Abstract
Soil fungi play indispensable roles in all ecosystems including the recycling of organic matter and interactions with plants, both as symbionts and pathogens. Past observations and experimental manipulations indicate that projected global change effects, including the increase of CO2 concentration, temperature, change of precipitation and nitrogen (N) deposition, affect fungal species and communities in soils. Although the observed effects depend on the size and duration of change and reflect local conditions, increased N deposition seems to have the most profound effect on fungal communities. The plant-mutualistic fungal guilds – ectomycorrhizal fungi and arbuscular mycorrhizal fungi – appear to be especially responsive to global change factors with N deposition and warming seemingly having the strongest adverse effects. While global change effects on fungal biodiversity seem to be limited, multiple studies demonstrate increases in abundance and dispersal of plant pathogenic fungi. Additionally, ecosystems weakened by global change-induced phenomena, such as drought, are more vulnerable to pathogen outbreaks. The shift from mutualistic fungi to plant pathogens is likely the largest potential threat for the future functioning of natural and managed ecosystems. However, our ability to predict global change effects on fungi is still insufficient and requires further experimental work and long-term observations.
Citation: Baldrian P, Bell-Dereske L, Lepinay C, Větrovský T, Kohout P (2022). Fungal communities in soils under global change. Studies in Mycology 103: 1–24. doi: 10.3114/sim.2022.103.01
Keywords: drought, elevated CO2, global change, mycorrhiza, nitrogen, deposition, warming
INTRODUCTION
Over the past century, CO2 levels have steadily increased, and global temperatures have risen accordingly. The climate is predicted to continue to change, with increased variability in rain and temperature extremes, both inter- and intra-annually (IPCC 2014, Lee et al. 2021), and affect the whole biosphere including soils. In addition to the changing climate, it is the change of global atmospheric nitrogen (N) deposition that is perhaps the most threatening global phenomenon. It has increased from 34 Tg N/y in 1860 to 93.6 Tg N/y in 2016 (Ackerman et al. 2019) and is predicted to continue increasing worldwide as the result of human activity. Whether soils will become a source or sink of greenhouse gases under future climate scenarios is difficult to predict due to unclear changes in soil carbon and nitrogen pools, and differences in microbial responses between ecosystems and locations (Jansson & Hofmockel 2020), but there is a justified concern that soils will be heavily affected.
Fungi are eukaryotic microorganisms that play multiple fundamental roles related to the future of soil health. As major decomposers of organic matter, mutualists, or pathogens, fungi significantly influence plant health, carbon mineralisation and sequestration, and act as important regulators of the soil carbon balance (Crowther et al. 2016). It is thus important to determine how climate and other global change factors affect future soil fungal communities. The responses of the plant associated guilds to global change factors will likely be of particular interest due to their effects on plant communities. Mycorrhizal fungi act as mutualistic symbionts to plants, providing access to critical nutrients and can ameliorate abiotic stressors associated with climate change, such as heat and drought (Redman et al. 2002, Kivlin et al. 2013). Plant pathogenic fungi, on the other hand, may opportunistically attack plant hosts that are under stress due to the rapid change in their environment (Juroszek et al. 2020, Desaint et al. 2021). Therefore, soil fungi, particularly plant associated guilds, mediate the effects of global change on natural vegetation and agricultural crops in multiple ways.
In addition to direct effects, climate change can indirectly affect soil fungi through shifts in soil chemistry and vegetation structure (Tedersoo et al. 2014, Větrovský et al. 2019, Zhou et al. 2020). It is thus important to understand how global change affects soil fungi. Even though this question has been repeatedly addressed in many contexts and settings in the past, it is still difficult to give a general answer. Soil is the habitat with the highest fungal diversity (Baldrian et al. 2021) and generalisations based on the observed response of individual species are difficult. This high diversity is associated with high levels of functional redundancy in the communities of saprotrophic as well as symbiotic fungi (Žifčáková et al. 2017). Consequently, loss of some species may in theory be replaced by other taxa. However, the critical level of species loss with consequences for ecosystem processes remains largely unknown. Additionally, the diversity, and dependence on plant hosts, of fungal lifestyles (i.e., free-living saprotrophs, mutualistic symbionts and plant pathogens) affect fungal species responses to climate change.
In this review, we will discuss the links between soils, plants, and fungi to explore the paths by which global change affects fungi and their roles in soils. We will also estimate taxon realised niche space to make predictions about the relative sensitivity of various fungi to global change. Lastly, we will use the accumulated information from experimental manipulations of ecosystems to find general patterns in fungal responses to individual global change factors. For simplicity, we will cover only selected global change processes, namely the increasing CO2 levels, warming, reduction in precipitation and N deposition (Fig. 1) since these effects are general and long-lasting. While there is a whole suite of other important phenomena linked with global change, such as land use change, biological invasions, increased fire frequency or increased phosphorus (P) input, these factors are very often geographically local or appear at limited temporal scales which makes the predictions of their effects on fungi difficult. This review adds to our knowledge of belowground communities’ responses to global change by focusing on soil fungi, comparing the possible and current responses of plant pathogens to that of mycorrhizal symbionts, leveraging estimates of fungal guilds realised niches to predict their responses, and only synthesising studies that impose realistic global change manipulations.
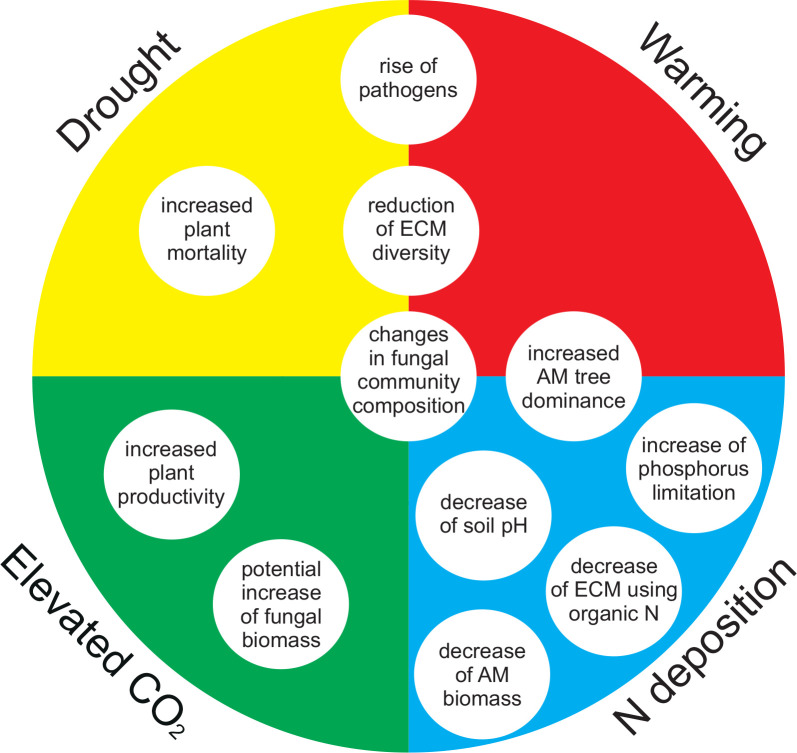
Fig. 1.
Major current and predicted responses to global change factors. Responses to each factor is represented by the location within each section with responses spanning multiple sections indicating the importance of multiple climate change factors.
Fungi and their climatic niche
Utilisation of the niche concept is one approach to predicting the response of fungi to climate change: if we understand the constraints for fungal life, we can identify and localise the environments where they can live. The concept of the ecological niche provides a framework for understanding resource partitioning by organisms and emergent patterns of coexistence and distribution (Macarthur & Levins 1967). Realised niches define the conditions under which organisms can survive and reproduce in the presence of biotic interactions while fundamental niches are defined in the absence of biotic interactions. While the realised niche can be derived from a species’ distribution and abundance across habitat properties (Veresoglou et al. 2012, Davison et al. 2021), characterisation of the fundamental niche is more difficult, because it requires experimental investigation of responses to environmental gradients (Lekberg et al. 2007). However, knowing parameters of the fundamental niches of species would be a valuable tool for the prediction of species’ responses to changing abiotic environments. The fundamental niche provides information on species’ potential responses without the influence of biotic interactions, which must also be expected to change along with abiotic changes (Blois et al. 2013).
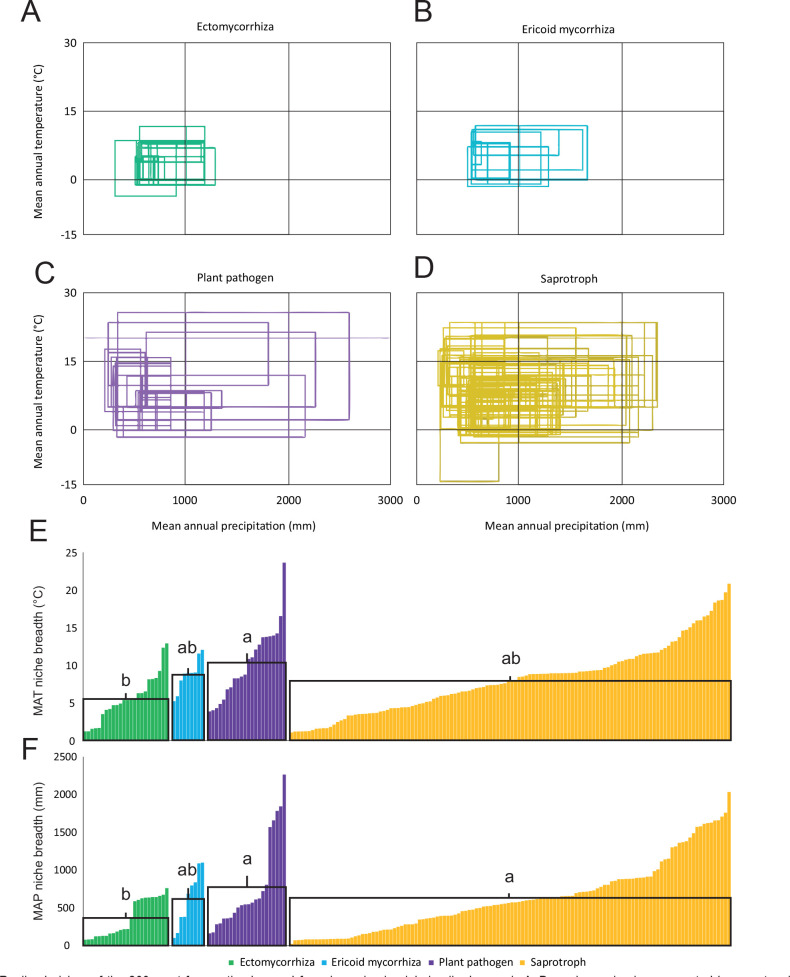
In a global metastudy of soil fungal occurrences using available high-throughput sequencing data, climatic factors contributed, on average, 40–80 % of total explained variability, substantially more than the soil and vegetation properties (Větrovský et al. 2019). Though climatic factors are generally found to be among the most important drivers of global fungal composition, their relative importance varies between studies. For example, Bahram et al. (2018) found that soil carbon-to-nitrogen ratio was the most important driver of fungal abundance, taxonomic and gene composition while Tedersoo et al. (2014) found that soil pH was a major driver of many fungal guilds. Of the climatic factors tested, Větrovský et al. (2019) found that mean temperature of driest quarter, precipitation seasonality, mean temperature of wettest quarter, precipitation of coldest quarter and diurnal temperature range were most often the strongest predictors of individual species distributions. Here we used mean annual temperature (MAT) and mean annual precipitation (MAP) to define species realised niches because these metrics are the most widely used and intuitive defining features of biomes and local climates, are known to affect both soil biota and plants (Jetz et al. 2012, Thompson et al. 2017) and MAT was identified as the strongest predictor of the local distribution of macrofungi within Norway (Wollan et al. 2008). If we define the breadth of the realised climatic niche as the range of MAT / MAP where 90 % of occurrences are observed, fungal species typically inhabit soils within 5–15 °C difference in MAT and 300–1 200 mm difference in MAP (Větrovský et al. 2019), although niche breadth varies largely among individual taxa (Fig. 2). When we compared the 200 most common soil fungi (taxa occurring in > 99 samples worldwide) based on their membership in ecological guilds, the mean annual temperature at the location of occurrence was lowest for ectomycorrhizal (ECM) fungi followed by ericoid mycorrhizal (ERM) fungi, saprotrophs, and plant pathogens while there was less variation between guilds in the observed mean annual precipitation (Table 1). More importantly, the size of the realised temperature and precipitation niche (the range of MAT and MAP between the first and the ninth decile of all observations) was smaller in ECM fungi than in saprotrophs, ERM fungi, and plant pathogens (Table 1; Fig. 2; Větrovský et al. 2019). Narrow breadth of the temperature niche in ECM fungi across climatic gradients was also observed within a smaller geographic extent spanning Japan (Miyamoto et al. 2018).
Fig. 2.
Realised niches of the 200 most frequently observed fungal species in global soils. In panels A–D, each species is represented by a rectangle representing the lower and upper decile of the mean annual precipitation (MAP) and mean annual temperature (MAT) of locations from where it was reported. Colours indicate ecological guild membership: A) green – ectomycorrhizal fungi (n = 24), B) blue – ericoid mycorrhizal fungi (n = 9), C) purple – plant pathogens (n = 22), and D) yellow – saprotrophs (n = 125). The distribution of fungal species niche breadth in E) MAT and F) MAP with color representing guilds and pairwise significant difference between means represented by letters. Individual species are represented with columns. Data from (Větrovský et al. 2019).
Table 1.
Realised niche of fungal guilds of the 200 most common soil fungi from Větrovský et al. (2019). The centre of the niche space is represented by the mean guild Mean Annual Temperature (MAT) and Mean Annual Precipitation (MAP) while the size is represented by the range of MAT and MAP between the first and the ninth decile of all observations.
| Fungal Guild | n | Mean MAT (mean ± SD °C) | Mean MAP (mean ± SD mm) | Range MAT (°C) | Range Mean Annual Precipitation (mm) |
|---|---|---|---|---|---|
| ectomycorrhizal fungi (ECM) | 24 | 4.8 ± 2.2 | 714 ± 124 | 5.5 | 365 |
| ericoid mycorrhizal (ERM) fungi | 9 | 4.9 ± 1.7 | 838 ± 194 | 8.7 | 616 |
| saprotrophs | 125 | 7.7 ± 3.3 | 809 ± 249 | 7.9 | 630 |
| plant pathogens | 22 | 8.1 ± 3.7 | 807 ± 316 | 10.3 | 774 |
Since plant pathogens tend to inhabit warmer areas, and individual species extend both into drier and wetter climates than the ECM fungi (Fig. 2), warming will likely more negatively affect plant-beneficial fungi than plant pathogens (Větrovský et al. 2019). Supporting our prediction of increased soil pathogens, a recent global model of current and projected distributions of plant pathogens showed likely increases in pathogen abundance with MAT predicted to be the major driver (Delgado-Baquerizo et al. 2020a). Furthermore, there is evidence that the niches of pathogens may lack trade-offs between biotic and abiotic niche breadths (Chaloner et al. 2020) and may be more labile than that of plant mutualists such as AM fungi (Bebber & Chaloner 2022) suggesting that pathogens may adapt more rapidly to future climates than plant mutualists. It should be noted that the niche concept can be, in theory, extended to other global change factors as well. For example, the response of ectomycorrhizal fungi to nitrogen availability is known for several taxa (van der Linde et al. 2018). However, the limited number of species with reasonable information on their niche breadth, and missing data on local N availability (which exhibits much higher spatial variability than climate), make this concept at present unusable for predicting responses to altered N.
Ecological guilds of fungi and global change
As already discussed, global surveys of soil fungal occurrences in the GlobalFungi database (Větrovský et al. 2020) show that members of various fungal guilds differ in the size of their climatic niche. Moreover, the level of dependence on vegetation varies from obligate biotrophs to free-living fungi. Due to this, global changes are expected to affect various ecological guilds of soil fungi (ECM fungi, AM fungi, ERM fungi, plant pathogens and saprotrophs) differently, affecting their relative share or community composition. These shifts may subsequently result in changes in various ecosystem processes such as decomposition rate or plant performance.
Importantly, climate change-driven shifts in plant communities may lead to shifts in the host availability affecting those fungi that have a narrow host range. With increasing warming, some alpine communities have seen the replacement of forbs with deep rooted grasses (Liu et al. 2018) and increasing nitrogen deposition can lead to reduced species richness though this effect depends on ecosystem characteristics, such as mean annual precipitation (Clark et al. 2007). Altered environmental conditions promote not only natural range shifts of plants species (Rudgers et al. 2014), but also enable naturalisation of alien plant species outside their native distribution range (Seebens et al. 2015). Such events can affect local ecosystems and their fungal components in several ways: by competition for resources, by the introduction of novel fungal species (such as mycorrhizal symbionts or pathogens), or by selective recruitment of root-associating fungal species already present in the local pool by the alien plants (Rudgers et al. 2020, Vlk et al. 2020a). Because of all these factors, changes in local fungal communities are expected as has been already observed for plant introductions (Vlk et al. 2020b).
Due to the complex effects of N on soil chemistry and vegetation, and the fact that mutualistic mycorrhizal fungi mediate its transfer to plants, change in atmospheric deposition is perhaps the factor with greatest importance for guild composition of soil fungi (Fig. 1). Indeed, nitrogen addition to 25 grasslands distributed across four continents led to the increase of fungal pathogens, although it did not significantly affect AM fungi and saprotrophs. These guild level responses were primarily mediated through nutrient-induced shifts in plant communities (Lekberg et al. 2021). On the other hand, no consistent shifts in guild composition were observed across N-supplemented forests in the USA (Moore et al. 2021).
Among the various aspects of global change, changes in climate lead to severe ecosystem alterations. Forests are already facing increasing lengths of heat waves with unprecedented increases of temperature in high latitudes combined with long drought periods. This high level of climate stress likely increases the vulnerability of forests to disturbances including tree dieback and forest fires (Fig. 1; Allen et al. 2010). These severe forest disturbances were shown to result in a shift of fungal communities from those dominated by ectomycorrhizal fungi in undisturbed forests to those dominated by saprotrophs in disturbed forests (Štursová et al. 2014, Rodriguez-Ramos et al. 2021) as a response to changes in primary productivity.
Mycorrhizal plant symbionts
Geographic distributions of plants with various mycorrhizal symbioses show climate-driven patterns. Temperature-related factors have been found to be the main predictors of the distributions of plant species forming AM, ECM, and ERM symbiosis. Recent models show AM plants to be favoured by warm climates, while dominance of ECM plants (and to some extent ERM plants) is more favoured by colder climates (Barcelo et al. 2019). Ectomycorrhizal symbiosis dominates forests in which seasonally cold and dry climates inhibit decomposition and is the predominant form of symbiosis at high latitudes and elevation. AM trees dominate in grasslands and the warm-and-wet climates of tropical forests where enhance decomposition is typical (Steidinger et al. 2019). Warming can significantly alter the distribution of mycorrhizal host plants, with likely subsequent impacts on the proportion of various guilds of mycorrhizal fungi. In addition to warm climates, AM fungal colonisation has been found to be strongly related to soil carbon-to-nitrogen ratio and highest at sites featuring continental climates with mild summers and a high availability of soil nitrogen (Soudzilovskaia et al. 2015). In contrast, the intensity of ectomycorrhizal infection in plant roots maybe more related to soil acidity, soil carbon-to-nitrogen ratio and seasonality of precipitation and is highest at sites with acidic soils and relatively constant precipitation levels (Soudzilovskaia et al. 2015). As such, root colonisation by both guilds is predicted to respond to climatic factors and N deposition.
AM fungi primarily rely on inorganic forms of N (Phillips et al. 2013) or small organic N compounds (Whiteside et al. 2012). In contrast, some ECM fungi are thought to rely more heavily on organic N sources (Phillips et al. 2013), having a greater capacity to invest in N-degrading extracellular enzymes that access complex organic forms of N in soil, such as proteins and chitin (Fernandez & Kennedy 2016). ECM fungi are thus more associated with slower decomposition of soil organic matter and increased soil carbon (C) storage (Averill et al. 2014, Averill & Hawkes 2016, Fernandez & Kennedy 2016), potentially by competing with free-living soil microbes for organic N resources. These distinctions between AM and ECM fungi lead to two important predictions: (a) that inorganic N inputs to ecosystems will favour AM-associated trees at the expense of ECM-associated trees, and (b) that inorganic N-driven declines in ECM fungal abundance will reduce the belowground C storage capacity of the forest biome (Fig. 1). Indeed, recent nitrogen deposition across USA favoured the expansion of AM trees at the expense of ectomycorrhizal trees, and was spatially correlated with reduced soil carbon stocks (Jo et al. 2019). This implies that future changes in nitrogen deposition may further turn the balance between AM and ECM fungi in forest ecosystems (Averill et al. 2018).
Ectomycorrhizal fungi
Despite the potential for climate change driven replacement of ECM with AM trees, most ecosystems are dominated by either ECM plant symbionts (in most temperate and boreal forests worldwide) or AM symbionts (in natural grasslands, croplands and tropical forests). Therefore, relative abundance of each guild or the change of within-guild species composition are the most likely responses. While shifts in dominant mycorrhizal type mediated by global changes will likely result in changes in nutrient cycles and soil carbon storage, consequences of potential shifts of within guild species composition are less clear.
Based on the assessment of present climatic drivers of ECM fungal distribution, under future climate scenarios North American Pinaceae forests are predicted to see as high as 26 % declines in ECM fungal species richness within 50 years, although there is a high level of regional variation (Steidinger et al. 2020). Furthermore, ECM fungal diversity across Japan was also demonstrated to significantly decrease with MAT (Miyamoto et al. 2018), suggesting potential decreases with warming. The observation of the ECM fungal community shift on Betula papyrifera and Abies balsamea saplings in a warming experiment (Fernandez et al. 2017) suggests that warming may change the future composition of the ECM fungal subcommunity.
Since N supply to plants is one of the major roles of ECM fungi, N deposition likely affects ECM fungal communities. With increasing nitrogen availability, fungi that obtain nitrogen from complex soil organic sources using metabolically costly pathways – e.g., Cortinarius, Piloderma and Tricholoma – are likely at a disadvantage compared to fungi that use inorganic nitrogen, such as Elaphomyces or Laccaria (Lilleskov et al. 2011). In a large survey of ECM fungi associated with forest trees in Europe, several ECM fungi responded to N throughfall deposition. Fungi that use organic nitrogen tended to be negative indicators for nitrogen deposition, while fungi that use inorganic nitrogen tended to be positive indicators. Conifer specialists – particularly those with abundant hyphae and rhizomorphs – were more negatively affected by increasing nitrogen than generalists and broad-leaf specialists (van der Linde et al. 2018). In the future, N deposition will likely affect ECM fungi and promote shifts from nitrophobic species (e.g., Russula vinosa, Lactarius rufus) to nitrophilic species (e.g., Scleroderma citrinum, Amanita rubescens, Russula ochroleuca) (Fig. 1; van der Linde et al. 2018).
In theory, mutualistic fungi could accompany host plants in climate-induced migration (Rudgers et al. 2020). In a study of the upward migration of tree individuals above the tree line, low ECM diversity was observed in the roots of migrating trees indicating that the altitudinal shift in the ECM fungal community lags behind climate-driven tree migration. ECM fungal dispersal limitation is thus an important factor controlling this process and possibly retarding vegetation shifts (Alvarez-Garrido et al. 2019). Similar conclusions were found in a study of invasive pines that clearly showed plant invasions can be limited by the dispersal of ECM fungi (Nunez et al. 2009).
Arbuscular mycorrhizal fungi
Similar to ECM fungi, AM fungi also fully depend on their symbiotic host plants as a sole source of carbon (Tisserant et al. 2013) and therefore any environmental shifts may affect abundance, species richness and AM fungal community composition directly as well as indirectly by altering their host plants. A recent review of the response of AM fungal species richness and community composition to various aspects of global change found that elevated CO2 will likely have no effect on AM fungal richness, and responses to N deposition, warming, and changed precipitation will likely be highly context dependent (Cotton 2018).
The effects of the above-mentioned extrinsic factors associated with global change are translated into community composition of AM fungi via differential responses of each species, which are determined by their intrinsic characteristics, such as specific growth patterns, morphology or anatomy. AM fungi greatly vary in root colonisation traits such as extent and structure (Klironomos & Hart 2002), and soil hyphal traits such as extent, density and structure (Powell et al. 2009). Interestingly, the increase of CO2 concentration, as well as increases in N availability, leads to lower relative abundance of AM fungal taxa from the Gigasporaceae and Diversisporaceae families, which produce high levels of extraradical mycelia, while relative abundance of the Glomeraceae taxa, which are characterised by extensive intraradical colonisation, tend to increase (Cotton 2018). This shift in community traits suggests lower investments in potentially costly nutrient acquisition traits with increasing nutrient availability.
The community level responses to environmental conditions combined with various intrinsic characteristics indicate that niche optima and niche width may differ among the species of AM fungi. Large sampling campaigns, enabled by an onset of high-throughput sequencing methods, provide sufficient data to model parameters of species ecological niches. While Acaulosporaceae has a realised niche optima in low temperature conditions, Gigasporaceae has a realised niche optima in high temperature and high precipitation conditions (Davison et al. 2021). Additionally, the width of the AM fungal temperature niche appears to be limiting, seeming to be narrower than in other fungal guilds (Větrovský et al. 2019, Davison et al. 2021). These findings indicate that changes of MAT and MAP can particularly affect the composition of AM fungal communities.
Contrary to diversity, the abundance of AM fungi seems to be more consistently affected by changes in N availability and shifts in CO2 concentration. While the majority of studies report a decrease in AM fungal abundance with enhanced nitrogen (e.g., Shen et al. 2014, Chen et al. 2017, Treseder et al. 2018, Zhang et al. 2018, Han et al. 2020, Jia et al. 2020a, Ma et al. 2021a), a few found no effect (Lilleskov et al. 2019, Karst et al. 2021). The addition of N can benefit AM fungi if it exacerbates plant P limitation (Johnson 2010), but may be suppressive if nitrophilic, ruderal plants replace plants that allocate more C to AM fungi (Isbell et al. 2013). Thus, the responses likely depend on the extent to which nutrient addition alleviates plant deficiencies and alters plant communities. A meta-analysis examining the global effects of nutrient enrichment on AM fungal and plant diversity showed that AM fungal diversity, rate of root colonisation, and extraradical biomass typically decreased with N addition, while spore abundance and hyphal length were unaffected. These results were consistent among forests, grasslands, and agro-ecosystems (Ma et al. 2021a).
The short-term fertilisation effect of elevated CO2 concentrations mostly stimulated AMF abundance (e.g., Treseder 2004, Antoninka et al. 2011, Zavalloni et al. 2012, Sun et al. 2017, Dong et al. 2018). Importantly, while stimulation of AM fungal abundance with increased CO2 is expected, considering that plant productivity depends on nutrient supply by AM fungi, the increase of temperature and shifts in precipitation will likely affect AM fungal abundance thanks to a greater climate niche partitioning of AM fungi.
Plant pathogens
Analyses of fungal guild niche breadth indicates that plant pathogens may better cope with climate change than other fungal guilds (Chaloner et al. 2020). Conditions that affect pathogen overwintering and dispersal are of essential importance due to pathogen lifestyles, survival in soils, and outbreaks triggered by climatic and plant host signals. Global warming in areas with seasonal temperature variation has increased pathogen survival during winters and increased the length of vegetation seasons leading to faster pathogen spread or stronger outbreaks (Harvell et al. 2002). As an ongoing consequence of warming, movement of crop pests to higher latitudes has already been observed. Since the 1960s, fungal crop pests were observed to move polewards at a pace of some 5 km/y, more rapidly than most other crop pests (Bebber et al. 2013).
Warming appears to be the most important driver of plant pathogen abundance. Climatic factors, especially the MAT and precipitation seasonality were the most important predictors of the relative abundance of plant pathogens across 235 global sites. Under future climate change and land-use scenarios, relative abundance of plant pathogens is predicted to increase (Delgado-Baquerizo et al. 2020a). A nine-year warming experiment in a dryland on the Iberian peninsula showed higher relative share of pathogens, higher relative abundance of Alternaria and higher absolute abundance of Alternaria in warmed plots (Delgado-Baquerizo et al. 2020a). While the increase in relative abundance, or sporulation, of plant pathogens may increase the risk of a disease outbreak, direct causal links may be difficult to find. It is possible that negative responses of mycorrhizal fungi and neutral or positive responses of pathogens to climate change can subsequently manifest in negative responses of vegetation. More importantly, climatic events seem to be predictive factors of fungal disease outbreaks with high humidity and high temperature being the most common factors (Romero et al. 2022). Pathogens may also use the opportunity to attack weakened host communities such as forest ecosystems after dieback caused by drought or heat stress (Fig. 1; Anderegg et al. 2013).
In natural systems, pathogens appear to be more abundant in resource-rich environments (Reynolds et al. 2003, Revillini et al. 2016), and nutrient addition (e.g. fertilisation) has been linked to increased disease incidence in plants (Walters & Bingham 2007, Veresoglou et al. 2013) which may increase the risk of pathogen spread or outbreaks at elevated atmospheric N deposition. The effect of CO2 increase on pathogens is less clear, however, concentrations of spores of several pathogens were increased by elevated atmospheric CO2 (eCO2) in a Populus tremuloides plantation in air and litter. Although the responses of fungi were not uniform, significant increases were found in the potential pathogenic genera Alternaria, Cladosporium and Fusarium (Klironomos et al. 1997).
Plant pathogen community composition may not intrinsically affect ecosystems because it is often individual taxa that cause disease outbreaks. The effects of global change on individual plant pathogen taxa may thus be more important than the guild-level effects. Based on historical observations of higher Alternaria spp. spore concentrations at warm temperatures, spore concentrations are predicted to increase with warming in the United Kingdom (Maya-Manzano et al. 2016) and future climate models suggest increased prevalence of Alternaria brassicae in North Germany (Siebold & Tiedemann 2012). In several instances, eCO2 increased spore production by Alternaria spp. several-fold (Klironomos et al. 1997, Wolf et al. 2010). Considering disease severity, both warming and eCO2 has been shown to increase Alternaria leaf spot severity on rocket, cauliflower and cabbage (Pugliese et al. 2012, Siciliano et al. 2017).
To conclude, while differential response of ECM fungal species to global changes such as N deposition can be predicted from their extracellular enzymatic capabilities related to organic nitrogen accessibility, response of AM fungi depends on their differential colonisation traits. Species traits of saprotrophs or pathogens related to their response to global changes are much less clear and therefore predictions of global change effects on these two guilds are much more difficult.
Fungal response to global change factors and lessons learned from manipulated studies
Our present understanding of the response of fungi to global change is based on several lines of support: (1) ecological theory and the predictions based on the known niches of fungal species, (2) predictions of responses to indirect factors affected by global change, such as the change of soil chemistry, vegetation composition, or ecosystem productivity, (3) extrapolation of observations of changes in fungal communities across time and space, (4) experimental simulation of future conditions and the analysis of fungal response. Since there is a lack of long-term observations on soil fungi under conditions of real-time climate change and the extrapolation of such observations may be problematic, experimental manipulations simulating global change factors appear to be the best tool to predict the future of soil fungi.
Experimental approaches have several limitations that must be considered when interpreting results. Each of the experiments has at least three important aspects that affect the observations: (1) the duration of treatment, (2) the intensity of manipulation, and (3) the local conditions. Over the duration of treatment, several components of the system respond so that direct, and/or indirect, effects change in time and adaptations emerge. The plant communities likely respond first with altered productivity, while change in composition comes later (Smith et al. 2009). Importantly, the effects of short-term warming and/or precipitation experiments can be eclipsed by site specific year-to-year variation in climatic conditions. The intensity of manipulation is another critical issue. In many experiments, especially those simulating N deposition, the magnitude of treatments is considerably larger than those predicted by current models. Equally important, the target biome and local condition at the experimental sites can interact with the global change treatments. Moreover, soil fungi as the responding community are extremely diverse in terms of alpha and beta diversity (Baldrian et al. 2021) which limits the cross-ecosystem interpretation of community effects. Unfortunately, the experimental results reported so far show high levels of geographic bias with most studies in forests and grasslands of the temperate zone (Tables 2–5). These biases in sampling mean that surprising results from underexplored biomes, such as massive CO2 fluxes from warmed plots recorded in the Panama tropical rainforest, cannot be ignored. Such fluxes largely exceeded model predictions and indicated high sensitivity of local soil C stocks to warming (Nottingham et al. 2020).
Table 2.
Effects of experimental CO2 enrichment on fungi. Manipulations of at least 1 y duration where CO2 enrichment was not combined with other factors were considered.
| Location | Experimental system | Duration of treatment (yr) | CO2 concentration applied (ppm) | Biomass | Diversity | Guilds share | Community composition | Reference1 |
|---|---|---|---|---|---|---|---|---|
| All fungi | ||||||||
| Asia (China) | cropland | 2 | 500 | + | change | Liu et al. (2014) | ||
| Asia (China) | cropland | 3 | 500 | + | 0 | change | Liu et al. (2017) | |
| North America (USA) | experimental grassland | 3 | 500 | 0 / + | change (more AMF) | change | Procter et al. (2014) | |
| Asia (China) | shrubland | 4 | 700 | 0 | change | Jia et al. (2020b) | ||
| Europe (Denmark) | shrubland | 5 | 510 | 0 | Haugwitz et al. (2014) | |||
| Australia (Australia) | grassland | 5 | 550 | + | change | Hayden et al. (2012) | ||
| North America (USA) | grassland | 6 | 680 | 0 | Gutknecht et al. (2012) | |||
| Europe (Italy) | forest plantation | 6 | 550 | 0 | change | Lagomarsino et al. (2007) | ||
| Asia (China) | cropland | 8 | 500 | + | Liu et al. (2021a) | |||
| North America (USA) | shrubland | 8 | 550 | + | + | change | Lipson et al. (2014) | |
| Europe (Switzerland) | experimental forest | 9 | 570 | 0 | no change | change | Solly et al. (2017) | |
| Europe (Germany) | grassland | 10 | 440 | 0 | Guenet et al. (2012) | |||
| North America (USA) | forest plantation | 11 | 560 | 0 | 0 | no change | Dunbar et al. (2014) | |
| North America (USA) | experimental field | 12 | 550 | + | change (more Basidiomycota, less Ascomycota) | Tu et al. (2015) | ||
| North America (USA) | forest plantation | 14 | 571 | change | Weber et al. (2013) | |||
| Arbuscular mycorrhizal fungi | ||||||||
| North America (USA) | experimental field | 2; 4; 6 | 550 | 0 | change (more Glomeraceae and Gigasporaceae) | Cotton et al. (2015) | ||
| North America (USA) | grassland | 3 | 680 | 0 | no change | Mueller & Bohannan (2015) | ||
| North America (USA) | shrubland | 3.4–3.9 | up to 750 | + | change (more Acaulospora and Scutellospora) | Treseder et al. (2003) | ||
| Europe (Switzerland) | experimental field | 7 | 600 | + (root colonisation) | Gamper et al. (2004) | |||
| North America (USA) | orchard | 7 | 670 | 0 | 0 | Kimball et al. (2007) | ||
| Asia (China) | grassland | 7 | 580 | 0 | - | no change | Zheng et al. (2022a) | |
| North America (USA) | grassland | 7 | 560 | + (soil hyphae) | Antoninka et al. (2011) | |||
| Asia (India) | experimental field | 8 | 550 | + | 0 | change | Panneerselvam et al. (2020) | |
| Europe (Germany) | grassland | 15 | 440 | 0 (root colonisation) | + | no change | Maček et al. (2019) | |
Table 5.
Effects of experimental N addition on fungi. Manipulations where N was added in a mineral form with the aim to simulate atmospheric deposition that lasted at least for 1 yr and where N addition was not combined with other factors were considered. Manipulations or treatments where N addition exceeded 75 kg N/ha/y were not considered as highly exceeding projected N deposition increase; + denotes that the experiment also included treatment(s) with higher N addition level(s).
| Location | Experimental system | Duration of treatment (yr) | N addition (kg N/ha/y) | Biomass | Diversity | Guilds share | Community composition | Reference |
|---|---|---|---|---|---|---|---|---|
| All fungi | ||||||||
| North America (USA) | shrubland | 1 | 7; 15 | 0 | no change | Mueller et al. (2015) | ||
| Asia (China) | grassland | 1 | 50; + | 0 | change | Li et al. (2020a) | ||
| North America (USA) | experimental field | 1 | 50 | 0 | no change | no change | Anthony et al. (2020) | |
| North America (Canada) | grassland | 1–2 | 20 | + | Bell et al. (2010) | |||
| Asia (China) | shrubland | 2 | 60 | 0 | change | She et al. (2018) | ||
| Asia (China) | forest | 2 | 30; 60; + | + | + | change (more AMF) | change (more Basidiomycota) | Li et al. (2019a) |
| Asia (China) | wetland | 2 | 30; 60; + | 0 | change | Li et al. (2020b) | ||
| Asia (China) | forest | 2 | 25 | + | change | Guo et al. (2021) | ||
| Asia (China) | grassland | 3 | 15; 30; 50; + | 0 | Zhang et al. (2018) | |||
| Europe (Czech Republic) | forest | 4 | 50 | 0 | no change | Choma et al. (2020) | ||
| Asia (China) | grassland | 5 | 35 | 0 | change (less AMF) | change | Wang et al. (2020b) | |
| Asia (China) | forest | 5 | 25 | + | + | change (more saprotrophs) | change | Zhao et al. (2020) |
| Asia (China) | forest | 6 | 50; + | - | 0 | change (less Ascomycota) | Wang et al. (2021b) | |
| North America (USA) | grassland | 6 | 70 | change (less AMF) | Gutknecht et al. (2012) | |||
| Asia (China) | forest | 6 | 50; + | 0 | no change | Li et al. (2019b) | ||
| Asia (China) | experimental field | 7 | 35; 70; + | + | - | change | Wang et al. (2015) | |
| North America (USA) | experimental grassland | 7 | 28; 56; + | 0 | change (less AMF) | change | Li et al. (2021) | |
| Asia (China) | forest | 8 | 50 | 0 | Yan et al. (2021) | |||
| North America (Canada) | forest | 10 | 30 | 0 | no change | no change | Wu et al. (2021) | |
| North America (Canada) | experimental field | 10 | 30; + | 0 | - | change | Tosi et al. (2021) | |
| Europe (United Kingdom) | wetland | 14 | 8; 25; 56 | change (less ERM) | Vesala et al. (2021) | |||
| Asia (China) | grassland | 15 | 18; 53; + | - | 0 | change (more Eurotiomycetes and Sordariomycetes) | Chen et al. (2019) | |
| North America (USA) | forest | 16 | 30 | 0 | no change | Hesse et al. (2015) | ||
| North America (USA) | forest | 20 | 30 | 0 | 0 | no change | Freedman et al. (2015) | |
| North America (USA) | forest | 20 | 50; + | change (less ECM, more saprotrophs) | change (more nitrophilic ECM) | Morrison et al. (2016) | ||
| Europe (Switzerland) | forest | 20 | 22 | 0 | 0 | change (less ECM) | change | Frey et al. (2020) |
| Europe (Sweden) | forest | 23; 46 | 34; 73 | 0 | change (less ECM) | Choma et al. (2017) | ||
| Europe (Sweden) | forest | 24 | 40 | + | 0 | change (more saprotrophs) | change (more nitrophilic ECM) | Tahovská et al. (2020) |
| Arbuscular mycorrhizal fungi | ||||||||
| North America (USA) | experimental field | 1 | 50 | 0 | no change | Anthony et al. (2020) | ||
| Asia (China) | forest | 2 | 30; 60 | + (root colonisation) | + | change | Liu et al. (2021c) | |
| North America (USA) | shrubland | 2.8 | 60 | - (spore density) | - | change (more Glomus, less Gigaspora and Scutellospora) | Egerton-Warburton & Allen (2000) | |
| Asia (China) | forest plantation | 3 | 40; + | + (root colonisation) | 0 | change (more Gigasporaceae) | Cao et al. (2020b) | |
| Asia (China) | grassland | 3 | 50; + | - (root colonisation) | + | change | Jiang et al. (2018) | |
| North America (USA) | grassland | 3 | 70 | 0 | no change | Mueller & Bohannan (2015) | ||
| Asia (China) | forest | 4 | 50 | 0 | no change | Zhao et al. (2018) | ||
| Asia (China) | experimental field | 5 | 50; + | - | - | change | Zhu et al. (2018) | |
| Asia (China) | grassland | 6 | 15; 75 | - (spore density) | + | no change | Zheng et al. (2014) | |
| North America (USA) | grassland | 6 | 70 | - (biomass) | Gutknecht et al. (2012) | |||
| Asia (China) | grassland | 7 | 50; + | - (root colonisation) | 0 | change | Lu et al. (2020) | |
| Asia (China) | grassland | 7 | 50; + | - (root colonisation, biomass) | - | change | Chen et al. (2017) | |
| Asia (China) | grassland | 8 | 25; 50 | 0 | 0 | no change | Li et al. (2015) | |
| North America (USA) | forest | 12 | 30 | change (more Glomus) | van Diepen et al. (2011) | |||
| North America (USA) | forest | 16 | 30 | 0 | no change | van Diepen et al. (2013) | ||
Here, we review the results from experimental simulations of climate change factors 1) elevated CO2, 2) warming, 3) reduction of precipitation, and 4) increased N deposition (Fig. 1). We ended up with 138 studies that applied realistic treatment types and levels (see each section) and reported at least one of the below response variables (Supp. S1). Though our survey is not exhaustive, we believe it is representative of the current state of knowledge. We decided to focus on the commonly studied fungal responses biomass, diversity, guild share, and changes in community composition. Though these responses are interconnected (e.g., changes in fungal diversity will likely lead to changes in composition), we decided to survey all factors to highlight the current focuses of research into the responses of fungi to climate change factors. The analyses of diversity, guild share, and changes in community composition largely rely on meta-barcoding sequencing, which we recognise as suffering from biases such as primer bias and the use of relative abundances (Quinn et al. 2018, Alteio et al. 2021), it is still the best tool for understanding fungal communities (Nilsson et al. 2018). All recorded responses are taken directly from the results sections and therefore represent current interests in the field.
Increase of CO2 concentration
Elevated CO2 partially underlies global increases in plant productivity (Nemani et al. 2003). Furthermore, experimentally elevated atmospheric CO2 concentrations (eCO2) have led to short term increases in plant biomass production, allocation of carbon to roots and to soil (Adair et al. 2011) and consequently soil respiration. The higher C allocation belowground can fuel the breakdown of labile organic matter by copiotrophic microorganisms. Therefore, microbial biomass and heterotrophic respiration will likely increase (Fig. 1; Naylor et al. 2020). At longer time scales, eCO2 has been shown to increase microbial decomposition of soil organic matter (SOM) through priming (van Groenigen et al. 2014). Direct effects on individual fungi are unlikely since CO2 concentration in soil pores is higher than in the atmosphere and varies in space and time.
Furthermore, eCO2 may affect fungal propagation and dispersal. Under an 2×-ambient CO2 treatment in a Populus tremuloides plantation, the concentration of airborne fungal propagules, mostly spores, increased fourfold. Analysis of decomposing leaf litter (likely the main source of airborne fungal propagules) indicated that fungi produced fivefold more spores (Klironomos et al. 1997). Furthermore, increased total sporocarp biomass was observed in an eCO2 experiment (Andrew & Lilleskov 2009). Since fruiting and sporulation is the main mode of dispersal of soil fungi, consequences of this observation – if confirmed in additional systems – may be important.
Across the studies we surveyed, eCO2 experiments report either no change or increased biomass and diversity of all fungi, and only single cases of reduced AM fungal diversity and change in guild composition. Most experiments report change in the fungal community composition but there were no consistent observations of enriched or suppressed taxa (Fig. 3, Table 2). Though we found no clear relationship between fungal responsiveness and experimental length (Figs 3, 4), a meta-analysis of 11 studies found a positive relationship between increased fungal richness due to eCO2 and experimental length (Veresoglou et al. 2016). A recent global meta-analysis found no relationship between experimental length and the responsiveness of fungal biomass, but found that eCO2 decreased the F/B ratio across 31 studies (Sun et al. 2021). In our survey, the longest experiments showed contrasting effects on soil chemistry. A forest-based experiment reported significant decreases in pH, organic matter content, and P and increased water content (Weber et al. 2013) which may all potentially affect fungi. However, a grassland experiment of a similar length reported no significant change in soil chemistry (Maček et al. 2019).
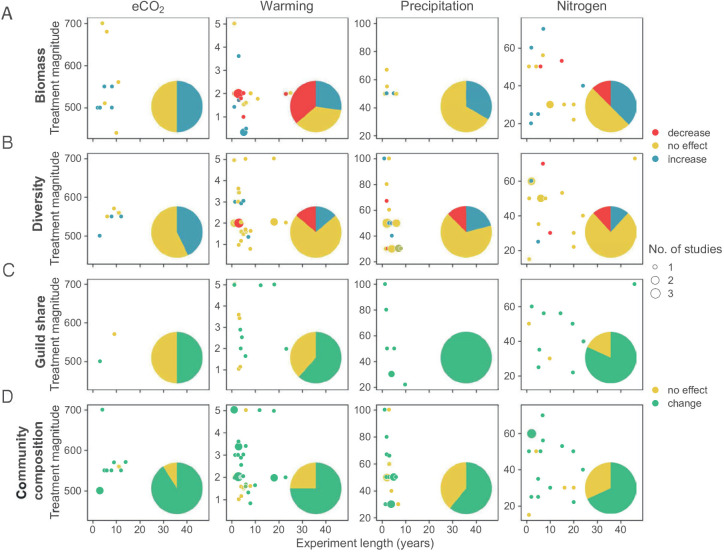
Fig. 3.
Observations of the effects of selected global change factors on the A) biomass, B) diversity, C) guild composition and D) community composition of total fungi in the context of experimental length and magnitude of treatment. The pie graphs indicate the total share of experiments reporting statistically significant effects (increase, decrease, no change). Treatment intensities are in ppm applied for CO2, increase in °C in temperature manipulation, percent reduction in precipitation and kg/ha/y in N addition. For the lists of experiments, see Tables 1–4.
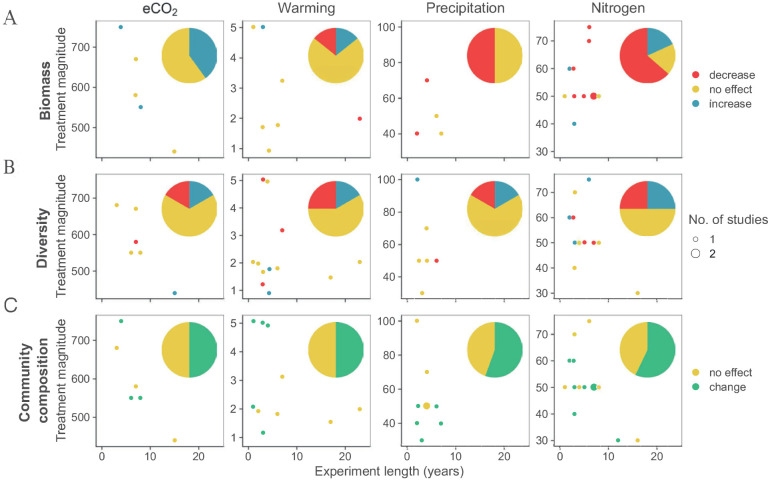
Fig. 4.
Observations of the effects of selected global change factors on the A) biomass, B) diversity and C) community composition of AM fungi in the context of experimental length and magnitude of treatment. The pie graphs indicate the total share of experiments reporting statistically significant effects (increase, decrease, no change). Treatment intensities are in ppm applied for CO2, increase in °C in temperature manipulation, percent reduction in precipitation and kg/ha/y in N addition. For the lists of experiments, see Tables 1–4.
Warming
In agreement with the increasing catalytic performance of soil enzymes with increasing temperature (Baldrian et al. 2013), C turnover across global biomes has been shown to increase with temperature (Carvalhais et al. 2014). Temperature sensitivity of soil C loss appears higher in cold regions (Crowther et al. 2016, Koven et al. 2017) and probably the most extreme response is expected in the permafrost where thawing dramatically increases organic matter transformation and the emissions of CO2 and CH4 (Jansson & Tas 2014). The expected C losses are large since the soils in cold regions host large C stocks (Crowther et al. 2019, García-Palacios et al. 2021). Additionally, warming has led to the loss of plant species unable to tolerate new environmental conditions (Freeman et al. 2018) or outcompeted by invaders better adapted to the new conditions (Alexander et al. 2015). These shifts in plant species composition may alter the quality of the carbon input into the system (Harte et al. 2015). Shifts in fungal saprotroph communities in response to both increased access to extant carbon and novel carbon inputs will have important implications for global responses to climate change (García-Palacios et al. 2021).
The responses of soil fungal communities to warming likely depends on the local climatic conditions, such as MAT. Not surprisingly, in the Antarctic, at the lower limit of fungal temperature tolerance, air temperature is the strongest and most consistent predictor of soil fungal diversity and, with current rates of warming, a 30 % increase in fungal diversity is predicted by 2100 (Newsham et al. 2016). However, this diversity response to warming is probably not universal since the highest level of fungal diversity is predicted in cold areas (Větrovský et al. 2019). Similar to soil fungi, the highest diversity of bacteria in global surveys has also been observed at locations with relatively low MAT (around 10 °C; Thompson et al. 2017) and temperate regions (Bahram et al. 2018), although bacterial biomass in soils does not seem to be affected by warming (Lladó et al. 2017).
Short-term and prolonged warming may have differing effects. An initial loss of labile soil carbon in one of the longest running warming experiments in the Harvard Forest was later followed by increased degradation of more recalcitrant carbon compounds. Sustained warming for 26 years resulted in the depletion of soil organic carbon (SOC) with corresponding reductions in microbial biomass (Melillo et al. 2017). Based on a meta-analysis, warming initially increases soil respiration, but the magnitude of observed effect declines significantly as warming progresses and in fact, after 10 years of warming, soil respiration in experimentally warmed plots was similar to controls. Microbial acclimation, community shifts, adaptation, or reductions in labile C may ameliorate warming effects on soil respiration in the long-term. Accordingly, long-term soil C losses might be smaller than those suggested by short-term warming studies. The share of experiments where fungal biomass increased versus decreased with warming have been found to be roughly equivalent and no significant change in the fungal to bacterial (F/B) biomass ratio were observed across studies (Romero-Olivares et al. 2017). The F/B ratio was also unaffected after 7–25 yr of warming across 12 experiments in the Alpine and Arctic tundra (Jeanbille et al. 2021).
Temperature also alters fungal fruiting with consequences for dispersal. Across Europe, timing of fruiting has been shown to vary by 25 d among latitudes and 30 d among altitudes suggesting a strong temperature effect (Andrew et al. 2018). Present-day autumn fruiting of fungi has been shown to occur later than in the past, and the fruiting season length has increased, similar to the vegetation season (Kauserud et al. 2012). There has also been shown to be a significant shift in fruiting of saprotrophic and ectomycorrhizal fungi towards higher altitudes in the Swiss Alps between 1960 and 2010 as a consequence of warming (Diez et al. 2020).
Warming was the most frequently applied treatment in our survey (47 % of studies) and as such gives the best opportunity for generalisations. Importantly, warming was most frequently reported to alter total fungal biomass and a substantial fraction of the observations indicate negative effects, especially between 3–5 yr of application. In longer-lasting experiments, however, the effects on fungal biomass were less pronounced and AM fungi seem to be even less affected. Both negative and positive effects on total fungal diversity were reported but no effects were reported for experiments running for more than three years; furthermore, the decrease of AM fungal diversity was also observed only in the short term (Figs 3, 4, Table 3). Many individual experiments reported significant effects on fungal guild composition, which were, however, context-dependent. The only exception is the effect on plant pathogens where all reports showed their increase (Table 3). Most warming experiments also reported change in fungal community composition, often within the ectomycorrhizal guild (Fernandez et al. 2017, van Nuland et al. 2020) and a decrease of the Glomeraceae was recorded within the AM fungi (Cao et al. 2020a, b). Interestingly, almost all studies with experimental lengths longer than 10 yr or any experimental length with warming treatments larger than 2 °C reported significant changes in fungal community composition. In partial support of our survey, a recent global meta-analysis found that warming decreased fungal richness but that there was no significant effect of experimental length on this response (Li et al. 2022). There were no reports of important changes in soil nutrient content or pH but some of the long-term experiments report the decrease of the F/B biomass ratio (Gutknecht et al. 2012) and lower transcription of hydrolytic enzymes (Romero-Olivares et al. 2019), two factors that may be connected since fungi are important producers of enzymes in soils (Starke et al. 2021).
Table 3.
Effects of experimental warming on fungi. Manipulations of at least 1 yr duration where warming was not combined with other factors were considered.
| Location | Experimental system | Duration of treatment (yr) | Temperature increase (°C) | Biomass | Diversity | Guilds share | Community composition | Reference |
|---|---|---|---|---|---|---|---|---|
| All fungi | ||||||||
| Asia (China) | grassland | 1 | 2.0 | 0 | no change | Zhang et al. (2016a) | ||
| North America (USA) | forest | 1 | 5.0 | 0 | change (more plant pathogens, less AMF) | change | Garcia et al. (2020) | |
| North America (USA) | experimental field | 1 | 5.0 | 0 | no change | change | Anthony et al. (2020) | |
| South America (Brazil) | experimental field | 1 | 2.0 | 0 | change (more Hypocreales, less Pleosporales) | de Oliveira et al. (2020) | ||
| Asia (China) | forest plantation | 1.2 | 1.4 | + | Liu et al. (2021b) | |||
| Asia (China) | grassland | 1.3 | 1.0; 2.0 | 0 | change | Xiong et al. (2014) | ||
| Asia (South Korea) | forest plantation | 1.5 | 3.0 | + | change | Li et al. (2017) | ||
| North America (Canada) | grassland | 1–2 | 2.0 | 0 | Bell et al. (2010) | |||
| Asia (China) | grassland | 1; 2; 4 | 1.6 | 0 | no change | Shi et al. (2020) | ||
| North America (USA) | grassland | 1–5 | 3.0 | + | change | Guo et al. (2019) | ||
| Asia (China) | cropland | 2 | 2.0 | variable | - | change | Liu et al. (2014) | |
| Asia (South Korea) | forest plantation | 2.7 | 3.0 | 0 | change | Li et al. (2018) | ||
| Asia (China) | experimental grassland | 3 | 1.5; 2.0 | - / 0 | change | Zhang et al. (2016b) | ||
| Asia (China) | cropland | 3 | 2.0 | - | - | change | Liu et al. (2017) | |
| Asia (China) | experimental grassland | 3 | 1.5; 2.0 | - / 0 | change | Zhang et al. (2017) | ||
| North America (USA) | forest | 3 | 1.7; 3.4 | no change | change (within ECM community) | Mucha et al. (2018) | ||
| North America (USA) | forest plantation | 3 | 1.7; 3.4 | 0 | change (within ECM community) | van Nuland et al. (2020) | ||
| North America (USA) | desert | 3 | 2.0 | - | Zelikova et al. (2012) | |||
| Asia (China) | grassland | 3 | 1.7 | + | Ding et al. (2020) | |||
| Asia (China) | grassland | 3 | 1.5; 2.0 | no change | Zhang et al. (2019) | |||
| Asia (Japan) | grassland | 3 | 2.0 | - | Yoshitake et al. (2015) | |||
| Asia (China) | grassland | 3 | 1.8 | - | Ma et al. (2011) | |||
| Europe (Switzerland) | forest plantation | 3 | 3.6 | + | 0 | no change | change | Solly et al. (2017) |
| North America (USA) | grassland | 3 | 1.0 | 0 | no change | no change | Jumpponen & Jones (2014) | |
| Australia (Australia) | shrubland | 4 | 2.9 | 0 / + | change (more plant pathogens) | change | Birnbaum et al. (2019) | |
| Europe (Spain) | shrubland | 4 | 2.5 | change (less ECM) | change (within ECM community) | León-Sánchez et al. (2018) | ||
| Asia (China) | grassland | 4 | 1.8 | - | Li et al. (2013) | |||
| Europe (Norway) | tundra | 4 | 0.6–1.1 | 0 | no change | no change | Ahonen et al. (2021) | |
| Europe (Spain) | shrubland | 4 | 2 | 0 | change (less ECM) | no change | Querejeta et al. (2021) | |
| Asia (China) | forest plantation | 5 | 1.5 | 0 | 0 | no change | Wang et al. (2019) | |
| Europe (Denmark) | shrubland | 5 | 0.3 | + | Haugwitz et al. (2014) | |||
| Asia (China) | grassland | 5 | 1.0 | - | Shao et al. (2018) | |||
| Asia (China) | grassland | 5 | 0.3 | + | Wang et al. (2017) | |||
| Australia (Australia) | grassland | 5 | 2.0 | - | change | Hayden et al. (2012) | ||
| North America (USA) | forest | 6 | 3.4 | change (within ECM community) | Fernandez et al. (2017) | |||
| Europe (Norway) | shrubland | 6 | 1.7 | 0 | no change | Lorberau et al. (2017) | ||
| Asia (China) | grassland | 6 | 1.6 | 0 | 0 | change (less AMF) | change (more Dothideomycetes) | Che et al. (2019) |
| North America (USA) | woodland | 6 | 5.0 | 0 | no change | Gehring et al. (2020) | ||
| North America (USA) | grassland | 6 | 1.0 | change (less AMF) | Gutknecht et al. (2012) | |||
| Asia (China) | shrubland | 6 | 0.5 | + | Song et al. (2021) | |||
| Asia (China) | grassland | 7 | 1.3 | 0 / + | change | Yu et al. (2019) | ||
| Many (Many) | 7–25 | 0.5–2.0 | 0 | Jeanbille et al. (2021) | ||||
| Asia (China) | cropland | 8 | 2.0 | 0 | Liu et al. (2021a) | |||
| Asia (China) | grassland | 8 | 1.6 | 0 | no change | Peng et al. (2020) | ||
| Antarctica (Antarctica) | desert | 8 | 0.8 | + | 0 | change | Kim et al. (2018) | |
| North America (USA) | forest | 10 | 1.6 | change | Romero-Olivares et al. (2019) | |||
| Asia (China) | grassland | 11 | 1.8 | 0 | Zhang et al. (2015) | |||
| North America (USA) | forest | 12 | 5.0 | - | change (less AMF) | change | Frey et al. (2008) | |
| North America (USA) | grassland | 18 | 1.5–2.0 | 0 | change | Geml et al. (2015) | ||
| North America (USA) | grassland | 18 | 1.0–5.0 | 0 | change (more plant pathogens and saprotrophs) | change (within Ascomycota) | Semenova et al. (2015) | |
| North America (USA) | grassland | 18 | 1.5–2.0 | 0 | change | Geml et al. (2021) | ||
| North America (USA) | grassland | 23 | 2.0 | 0 / - | 0 | change (more AMF) | change | Kazenel et al. (2019) |
| Arbuscular mycorrhizal fungi | ||||||||
| North America (USA) | experimental field | 1 | 5.0 | 0 | change | Anthony et al. (2020) | ||
| Europe (Germany) | cropland | 1 | 2.0 | 0 | change | Wahdan et al. (2021) | ||
| Asia (China) | grassland | 2 | 2.0 | 0 | no change | Wei et al. (2021) | ||
| Asia (China) | grassland | 3 | 0.5–1.2 | - | change | Shi et al. (2017) | ||
| Asia (China) | forest plantation | 3 | 5.0 | + (root colonisation) | - | change (less Glomeraceae) | Cao et al. (2020b) | |
| Asia (China) | grassland | 3 | 1.2–1.7 | 0 | 0 | Yang (2013) | ||
| Asia (China) | forest plantation | 4 | 5.0 | 0 | change (more Gigasporaceae, less Glomeraceae) | Cao et al. (2020a) | ||
| Asia (China) | grassland | 4.3 | 1.8 | + | Kim et al. (2015) | |||
| Asia (China) | grassland | 4.3 | 0.9 | 0 (soil hyphal density) | + | Kim et al. (2014) | ||
| Asia (China) | grassland | 6 | 1.8 | 0 (soil hyphal and spore density) | 0 | no change | Gao et al. (2016) | |
| Asia (China) | grassland | 7 | 1.5–3.2 | 0 | - | no change | Zheng et al. (2022a) | |
| Asia (China) | grassland | 17 | 1.5 | 0 | no change | Shi et al. (2021) | ||
| North America (USA) | grassland | 23 | 2.0 | 0 / - | 0 | no change | Kazenel et al. (2019) | |
Reduction of precipitation
Since soil C turnover across global biomes increases with precipitation (Carvalhais et al. 2014), any change in precipitation likely affects C cycling. Responses of plant communities to increased variability in precipitation have ranged from high ecosystem stability in the face of intra-annual variability (Jones et al. 2016) to increasing functional diversity with increased inter-annual variability (Gherardi & Sala 2015). Even when there is very little recorded change in plant community diversity, significant changes in species composition through reordering have been recorded (Jones et al. 2017). While climate models predict both decreases and increases in precipitation across global locations (IPCC 2014), drought effects on ecosystems are likely much more dramatic. Increases in the durations of drought are expected to be a major consequence of future climate and increased desertification is predicted for most semi-arid or arid regions in the coming decades (Huang et al. 2016). Based on a recent meta-analysis, terrestrial ecosystem productivity was decreased by drought across all ecosystems (Wang et al. 2021a). The response of productivity to drought are more pronounced with higher drought intensity and longer duration, and consistent across biomes and climates. Drought can significantly decrease soil moisture, soil C content, soil C:N ratios, and microbial biomass C, whereas it tends to increase soil pH. The relative proportion of fungal biomass (F/B ratio) however, frequently increases with drought (Delgado-Baquerizo et al. 2020b, Wang et al. 2021a). The diversity and abundance of soil bacteria and fungi have been shown to decrease in drylands as aridity increased, being largely driven by the negative impacts of aridity on soil organic carbon content (Maestre et al. 2015).
Since most global change models predict changes in precipitation, experimental manipulations of precipitation are relatively frequent. Unfortunately, such manipulations are highly diverse and range from reduction and addition to redistribution. Both reduction and addition are frequently combined often without a clear link to a model prediction for the ecosystem under study (Knapp et al. 2015). Moreover, many experiments use manipulations that are likely outside the model predictions with reductions or additions > 50 % and the relevance of such manipulations is thus unclear. For simplicity, we surveyed the effects of precipitation reduction since drought seemed to have more profound ecosystem consequences (Table 4).
Table 4.
Effects of experimental reduction of precipitation on fungi. Manipulations of at least 1 y duration where reduction of precipitation was not combined with other factors were considered.
| Location | Experimental system | Duration of treatment (yr) | Reduction of precipitation (%) | Biomass | Diversity | Guilds share | Community composition | Reference1 |
|---|---|---|---|---|---|---|---|---|
| All fungi | ||||||||
| North America (USA) | grassland | 1 | 100 | 0 | change | McHugh & Schwartz (2015) | ||
| Asia (China) | grassland | 1 | 50 | 0 | no change | Zhang et al. (2016a) | ||
| North America (Brazil) | experimental field | 1 | 100 | + | change (more selected plant pathogens) | no change | de Oliveira et al. (2020) | |
| Asia (China) | forest plantation | 1.2 | 50 | 0 | Liu et al. (2021b) | |||
| Asia (Korea) | forest plantation | 1.5 | 30 | 0 | change | Li et al. (2017) | ||
| North America/Australia (USA/Australia) | grassland | 1–2 | 50 | 0 / + | variable | change (less AMF) | change | Ochoa-Hueso et al. (2018) |
| Asia (China) | grassland | 1–2 | 30; 50 | 0 | no change | Yang et al. (2021b) | ||
| Asia (China) | cropland | 1–2 | 30; 50 | 0 | no change | Sun et al. (2020) | ||
| Asia (China) | grassland | 1–2 | 20; 40; 60 | change | Zhao et al. (2016) | |||
| Australia (Australia) | grassland | 1; 2; 3 | 50 | + | change | Ochoa-Hueso et al. (2020) | ||
| Asia (China) | grassland | 1; 2; 4 | 50 | + | change | Shi et al. (2020) | ||
| North America (USA) | grassland | 1–5 | 50 | - | change | Guo et al. (2019) | ||
| Asia (China) | forest | 2 | 67 | 0 | - | change (more Basidiomycota, less Ascomycota) | Zhao et al. (2017) | |
| Asia (China) | grassland | 2 | 40; 80 | 0 | change (more pathogens, less AMF) | change | Huang et al. (2021) | |
| Europe (Belgium) | forest plantation | 2 | 45–55 | 0 | Hicks et al. (2018) | |||
| Asia (South Korea ) | forest plantation | 2.7 | 30 | - | change | Li et al. (2018) | ||
| North America (USA) | grassland | 2–3 | 66 | change | Lagueux et al. (2021) | |||
| Asia (China) | grassland | 3 | 0–100 | 0 | no change | Wu et al. (2020) | ||
| Asia (China) | grassland | 3 | 30; 60 | 0 | no change | Wang et al. (2020a) | ||
| Australia (Australia) | shrubland | 4 | 30 | 0 | change (more ECM and plant pathogens) | change | Birnbaum et al. (2019) | |
| Europe (Spain) | shrubland | 4 | 30 | 0 | change (less ECM) | change (within EMF community) | León-Sánchez et al. (2018) | |
| North America (USA) | grassland | 4 | 40 | 0/+ | no change | Narayanan et al. (2021) | ||
| Asia (China) | grassland | 5 | 50 | 0 | change (more plant pathogens) | change | Wang et al. (2020b) | |
| Europe (Denmark) | shrubland | 5 | 50 | + | Haugwitz et al. (2014) | |||
| North America (USA) | woodland | 6 | 50 | 0 | 0 | no change | Gehring et al. (2020) | |
| Asia (China) | grassland | 6 | 50 | 0 | change | Xiao et al. (2020) | ||
| Asia (China) | forest | 7 | 30 | 0 | no change | Zhang et al. (2021) | ||
| Asia (China) | grassland | 7 | 30 | 0 | Jia et al. (2017) | |||
| Asia (China) | forest | 8 | 30 | + | Yan et al. (2021) | |||
| North America (USA) | forest | 10 | 22 | change | Romero-Olivares et al. (2019) | |||
| Arbuscular mycorrhizal fungi | ||||||||
| Australia (Australia) | experimental grassland | 1; 2; 3; 4 | 50 | 0 | Deveautour et al. (2020) | |||
| Asia (China) | experimental field | 2 | 100 | + | 0 | Zhong et al. (2021) | ||
| North America (USA) | experimental grassland | 2 | 40 | - (root colonisation) | change | Emery et al. (2022) | ||
| Australia (Australia) | experimental grassland | 2.3 | 50 | 0 | change | Deveautour et al. (2018) | ||
| Asia (China) | grassland | 3 | 30 | 0 | change | Wang et al. (2021c) | ||
| Asia (China) | forest | 4 | 70 | - (hyphal and spore density, root colonisation) | 0 | 0 | Maitra et al. (2019) | |
| Asia (China) | forest plantation | 4 | 50 | 0 | 0 | Cao et al. (2020a) | ||
| Asia (China) | grassland | 6 | 50 | 0 | - | change | Zheng et al. (2022b) | |
| North America (USA) | shrubland | 7 | 40 | 0 | change | Weber et al. (2019) | ||
In our survey, no negative effects of precipitation on total fungal biomass were reported with most experiments reporting no effect on any response variable (Fig. 3, Table 4). In studies of AMF, there was decreased hyphal, spore density, and root colonisation in a forest system in connection with soil acidification (Maitra et al. 2019) and a reduction in root colonisation in a perennial cropping system (Emery et al. 2022). Reduction of precipitation most frequently did not affect the diversity of fungi and AM fungi and decrease of total fungal diversity was never observed in manipulations lasting three or more years (Figs 3, 4, Table 4). Contrary to our survey, a recent global meta-analysis found that precipitation reduction led to increased fungal richness with the effect size increasing with experimental length, though precipitation reduction had no effect on fungal diversity (Li et al. 2022). Importantly, precipitation reduction typically shifted the share of fungal guilds with the reduction of AM fungi and increase of plant pathogens being frequently reported. Changes in fungal community composition were also relatively frequent (Table 4). Increase of the F/B ratio was observed in a heathland experiment (Haugwitz et al. 2014). Changes in soil chemistry were typically not found, not even for the long-lasting experiments.
Increased atmospheric N deposition
Many plant communities are N limited (LeBauer & Treseder 2008), and additional N can thus promote plant productivity if P content is non-limiting (Fay et al. 2015). Additionally, N deposition may reduce plant species richness though this effect depends on ecosystem characteristics, such as MAP (Clark et al. 2007). For example, N addition may increase plant species richness in ecosystems with high MAP (Komatsu et al. 2019). In addition to effects on vegetation, N has multiple effects on soil chemistry, including acidification (Lekberg et al. 2021). Though a recent global meta-analysis found that N reduced overall soil fungal richness (Zhou et al. 2020), the effects of N deposition on soil fungi can be, like plant community responses, context dependent. Across N-addition studies in the US forests, fungal biomass and richness increased with simulated N deposition at sites with low ambient deposition but decreased at sites with high ambient deposition (Moore et al. 2021). Along local fertility gradients, total fungal biomass was highest in soils with the lowest nutrient availability and tree productivity (Nilsson et al. 2005). Higher N availability promotes bacterial growth due to their higher N demand. Especially in the N-limited boreal soils, N addition results in a decrease of the F/B ratio by 25–70 % (Frey et al. 2004, Wallenstein et al. 2006, Maaroufi et al. 2015).
There appears to be a general consensus that N deposition increases soil C sequestration due to the decline in SOM decomposition via the reduction of fungal abundance and decomposer activity in many different soil environments, including temperate and boreal forests (Frey et al. 2014, Maaroufi et al. 2015). Since, similar to plants, many fungi respond to P availability in soil and it is an important driver of fungal abundance in soils without N limitations (Odriozola et al. 2021), increased N content may act on fungal productivity and community composition indirectly through P limitation (Fig. 1).
Within our survey, the goal of the majority of N addition experiments was to simulate predicted increases in atmospheric deposition, but many used unreasonably high amounts of fertilizer, ignored ambient N deposition rates, and virtually none of them referenced a model that predicts future deposition, whose extent shows high local variation. It is currently estimated that the vast majority of forests are subject to total N deposition lower than 25 kg N/ha/y (Schwede et al. 2018) and it is unrealistic to expect that the increase in future is several-fold. We have thus considered only the results of experiments where N addition was lower than 75 kg N/ha/y.
The effects of N addition on fungal biomass in soil were variable. For AM fungi, decreased spore density, root colonisation, and biomass were much more frequent than positive effects (Fig. 4). In forest ecosystems, decrease of fungal biomass and root colonisation appears typical (Ma et al. 2021b). Both increases and decreases in diversity of fungi or AM fungi were observed (Figs 3, 4, Table 5). This lack of consistency in diversity responses is somewhat supported by the effects of increased N on fungal richness varying between global meta-analyses with increased N either decreasing richness or having no effect (Zhou et al. 2020, Li et al. 2022). Changes in the representation of fungal guilds were a common consequence of N addition. In most long-term N addition experiments, the share of ECM fungi was significantly reduced (Table 5) with a shift to nitrophilic taxa such as Rusula vinacea (Morrison et al. 2016, Tahovská et al. 2020). The consequences of longer N enrichment (> 4 yr) were relatively complex and include acidification and increased N availability (Choma et al. 2017, Wang et al. 2021b), decreased F/B ratio (Gutknecht et al. 2012, Wang et al. 2015) and decreased activity of enzymes decomposing recalcitrant plant biopolymers lignin and cellulose (Freedman et al. 2015, Hesse et al. 2015). Although vegetation responds to N addition as well, the change of soil chemistry appeared to be the immediate driver of fungal community composition (Zheng et al. 2014, Zhou et al. 2020, Wang et al. 2021b).
Combined effects and model predictions
Current models predict that the effects of global change factors will act simultaneously in most terrestrial habitats and the resulting effect of global change thus reflects their combination. Furthermore, shifts in plant community composition are likely determined by interactions between multiple climate change drivers (Avolio et al. 2021). Between 1990 and 2014, global heterotrophic soil respiration and its ratio to total soil respiration increased, probably in response to the combined effects of global change factors (Bond-Lamberty et al. 2018). This suggests that climate-driven losses of soil carbon are currently occurring across many ecosystems, with a detectable and sustained trend emerging at the global scale, although the underlying mechanisms cannot be easily identified. Simulation of the global change effects until the year 2090 using available data from 1950 indicates that climate change acts mostly indirectly, through other environmental variables, e.g., changes in the soil pH (Guerra et al. 2021). The effects of global change factors on fungi thus may depend either on the relative importance of each individual factor under local conditions or on the combined effects of multiple factors.
CONCLUSIONS
While ongoing climate change has had seemingly no dramatic effects on soil fungal communities, and neither fungal biomass nor fungal diversity in soils appear to be dramatically affected, experiments simulating the main global change effects predict significant shifts in fungal community composition and the share of fungal guilds. The differences in the size of the realised niche of plant-beneficial ECM fungi compared to that of plant pathogens suggests that the fitness of vegetation may decrease as ecosystems experience increased spread of plant pathogens and potentially higher frequencies of outbreaks. This issue is perhaps the one that deserves most attention (Fig. 1). Interestingly, responses of soil fungi to various aspects of global change can be predicted based on different ecological features. While differential responses of ECM fungal species to global changes such as N deposition can be predicted from their extracellular enzymatic capabilities related to organic nitrogen accessibility, response of AM fungal species depends on their differential colonisation traits.
Global change effects on ecosystems are highly context dependent and there are undoubtedly ecosystems where changes will be more pronounced. Where global change relieves existing limitations, such as the coldest or N-limited areas, novel limitations will arise, such as increased desertification or induced P-limitation, respectively. Unfortunately, these systems are rarely the subject of research. Experimental manipulations in underexplored systems are thus most welcome.
Although the experiments combining multiple factors are relatively frequent (Yang et al. 2021a), they are in most cases applying unrealistic treatment intensities and so far too rare to allow generalisations. Since global change factors act in combination and their effects are not simply additive (Rillig et al. 2019), it would be more than welcome to see results of long-term manipulations based on complex predictions of multiple global change factors for given localities. Since it will never be possible to perform manipulations everywhere, long term collection of observational data is needed that would help to describe trends in the soil mycobiome. Global and regional initiatives intending to capture all available types of fungal community data, combined with paired environmental metadata, across time (Andrew et al. 2017, Větrovský et al. 2020) have the potential to scale our understanding of global change effects on soil fungi to a global level.
Acknowledgments
This work was supported by the Czech Science Foundation (21-17749S). LBD was supported by the Ministry of Education, Youth and Sports of the Czech Republic (CZ02.2.69/0.0/0.0/18_053/0017705).
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Selection criteria for the inclusion of studies describing effects of global change manipulation on fungi.
REFERENCES
- Ackerman D, Millet DB, Chen X. (2019). Global estimates of inorganic nitrogen deposition across four decades. Global Biogeochemical Cycles 33: 100–107. [Google Scholar]
- Adair EC, Reich PB, Trost JJ, et al. (2011). Elevated CO2 stimulates grassland soil respiration by increasing carbon inputs rather than by enhancing soil moisture. Global Change Biology 17: 3546–3563. [Google Scholar]
- Ahonen SHK, Ylänne H, Väisänen M, et al. (2021). Reindeer grazing history determines the responses of subarctic soil fungal communities to warming and fertilization. New Phytologist 232: 788–801. [DOI] [PubMed] [Google Scholar]
- Alexander JM, Diez JM, Levine JM. (2015). Novel competitors shape species’ responses to climate change. Nature 525: 515–518. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Alteio LV, Séneca J, Canarini A, et al. (2021). A critical perspective on interpreting amplicon sequencing data in soil ecological research. Soil Biology and Biochemistry 160: 108357. [Google Scholar]
- Alvarez-Garrido L, Vinegla B, Hortal S, et al. (2019). Distributional shifts in ectomycorrizhal fungal communities lag behind climate-driven tree upward migration in a conifer forest-high elevation shrubland ecotone. Soil Biology and Biochemistry 137: 107545. [Google Scholar]
- Anderegg WRL, Kane JM, Anderegg LDL. (2013). Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change 3: 30–36. [Google Scholar]
- Andrew C, Heegaard E, Hoiland K, et al. (2018). Explaining European fungal fruiting phenology with climate variability. Ecology 99: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Andrew C, Heegaard E, Kirk PM, et al. (2017). Big data integration: Pan-European fungal species observations’ assembly for addressing contemporary questions in ecology and global change biology. Fungal Biology Reviews 31: 88–98. [Google Scholar]
- Andrew C, Lilleskov EA. (2009). Productivity and community structure of ectomycorrhizal fungal sporocarps under increased atmospheric CO2 and O3. Ecology Letters 12: 813–822. [DOI] [PubMed] [Google Scholar]
- Anthony MA, Stinson KA, Moore JAM, et al. (2020). Plant invasion impacts on fungal community structure and function depend on soil warming and nitrogen enrichment. Oecologia 194: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoninka A, Reich PB, Johnson NC. (2011). Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytologist 192: 200–214. [DOI] [PubMed] [Google Scholar]
- Averill C, Dietze MC, Bhatnagar JM. (2018). Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Global Change Biology 24: 4544–4553. [DOI] [PubMed] [Google Scholar]
- Averill C, Hawkes CV. (2016). Ectomycorrhizal fungi slow soil carbon cycling. Ecology Letters 19: 937–947. [DOI] [PubMed] [Google Scholar]
- Averill C, Turner BL, Finzi AC. (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505: 543–545. [DOI] [PubMed] [Google Scholar]
- Avolio ML, Komatsu KJ, Collins SL, et al. (2021). Determinants of community compositional change are equally affected by global change. Ecology Letters 24: 1892–1904. [DOI] [PubMed] [Google Scholar]
- Bahram M, Hildebrand F, Forslund SK, et al. (2018). Structure and function of the global topsoil microbiome. Nature 560: 233–237. [DOI] [PubMed] [Google Scholar]
- Baldrian P, Větrovský T, Lepinay C, et al. (2021). High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Diversity 114: 539–547. [Google Scholar]
- Baldrian P, Šnajdr J, Merhautová V, et al. (2013). Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biology and Biochemistry 56: 60–68. [Google Scholar]
- Barcelo M, van Bodegom PM, Soudzilovskaia NA. (2019). Climate drives the spatial distribution of mycorrhizal host plants in terrestrial ecosystems. Journal of Ecology 107: 2564–2573. [Google Scholar]
- Bebber DP, Chaloner TM. (2022). Specialists, generalists and the shape of the ecological niche in fungi. New Phytologist 234: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebber DP, Ramotowski MAT, Gurr SJ. (2013). Crop pests and pathogens move polewards in a warming world. Nature Climate Change 3: 985–988. [Google Scholar]
- Bell TH, Klironomos JN, Henry HAL. (2010). Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Science Society of America Journal 74: 820–828. [Google Scholar]
- Birnbaum C, Hopkins AJM, Fontaine JB, et al. (2019). Soil fungal responses to experimental warming and drying in a Mediterranean shrubland. Science of the Total Environment 683: 524–536. [DOI] [PubMed] [Google Scholar]
- Blois JL, Zarnetske PL, Fitzpatrick MC, et al. (2013). Climate change and the past, present, and future of biotic interactions. Science 341: 499–504. [DOI] [PubMed] [Google Scholar]
- Bond-Lamberty B, Bailey VL, Chen M, et al. (2018). Globally rising soil heterotrophic respiration over recent decades. Nature 560: 80–83. [DOI] [PubMed] [Google Scholar]
- Cao JL, Xie L, Zheng YX, et al. (2020a). Drought intensify the effects of warming on root-colonizing arbuscular mycorrhizal fungal community in subtropical Chinese fir plantation. Forest Ecology and Management 464: 118078. [Google Scholar]
- Cao JL, Lin TC, Yang ZJ, et al. (2020b). Warming exerts a stronger effect than nitrogen addition on the soil arbuscular mycorrhizal fungal community in a young subtropical Cunninghamia lanceolata plantation. Geoderma 367: 114273. [Google Scholar]
- Carvalhais N, Forkel M, Khomik M, et al. (2014). Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 514: 213–217. [DOI] [PubMed] [Google Scholar]
- Chaloner TM, Gurr SJ, Bebber DP. (2020). Geometry and evolution of the ecological niche in plant-associated microbes. Nature Communications 11: 2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che RX, Wang SP, Wang YF, et al. (2019). Total and active soil fungal community profiles were significantly altered by six years of warming but not by grazing. Soil Biology and Biochemistry 139: 107611. [Google Scholar]
- Chen DM, Xing W, Lan ZC, et al. (2019). Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Functional Ecology 33: 175–187. [Google Scholar]
- Chen YL, Xu ZW, Xu TL, et al. (2017). Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biology and Biochemistry 115: 233–242. [Google Scholar]
- Choma M, Tahovská K, Kaštovská E, et al. (2020). Bacteria but not fungi respond to soil acidification rapidly and consistently in both a spruce and beech forest. FEMS Microbiology Ecology 96: fiaa174. [DOI] [PubMed] [Google Scholar]
- Choma M, Rappe-George MO, Bárta J, et al. (2017). Recovery of the ectomycorrhizal community after termination of long-term nitrogen fertilisation of a boreal Norway spruce forest. Fungal Ecology 29: 116–122. [Google Scholar]
- Clark CM, Cleland EE, Collins SL, et al. (2007). Environmental and plant community determinants of species loss following nitrogen enrichment. Ecology Letters 10: 596–607. [DOI] [PubMed] [Google Scholar]
- Cotton TEA. (2018). Arbuscular mycorrhizal fungal communities and global change: an uncertain future. FEMS Microbiology Ecology 94: fiy179. [DOI] [PubMed] [Google Scholar]
- Cotton TEA, Fitter AH, Miller RM, et al. (2015). Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytologist 205: 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther TW, Van den Hoogen J, Wan J, et al. (2019). The global soil community and its influence on biogeochemistry. Science 365: eaav0550. [DOI] [PubMed] [Google Scholar]
- Crowther TW, Todd-Brown KEO, Rowe CW, et al. (2016). Quantifying global soil carbon losses in response to warming. Nature 540: 104–108. [DOI] [PubMed] [Google Scholar]
- Davison J, Moora M, Semchenko M, et al. (2021). Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytologist 231: 763–776. [DOI] [PubMed] [Google Scholar]
- de Oliveira TB, de Lucas RC, Scarcella ASD, et al. (2020). Fungal communities differentially respond to warming and drought in tropical grassland soil. Molecular Ecology 29: 1550–1559. [DOI] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Guerra CA, Cano-Díaz C, et al. (2020a). The proportion of soil-borne pathogens increases with warming at the global scale. Nature Climate Change 10: 550–559 [Google Scholar]
- Delgado-Baquerizo M, Doulcier G, Eldridge DJ, et al. (2020b). Increases in aridity lead to drastic shifts in the assembly of dryland complex microbial networks. Land Degradation & Development 31: 346–355. [Google Scholar]
- Desaint H, Aoun N, Deslandes L, et al. (2021). Fight hard or die trying: when plants face pathogens under heat stress. New Phytologist 229: 712–734. [DOI] [PubMed] [Google Scholar]
- Deveautour C, Power SA, Barnett KL, et al. (2020). Temporal dynamics of mycorrhizal fungal communities and co-associations with grassland plant communities following experimental manipulation of rainfall. Journal of Ecology 108: 515–527. [Google Scholar]
- Deveautour C, Donn S, Power SA, et al. (2018). Experimentally altered rainfall regimes and host root traits affect grassland arbuscular mycorrhizal fungal communities. Molecular Ecology 27: 2152–2163. [DOI] [PubMed] [Google Scholar]
- Diez J, Kauserud H, Andrew C, et al. (2020). Altitudinal upwards shifts in fungal fruiting in the Alps. Proceedings of the Royal Society B: Biological Sciences 287: 20192348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Chen S, Zhang B, et al. (2020). Warming yields distinct accumulation patterns of microbial residues in dry and wet alpine grasslands on the Qinghai-Tibetan Plateau. Biology and Fertility of Soils 56: 881–892. [Google Scholar]
- Dong Y, Wang Z, Sun H, et al. (2018). The response patterns of arbuscular mycorrhizal and ectomycorrhizal symbionts under elevated CO2: A meta-analysis. Frontiers in Microbiology 9: 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar J, Gallegos-Graves L, Steven B, et al. (2014). Surface soil fungal and bacterial communities in aspen stands are resilient to eleven years of elevated CO2 and O3. Soil Biology and Biochemistry 76: 227–234. [Google Scholar]
- Egerton-Warburton LM, Allen EB. (2000). Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecological Applications 10: 484–496. [Google Scholar]
- Emery SM, Bell-Dereske L, Stahlheber KA, et al. (2022). Arbuscular mycorrhizal fungal community responses to drought and nitrogen fertilization in switchgrass stands. Applied Soil Ecology 169: 104218. [Google Scholar]
- Fay PA, Prober SM, Harpole WS, et al. (2015). Grassland productivity limited by multiple nutrients. Nature Plants 1: 15080. [DOI] [PubMed] [Google Scholar]
- Fernandez CW, Nguyen NH, Stefanski A, et al. (2017). Ectomycorrhizal fungal response to warming is linked to poor host performance at the boreal-temperate ecotone. Global Change Biology 23: 1598–1609. [DOI] [PubMed] [Google Scholar]
- Fernandez CW, Kennedy PG. (2016). Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New phytologist 209: 1382–1394. [DOI] [PubMed] [Google Scholar]
- Freedman ZB, Romanowicz KJ, Upchurch RA, et al. (2015). Differential responses of total and active soil microbial communities to long-term experimental N deposition. Soil Biology and Biochemistry 90: 275–282. [Google Scholar]
- Freeman BG, Lee-Yaw JA, Sunday JM, et al. (2018). Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Global Ecology and Biogeography 27: 1268–1276. [Google Scholar]
- Frey B, Carnol M, Dharmarajah A, et al. (2020). Only minor changes in the soil microbiome of a sub-alpine forest after 20 years of moderately increased nitrogen loads. Frontiers in Forests and Global Change 3: 77. [Google Scholar]
- Frey SD, Ollinger S, Nadelhoffer KE, et al. (2014). Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121: 305–316. [Google Scholar]
- Frey SD, Drijber R, Smith H, et al. (2008). Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biology and Biochemistry 40: 2904–2907. [Google Scholar]
- Frey SD, Knorr M, Parrent JL, et al. (2004). Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecology and Management 196: 159–171. [Google Scholar]
- Gamper H, Peter M, Jansa J, et al. (2004). Arbuscular mycorrhizal fungi benefit from 7 years of free air CO2 enrichment in well-fertilized grass and legume monocultures. Global Change Biology 10: 189–199. [Google Scholar]
- Gao C, Kim YC, Zheng Y, et al. (2016). Increased precipitation, rather than warming, exerts a strong influence on arbuscular mycorrhizal fungal community in a semiarid steppe ecosystem. Botany 94: 459–469. [Google Scholar]
- García-Palacios P, Crowther TW, Dacal M, et al. (2021). Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nature Reviews Earth & Environment 2: 507–517. [Google Scholar]
- Garcia MO, Templer PH, Sorensen PO, et al. (2020). Soil microbes trade-off biogeochemical cycling for stress tolerance traits in response to year-round climate change. Frontiers in Microbiology 11: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring C, Sevanto S, Patterson A, et al. (2020). Ectomycorrhizal and dark septate fungal associations of pinyon pine are differentially affected by experimental drought and warming. Frontiers in Plant Science 11: 582574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geml J, Morgado LN, Semenova-Nelsen TA. (2021). Tundra type drives distinct trajectories of functional and taxonomic composition of arctic fungal communities in response to climate change–results from long-term experimental summer warming and increased snow depth. Frontiers in Microbiology 12: 628746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geml J, Morgado LN, Semenova TA, et al. (2015). Long-term warming alters richness and composition of taxonomic and functional groups of arctic fungi. FEMS Microbiology Ecology 91: fiv095. [DOI] [PubMed] [Google Scholar]
- Gherardi LA, Sala OE. (2015). Enhanced interannual precipitation variability increases plant functional diversity that in turn ameliorates negative impact on productivity. Ecology Letters 18: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Guenet B, Lenhart K, Leloup J, et al. (2012). The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 170: 331–336. [Google Scholar]
- Guerra CA, Delgado-Baquerizo M, Duarte E, et al. (2021). Global projections of the soil microbiome in the Anthropocene. Global Ecology and Biogeography 30: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Ding J, Wang Q, et al. (2021). Soil fertility controls ectomycorrhizal mycelial traits in alpine forests receiving nitrogen deposition. Soil Biology and Biochemistry 161: 108386. [Google Scholar]
- Guo X, Zhou XS, Hale L, et al. (2019). Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nature Ecology & Evolution 3: 612–619. [DOI] [PubMed] [Google Scholar]
- Gutknecht JLM, Field CB, Balser TC. (2012). Microbial communities and their responses to simulated global change fluctuate greatly over multiple years. Global Change Biology 18: 2256–2269. [Google Scholar]
- Han Y, Feng J, Han M, et al. (2020). Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Global Change Biology 26: 7229–7241. [DOI] [PubMed] [Google Scholar]
- Harte J, Saleska SR, Levy C. (2015). Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate–soil carbon feedback. Global Change Biology 21: 2349–2356. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, et al. (2002). Climate warming and disease risks for terrestrial and marine biota. Science 296: 2158–2162. [DOI] [PubMed] [Google Scholar]
- Haugwitz MS, Bergmark L, Priemé A, et al. (2014). Soil microorganisms respond to five years of climate change manipulations and elevated atmospheric CO2 in a temperate heath ecosystem. Plant and Soil 374: 211–222. [Google Scholar]
- Hayden HL, Mele PM, Bougoure DS, et al. (2012). Changes in the microbial community structure of bacteria, archaea and fungi in response to elevated CO2 and warming in an Australian native grassland soil. Environmental Microbiology 14: 3081–3096. [DOI] [PubMed] [Google Scholar]
- Hesse CN, Mueller RC, Vuyisich M, et al. (2015). Forest floor community metatranscriptomes identify fungal and bacterial responses to N deposition in two maple forests. Frontiers in Microbiology 6: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LC, Rahman MM, Carnol M, et al. (2018). The legacy of mixed planting and precipitation reduction treatments on soil microbial activity, biomass and community composition in a young tree plantation. Soil Biology and Biochemistry 124: 227–235. [Google Scholar]
- Huang Q, Jiao F, Huang YM, et al. (2021). Response of soil fungal community composition and functions on the alteration of precipitation in the grassland of Loess Plateau. Science of the Total Environment 751: 142273. [DOI] [PubMed] [Google Scholar]
- Huang JP, Yu HP, Guan XD, et al. (2016). Accelerated dryland expansion under climate change. Nature Climate Change 6: 166–171. [Google Scholar]
- IPCC (2014). Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, United Kingdom. [Google Scholar]
- Isbell F, Reich PB, Tilman D, et al. (2013). Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proceedings of the National Academy of Sciences of the United States of America 110: 11911–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson JK, Hofmockel KS. (2020). Soil microbiomes and climate change. Nature Reviews Microbiology 18: 35–46. [DOI] [PubMed] [Google Scholar]
- Jansson JK, Tas N. (2014). The microbial ecology of permafrost. Nature Reviews Microbiology 12: 414–425. [DOI] [PubMed] [Google Scholar]
- Jeanbille DM, Clemmensen DK, Juhanson DJ, et al. (2021). Site-specific responses of fungal and bacterial abundances to experimental warming in litter and soil across arctic and alpine tundra. Arctic Science. Doi:10.1139/as-2020-0053. [Google Scholar]
- Jetz W, McPherson JM, Guralnick RP. (2012). Integrating biodiversity distribution knowledge: toward a global map of life. Trends in Ecology & Evolution 27: 151–159. [DOI] [PubMed] [Google Scholar]
- Jia X, Zhong Y, Liu J, et al. (2020a). Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 366: 114256. [Google Scholar]
- Jia X, Wang L, Zhao Y, et al. (2020b). Soil microbial communities in the rhizosphere of Robinia pseudoacacia L. after being exposed to elevated atmospheric CO2 and cadmium for 4 years. Applied Soil Ecology 154: 103661. [Google Scholar]
- Jia M, Liu C, Li Y, et al. (2017). Response of fungal composition and diversity to simulated nitrogen deposition and manipulation of precipitation in soils of an Inner Mongolia desert steppe of northern China. Canadian Journal of Soil Science 97: 613–625. [Google Scholar]
- Jiang SJ, Liu YJ, Luo JJ, et al. (2018). Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytologist 220: 1222–1235. [DOI] [PubMed] [Google Scholar]
- Jo I, Fei S, Oswalt CM, et al. (2019). Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Science Advances 5: eaav6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC. (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist 185: 631–647. [DOI] [PubMed] [Google Scholar]
- Jones SK, Ripplinger J, Collins SL. (2017). Species reordering, not changes in richness, drives long-term dynamics in grassland communities. Ecology Letters 20: 1556–1565. [DOI] [PubMed] [Google Scholar]
- Jones SK, Collins SL, Blair JM, et al. (2016). Altered rainfall patterns increase forb abundance and richness in native tallgrass prairie. Scientific Reports 6: 20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumpponen A, Jones KL. (2014). Tallgrass prairie soil fungal communities are resilient to climate change. Fungal Ecology 10: 44–57. [Google Scholar]
- Juroszek P, Racca P, Link S, et al. (2020). Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant Pathology 69: 179–193. [Google Scholar]
- Karst J, Wasyliw J, Birch JD, et al. (2021). Long-term nitrogen addition does not sustain host tree stem radial growth but doubles the abundance of high-biomass ectomycorrhizal fungi. Global Change Biology 27: 4125–4138. [DOI] [PubMed] [Google Scholar]
- Kauserud H, Heegaard E, Buntgen U, et al. (2012). Warming-induced shift in European mushroom fruiting phenology. Proceedings of the National Academy of Sciences of the United States of America 109: 14488–14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenel MR, Kivlin SN, Taylor DL, et al. (2019). Altitudinal gradients fail to predict fungal symbiont responses to warming. Ecology 100: e02740. [DOI] [PubMed] [Google Scholar]
- Kim D, Park HJ, Kim JH, et al. (2018). Passive warming effect on soil microbial community and humic substance degradation in maritime Antarctic region. Journal of Basic Microbiology 58: 513–522. [DOI] [PubMed] [Google Scholar]
- Kim YC, Gao C, Zheng Y, et al. (2015). Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25: 267–276. [DOI] [PubMed] [Google Scholar]
- Kim YC, Gao C, Zheng Y, et al. (2014). Different responses of arbuscular mycorrhizal fungal community to day-time and night-time warming in a semiarid steppe. Chinese Science Bulletin 59: 5080–5089. [Google Scholar]
- Kimball BA, Idso SB, Johnson S, et al. (2007). Seventeen years of carbon dioxide enrichment of sour orange trees: Final results. Global Change Biology 13: 2171–2183. [Google Scholar]
- Kivlin SN, Emery SM, Rudgers JA. (2013). Fungal symbionts alter plant responses to global change. American Journal of Botany 100: 1445–1457. [DOI] [PubMed] [Google Scholar]
- Klironomos JN, Hart MM. (2002). Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12: 181–184. [DOI] [PubMed] [Google Scholar]
- Klironomos J, Rillig MC, Allen MF, et al. (1997). Increased levels of airborne fungal spores in response to Populus tremuloides grown under elevated atmospheric CO2. Canadian Journal of Botany 75: 1670–1673. [Google Scholar]
- Knapp AK, Hoover DL, Wilcox KR, et al. (2015). Characterizing differences in precipitation regimes of extreme wet and dry years: implications for climate change experiments. Global Change Biology 21: 2624–2633. [DOI] [PubMed] [Google Scholar]
- Komatsu KJ, Avolio ML, Lemoine NP, et al. (2019). Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proceedings of the National Academy of Sciences of the United States of America 116: 17867–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven CD, Hugelius G, Lawrence DM, et al. (2017). Higher climatological temperature sensitivity of soil carbon in cold than warm climates. Nature Climate Change 7: 817–822. [Google Scholar]
- Lagomarsino A, Knapp BA, Moscatelli MC, et al. (2007). Structural and functional diversity of soil microbes is affected by elevated CO2 and N addition in a poplar plantation. Journal of Soils and Sediments 7: 399–405. [Google Scholar]
- Lagueux D, Jumpponen A, Porras-Alfaro A, et al. (2021). Experimental drought re-ordered assemblages of root-associated fungi across North American grasslands. Journal of Ecology 109: 776–792. [Google Scholar]
- LeBauer DS, Treseder KK. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89: 371–379. [DOI] [PubMed] [Google Scholar]
- Lee JY, Marotzke J, Bala G, et al. (2021). Future global climate: scenario-based projections and near-term information. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Masson-Delmotte V, Zhai P, Pirani A, et al. eds). Cambridge University Press, United Kingdom: 1–195. [Google Scholar]
- Lekberg Y, Arnillas CA, Borer ET, et al. (2021). Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nature Communications 12: 3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekberg Y, Koide RT, Rohr JR, et al. (2007). Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. Journal of Ecology 95: 95–105. [Google Scholar]
- León-Sánchez L, Nicolás E, Goberna M, et al. (2018). Poor plant performance under simulated climate change is linked to mycorrhizal responses in a semi-arid shrubland. Journal of Ecology 106: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ma J, Yu Y, et al. (2022). Effects of multiple global change factors on soil microbial richness, diversity and functional gene abundances: A meta-analysis. Science of the Total Environment 815: 152737. [DOI] [PubMed] [Google Scholar]
- Li B-B, Roley SS, Duncan DS, et al. (2021). Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biology and Biochemistry 160: 108349. [Google Scholar]
- Li JJ, Yang C, Zhou HK, et al. (2020a). Responses of plant diversity and soil microorganism diversity to water and nitrogen additions in the Qinghai-Tibetan Plateau. Global Ecology and Conservation 22: e01003. [Google Scholar]
- Li W, Sheng H, Liu Y, et al. (2020b). Ecostoichiometry reveals the separation of microbial adaptation strategies in a bamboo forest in an urban wetland under simulated nitrogen deposition. Forests 11: 428. [Google Scholar]
- Li WC, Sheng HY, Ekawati D, et al. (2019a). Variations in the compositions of soil bacterial and fungal communities due to microhabitat effects induced by simulated nitrogen deposition of a bamboo forest in wetland. Forests 10: 1098. [Google Scholar]
- Li L, McCormack ML, Chen F, et al. (2019b). Different responses of absorptive roots and arbuscular mycorrhizal fungi to fertilization provide diverse nutrient acquisition strategies in Chinese fir. Forest Ecology and Management 433: 64–72. [Google Scholar]
- Li G, Kim S, Han SH, et al. (2018). Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biology and Biochemistry 120: 212–221. [Google Scholar]
- Li G, Kim S, Park M, et al. (2017). Short-term effects of experimental warming and precipitation manipulation on soil microbial biomass C and N, community substrate utilization patterns and community composition. Pedosphere 27: 714–724. [Google Scholar]
- Li X, Zhu T, Peng F, et al. (2015). Inner Mongolian steppe arbuscular mycorrhizal fungal communities respond more strongly to water availability than to nitrogen fertilization. Environmental Microbiology 17: 3051–3068. [DOI] [PubMed] [Google Scholar]
- Li Q, Bai H, Liang W, et al. (2013). Nitrogen addition and warming independently influence the belowground micro-food web in a temperate steppe. PLoS ONE 8: e60441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleskov EA, Kuyper TW, Bidartondo MI, et al. (2019). Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review. Environmental Pollution 246: 148–162. [DOI] [PubMed] [Google Scholar]
- Lilleskov EA, Hobbie EA, Horton TR. (2011). Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecology 4: 174–183. [Google Scholar]
- Lipson DA, Kuske CR, Gallegos-Graves LV, et al. (2014). Elevated atmospheric CO2 stimulates soil fungal diversity through increased fine root production in a semiarid shrubland ecosystem. Global Change Biology 20: 2555–2565. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu X, Wu X, et al. (2021a). Long-term elevated CO2 and warming enhance microbial necromass carbon accumulation in a paddy soil. Biology and Fertility of Soils 57: 673–684. [Google Scholar]
- Liu Y, Tian H, Li J, et al. (2021b). Reduced precipitation neutralizes the positive impact of soil warming on soil microbial community in a temperate oak forest. Science of the Total Environment 806: 150957. [DOI] [PubMed] [Google Scholar]
- Liu M, Shen Y, Li Q, et al. (2021c). Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C:N:P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant and Soil 461: 421–440. [Google Scholar]
- Liu H, Mi Z, Lin L, et al. (2018). Shifting plant species composition in response to climate change stabilizes grassland primary production. Proceedings of the National Academy of Sciences of the United States of America 115: 4051–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang H, Xiong M, et al. (2017). Abundance and composition response of wheat field soil bacterial and fungal communities to elevated CO2 and increased air temperature. Biology and Fertility of Soils 53: 3–8. [Google Scholar]
- Liu Y, Li M, Zheng JW, et al. (2014). Short-term responses of microbial community and functioning to experimental CO2 enrichment and warming in a Chinese paddy field. Soil Biology and Biochemistry 77: 58–68. [Google Scholar]
- Lladó S, López-Mondéjar R, Baldrian P. (2017). Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiology and Molecular Biology Reviews 81: e00063–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberau KE, Botnen SS, Mundra S, et al. (2017). Does warming by open-top chambers induce change in the root-associated fungal community of the arctic dwarf shrub Cassiope tetragona (Ericaceae)? Mycorrhiza 27: 513–524. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liu X, Chen F, et al. (2020). Shifts in plant community composition weaken the negative effect of nitrogen addition on community-level arbuscular mycorrhizal fungi colonization. Proceedings of the Royal Society B: Biological Sciences 287: 20200483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Geng QH, Zhang HG, et al. (2021a). Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytologist 229: 2957–2969. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhu B, Nie Y, et al. (2021b). Root and mycorrhizal strategies for nutrient acquisition in forests under nitrogen deposition: A meta-analysis. Soil Biology and Biochemistry 163: 108418. [Google Scholar]
- Ma LN, Lü XT, Liu Y, et al. (2011). The effects of warming and nitrogen addition on soil nitrogen cycling in a temperate grassland, northeastern China. PLoS One 6: e27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaroufi NI, Nordin A, Hasselquist NJ, et al. (2015). Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Global Change Biology 21: 3169–3180. [DOI] [PubMed] [Google Scholar]
- Macarthur R, Levins R. (1967). The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385. [Google Scholar]
- Maček I, Clark DR, Šibanc N, et al. (2019). Impacts of long-term elevated atmospheric CO2 concentrations on communities of arbuscular mycorrhizal fungi. Molecular Ecology 28: 3445–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Delgado-Baquerizo M, Jeffries TC, et al. (2015). Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proceedings of the National Academy of Sciences of the United States of America 112: 15684–15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra P, Zheng Y, Chen L, et al. (2019) Effect of drought and season on arbuscular mycorrhizal fungi in a subtropical secondary forest. Fungal Ecology 41: 107–115. [Google Scholar]
- Maya-Manzano JM, Muñoz-Triviño M, Fernández-Rodríguez S, et al. (2016). Airborne Alternaria conidia in Mediterranean rural environments in SW of Iberian Peninsula and weather parameters that influence their seasonality in relation to climate change. Aerobiologia 32: 95–108. [Google Scholar]
- McHugh TA, Schwartz E. (2015). Changes in plant community composition and reduced precipitation have limited effects on the structure of soil bacterial and fungal communities present in a semiarid grassland. Plant and Soil 388: 175–186. [Google Scholar]
- Melillo JM, Frey SD, DeAngelis KM, et al. (2017). Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 358: 101–104. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Terashima Y, Nara K. (2018). Temperature niche position and breadth of ectomycorrhizal fungi: Reduced diversity under warming predicted by a nested community structure. Global Change Biology 24: 5724–5737. [DOI] [PubMed] [Google Scholar]
- Moore JAM, Anthony MA, Pec GJ, et al. (2021). Fungal community structure and function shifts with atmospheric nitrogen deposition. Global Change Biology 27: 1349–1364. [DOI] [PubMed] [Google Scholar]
- Morrison EW, Frey SD, Sadowsky JJ, et al. (2016). Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecology 23: 48–57. [Google Scholar]
- Mucha J, Peay KG, Smith DP, et al. (2018). Effect of simulated climate warming on the ectomycorrhizal fungal community of boreal and temperate host species growing near their shared ecotonal range limits. Microbial Ecology 75: 348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller RC, Bohannan BJM. (2015). Shifts in the phylogenetic structure of arbuscular mycorrhizal fungi in response to experimental nitrogen and carbon dioxide additions. Oecologia 179: 175–185. [DOI] [PubMed] [Google Scholar]
- Mueller RC, Belnap J, Kuske CR. (2015). Soil bacterial and fungal community responses to nitrogen addition across soil depth and microhabitat in an arid shrubland. Frontiers in Microbiology 6: 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Ismert KJ, Smith MD, et al. (2021). Soil fungal communities are compositionally resistant to drought manipulations – Evidence from culture-dependent and culture-independent analyses. Fungal Ecology 51: 101062. [Google Scholar]
- Naylor D, Sadler N, Bhattacharjee A, et al. (2020). Soil microbiomes under climate change and implications for carbon cycling. Annual Review of Environment and Resources 45: 29–59. [Google Scholar]
- Nemani RR, Keeling CD, Hashimoto H, et al. (2003). Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300: 1560–1563. [DOI] [PubMed] [Google Scholar]
- Newsham KK, Hopkins DW, Carvalhais LC, et al. (2016). Relationship between soil fungal diversity and temperature in the maritime Antarctic. Nature Climate Change 6: 182–186. [Google Scholar]
- Nilsson RH, Anslan S, Bahram M, et al. (2018). Mycobiome diversity: high-throughput sequencing and identification of fungi. Nature Reviews Microbiology 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Nilsson LO, Giesler R, Bååth E, et al. (2005). Growth and biomass of mycorrhizal mycelia in coniferous forests along short natural nutrient gradients. New Phytologist 165: 613–622. [DOI] [PubMed] [Google Scholar]
- Nottingham AT, Meir P, Velasquez E, et al. (2020). Soil carbon loss by experimental warming in a tropical forest. Nature 584: 234–237. [DOI] [PubMed] [Google Scholar]
- Nunez MA, Horton TR, Simberloff D. (2009). Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90: 2352–2359. [DOI] [PubMed] [Google Scholar]
- Ochoa-Hueso R, Arca V, Delgado-Baquerizo M, et al. (2020). Links between soil microbial communities, functioning, and plant nutrition under altered rainfall in Australian grassland. Ecological Monographs 90: e01424. [Google Scholar]
- Ochoa-Hueso R, Collins SL, Delgado-Baquerizo M, et al. (2018). Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Global Change Biology 24: 2818–2827. [DOI] [PubMed] [Google Scholar]
- Odriozola I, Navrátilová D, Tláskalová P, et al. (2021). Predictors of soil fungal biomass and community composition in temperate mountainous forests in Central Europe. Soil Biology and Biochemistry 161: 108366. [Google Scholar]
- Panneerselvam P, Kumar U, Senapati A, et al. (2020). Influence of elevated CO2 on arbuscular mycorrhizal fungal community elucidated using Illumina MiSeq platform in sub-humid tropical paddy soil. Applied Soil Ecology 145: 103344. [Google Scholar]
- Peng F, Zhang W, Li C, et al. (2020). Sustained increase in soil respiration after nine years of warming in an alpine meadow on the Tibetan Plateau. Geoderma 379: 114641. [Google Scholar]
- Phillips RP, Brzostek E, Midgley MG. (2013). The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist 199: 41–51. [DOI] [PubMed] [Google Scholar]
- Powell JR, Parrent JL, Hart MM, et al. (2009). Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proceedings of the Royal Society B: Biological Sciences 276: 4237–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procter AC, Ellis JC, Fay PA, et al. (2014). Fungal community responses to past and future atmospheric CO2 differ by soil type. Applied and Environmental Microbiology 80: 7364–7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese M, Cogliati E, Gullino ML, et al. (2012). Effect of climate change on Alternaria leaf spot of rocket salad and black spot of basil under controlled environment. Communications in Agricultural and Applied Biological Sciences 77: 241–244. [PubMed] [Google Scholar]
- Querejeta JI, Schlaeppi K, López-García Á, et al. (2021). Lower relative abundance of ectomycorrhizal fungi under a warmer and drier climate is linked to enhanced soil organic matter decomposition. New Phytologist 232: 1399–1413. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Erb I, Richardson MF, et al. (2018). Understanding sequencing data as compositions: an outlook and review. Bioinformatics 34: 2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, et al. (2002). Thermotolerance generated by plant/fungal symbiosis. Science 298: 1581–1581. [DOI] [PubMed] [Google Scholar]
- Revillini D, Gehring CA, Johnson NC. (2016). The role of locally adapted mycorrhizas and rhizobacteria in plant-soil feedback systems. Functional Ecology 30: 1086–1098. [Google Scholar]
- Reynolds HL, Packer A, Bever JD, et al. (2003). Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 84: 2281–2291. [Google Scholar]
- Rillig MC, Ryo M, Lehmann A, et al. (2019). The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366: 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ramos JC, Cale JA, Cahill JF, et al. (2021). Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytologist 229: 1105–1117. [DOI] [PubMed] [Google Scholar]
- Romero-Olivares AL, Melendrez-Carballo G, Lago-Leston A, et al. (2019). Soil metatranscriptomes under long-term experimental warming and drying: fungi allocate resources to cell metabolic maintenance rather than decay. Frontiers in Microbiology 10: 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Olivares AL, Allison SD, Treseder KK. (2017). Soil microbes and their response to experimental warming over time: A meta-analysis of field studies. Soil Biology and Biochemistry 107: 32–40. [Google Scholar]
- Romero F, Cazzato S, Walder F, et al. (2022). Humidity and high temperature are important for predicting fungal disease outbreaks worldwide. New Phytologist 234: 1553–1556. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Afkhami ME, Bell-Dereske L, et al. (2020). Climate disruption of plant-microbe interactions. Annual Review of Ecology, Evolution, and Systematics 51: 561–586. [Google Scholar]
- Rudgers JA, Kivlin SN, Whitney KD, et al. (2014). Responses of high-altitude graminoids and soil fungi to 20 years of experimental warming. Ecology 95: 1918–1928. [DOI] [PubMed] [Google Scholar]
- Schwede DB, Simpson D, Tan J, et al. (2018). Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environmental Pollution 243: 1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebens H, Essl F, Dawson W, et al. (2015). Global trade will accelerate plant invasions in emerging economies under climate change. Global Change Biology 21: 4128–4140. [DOI] [PubMed] [Google Scholar]
- Semenova TA, Morgado LN, Welker JM, et al. (2015). Long-term experimental warming alters community composition of ascomycetes in Alaskan moist and dry arctic tundra. Molecular Ecology 24: 424–437. [DOI] [PubMed] [Google Scholar]
- Shao P, He H, Zhang X, et al. (2018). Responses of microbial residues to simulated climate change in a semiarid grassland. Science of the Total Environment 644: 1286–1291. [DOI] [PubMed] [Google Scholar]
- She W, Bai Y, Zhang Y, et al. (2018). Resource availability drives responses of soil microbial communities to short-term precipitation and nitrogen addition in a desert shrubland. Frontiers in Microbiology 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RC, Xu M, Chi YG, et al. (2014) Soil microbial responses to experimental warming and nitrogen addition in a temperate steppe of Northern China. Pedosphere 24: 427–436. [Google Scholar]
- Shi G, Yao B, Liu Y, et al. (2021). The effects of long-term warming on arbuscular mycorrhizal fungal communities depend on habitat type on the Qinghai-Tibet Plateau. Applied Soil Ecology 167: 104030. [Google Scholar]
- Shi Y, Zhang K, Li Q, et al. (2020). Interannual climate variability and altered precipitation influence the soil microbial community structure in a Tibetan Plateau grassland. Science of the Total Environment 714: 136794. [DOI] [PubMed] [Google Scholar]
- Shi G, Yao B, Liu Y, et al. (2017). The phylogenetic structure of AMF communities shifts in response to gradient warming with and without winter grazing on the Qinghai-Tibet Plateau. Applied Soil Ecology 121: 31–40. [Google Scholar]
- Siciliano I, Berta F, Bosio P, et al. (2017). Effect of different temperatures and CO2 levels on Alternaria toxins produced on cultivated rocket, cabbage and cauliflower. World Mycotoxin Journal 10: 63–71. [Google Scholar]
- Siebold M, Tiedemann AV. (2012). Potential effects of global warming on oilseed rape pathogens in Northern Germany. Fungal Ecology 5: 62–72. [Google Scholar]
- Smith MD, Knapp AK, Collins SL. (2009). A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90: 3279–3289. [DOI] [PubMed] [Google Scholar]
- Solly EF, Lindahl BD, Dawes MA, et al. (2017). Experimental soil warming shifts the fungal community composition at the alpine treeline. New Phytologist 215: 766–778. [DOI] [PubMed] [Google Scholar]
- Song Y, Jiang L, Song C, et al. (2021). Microbial abundance and enzymatic activity from tussock and shrub soil in permafrost peatland after 6-year warming. Ecological Indicators 126: 107589. [Google Scholar]
- Soudzilovskaia NA, Douma JC, Akhmetzhanova AA, et al. (2015). Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Global Ecology and Biogeography 24: 371–382. [Google Scholar]
- Starke R, Mondéjar RL, Human ZR, et al. (2021). Niche differentiation of bacteria and fungi in carbon and nitrogen cycling of different habitats in a temperate coniferous forest: A metaproteomic approach. Soil Biology and Biochemistry 155: 108170. [Google Scholar]
- Steidinger BS, Bhatnagar JM, Vilgalys R, et al. (2020). Ectomycorrhizal fungal diversity predicted to substantially decline due to climate changes in North American Pinaceae forests. Journal of Biogeography 47: 772–782. [Google Scholar]
- Steidinger BS, Crowther TW, Liang J, et al. (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569: 404–408. [DOI] [PubMed] [Google Scholar]
- Štursová M, Šnajdr J, Cajthaml T, et al. (2014). When the forest dies: the response of forest soil fungi to a bark beetle-induced tree dieback. Isme Journal 8: 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang C, Yang J, et al. (2021). Elevated CO2 shifts soil microbial communities from K- to r-strategists. Global Ecology and Biogeography 30: 961–972. [Google Scholar]
- Sun Y, Chen HYH, Jin L, et al. (2020). Drought stress induced increase of fungi: bacteria ratio in a poplar plantation. Catena 193: 104607. [Google Scholar]
- Sun J, Xia Z, He T, et al. (2017). Ten years of elevated CO2 affects soil greenhouse gas fluxes in an open top chamber experiment. Plant and Soil 420: 435–450. [Google Scholar]
- Tahovská K, Choma M, Kaštovská E, et al. (2020). Positive response of soil microbes to long-term nitrogen input in spruce forest: Results from Gardsjon whole-catchment N-addition experiment. Soil Biology and Biochemistry 143: 107732. [Google Scholar]
- Tedersoo L, Bahram M, Polme S, et al. (2014). Global diversity and geography of soil fungi. Science 346: 1256688. [DOI] [PubMed] [Google Scholar]
- Thompson LR, Sanders JG, McDonald D, et al. (2017). A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserant E, Malbreil M, Kuo A, et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America 110: 20117–20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M, Deen W, Drijber R, et al. (2021). Long-term N inputs shape microbial communities more strongly than current-year inputs in soils under 10-year continuous corn cropping. Soil Biology and Biochemistry 160: 108361. [Google Scholar]
- Treseder KK, Allen EB, Egerton-Warburton LM, et al. (2018). Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. Journal of Ecology 106: 480–489. [Google Scholar]
- Treseder KK. (2004). A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytologist 164: 347–355. [DOI] [PubMed] [Google Scholar]
- Treseder KK, Egerton-Warburton LM, Allen MF, et al. (2003). Alteration of soil carbon pools and communities of mycorrhizal fungi in chaparral exposed to elevated carbon dioxide. Ecosystems 6: 786–796. [Google Scholar]
- Tu Q, Yuan M, He Z, et al. (2015). Fungal communities respond to long-term CO2 elevation by community reassembly. Applied and Environmental Microbiology 81: 2445–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde S, Suz LM, Orme CDL, et al. (2018). Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 558: 243–248. [DOI] [PubMed] [Google Scholar]
- van Diepen LTA, Entwistle EM, Zak DR. (2013). Chronic nitrogen deposition and the composition of active arbuscular mycorrhizal fungi. Applied Soil Ecology 72: 62–68. [Google Scholar]
- van Diepen LTA, Lilleskov EA, Pregitzer KS. (2011). Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Molecular Ecology 20: 799–811. [DOI] [PubMed] [Google Scholar]
- van Groenigen KJ, Qi X, Osenberg CW, et al. (2014). Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 344: 508–509. [DOI] [PubMed] [Google Scholar]
- van Nuland ME, Smith DP, Bhatnagar JM, et al. (2020). Warming and disturbance alter soil microbiome diversity and function in a northern forest ecotone. FEMS Microbiology Ecology 96: fiaa108. [DOI] [PubMed] [Google Scholar]
- Veresoglou SD, Anderson IC, de Sousa NMF, et al. (2016). Resilience of fungal communities to elevated CO2. Microbial Ecology 72: 493–495. [DOI] [PubMed] [Google Scholar]
- Veresoglou SD, Barto EK, Menexes G, et al. (2013). Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathology 62: 961–969. [Google Scholar]
- Veresoglou SD, Caruso T, Rillig MC. (2012). Modelling the environmental and soil factors that shape the niches of two common arbuscular mycorrhizal fungal families. Plant and Soil 368: 507–518. [Google Scholar]
- Vesala R, Kiheri H, Hobbie EA, et al. (2021). Atmospheric nitrogen enrichment changes nutrient stoichiometry and reduces fungal N supply to peatland ericoid mycorrhizal shrubs. Science of the Total Environment 794: 148737. [DOI] [PubMed] [Google Scholar]
- Větrovský T, Morais D, Kohout P, et al. (2020). GlobalFungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Scientific Data 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Větrovský T, Kohout P, Kopecký M, et al. (2019). A meta-analysis of global fungal distribution reveals climate-driven patterns. Nature Communications 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlk L, Tedersoo L, Antl T, et al. (2020a). Alien ectomycorrhizal plants differ in their ability to interact with co-introduced and native ectomycorrhizal fungi in novel sites. The ISME Journal 14: 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlk L, Tedersoo L, Antl T, et al. (2020b). Early successional ectomycorrhizal fungi are more likely to naturalize outside their native range than other ectomycorrhizal fungi. New Phytologist 227: 1289–1293. [DOI] [PubMed] [Google Scholar]
- Wahdan SFM, Reitz T, Heintz-Buschart A, et al. (2021). Organic agricultural practice enhances arbuscular mycorrhizal symbiosis in correspondence to soil warming and altered precipitation patterns. Environmental Microbiology 23: 6163–6176. [DOI] [PubMed] [Google Scholar]
- Wallenstein MD, McNulty S, Fernandez IJ, et al. (2006). Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. Forest Ecology and Management 222: 459–468. [Google Scholar]
- Walters DR, Bingham IJ. (2007). Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Annals of Applied Biology 151: 307–324. [Google Scholar]
- Wang C, Sun Y, Chen HYH, et al. (2021a). Meta-analysis shows non-uniform responses of above- and belowground productivity to drought. Science of the Total Environment 782: 146901. [DOI] [PubMed] [Google Scholar]
- Wang J, Shi X, Zheng C, et al. (2021b). Different responses of soil bacterial and fungal communities to nitrogen deposition in a subtropical forest. Science of the Total Environment 755: 142449. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang J, Wang C, et al. (2021c). Precipitation exerts a strong influence on arbuscular mycorrhizal fungi community and network complexity in a semiarid steppe ecosystem. European Journal of Soil Biology 102: 103268. [Google Scholar]
- Wang N, Li L, Zhang B, et al. (2020a). Population turnover promotes fungal stability in a semi-arid grassland under precipitation shifts. Journal of Plant Ecology 13: 499–509. [Google Scholar]
- Wang H, Ta N, Jin K, et al. (2020b). Interactive effects of nitrogen fertilizer and altered precipitation on fungal communities in arid grasslands of northern China. Journal of Soils and Sediments 20: 1344–1356. [Google Scholar]
- Wang H, Liu S, Schindlbacher A, et al. (2019). Experimental warming reduced topsoil carbon content and increased soil bacterial diversity in a subtropical planted forest. Soil Biology and Biochemistry 133: 155–164. [Google Scholar]
- Wang C, Zhao X, Zi H, et al. (2017). The effect of simulated warming on root dynamics and soil microbial community in an alpine meadow of the Qinghai-Tibet Plateau. Applied Soil Ecology 116: 30–41. [Google Scholar]
- Wang J, Bao J, Su J, et al. (2015). Impact of inorganic nitrogen additions on microbes in biological soil crusts. Soil Biology and Biochemistry 88: 303–313. [Google Scholar]
- Weber SE, Diez JM, Andrews LV, et al. (2019). Responses of arbuscular mycorrhizal fungi to multiple coinciding global change drivers. Fungal Ecology 40: 62–71. [Google Scholar]
- Weber CF, Vilgalys R, Kuske CR. (2013). Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Frontiers in Microbiology 4: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Shi Y, Qin F, et al. (2021). Effects of experimental warming, precipitation increase and their interaction on AM fungal community in an alpine grassland of the Qinghai-Tibetan Plateau. European Journal of Soil Biology 102: 103272. [Google Scholar]
- Whiteside MD, Digman MA, Gratton E, et al. (2012). Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest. Soil Biology and Biochemistry 55: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, O’Neill NR, Rogers CA, et al. (2010). Elevated atmospheric carbon dioxide concentrations amplify Alternaria alternata sporulation and total antigen production. Environmental Health Perspectives 118: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollan AK, Bakkestuen V, Kauserud H, et al. (2008). Modelling and predicting fungal distribution patterns using herbarium data. Journal of Biogeography 35: 2298–2310. [Google Scholar]
- Wu Y, Kwak JH, Karst J, et al. (2021). Long-term nitrogen and sulfur deposition increased root-associated pathogen diversity and changed mutualistic fungal diversity in a boreal forest. Soil Biology and Biochemistry 155: 108163. [Google Scholar]
- Wu Y, Wu J, Saleem M, et al. (2020). Ecological clusters based on responses of soil microbial phylotypes to precipitation explain ecosystem functions. Soil Biology and Biochemistry 142: 107717. [Google Scholar]
- Xiao Y, Li C, Yang Y, et al. (2020). Soil fungal community composition, not assembly process, was altered by nitrogen addition and precipitation changes at an alpine steppe. Frontiers in Microbiology 11: 579072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Peng F, Sun H, et al. (2014). Divergent responses of soil fungi functional groups to short-term warming. Microbial Ecology 68: 708–715. [DOI] [PubMed] [Google Scholar]
- Yan G, Han S, Wang Q, et al. (2021). Variations of the effects of reduced precipitation and N addition on microbial diversity among different seasons in a temperate forest. Applied Soil Ecology 166: 103995. [Google Scholar]
- Yang Y, Li T, Wang Y, et al. (2021a). Negative effects of multiple global change factors on soil microbial diversity. Soil Biology and Biochemistry 156: 108229. [Google Scholar]
- Yang X, Zhu K, Loik ME, et al. (2021b). Differential responses of soil bacteria and fungi to altered precipitation in a meadow steppe. Geoderma 384: 114812. [Google Scholar]
- Yoshitake S, Tabei N, Mizuno Y, et al. (2015). Soil microbial response to experimental warming in cool temperate semi-natural grassland in Japan. Ecological Research 30: 235–245. [Google Scholar]
- Yu CQ, Han FS, Fu G. (2019). Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Science of the Total Environment 655: 814–822. [DOI] [PubMed] [Google Scholar]
- Zavalloni C, Vicca S, Büscher M, et al. (2012). Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant and Soil 359: 121–136. [Google Scholar]
- Zelikova TJ, Housman DC, Grote EE, et al. (2012). Warming and increased precipitation frequency on the Colorado Plateau: implications for biological soil crusts and soil processes. Plant and Soil 355: 265–282. [Google Scholar]
- Zhang J, Liu S, Liu C, et al. (2021). Soil bacterial and fungal richness and network exhibit different responses to long-term throughfall reduction in a warm-temperate oak forest. Forests 12: 165. [Google Scholar]
- Zhang Y, Dong S, Gao Q, et al. (2019). “Rare biosphere” plays important roles in regulating soil available nitrogen and plant biomass in alpine grassland ecosystems under climate changes. Agriculture, Ecosystems & Environment 279: 187–193. [Google Scholar]
- Zhang H, Wang L, Liu H, et al. (2018). Nitrogen deposition combined with elevated precipitation is conducive to maintaining the stability of the soil fungal diversity on the Stipa baicalensis steppe. Soil Biology and Biochemistry 117: 135–138. [Google Scholar]
- Zhang Y, Dong SK, Gao QZ, et al. (2017). Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Scientific Reports 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shi Y, Jing X, et al. (2016a). Effects of short-term warming and altered precipitation on soil microbial communities in alpine grassland of the Tibetan Plateau. Frontiers in Microbiology 7: 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong SK, Gao QZ, et al. (2016b). Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Science of the Total Environment 562: 353–363. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wan S, Guo J, et al. (2015). Precipitation modifies the effects of warming and nitrogen addition on soil microbial communities in northern Chinese grasslands. Soil Biology and Biochemistry 89: 12–23. [Google Scholar]
- Zhao A, Liu L, Chen B, et al. (2020). Soil fungal community is more sensitive to nitrogen deposition than increased rainfall in a mixed deciduous forest of China. Soil Ecology Letters 2: 20–32. [Google Scholar]
- Zhao AH, Liu L, Xu TL, et al. (2018). Influences of canopy nitrogen and water addition on AM fungal biodiversity and community composition in a mixed deciduous forest of China. Frontiers in Plant Science 9: 1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Jian SG, Nunan N, et al. (2017). Altered precipitation seasonality impacts the dominant fungal but rare bacterial taxa in subtropical forest soils. Biology and Fertility of Soils 53: 231–245. [Google Scholar]
- Zhao C, Miao Y, Yu C, et al. (2016). Soil microbial community composition and respiration along an experimental precipitation gradient in a semiarid steppe. Scientific Reports 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Cui M, Wang C, et al. (2022a). Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Science of the Total Environment 806: 150522. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Ma X, Zhang Y, et al. (2022b). Soil properties and plant community-level traits mediate arbuscular mycorrhizal fungal response to nitrogen enrichment and altered precipitation. Applied Soil Ecology 169: 104245. [Google Scholar]
- Zheng Y, Kim YC, Tian XF, et al. (2014). Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiology Ecology 89: 594–605. [DOI] [PubMed] [Google Scholar]
- Zhong R, Xia C, Ju YW, et al. (2021). A foliar Epichloë endophyte and soil moisture modified belowground arbuscular mycorrhizal fungal biodiversity associated with Achnatherum inebrians. Plant and Soil 458: 105–122. [Google Scholar]
- Zhou Z, Wang C, Luo Y. (2020). Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nature Communications 11: 3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Tian GL, Luo GW, et al. (2018). N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agriculture, Ecosystems & Environment 254: 191–201. [Google Scholar]
- Žifčáková L, Větrovský T, Lombard V, et al. (2017). Feed in summer, rest in winter: microbial carbon utilization in forest topsoil. Microbiome 5: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection criteria for the inclusion of studies describing effects of global change manipulation on fungi.