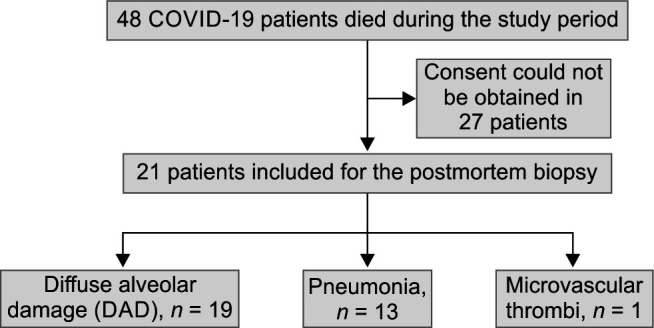
Flow diagram of the study
Pulmonary Histopathology
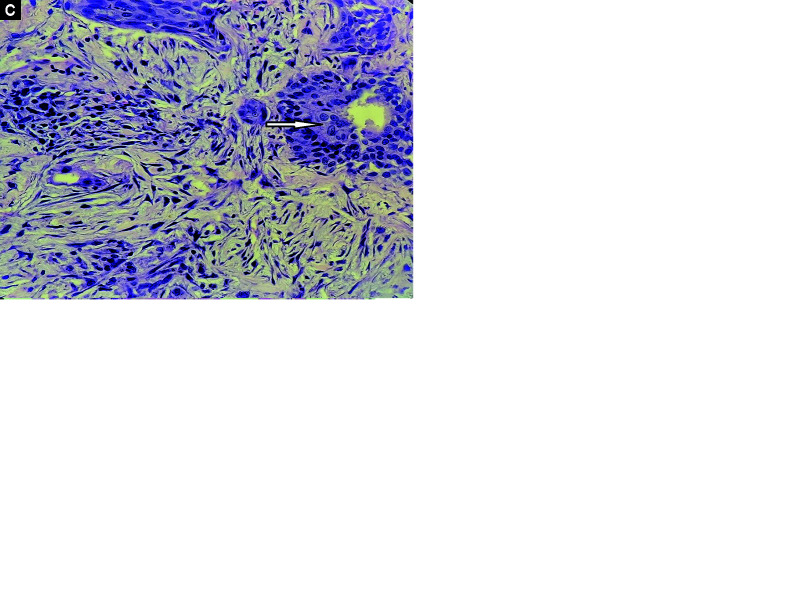
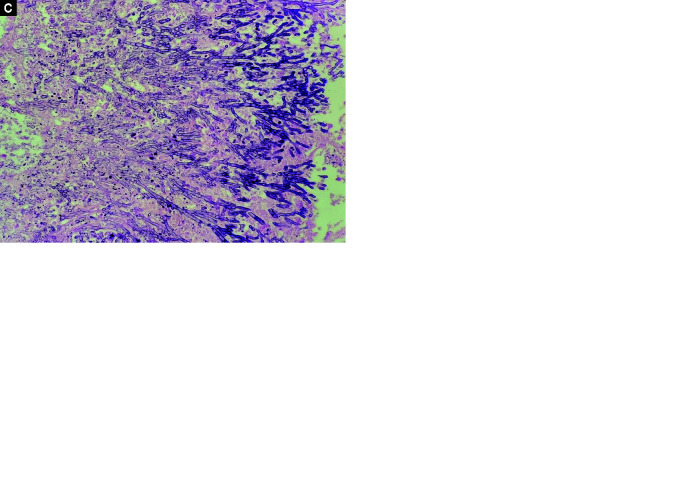
Histopathological data are presented in Table 3. Diffuse alveolar damage was the predominant feature in most patients (19/21), followed by pneumonia (13/21). Exudative DAD, characterized by intra-alveolar hyaline membrane with inflammatory infiltrates in the septum, was seen in 2 patients (Fig. 1A). Intermediate DAD represented by intra-alveolar hyaline membrane with interstitial fibroblastic proliferation was seen in 11 patients, and advanced DAD represented by marked interstitial fibrosis, absent hyaline membrane, with or without areas of squamous metaplasia, was noted in 6 patients (Figs 1B and C). The disease duration and its correlation to DAD are mentioned in Table 4. Pneumonia findings were associated with positive respiratory or blood cultures, and 2 patients also had microscopic findings of gram-negative bacilli (Figs 2A and B). One patient exhibited microvascular thrombi findings, and one had microscopic findings of septate hyphae suggestive of aspergillosis (Figs 2C and D). No patient had microscopic findings of alveolar hemorrhage.
Table 3.
Histopathological findings
| Subject | Type DAD | Pneumonia | Micro-organism | Microvascular thrombi | Alveolar hemorrhage | Duration of illness | Anticoagulation |
|---|---|---|---|---|---|---|---|
| 1 | Advanced | No | Nil | No | Nil | 25 | Yes |
| 2 | Nil | Yes | GNB | No | Nil | 15 | No |
| 3 | Exudative | Yes | Nil | No | Nil | 7 | Yes |
| 4 | Intermediate | No | Nil | No | Nil | 24 | Yes |
| 5 | Nil | Yes | Nil | No | Nil | 10 | Yes |
| 6 | Intermediate | Yes | Nil | No | Nil | 21 | Yes |
| 7 | Intermediate | Yes | Nil | No | Nil | 21 | Yes |
| 8 | Advanced | Yes | Nil | Yes | Nil | 25 | Yes |
| 9 | Intermediate | Aspergillosis | Nil | No | Nil | 18 | Yes |
| 10 | Advanced | Yes | Nil | No | Nil | 30 | Yes |
| 11 | Intermediate | No | Nil | No | Nil | 11 | Yes |
| 12 | Intermediate | No | Nil | No | Nil | 22 | Yes |
| 13 | Exudative | No | Nil | No | Nil | 14 | Yes |
| 14 | Intermediate | Yes | Nil | No | Nil | 9 | Yes |
| 15 | Intermediate | Yes | Nil | No | Nil | 16 | Yes |
| 16 | Advanced | Yes | Nil | No | Nil | 35 | Yes |
| 17 | Intermediate | Yes | GNB | No | Nil | 16 | No |
| 18 | Advanced | Yes | Nil | No | Nil | 38 | Yes |
| 19 | Intermediate | Yes | Nil | No | Nil | 12 | Yes |
| 20 | Intermediate | No | Nil | No | Nil | 21 | Yes |
| 21 | Advanced | No | Nil | No | Nil | 27 | Yes |
DAD, diffuse alveolar damage; GNB, gram-negative bacilli
Figs 1A to C.
(A) Exudative DAD, intra-alveolar hyaline membrane (arrow) with minimal inflammatory infiltrate in septa; (B) Intermediate DAD, intra-alveolar hyaline membrane with interstitial fibroblastic proliferation (arrow); (C) Advanced DAD, advanced diffuse alveolar damage: marked interstitial fibrosis with areas of squamous metaplasia (arrow)
Table 4.
Duration of illness and correlation to DAD
| DAD type | Number n (%) | Mean duration of illness ± SD (days) |
|---|---|---|
| Exudative | 2 (10) | 10 ± 5 |
| Intermediate | 11 (52) | 17 ± 5 |
| Advanced | 6 (28) | 30 ± 5 |
| Nil | 2 (10) | 12 ± 6 |
DAD, diffuse alveolar damage; SD, standard deviation
Figs 2A to D.
(A) DAD with superadded pneumonia showing dense intra-alveolar and interstitial acute inflammatory cells; (B) Gram stain highlighting GNB colonies; (C) Fibrin thrombi (arrow); (D) Aspergillosis-branching septate hyphae
Discussion
Our study of 21 patients describes the histopathological findings of COVID-19 infection; the predominant finding was DAD and pneumonia. Also, there was only one case of microvascular thrombi, and no case of alveolar hemorrhage was noted during the histological examination. Clinically patients had a longer duration of illness and higher secondary infection.
The histological presentation of DAD in our study correlated with the duration of illness; exudative DAD was seen in patients with illness duration of less than 10 days, intermediate DAD in patients with an illness of 10–20 days, and advanced DAD in patients with duration of illness of more than 20 days. This finding is in correlation to other studies by Kyada et al. and Li et al.10,13 Mauad et al. detected the incidence of exudative, intermediate, and advanced DAD patterns in 75.6%, 82.9%, and 39% of cases, respectively.11 Compared with other studies, predominant patients in our study had intermediate and advanced DAD, which can be attributed to longer duration of illness in our patient (Table 4).10,11,13 A better understanding of the disease process and the availability of growing evidence for the treatment of COVID-19 might have caused the longer duration of illness in our patients.14–16
Our study showed evidence of microvascular thrombosis in only one patient, which is less compared with previous studies.8–11 The reason for this finding could be multifactorial, including treatment with anticoagulation in most patients. Our hospital policy at the time of this study was to give therapeutic dose anticoagulation for all patients with severe COVID-19. Anticoagulation was achieved at our hospital by unfractionated heparin or low-molecular-weight heparin (LMWH). Anticoagulation has been deemed to reduce mortality across various studies, and heparin/LMWH might also provide anti-inflammatory and antiviral properties.17–19 The low detection of microvascular thrombi might also be due to variation in the disease presentation across geographic locations. Moreover, we conducted only a minimally invasive biopsy, and the whole lung gross examination and biopsy were not conducted, which could have also led to lower detection.
We detected pneumonia in 13 patients during the histological examination: two had Gram-negative colonies during the histological examination, 10 patients had growth of Gram-negative organisms from the respiratory cultures, and 1 also had positive blood culture. From these findings, we assume pneumonia findings were predominantly due to secondary bacterial infection. Most commonly, the organisms isolated were extensively drug-resistant (XDR) except in two culture reports, where they were sensitive to third-generation cephalosporin.
All the patients in our study received empirical antibiotics at admission. The critical condition of the patients would have prompted the initiation of empirical antibiotics, and it was difficult to differentiate bacterial co-infection in such patients at initial presentation. Use of empiric antimicrobials and steroids might have predisposed the patients to secondary bacterial infections with XDR organisms. Extensively drug-resistant was defined as the nonsusceptibility of organisms to at least one agent in all but two or fewer antimicrobial categories, i.e., bacterial isolates remain susceptible to only one or two categories.20 We used higher antibiotics like Polymyxin B and Tigecycline to treat predominant secondary infections based on culture sensitivity reports. Treating XDR organisms was an added challenge in these patients and could have contributed to mortality. The average length of ICU stay in our study was around 14 days, which is high compared with other studies.10,11,13 About 90% of our study population also had one or more comorbidities. Thus, the longer length of ICU stay and higher comorbidities could have also conspired to higher secondary infection.
Many of our patients had leukocytosis, and procalcitonin was used to identify evidence of infection along with appropriate cultures. At our institute, the procalcitonin value greater than 0.5 (µg/L) is considered positive, and we paired the testing with respiratory and blood cultures. We had positive cultures in 12 patients, and procalcitonin was positive in 8 patients. Four patients had negative procalcitonin values, despite positive culture growth in blood and respiratory secretions. The negative procalcitonin value, despite culture positivity, might be due to the use of steroids in these patients, which can cause false-negative values. These findings need further evaluation as previous studies have shown that corticosteroids do not affect procalcitonin values.21
We used serum galactomannan assay to identify Aspergillus infection, which was positive in 7 patients. Aspergillosis was detected during histological examination in only 1 patient. These findings indicate that serum galactomannan might cause over-estimation of Aspergillus infection (false positive). Fungal Candida growth was noted in 1 patient during blood cultures. Whole lung biopsy could have helped in better understanding of Aspergillus or other fungal infections, as few areas could have been missed during core biopsy sampling.
The salient feature of our study is that it reflected the pathological findings with clinical presentation and mortality. The DAD findings correlated with the duration of illness and the predominant findings of our study were intermediate and advanced DAD, in contrast to exudative DAD in other studies. Our study also showed limited evidence of microvascular thrombosis compared with other biopsy studies, and it might reflect the effect of treatment like anticoagulation. Our study also detected a higher incidence of pneumonia compared with other studies, and it was ascribed to secondary infection. The use of empirical antibiotics, steroids, and longer ICU stay could have led to high secondary infection and contributed to mortality along with DAD. This suggests rationale use of antibiotics and steroids along with strict infection control measures can improve morbidity and mortality associated with severe COVID-19 infection. Position paper implementation of the Indian Society of Critical Care Medicine regarding infection prevention and control can also help in the reduction of infectious complications.22,23
Our study had many limitations, it was a single-center observational study, and the sample size was small to get concluding results for the general population. We could not do a whole lung biopsy, hence, we could not see evidence of large-vessel thrombosis. Nevertheless, our study also identifies a few distinct features that emphasize the need for further research as the disease and treatment process keeps evolving.
Conclusion
Our study showed that the predominant pathological findings in COVID-19 patients were DAD and pneumonia. The DAD type correlated with the duration of illness, and we attributed pneumonia findings to secondary infection. The incidence of microvascular thrombi was low, and it might reflect the effect of treatment like anticoagulation.
Acknowledgments
The authors would like to thank all the healthcare workers who provided care to COVID-19 patients at our institute.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Sagar Shanmukhappa Maddani https://orcid.org/0000-0003-0700-0532
Raghavendra Rao https://orcid.org/0000-0001-7366-3217
HC Deepa https://orcid.org/0000-0002-6420-5705
Adrian Keith Noronha https://orcid.org/0000-0002-9262-4579
Souvik Chaudhuri https://orcid.org/0000-0001-8392-2366
Vishwas P https://orcid.org/0000-0002-6149-9509
References
- 1.Pijls BG, Jolani S, Atherley A, Derckx RT, Dijkstra JIR, Franssen GHL, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open. 2021;11:e044640. doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lian J, Jin X, Hao S, Jia H, Cai H, Zhang X, et al. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other Respir Viruses. 2020;14(5):564–574. doi: 10.1111/irv.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey P, Agarwal S, Rajkumar Lung pathology in COVID-19: A systematic review. Int J Appl Basic Med Res. 2020;10(4):226–233. doi: 10.4103/ijabmr.IJABMR_381_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, et al. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory syndrome and H1N1 influenza: A systematic review. Chest. 2021;159(1):73–84. doi: 10.1016/j.chest.2020.09.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari S, Solanki R, Jindal A, Rankawat G, Pathak D, Bagarhatta M, et al. Demystifying COVID-19 lung pathology: A clinicopathological study of postmortem core needle biopsy. Lung India. 2021;38(4):343–349. doi: 10.4103/lungindia.lungindia_919_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary postmortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, et al. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyada HC, Bhalara RV, Vadgama DK, Varu PR, Trangadia MM, Manvar PJ, et al. Pathological findings in COVID-19: A conventional autopsy-based study from India. Indian J Med Res. 2022;155(1):178–188. doi: 10.4103/ijmr.IJMR_677_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauad T, Duarte-Neto AN, da Silva LFF, de Oliveira EP, de Brito JM, do Nascimento ECT, et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res. 2021;22(1):1–11. doi: 10.1186/s12931-021-01628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray A, Jain D, Goel A, Agarwal S, Swaroop S, Das P, et al. Clinico-pathological features in fatal COVID-19 infection: A preliminary experience of a tertiary care center in North India using postmortem minimally invasive tissue sampling. Expert Rev Respir Med. 2021;15(10):1367–1375. doi: 10.1080/17476348.2021.1951708. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wu J, Wang S, Li X, Zhou J, Huang B, et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2021;78(4):542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for COVID-19 — Interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ionescu F, Jaiyesimi I, Petrescu I, Lawler PR, Castillo E, Munoz-Maldonado Y, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: A retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106(2):165–174. doi: 10.1111/ejh.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: A study. J Pathog. 2016;2016:4065603. doi: 10.1155/2016/4065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kruif MD, Lemaire LC, Giebelen IA, Struck J, Morgenthaler NG, Papassotiriou J, et al. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34(3):518–522. doi: 10.1007/s00134-007-0955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma J, Nasa P, Reddy KS, Kuragayala SD, Sahi S, Gopal P, et al. Infection prevention and control for ICU during COVID-19 pandemic: Position paper of the Indian Society of Critical Care Medicine. Indian J Crit Care Med. 2020;24(Suppl 5):S280–S289. doi: 10.5005/jp-journals-10071-23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juneja D, Savio RD, Srinivasan S, Pandit RA, Ramasubban S, Reddy PK, et al. Basic critical care for management of COVID-19 patients: Position paper of the Indian Society of Critical Care Medicine, Part II. Indian J Crit Care Med. 2020;24(Suppl 5):S254–S262. doi: 10.5005/jp-journals-10071-23593. [DOI] [PMC free article] [PubMed] [Google Scholar]