Abstract
Background:
Subintimal angioplasty is a common treatment for chronic total occlusions (CTOs) in the iliac and infrainguinal arteries. Although technical success has been described using intravascular ultrasound-guided reentry devices (IVUS-RED), outcomes are still not well defined. This report describes the technical aspects and longitudinal follow-up after intravascular ultrasound-guided reentry of iliac and infrainguinal CTOs.
Methods:
A retrospective review was performed of 20 patients with lower extremity CTO treated with IVUS-RED from 2011 to 2013. A matched cohort of patients who underwent lower extremity interventions without the use of IVUS-RED was also identified. Procedural success, patency estimates, ankle-brachial indices (ABIs), complications, and limb salvage were analyzed.
Results:
Twenty patients (mean age, 69 ± 13 years), including 11 men and 9 women, underwent attempted IVUS-RED–guided recanalization. Median follow-up was 4.3 months (range, 0.4–24). Eleven patients presented with critical limb ischemia (CLI), and 9 presented with claudication. Technical success was achieved in 18 (90%) patients. Ten common iliac arteries, 3 external iliac arteries, and 5 superficial femoral arteries (SFA) were treated. No intraoperative complications resulted from device use. After procedure, ABIs significantly increased (0.5–0.9; P < 0.01) in the 13 patients with follow-up. Primary patency for the entire cohort was 62% at 12 months. No patient treated for claudication required reintervention, whereas 3 (27%) of those treated for CLI required repeat interventions. During follow-up, 2 patients died unrelated to the procedure, 1 patient required an amputation, and 1 patient eventually required open revascularization. When the IVUS-RED group was compared with a cohort matched on Trans-Atlantic Inter-Society Consensus and age, no difference was found in runoff scores and patency between the 2 groups during follow-up (P > 0.05).
Conclusions:
Recanalization of CTO using IVUS-RED is safe and effective. Use of IVUS-RED does not adversely impact outcomes in conjunction with other endovascular techniques. Early follow-up demonstrates acceptable patency, especially in patients with claudication, and freedom from reintervention.
INTRODUCTION
Endovascular therapy for the treatment of aortoiliac and infrainguinal arterial occlusive disease is considered first line therapy, and techniques to improve outcomes continue to evolve.1–3 The development of numerous tools and techniques has allowed this rapid evolution. One such technique is subintimal angioplasty, which allows for the creation of a new channel for blood to flow around a chronically occluded native lumen. Such an approach is associated with limb salvage and low morbidity when combined with adjunctive balloon angioplasty and/or stenting.4–13
One limitation of subintimal angioplasty is inability to reenter the true lumen. In addition, reentry using standard catheter and wire techniques alone may result in reentry distal to the zone of vessel reconstitution, thereby inadvertently sacrificing important collateral vessels. As a result, reentry devices including the Outback LTD (Cordis Corp., Bridgewater, NJ) and the Pioneer Plus (Volcano Corp., San Diego, CA) were developed. Although both catheter devices use a curved hollow retractable nitinol needle to reenter the true lumen, the Outback uses 2-dimensional fluoroscopy to guide the needle whereas the Pioneer uses intravascular ultrasound.
Although the technical success of intravascular ultrasound-guided reentry devices (IVUS-RED) have been described, outcomes are still not well defined. More specifically, outcomes of IVUS-RED in lower extremity revascularization have not been compared with when reentry catheters were not needed.14–18 In this study, both the technical aspects and longitudinal follow-up are described after intravascular ultrasound-guided reentry in chronic total occlusions (CTOs). The overall goal of our study was to review the safety and effectiveness of IVUS-RED in management of lower extremity arterial CTOs.
METHODS
The University of California Davis maintains a retrospective database of all patients treated for lower extremity arterial disease from June 2006 to December 2013. The database collects demographic, procedural, and outcomes data for patients who undergo endovascular and/or surgical treatment. Maintenance and analysis of the database is approved by the University of California Davis’s Institutional Review Board. Additional patients who required IVUS-RED at David Grant Medical Center were also compiled for this review. Approval from the David Grant Medical Center’s Institutional Review Board was obtained.
IVUS-RED Cohort
Twenty patients with lower extremity CTOs treated with IVUS-RED were identified from 2011 to 2013. Variables reviewed from the database included gender, age, comorbidities, preprocedure symptoms, indications for the intervention, Rutherford classification of disease, medications, and assessment of prior treatment. At the time of entry into the database, a board-certified vascular surgeon or interventional cardiologist with peripheral arterial experience reviews all angiographic imaging to assign the appropriate Trans-Atlantic Inter-Society Consensus (TASC) II classification of disease. Resting ankle-brachial indices (ABI) and/or toe-brachial indices (TBI) are routinely obtained preoperatively and at clinical follow-up exams. Surveillance protocols also include a duplex ultrasound (DUS) and/or ABI/TBIs within 1 month of the procedure and at 3 month intervals in the first year. Patients are typically seen every 6 months in the second year and then yearly thereafter. At each clinical visit, noninvasive testing with DUS and/or ABI/TBIs is obtained. Procedural success, patency estimates, ABIs, complications, and limb salvage were analyzed.
Peripheral Arterial Disease Cohort Match
Using TASC classification, lesion location, age ± 5 years, and gender, an additional cohort of 20 patients who underwent endovascular treatment for peripheral arterial disease (PAD) without the use of IVUS-RED were identified from the database and matched to the IVUS-RED cases. The matched patients were treated between 2006 and 2012. Review of angiographic images, TASC classification, and follow-up protocols were performed in the same manner as the IVUS-RED patients. In 11 patients, more than 1 match was identified using TASC, lesion location, and age. In this scenario, a best match was further identified by aligning the most number of shared comorbidities including coronary artery disease (CAD), diabetes mellitus (DM), end-stage renal disease (ESRD), smoking history, hypertension (HTN), history of stroke, transient ischemic attack (TIA), abdominal aortic aneurysm, or congestive heart failure (CHF).
Interventions
A board-certified vascular surgeon or an interventional cardiologist specializing in peripheral artery disease performed all the interventions. All treatments were performed with fixed imaging under local anesthesia with conscious sedation, except in cases where a hybrid surgical procedure necessitated general anesthesia. Heparin is routinely administered throughout the case to maintain the activated clotting time above 250 seconds. All CTOs that were successfully crossed using IVUS-RED had balloon angioplasty and stenting performed. Choice of stent (covered, bare metal, and drug eluting) was decided on a case-by-case basis at the discretion of the treating physician. Although some patients underwent treatment of multiple arterial segments, including the femoropopliteal segment or tibial vessels during the index procedure, only the segments where IVUS-RED was used were analyzed.
The Pioneer catheter was used for all patients in the IVUS-RED group. The 7F catheter contains intravascular ultrasound in the tip and integrates with an IVUS console (Volcano, San Diego, CA). A 0.035-in wire is used to cross the occlusion by creating a subintimal dissection. The 0.035 wire is then exchanged for a 0.014-in wire on which the Pioneer monorail is advanced to the point of desired reentry along the dissection plane. Using chromoflow imaging, the true lumen is visualized by IVUS (Fig. 1). A hollow, curved, retractable, nitinol needle is then advanced in a controlled fashion into the true lumen. After reentering the true lumen, an exchange length 0.014-in wire is passed from the end of the catheter and the nitinol needle is retracted. The Pioneer is then withdrawn. Typically, the point where intima was punctured requires balloon angioplasty to allow successful exchange for a 0.035-in wire.
Fig. 1.
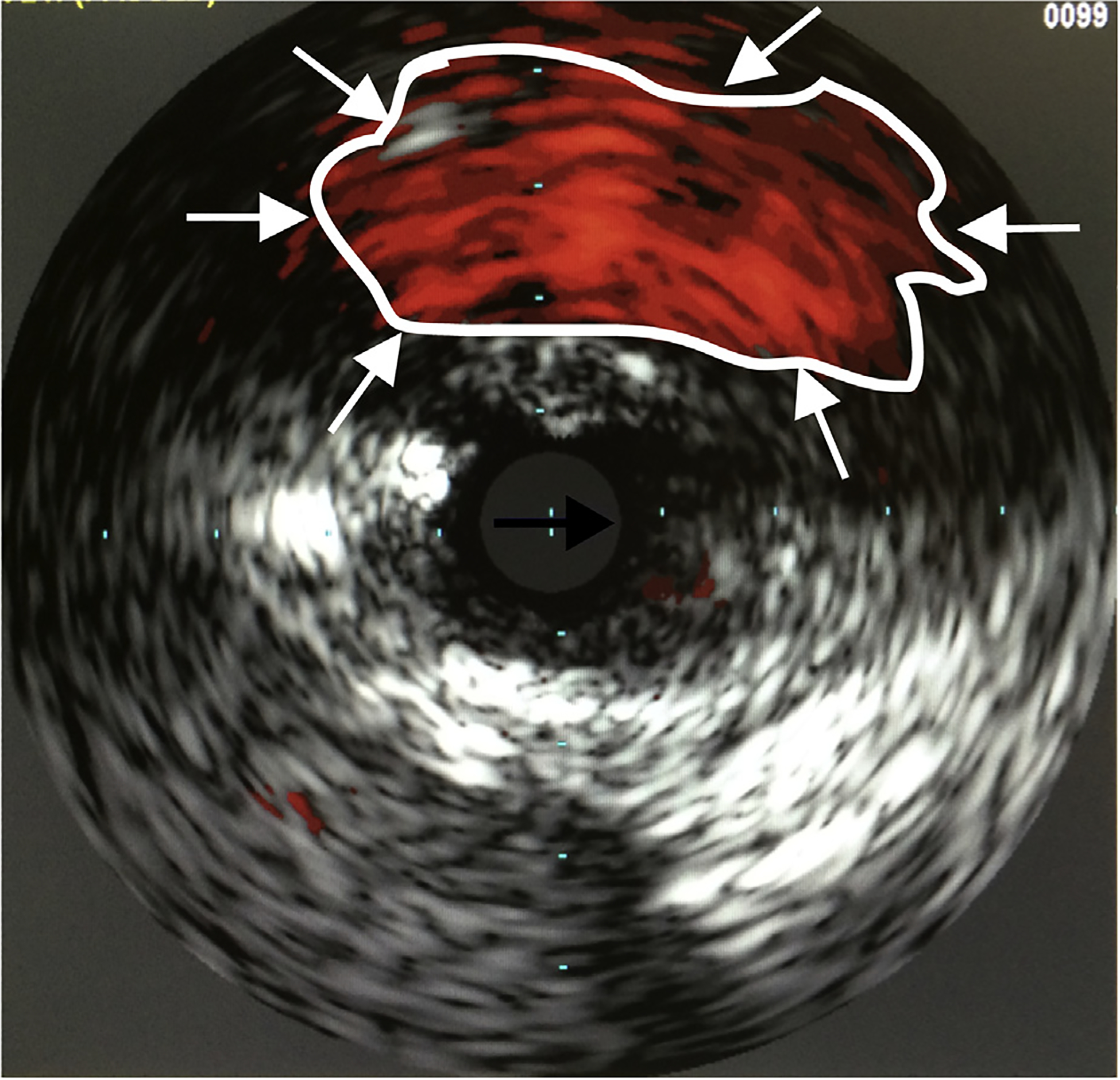
The true lumen is visualized by using chromoflow imaging (white arrows/outlined) and IVUS. The reentry needle (black arrow) is then positioned at the 12-o’clock position and deployed into the true lumen.
All patients are typically prescribed aspirin after procedure, at a dose ranging from 81 to 325 mg. Clopidogrel 75 mg daily after procedure is also prescribed with dual-antiplatelet therapy continued for 6 weeks. At the discretion of the treating physician, patients were loaded with 300 mg of clopidogrel at the time of the procedure. Patients on anticoagulation therapy were prescribed clopidogrel for 6 weeks in addition to the anticoagulation. After this time, patients were transitioned to long-term anticoagulation and aspirin therapy.
Outcomes
Technical success was defined as less than 30% residual stenosis within the intervened segment. Primary patency was defined as the absence of occlusion or greater than 50% stenosis during follow-up. The need for reintervention was determined by a change in a previously palpable pulse, recurrent symptoms, a drop in the ABI > 0.15, Doppler ultrasound findings indicating a > 50% stenosis, or any combination of the previously mentioned findings. Decision for reintervention was made on a case-by-case basis. Only major amputations, defined as at the level of the ankle or more proximal, were considered for outcomes. Runoff scores were calculated for every patient using the Society for Vascular Surgery (SVS) grading system.19 Overall survival was recorded for all patients using hospital charts and the Social Security Death Index.
Statistical Analysis
Data were analyzed using statistical software R (www.R-project.org). Descriptive statistics were used to evaluate demographic data. Patency estimates were determined using Kaplan–Meier analysis. Comparison of patency estimates was performed using a log-rank and Cox proportional hazard. Chi-squared test and Student’s t-test were used for 2-way comparisons using Microsoft Excel (Redmond, WA). Comparisons were deemed significant at P < 0.05.
RESULTS
IVUS-RED Cohort Outcomes
Twenty patients (mean age, 69 ± 13 years), including 11 men and 9 women, underwent attempted IVUS-RED–guided recanalization (Table I). Eleven patients presented with critical limb ischemia (CLI) and 9 patients with lifestyle-limiting claudication; all patients presented with TASC II B or greater lesions (Table I). Seventeen procedures were first-time attempts at revascularization, and 3 procedures were reinterventions for previously failed endovascular attempts. The reinterventions did not use a reentry device during the initial endovascular procedure. Technical success was achieved in 18 (90%) patients. Ten common iliac arteries (CIAs), 3 external iliac arteries (EIAs), and 5 superficial femoral arteries (SFAs) were treated. All CTOs that were crossed underwent angioplasty and stenting. Mean fluoroscopy time was 42.6 min (range, 13.9–112 min). No intraoperative complications resulted from device use. Balloon-expandable stents were used in CIA lesions. This included 4 bare metal balloon-expandable and 6 covered balloon-expandable stents. Seven uncovered self-expanding stents were used in the EIAs and SFAs. A single drug eluding stent was used in an EIA.
Table I.
Clinical characteristics of study patients
| Variable | IVUS-RED (N = 20) | No IVUS-RED (N = 20) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 69 ± 13 | 69 ± 12 | 0.92 |
| Male | 11 | 11 | 1 |
| Female | 9 | 9 | 1 |
| BMI (kg/m2) | 26.5 ± 6.9 | 25.7 ± 4.5 | 0.98 |
| Ethnicity, % (n) | |||
| Caucasian | 65 (13) | 65 (13) | |
| African-American | 10 (2) | 0 | |
| Asian | 20 (4) | 5 (1) | |
| Hispanic | 5 (1) | 0 | |
| Unknown | 0 | 30 (6) | |
| Risk factors, % (n) | |||
| Smoker | 75 (15) | 85 (17) | 0.43 |
| Diabetes mellitus | 50 (10) | 35 (7) | 0.34 |
| Hypertension | 80 (16) | 90 (18) | 0.38 |
| Coronary artery disease | 50 (10) | 50 (10) | 1 |
| End-stage renal disease | 20 (4) | 15 (3) | 0.68 |
| Congestive heart failure | 20 (4) | 10 (2) | 0.38 |
| Stroke/TIA | 25 (5) | 10 (2) | 0.21 |
| Preintervention, % (n) | |||
| Statin | 80 (16) | 75 (15) | 0.71 |
| ASA | 70 (14) | 70 (14) | 1 |
| Clopidogrel | 15 (3) | 20 (4) | 0.67 |
| Coumadin | 5 (1) | 15 (3) | 0.29 |
| Postintervention, % (n) | |||
| Statin | 80 (16) | 80 (16) | 1 |
| ASA | 100 (20) | 95 (19) | 0.31 |
| Clopidogrel | 90 (18) | 65 (13) | 0.06 |
| Coumadin | 10 (2) | 20 (4) | 0.37 |
| Fluoroscopy time | 42.6 ± 22.8 | 29.7 ± 20.1 | 0.12 |
| TASC B, % (n) | |||
| Common iliac artery | 25 (5) | 25 (5) | |
| External iliac artery | 10 (2) | 10 (2) | |
| Superficial femoral artery | 0 | 0 | |
| TASC C, % (n) | |||
| Common iliac artery | 15 (3) | 15 (3) | |
| External iliac artery | 5 (1) | 5 (1) | |
| Superficial femoral artery | 10 (2) | 10 (2) | |
| TASC D, % (n) | |||
| Common iliac artery | 10 (2) | 10 (2) | |
| External iliac artery | 0 | 0 | |
| Superficial femoral artery | 25 (5) | 25 (5) | |
| Claudication, % (n) | 45 (9) | 35 (7) | |
| Critical limb ischemia, % (n) | 55 (11) | 55 (11) | |
| Rest pain | 3 | 8 | |
| Tissue loss | 8 | 3 | |
| Acute limb ischemia, % (n) | 0 | 10 (2) |
SD, standard deviation; BMI, body mass index.
Most patients (80%; Table I) were on a statin and antiplatelet agent before the intervention. Only 6 (30%) patients were not on any antiplatelet therapy before the procedure. After intervention, all 18 patients who had angioplasty and stenting across CTOs were prescribed dual-antiplatelet therapy.
Two SFA CTOs could not be successfully crossed and treated. These 2 occlusions were both TASC D SFA occlusions with CLI and tissue loss. In both cases, the Pioneer catheter could not be advanced into position. One patient required a below-knee amputation 14 days later. The other patient eventually had another failed endovascular attempt because there were not any distal targets for open revascularization; eventually this patient went on to heal the toe ulcers with conservative measures.
Median follow-up for the IVUS-RED cohort was 4.3 months (range, 0.4–24). In patients with noninvasive vascular lab follow-up, postprocedure ABIs (n = 13) significantly increased (0.54–0.9;P < 0.01) and TBIs (n = 10) significantly increased (0.29–0.56; P < 0.01; Fig. 2).
Fig. 2.

Toe-brachial indices improved to an average of 0.6 from 0.3, and ankle-brachial indices improved to an average of 0.9 from 0.5 after intervention using IVUS-RED.
During follow-up, 2 patients who had successful intervention died from causes unrelated to the procedure. Two other patients required reintervention for in-stent stenosis and required balloon angioplasty. Both of these patients had initially presented with CLI and TASC C and D lesions, respectively, of the SFA—1 of these patients required an open bypass after one additional endovascular reintervention for recurrent in-stent restenosis 1 year after his IVUS-RED procedure. Finally, one other patient, as previously mentioned, required a below-knee amputation.
The primary patency for the entire cohort was 62% at 12 months (Figs. 3 and 4). No patients with claudication required reintervention, but 27% of patients who presented with CLI required reintervention. A similar patency trend was identified based on TASC II classification—TASC B lesions did not require reintervention, but 33% of TASC C and D lesions required reintervention (Fig. 3).
Fig. 3.

The primary patency in those presenting with TASC B and TASC C and D lesions are shown in the figure above. The primary patency rate for the entire cohort was 62% at 12 months.
Fig. 4.

The primary patency rate for the IVUS-RED cohort compared with that for the matched PAD cohort showed no difference (P = 0.82).
PAD-Matched Cohort Outcomes
A cohort of patients who underwent endovascular intervention for lower extremity PAD who did not require IVUS-RED were matched with the IVUS-RED patients on lesion location, TASC classification, gender, and age ±5 years (Table I). No differences were observed between the IVUS-RED and no IVUS-RED cohorts with regard to comorbidities including smoking, DM, HTN, CAD, ESRD, CHF, and stroke/TIA (Table I). The matched cohort had a similar initial presentation as compared with the IVUS-RED cohort (45% vs. 35% for claudication and 55% for both groups for CLI, Table I). In addition, 2 patients in the matched cohort presented with acute limb ischemia whereas no patients in the IVUS-RED group presented with acute limb ischemia.
Nineteen of the 20 patients in the matched cohort had a successful endovascular procedure performed. One patient’s CTO in the SFA could not be crossed, and eventually, required an open bypass procedure. Sixteen of the 19 patients who underwent an endovascular intervention had a stent deployed, whereas 3 patients had balloon angioplasty alone. Nine CIAs were treated with balloon-expandable stents including 4 covered and 5 uncovered balloon-expandable stents. Seven self-expanding stents were used to treat EIA and SFA lesions, which included 1 covered self-expanding stent used to treat a SFA lesion. Mean fluoroscopy was 29.7 ± 20.1 min and was not significantly different from the mean fluoroscopy time in the IVUS-RED group (P = 0.12; Table I).
Most patients in the matched cohort were on statin and antiplatelet therapy before the procedure. All patients received antiplatelet therapy after the intervention. No difference was found between the preprocedure and postprocedure use of statin, antiplatelet, and Coumadin use between the IVUS-RED and the no IVUS-RED cohorts (Table I).
Median follow-up for the matched cohort was 10.5 months (range, 0.5–80). In patients with noninvasive vascular laboratory follow-up, postprocedure ABIs (n = 19) significantly increased (0.63–0.93; P < 0.01), which was similar to the results in the IVUS-RED group. One patient was lost to follow-up after a technically successful intervention. No patients required amputation during the follow-up period, but 3 patients died from causes unrelated to the procedure.
SVS runoff scores were calculated for the IVUS-RED and non–IVUS-RED groups. The average runoff score for the IVUS-RED group after treatment was 3.3 ± 2.9 and 3.8 ± 3 for the non–IVUS-RED group. There was no significant difference between the scores of the 2 groups (P = 0.7). The primary patency for the matched cohort was 71% at 12 months (Fig. 4). When compared with the primary patency of the IVUS-RED cohort, there was no difference in primary patency at 12 months between the 2 groups (Fig. 4; P = 0.82).
DISCUSSION
An endovascular-first approach for lower extremity arterial occlusive disease has been widely adopted due in part to advances in wires, support catheters, and crossing devices. Exploitation of the subintimal plane in crossing complex lesions and occlusions and subintimal angioplasty has emerged as a reasonable technique to achieve limb salvage with low morbidity. However, 2 of the major impediments in using subintimal angioplasty are reentry into the vessel true lumen after crossing the occlusion and reentry into the true lumen at a level as close to native vessel reconstitution as to not compromise feeding collaterals. Although a number of studies have evaluated the use of nonultrasound-guided reentry, only a small number of reports have evaluated the use of IVUS-RED.
Previous reports on the application of intravascular ultrasound-guided reentry catheters have been published and have mainly focused on the immediate technical outcomes. In 1 report, Al-ameri et al.14 reported a 95% success using the Pioneer catheter in 21 patients with CTOs, but did not report any data regarding follow-up for the cohort. In another report, Krishnamurthy et al. showed a 100% technical success using Pioneer reentry rate in a retrospective review of 11 patients (9 with CLI and 2 with claudication) with CTO of the iliac arteries. In follow-up, this study showed significant improvement in ABIs, resolution of symptoms, and healing of foot ulcer wounds in all patients.16
In larger series focused on the results of subintimal angioplasty with and without stenting, Scott et al.12 reported a primary patency rate of 55%, 43%, and 35% at 12, 24, and 36 months in a mixed cohort of patients with CLI and claudication. When the same group evaluated the role of reintervention after subintimal angioplasty, they found the patency estimate after reintervention was much lower at 33% at 1 year.10
Our study reviews 20 patients who had attempted true lumen reentry during a lower extremity endovascular procedure for either CLI or claudication. Similar to the other reports, our technical success was 90%, and demonstrated improvement in both ABI and TBIs in follow-up (Fig. 3). One major difference from the other reports of the Pioneer use is that we evaluated the early outcomes after the intervention and showed primary patency estimate of 67% at 12 months. However, given the heterogeneity in levels of disease, initial TASC stage, type of stent deployed, and runoff, the outcomes and durability cannot be attributed only to the use of the reentry device. Regardless, the results from this analysis show in an early follow-up period, the patency rates are congruent with prior studies where subintimal angioplasty with and without stents was employed. Thus, use of ultrasound-guided reentry when wire and catheter techniques alone are unsuccessful can achieve reasonable results, and does not affect short-term patency.
This present study also includes a comparison with a matched cohort who underwent endovascular revascularization for lower extremity arterial occlusive disease without the use of a reentry device. Although the 2 groups are small, and the match has some inequalities including clinical presentation and type of endovascular therapy, the comparison does demonstrate that application of IVUS-RED in management of CTOs is safe and effective with reasonable patency over short-term follow-up similar to that of patients who were successfully treated with other modalities.
In addition to being a retrospective report of a small sample size, the other major limitation of this study is the relatively short follow-up. Although subintimal angioplasty is becoming more commonplace, reentry to the true lumen can usually be achieved without the assistance of a reentry device, and thus, the use of reentry device is overall a relatively rare occurrence. For now, the best method for evaluating the outcomes of reentry devices will be through case series. Potentially, combining results from multiple institutions could serve as a more effective method for evaluating results.
In conclusion, application of IVUS-RED in achieving true lumen reentry is safe and effective because it does not adversely impact short-term outcomes. The early follow-up results when IVUS-RED is used in combination with other wire, catheter, and stenting modalities show an acceptable primary patency and freedom from reintervention, especially in patients with claudication. Ongoing evaluation of IVUS-RED over longer periods of time is justified to better evaluate the use of this technology in the overall durability of this approach.
REFERENCES
- 1.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925–34. [DOI] [PubMed] [Google Scholar]
- 2.Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg 2008;48:1451–7, 7.e1–3. [DOI] [PubMed] [Google Scholar]
- 4.Bolia A, Miles KA, Brennan J, et al. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol 1990;13:357–63. [DOI] [PubMed] [Google Scholar]
- 5.Bown MJ, Bolia A, Sutton AJ. Subintimal angioplasty: meta-analytical evidence of clinical utility. Eur J Vasc Endovasc Surg 2009;38:323–37. [DOI] [PubMed] [Google Scholar]
- 6.Chen BL, Holt HR, Day JD, et al. Subintimal angioplasty of chronic total occlusion in iliac arteries: a safe and durable option. J Vasc Surg 2011;53:367–73. [DOI] [PubMed] [Google Scholar]
- 7.Hynes N, Mahendran B, Manning B, et al. The influence of subintimal angioplasty on level of amputation and limb salvage rates in lower limb critical ischaemia: a 15-year experience. Eur J Vasc Endovasc Surg 2005;30:291–9. [DOI] [PubMed] [Google Scholar]
- 8.Lazaris AM, Salas C, Tsiamis AC, et al. Factors affecting patency of subintimal infrainguinal angioplasty in patients with critical lower limb ischemia. Eur J Vasc Endovasc Surg 2006;32:668–74. [DOI] [PubMed] [Google Scholar]
- 9.Schmieder GC, Richardson AI, Scott EC, et al. Selective stenting in subintimal angioplasty: analysis of primary stent outcomes. J Vasc Surg 2008;48:1175–80. discussion 80–1. [DOI] [PubMed] [Google Scholar]
- 10.Schmieder GC, Richardson AI, Scott EC, et al. Outcomes of reinterventions after subintimal angioplasty. J Vasc Surg 2010;52:375–82. [DOI] [PubMed] [Google Scholar]
- 11.Scott EC, Biuckians A, Light RE, et al. Subintimal angioplasty: our experience in the treatment of 506 infrainguinal arterial occlusions. J Vasc Surg 2008;48:878–84. [DOI] [PubMed] [Google Scholar]
- 12.Scott EC, Biuckians A, Light RE, et al. Subintimal angioplasty for the treatment of claudication and critical limb ischemia: 3-year results. J Vasc Surg 2007;46:959–64. [DOI] [PubMed] [Google Scholar]
- 13.Treiman GS, Treiman R, Whiting J. Results of percutaneous subintimal angioplasty using routine stenting. J Vasc Surg 2006;43:513–9. [DOI] [PubMed] [Google Scholar]
- 14.Al-Ameri H, Shin V, Mayeda GS, et al. Peripheral chronic total occlusions treated with subintimal angioplasty and a true lumen re-entry device. J Invasive Cardiol 2009;21:468–72. [PubMed] [Google Scholar]
- 15.Jacobs DL, Motaganahalli RL, Cox DE, et al. True lumen re-entry devices facilitate subintimal angioplasty and stenting of total chronic occlusions: initial report. J Vasc Surg 2006;43:1291–6. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy VN, Eliason JL, Henke PK, et al. Intravascular ultrasound-guidedtruelumenreentry deviceforrecanalization of unilateral chronic total occlusion of iliac arteries: technique and follow-up. Ann Vasc Surg 2010;24:487–97. [DOI] [PubMed] [Google Scholar]
- 17.Saket RR, Razavi MK, Padidar A, et al. Novel intravascular ultrasound-guided method to create transintimal arterial communications: initial experience in peripheral occlusive disease and aortic dissection. J Endovasc Ther 2004;11:274–80. [DOI] [PubMed] [Google Scholar]
- 18.Schneider PA, Caps MT, Nelken N. Re-entry into the true lumen from the subintimal space. J Vasc Surg 2013;58: 529–34. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]


