Abstract
Background
Trichilemmal carcinoma (TLC) is a rare malignant cutaneous adnexal neoplasm, with no relatively comprehensive research.
Objective
The aim of this study is to perform an updated statistical analysis so as to better understand TLC’s epidemiology, clinical features, diagnosis, and treatment.
Methods
The diagnosis and treatment of three TLC cases in our department were summarized. Then, all TLC cases published in the literature were retrieved for a comprehensive analysis, followed by the analysis of global trends and regional distribution, demographic characteristics, clinical features, pathogenesis, histopathological features, and treatment and prognosis of TLC.
Results
Of the 231 cases, the incidence of TLC has shown an upward trend recently, especially in China, in Asia. The susceptible population is men aged 60–80 and women over 80, and the most prone location is head and neck. The phenotype of TLC is not always typical and may be misdiagnosed because of the coexistence of other diseases. There is a linear relationship between the diameter and its duration or thickness. UV, locally present skin lesions, trauma, scarring, organ transplantation, and genetic disorders may trigger the occurrence of TLC. Periodic acid–Schiff staining and CD34, but not Epithelial Membrane Antigen (EMA), were helpful in the diagnosis of TLC. Although effective, surgical excision and Mohs micrographic surgery need further improvement to reduce recurrence of TLC. Carcinoma history is an independent risk factor for TLC recurrence.
Limitations
The limitation of this study is the lack of randomized controlled trial on TLC treatment and recurrence.
Conclusion
TLC has the possibility of invasive growth and recurrence, especially in patients with longer duration and carcinoma history.
Keywords: trichilemmal carcinoma, epidemiology, Mohs micrographic surgery, metastasis, recurrence, immunohistochemistry
Introduction
As first described and defined by Headington, trichilemmal carcinoma (TLC) is a rare malignant adnexal neoplasm and exhibits features of “outer root sheath differentiation and atypical clear cell neoplasm” (1, 2). Clinically, TLC usually manifests as an asymptomatic exophytic or polypoid masses and may be misdiagnosed as basal cell carcinoma (BCC), squamous cell carcinoma (SCC), keratoacanthoma, or proliferating pilar cyst (3). Generally manifested as an indolent course, TLC may still be locally destructive and may have the possibility of recurrence or metastasis, which implies the significance of accurate diagnosis and careful management.
Given the fact that there was still no relatively comprehensive research on TLC, so on the basis of the questions that we encountered during the diagnosis and treatment of three TLC cases, we further retrieved all TLC cases in the published literature for a comprehensive analysis, aiming to provide a more thorough understanding of TLC. Starting with the global prevalence of TLC over time and the regional distribution, we summarized the demographic characteristics of TLC, including the analysis of age distribution and susceptibility location. The clinical features of TLC were further systematically exhibited, from the possible concomitant diseases to the size and duration of TLC with the potential associated factors analyzed. To explore the possible pathogenesis, the etiology of TLC was systematically summed up, which may have a prompting effect on the prevention of TLC. In view of the diverse manifestations of TLC, which may lead to the misdiagnosis or missed diagnosis, we gathered the pathological features of TLC and proposed key pathological indicators that may be helpful for the differential diagnosis of TLC via the statistical analysis of the staining results of all cases. Various treatment methods for TLC were summarized, and the recurrence-related risk factors were analyzed, which may provide a basis for improving the prognosis of patients with TLC.
Here, we reported three cases of TLC treated in our department. Then, we summarized all reported cases in literature so as to better understand the epidemiology, clinical features, diagnosis, and treatment of TLC. The comprehensive analysis of TLC has good auxiliary significance for the prevention, diagnosis, treatment, and prognosis of TLC.
Materials and methods
Ethics and specimen acquisition
The study was approved by the Ethical Review Committee of Fourth Medical Center of PLA General Hospital. Written informed consent was signed after the patients were being informed of the research content and research methods.
By fully searching the clinical database of our admitted patients, a total of three cases were included in the study that met the diagnostic criteria of TLC with pathological confirmation. Clinical data and photographs were obtained and desensitized for the protection of patients’ privacy. All the three patients had previously undergone tissue biopsy for the differential diagnosis of the lesions. The biopsy tissues were fixed in formaldehyde, embedded in paraffin, and preserved in the Department of Pathology of Fourth Medical Center of PLA General Hospital. With the patients’ consent, the specimens were obtained and sliced for further immunohistochemical staining.
Immunohistochemical staining
For the obtained specimens, sections were incubated with DNase-free proteinase K (20 μg/ml; P1120, Solarbio, China) at 37°C for 30 min for antigen retrieval. Then, sections were incubated with 0.1% Triton X-100 (HFH10, Invitrogen, USA) in phosphate buffered saline (PBS) for 30 min to penetrate cell membrane. After blocking with 5% goat serum (16210064, Gibco, USA) in PBS for 30 min, the sections were incubated with corresponding antibodies overnight at 4°C. After incubation, sections were rinsed three times in PBS for 5 min each and then incubated with corresponding goat anti-rabbit or goat anti-mouse secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. Sections were washed with PBS and then exposed to 3,3′-diaminobenzidine (DAB) staining (DA1016, Solarbio, China) following the manufacturer’s instructions. Finally, the slices were observed and photographed using a panoramic scanner from 3DHISTECH CaseViewer.
Literature review and cases retrieval
A comprehensive literature review was conducted by searching the publications in PubMed (http://www.ncbi.nlm.nih.gov) and Embase (https://www.embase.com/) published between 1977 and 2022. The search terms used were “trichilemmal carcinoma”, “tricholemmal carcinoma”, and “tricholemmocarcinoma”. References not indexed in PubMed or Embase were also traced for a complete record. Specifically, the Cochrane Library (https://www.cochranelibrary.com/) and Prospero (https://www.crd.york.ac.uk/prospero/) were explored to obtain the TLC-relevant meta-analyses. The databases of ClinicalTrials.gov (https://clinicaltrials.gov/) and National Cancer Institute (https://www.cancer.gov/) were searched for any clinical trials on TLC. Translation of articles in languages other than English was executed via Google Translate (http://translate.google.com). The cutoff date was 7 July 2022. Eighty-six publications worldwide were identified from PubMed and Embase containing a total of 228 cases. No TLC-relevant meta-analyses or clinical trials were identified.
Statistical analysis
Excel and SPSS were utilized for processing information. Missing values were ignored. Descriptive statistics were utilized. For categorical variables, frequencies and the percentage were computed, and Chi-square test or two-sided Fisher’s exact tests were performed. Continuous variables were first tested for normality by the Kolmogorov–Smirnov test and then for homogeneity by the variance test. Normally distributed data were presented as mean ± standard deviation and tested using t test. Non-normally distributed data were presented as the median and interquartile range and compared using the Mann–Whitney U-test. Covariates with a P-value < 0.05 in the univariate analysis were chosen for the multivariate analysis, where logistic regression was used to determine each variable with the recurrence of TLC. The 0.05 level of confidence was accepted as a significant difference.
Results
Case series
Case 1
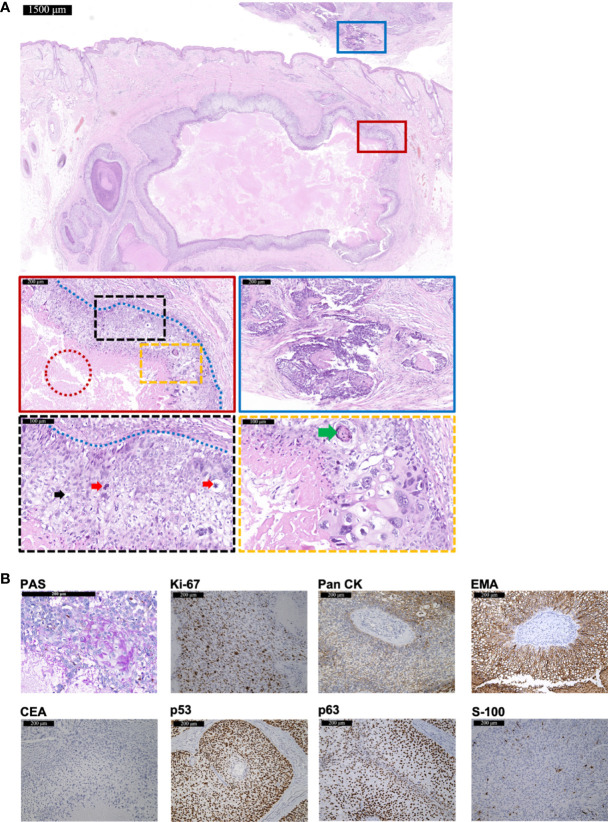
A 48-year-old man presented with a lesion on right temporal without itching, pain, or ulcers for 8 months. The 1.5-cm exophytic lesion was red and soft with multiple dilated capillaries on the surface, without pruritus or fluctuation. Any past medical history or hereditary disease were denied. Surgical excision was performed for further diagnosis. Hematoxylin-eosin (HE) staining revealed the infiltrative growth of the lesion ( Figure 1A ). The cytoplasm of tumor cells was clear (indicated by black arrows) and PAS-positive ( Figure 1B ), accompanied by scattered atypical nuclei and high mitotic index (indicated by red arrows). The blue dashed line indicated the peripheral palisading of clear cells. The typical histopathology of trichilemmal keratinization was indicated by the green arrows. In addition, focal necrotic area was visible within the tumor (indicated by red dashed circle). Immunohistochemistry showed positivity for Pan cytokeratin (Pan CK), EMA, Ki-67 (60%+), p53, and p63 but negativity for carcinoembryonic antigen (CEA) and S-100 ( Figure 1B ). For tumor cells seen at the bottom margins, further extended resection was performed and achieved tumor-free margins. A 54-month follow-up showed no recurrence or metastasis.
Figure 1.
The histopathological and immunohistochemical features of Case 1 diagnosed as TLC. (A) The histopathological features of the surgical resection specimen of Case 1. The scale bar of the pathological overview picture is 1,500 μm, and the scale bars of the enlarged field of view are 200 and 100 μm, respectively. The pathological features in the solid and dashed boxes have been further zoomed in and displayed. The red dashed circle indicates the focal necrotic area visible within the tumor. The black dashed box shows mitosis (red arrows) and a large number of polygonal, transparent tumor cells (black arrows). The yellow dashed box shows a typical histopathology of trichilemmal keratinization (green arrows) and cellular atypia. The blue dashed line indicates the peripheral palisading of clear cells. The blue solid box shows the tumor invasion. (B) The staining features of Case 1. Case 1 showed positivity for PAS staining. As for immunohistochemical staining, Case 1 showed positivity for Ki-67 (60%+), Pan CK, EMA, p53, and p63 but negativity for CEA and S-100. The scale bar is 200 μm.
Case 2
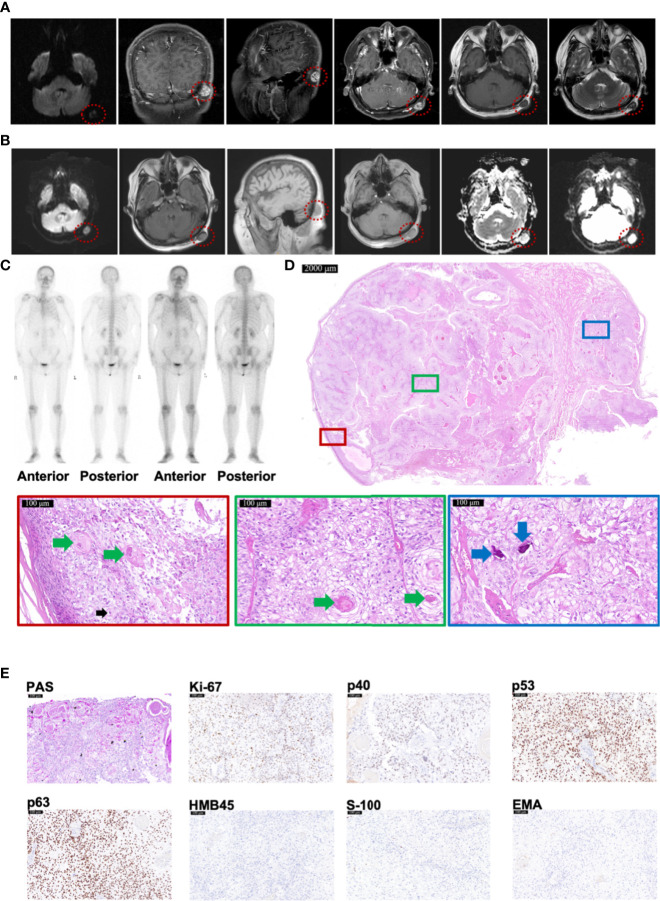
A woman suffered invasive ductal carcinoma of left breast with vessels infiltrated at age 55. Two years after the resection, head magnetic resonance imaging (MRI) detected an abnormal signal of 1.7 × 1.2 cm in left occipital subcutaneous without invasion to the skull or brain ( Figure 2A ). Another year later, MRI reported an abnormal signal of 1.8 × 1.3 cm in left occipital subcutaneous, whose imaging appearance was similar to that of 1 year ago ( Figure 2B ). No bone metastases were found by emission computed tomography (CT) ( Figure 2C ). After resection, pathological examination revealed that the tumor was attached to the epidermis with 2.2 × 2.0 × 1.5 cm in size ( Figure 2D ). The lesion had a classic presentation of TLC with many polygonal transparent tumor cells (indicated by black arrows), trichilemmal keratinization (indicated by green arrows), and calcification (indicated by blue arrows) ( Figure 2D ). PAS staining was positive ( Figure 2E ). Immunohistochemistry staining showed positivity for Ki-67 (35%), p53, and p63 but negativity for p40, HMB45, S-100, and EMA ( Figure 2E ). All resection margins were negative. A 24-month follow-up showed no recurrence.
Figure 2.
Radiology and histopathological features of Case 2 diagnosed as TLC. (A, B) MRI images of Case 2, taken respectively at her 57 (A) and 58 (B) years old, showing TLC nodules in the occipital region inside the red dotted circle. (C) The Emission Computed Tomography image of Case 2 showed that the bones of the whole body were not invaded by any tumor. (D) The histopathological features of Case 2. The scale bar of the pathological overview picture is 2,000 μm, and the scale bar of the enlarged field of view is 100 μm. The pathological features in the solid boxes have been further zoomed in and displayed. The lesion had many polygonal transparent tumor cells (indicated by black arrows), trichilemmal keratinization (indicated by green arrows), and calcification (indicated by blue arrows). (E) The staining features of Case 2. Case 2 showed positivity for PAS staining. As for immunohistochemical staining, Case 2 showed positivity for Ki-67 (35%), p53, and p63 but negativity for p40, HMB45, S-100, and EMA. The scale bar is 100 μm.
Case 3
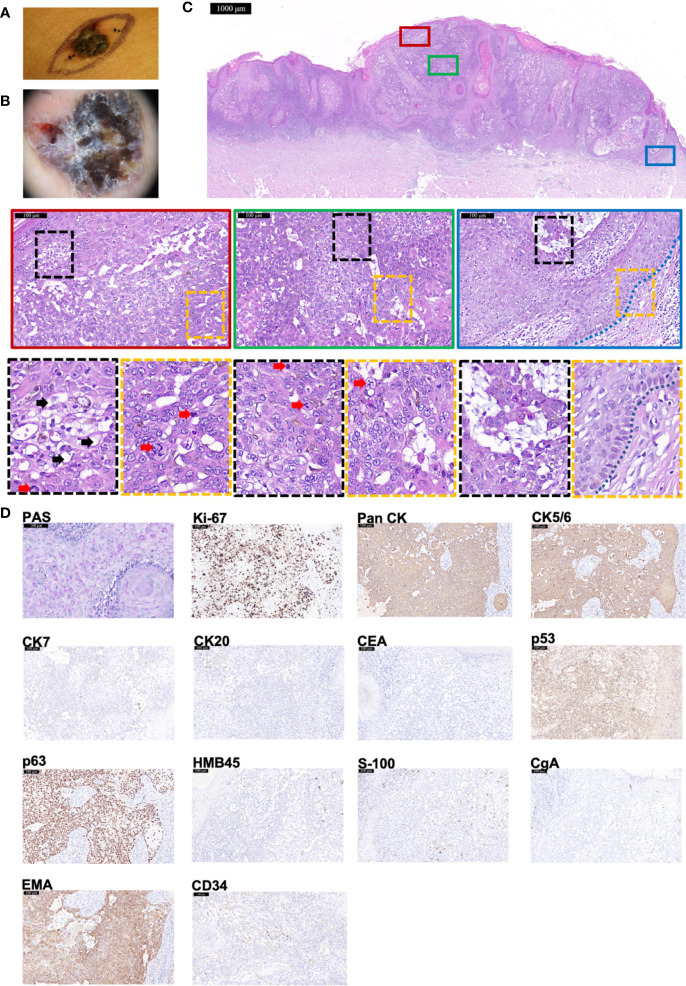
An 84-year-old woman presented with a dark-brown papule on right breast, with rough surface, uneven pigmentation, and clear boundaries ( Figure 3A ). Dermoscopy showed the nodule was an asymmetrical, multi-lobed lesion with crystalline pupa-like structures distributed ( Figure 3B ). There was no palpable lymphadenopathy. After the initial diagnosis of suspicious BCC, wide local excision was executed. HE staining manifested several features supporting a diagnosis of TLC—predominantly clear cytoplasm within the tumor cells (indicated by black arrows), many cells in mitosis (indicated by red arrows), and focal peripheral palisading (indicated by blue dashed line) ( Figure 3C ). PAS staining was positive ( Figure 3D ). Immunohistochemistry staining ( Figure 3D ) showed positivity for Ki-67 (80%+), Pan CK, EMA, CK5/6, p53, and p63 but negativity for CEA, CgA, CK7, CK20, HMB45, and S-100. In addition, CD34 was diffusely expressed. All of these supported the diagnosis of TLC. At 6-month follow-up, there were no signs of recurrence.
Figure 3.
The histopathological and immunohistochemical features of Case 3 diagnosed as TLC. (A, B) Macroscopic and dermoscopic view of the TLC lesion of Case 3. (C) The histopathological features of Case 3. The scale bar of the pathological overview picture is 1,000 μm, and the scale bar of the enlarged field of view is 100 μm. The pathological features in the solid and dashed boxes have been further zoomed in and displayed. The lesion had many polygonal transparent tumor cells (indicated by black arrows) and pathological mitosis (indicated by red arrows). The blue dashed line indicates the peripheral palisading of cells. (D) The staining features of Case 3. Case 3 showed positivity for PAS staining. As for immunohistochemical staining, Case 3 showed positivity for Ki-67 (80%+), Pan CK, CK5/6, p53, p63, and EMA but negativity for CK7, CK20, CEA, HMB45, S-100, and CgA. CD34 was diffusely expressed. The scale bar is 100 μm.
Global trends and regional distribution
All the reported cases were collected to analyze the characteristics of TLC. In total, 231 cases (including three cases in this article) were included. Because of the infrequent reporting or tracking of TLC occurrences, the actual incidence could not be accurately determined. The cumulative number of cases has grown slowly since the mid-1990s but been increasing rapidly since 2014 with a gradually upward growth curve ( Figure 4A ). The year 2020 (45, 19.48%), 2018 (37, 16.02%), and 1994 (28, 12.12%) were the top three years with the highest volume of published TLC cases ( Figure 4A ). All continents except Antarctica have cases, with most of the cases located in Asian (112, 48.48%) ( Figure 4B ). China ranked first (50, 21.65%), followed by the USA (41, 17.75%), UK (40, 17.32%), Japan (30, 12.99%), and Korea (12, 5.19%).
Figure 4.
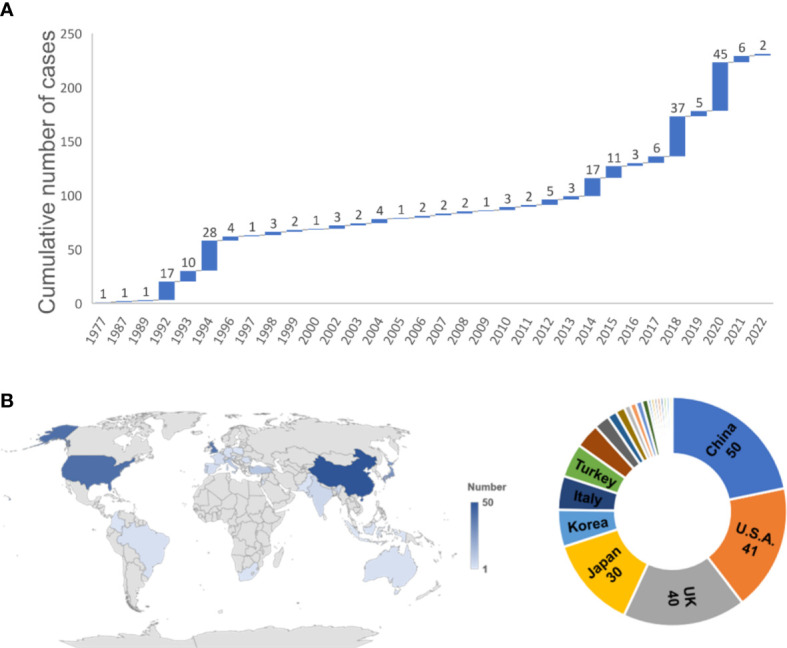
Temporal and spatial distribution of 231 TLC cases. (A) The annual and cumulative number of TLC cases shown in a waterfall chart from 1977 to 2022. (B) Regional distribution of the 231 TLC cases worldwide.
Demographic characteristics
The clinical demographic information of the 231 cases is summarized in Table 1 . A total of 121 cases were men, slightly more than 110 of women ( Table 1 ). The age ranged from 9 to 95 years, and there was no statistical difference between genders (t = 1.623, P = 0.1064). However, when grouped by every 20 years, the age distribution between genders was inconsistent (c2 = 11.255, P = 0.016). The age group with the highest proportion of men was 61–80 with 51 cases (53.68%), higher than that of the women (24 cases, 32.43%, P < 0.05). Meanwhile, the proportion of female patients over 80 years old was the highest (31 cases, 41.89%), which is statistically different from that of the male patients (19 cases, 20.00%, P < 0.05).
Table 1.
The demographic information of 231 TLC cases.
| Total (n = 231) | Male (n = 121) | Female (n = 110) | t/c2/Z-value | P-value | |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 65.70 ± 16.45 | 66.85 ± 16.05 | 71.18 ± 18.53 | 1.623 | 0.1064 |
| 0–20, mean (%) | 2 (1.18) | 1 (1.05) | 1 (1.35) | 11.255 | 0.016 |
| 21–40, mean (%) | 12 (7.10) | 7 (7.37) | 5 (6.76) | ||
| 41–60, mean (%) | 30 (17.75) | 17 (17.89) | 13 (17.57) | ||
| 61–80, mean (%) | 75 (44.38) | 51 (53.68) | 24 (32.43)* | ||
| 81–100, mean (%) | 50 (29.59) | 19 (20.00) | 31 (41.89)* | ||
| Location, Freq (%) | |||||
| Head and Neck | 194 (83.98) | 99 (81.82) | 95 (86.36) | 8.288 | 0.508 |
| Leg | 15 (6.49) | 7 (5.79) | 8 (7.27) | ||
| Chest | 6 (2.60) | 4 (3.31) | 2 (1.82) | ||
| Hand | 5 (2.16) | 4 (3.31) | 1 (0.91) | ||
| Arm | 2 (0.87) | 0 (0.00) | 2 (1.82) | ||
| Abdomen | 2 (0.87) | 1 (0.83) | 1 (0.91) | ||
| Shoulder | 2 (0.87) | 2 (1.65) | 0 (0.00) | ||
| Armpit | 2 (0.87) | 2 (1.65) | 0 (0.00) | ||
| Buttocks and perineum | 2 (0.87) | 1 (0.83) | 1 (0.91) | ||
| Back | 1 (0.43) | 1 (0.83) | 0 (0.00) | ||
| Diameter, cm, M (P25, P75) | 2.00 (1.00, 4.08) | 2.00 (1.00, 4.88) | 2.00 (1.28, 3.50) | 0.442 | 0.658 |
| Thickness, cm, M (P25, P75) | 2.25 (0.78,4.13) | 2.50 (2.00, 5.25) | 0.80 (0.50, 3.50) | 2.106 | 0.035 |
| Duration, months, M (P25, P75) | 13.0 (6.0, 36.0) | 12.0 (5.0, 25.5) | 24.0 (6.0, 42.0) | 1.525 | 0.127 |
| Regional lymph node metastasis, Freq (%) | 9 (3.90) | 5 (4.13) | 4 (3.64) | 0.000 | 1.000 |
| Carcinoma history, Freq (%) | 10 (4.33) | 4 (3.31) | 6 (5.45) | 0.228 | 0.633 |
| Recurrence, Freq (%) | 19 (8.23) | 12 (9.92) | 7 (6.36) | 0.964 | 0.326 |
| Follow-up, months, M (P25, P75) | 24.00 (12.00, 48.00) | 24.00 12.0, 40.75) | 24.00 (15.00, 60.00) | 1.207 | 0.228 |
Asterisks indicated a statistically significant difference in the ratio of male and female patients in that age group. M, Median.
Most common location was the head and neck (194 cases, 83.98%). The remaining cases were distributed on leg, chest, hand, arm, abdomen, shoulder, armpit, buttocks and perineum, and back. There was no statistical difference in the distribution between genders (c2 = 8.288, P = 0.508).
Clinical features
The phenotype of TLC was not always typical. In addition, TLC may be misdiagnosed or overlooked due to the co-occurrence of other diseases. Three cases presented with actinic keratosis simultaneously, two cases on ear, and one case on temple (4–6). The lesion on the abdomen of a 76-year-old woman was diagnosed as TLC arising in seborrheic keratosis (7). A case of TLC in facial sebaceous glands was accompanied by Syringocystadenoma papilliferum (8). There were also cases of TLC with primary cutaneous anaplastic large-cell lymphoma, a ductal eccrine component, nevus sebaceous, or a proliferating trichilemmal cyst (9–12). Some cases were combined with other carcinoma at the lesions, four cases with BCC (13, 14), two cases with Bowen’s disease (15, 16), and one case with Merkel cell carcinoma (17).
Manifestations of TLC ranged in diameter from 0.3 to 37 cm, and the thickness ranged from 0.30 to 10.00 cm. Diameter was not statistically different between genders (Z = 0.442, P = 0.658). On the contrary, male cases appear to have superiority in thickness (median, 2.50 cm) than that of female cases (median, 0.80 cm, Z = 2.106, P = 0.035). The duration had a median time of 13.0 months; 12.0 months in men and 24.0 months in women but with no statistical difference (Z = 1.525, P = 0.127).
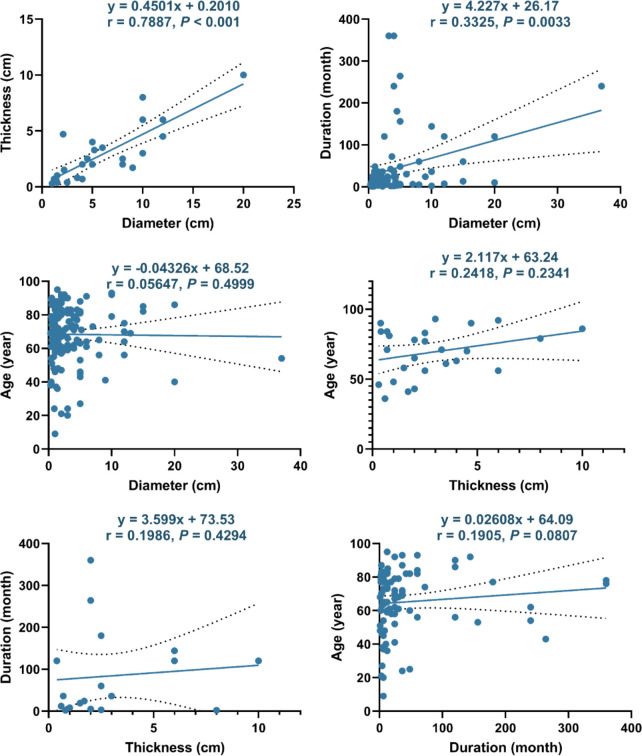
Furthermore, we explored factors that may be related to the size or duration of TLC ( Figure 5 ). The diameter of TLC had an obvious linear relationship with its thickness (r = 0.7887, P < 0.001) and a relatively weak linear relationship with the duration (r = 0.3325, P = 0.0033) but had no linear relationship with the age (r = 0.0565, P = 0.4999). In addition, no linear relationship was found between thickness with age (r = 0.2418, P = 0.2341), thickness with duration (r = 0.1986, P = 0.4294), or duration with age (r = 0.1905, P = 0.0807).
Figure 5.
Linear correlations between different factors of TLC. The dotted line represents the 95% confidence interval.
Another thing to note is that nine cases were accompanied by regional lymph node metastasis, with five men and four women.
Pathogenesis
The pathogenesis of TLC has not been clearly elucidated. A total of 87.01% of TLCs (201 of the 231 cases) occurred in the sun-exposed sites (head, neck, arm, and hand), 6.7 times of the non-exposed sites, suggesting the significant role of UV in the pathogenesis of TLC. Ten cases (4.33%) had previous carcinoma: four cases with breast cancer (including Case 2) (18–20), five cases with BCC (two of whom also had SCC) (13,) (21–24), and one case with both SCC and Bowen’s disease (25). There were four and six cases in men and women, respectively (c2 = 0.228, P = 0.633). Moreover, locally present skin lesions, trauma, or scarring may also be associated with the progression of TLC. Two cases were secondary to a previous site of actinic keratosis in neck and forehead, respectively (13, 25). TLC was also secondary to a previous proliferating trichilemmal (26), psoriasis (21), or cutaneous horn of the location (27). Local inappropriate physical or chemical stimulation may trigger the occurrence of TLC. A 36-year-old woman developed TLC after receiving laser treatment in the postauricular area (28). Prolonged topical use of hair removal cream on armpits also triggered TLC, with metastases to regional lymph nodes (29). In two cases, TLC was secondary to scars caused by burns 5 years or even 42 years ago (30). Ionizing radiation could induce carcinogenesis by inducing DNA damage and gene mutation. A 67-year-old man, who had been exposed to multiple x-rays (50–60 times in total) and CT of the chest, developed a 1.5-cm TLC with regional recurrence and distant metastasis after resection (31). However, it should be noted that the role of these factors in the occurrence and development of TLC may only be hypothesized now and remains to be further determined, given that the number of related cases was all small, which could very well be a random event.
Studies have shown that advanced age, Caucasian, male sex, and a chest organ transplant are the risk factors for skin carcinoma after solid organ transplantation (32). Here, a total of four cases from 39 to 63 years underwent transplantation [two kidney transplants (33, 34), one heart transplant (35), and one unknown (36)], all were men with three Caucasians and one Asian. Because immunosuppression may affect the phenotype of TLC, the true incidence of TLC secondary to solid organ transplantation may be higher (37).
Additional risk factors of TLC also include genetic disorders. As an autosomal recessive genetic skin disorder, patients with xeroderma pigmentosum (XP) have impaired DNA repair ability (38, 39). A 25-year-old man with XP presented with TLC in cheek and eyelid (40). Another 9-year-old girl with XP also suffered from TLC in the nose (41). Both cases were much younger than the average age, suggesting XP as a strong risk factor for TLC. The impaired DNA repair ability of XP was considered to be related to the changes of p53 to some extent (42). As a key anti-cancer protein with growth inhibitory function, p53 can regulate the cell cycle and promote cell apoptosis or senescence, thus inhibiting tumorigenesis (43). Studies have found that the total loss of chromosome arm 17p (where the TP53 gene resides) was found in a TLC case, leading to the blocked expression of p53, which may be associated with the marked increase of the proliferation fraction and thus contribute to the development of TLC (12).
Histopathological features
With the same tissue origin as trichilemmoma, TLC has similar histologic features but varies by atypical nuclei and high mitotic index (44). Centered on a pilosebaceous unit, TLC generally presents a typical histopathology of trichilemmal keratinization, a peripheral palisading pattern and focal necrosis (41, 45). The growth of TLC always presents lobular and invasive, characterized by classical large, polygonal, transparent tumor cells with eccentric nuclei but without hair follicle differentiation (41, 46). In some cases, TLC was found attached to the epidermis and infiltrating the hair follicle within the dermis, with the infiltrative lobules of clear cells where trichilemmal keratinization can be found (47).
In general, the pathological diagnostic criteria of TLC are not uniform and varied among different authors and doctors, but the following criteria for diagnosing are accepted and recognized by the majority: (1) PAS-positive glycogen within neoplastic cells, (2) folliculocentricity, (3) peripheral palisading of clear cells, (4) a prominent Periodic Acid Schiff Diastase (D-PAS)–positive basement membrane, (5) trichilemmal keratinization, (6) lobular architecture, and (7) the presence of pre-existing trichilemmoma (30, 48). TLC may be confused with other skin cancer, such as SCC with clear cell differentiation, BCC with keratin cysts and peripheral palisading cells within the basaloid islands, or even the keratoacanthoma, which was benign and could resolve spontaneously (27, 49–51). Given the similarity of cell origin and pathological phenotype, the accurate diagnosis of TLC from other tumors originated from the skin and the adnexa may sometimes be difficult and relies further on stains other than HE staining.
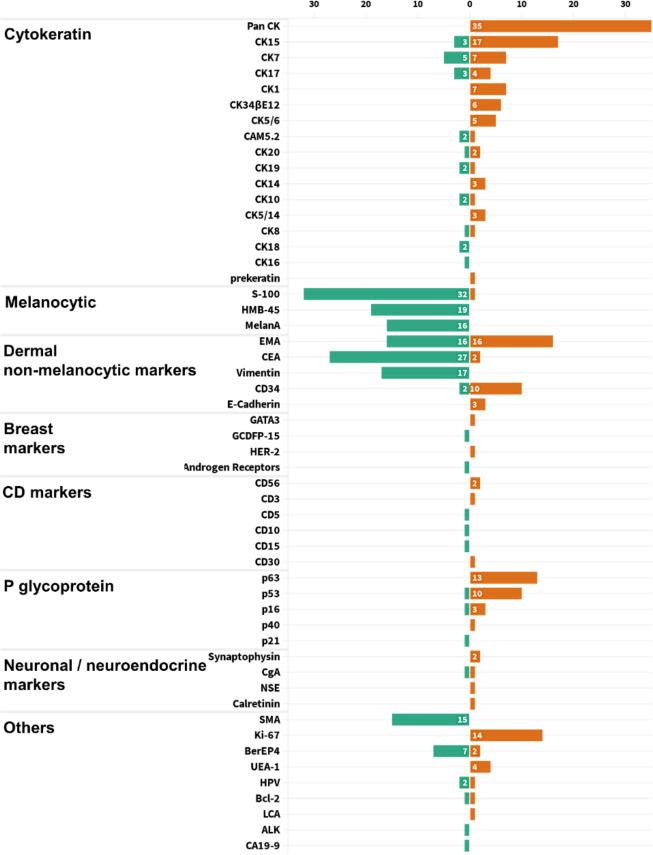
In general, stains were not performed unless the differential diagnosis from other mimickers was necessary. From the statistics of all published cases, PAS staining was used in 73 cases, all of which were positive. Mucicarmine staining was applied in 23 cases, and all samples were negative. All the other 53 pathological indicators were counted and sorted into eight categories, with an average frequency of 7.06 ( Figure 6 ). Using the frequency ≥ 7 as filter criterion, there were 17 remained indicators. Furthermore, the top six indicators of positive and negative rates, respectively, are listed in Figure 7 . It is worth noting that Pan CK ranked first with a frequency of 35 times, but its differential diagnostic value for skin tumors with clear cell changes was limited, because Pan CK was always positive in the mimickers, except for tumors arising from different lines of differentiation, such as melanoma, which, in turn, may not be difficult to diagnose due to its distinct appearances in HE staining (52).
Figure 6.
Statistical chart of pathological indicators used in all 231 TLC cases, divided into eight categories for display.
Figure 7.
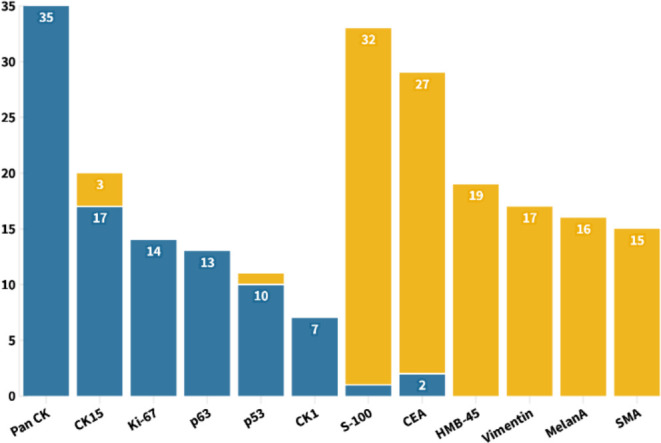
With the frequency no less than 7 as the screening criteria, the six pathological indicators with the highest positive and negative rates were shown for the diagnosis of TLC.
As a glycoprotein localized on the surface of cell membrane, CD34 is involved in the adhesion of cell–cell and cell–extracellular matrix (53). In addition to being used as a marker of hematopoietic stem cells, CD34 is also a clue to the differentiation from the outer root sheath and follicular, which is of great significance for the diagnosis of TLC (54–56). Here, a total of 12 cases of TLC were stained for CD34, of which 10 cases (83.33%) were positive. The staining of CEA in TLC was usually negative, and, of the 29 cases, only two cases were positive ( Figure 7 ). Moreover, EMA was widely adopted with a frequency up to 32 times, with half positive and half negative, suggesting that EMA may not be a suitable marker for identifying TLC ( Figure 6 ).
Treatment and prognosis
A total of 212 cases were treated by surgical excision, among which 16 cases (7.55%) experienced a recurrence. Mohs micrographic surgery (MMS) has been widely used in the resection of carcinoma especially in the head and face (57, 58). Sixteen cases of TLC were resected by MMS, of which two cases had tumor recurrence, namely, 77-year-old man and 65-year-old man both in cheek (23, 59). The recurrence rates of surgical excision and MMS were not statistically different (c2 = 0.052, P = 0.820). Another recurrence occurred in a 64-year-old man who refused treatment (60). Excision margins ranged from 1 to 20 mm, with no linear relationship with TLC diameter or thickness (r = 0.2852 and 0.3395, P = 0.1982 and 0.4028, Figure 8 ). As for MMS, there was only one documented resection margin, which was 8 mm.
Figure 8.
Linear relationship between the excision margin and the diameter or thickness of the TLC. The dotted line represents the 95% confidence interval.
Other interventions have also been reported. Imiquimod cream (5%) successfully healed a 90-year-old woman with a lesion of 2.5 × 2.0 × 0.39 cm on the cheek (61). Chemotherapy was used to treat a patient with TLC with primary cutaneous anaplastic large-cell lymphoma (9). Three cases underwent additional radiotherapy after excision (62, 63). Two cases received both chemotherapy and radiotherapy after excision. A 27-year-old man successfully relieved his 5-cm lesion on the neck (26). However, the lesion in 64-year-old man’s leg still recurred after treatment (58).
Nineteen cases were recorded with recurrence of TLC. We further analyzed the risk factors for TLC recurrence with cases divided into recurrence or non-recurrence groups ( Table 2 ). There was no statistically difference in sex, age, location, thickness, regional lymph node metastasis, or regional non-carcinoma lesions (P > 0.05). Diameters and duration were much higher in the recurrence group (Z = 2.711 and 2.073, P = 0.007 and 0.038), with carcinoma history more frequently occurred (c2 = 4.992, P = 0.025). Further logistic regression analysis revealed that carcinoma history was the independent risk factors for the recurrence of TLC, with odds ratio (OR) of 5.448 and 95% CI of 1.428–20.778 (P = 0.013, Table 3 ).
Table 2.
Statistical information on risk factors associated for TLC recurrence.
| Characteristics | Recurrence (n = 19) | Non-recurrence (n = 150) | t/c2/Z-value | P-value |
|---|---|---|---|---|
| Male, Freq (%) | 12 (63.16) | 83 (55.33) | 0.419 | 0.517 |
| Age, years, mean ± SD | 64.84 ± 17.95 | 69.24 ± 17.17 | 1.103 | 0.27 |
| 0–20, mean (%) | 20 (5.26) | 9(0.67) | 0.35 | 0.726 |
| 21–40, mean (%) | 35 (5.26) | 31.91 (7.33) | ||
| 41–60, mean (%) | 46 (10.53) | 53.82 (18.67) | ||
| 61–80, mean (%) | 62.75 (57.89) | 70.77 (42.67) | ||
| 81–100, mean (%) | 85.75 (21.05) | 86.74 (30.37) | ||
| Location, Freq (%) | 7.525 | 0.529 | ||
| Head and Neck | 13 (68.42) | 119 (79.33) | ||
| Leg | 3 (15.79) | 12 (8.00) | ||
| Chest | 2 (10.53) | 4 (2.67) | ||
| Hand | 1 (5.26) | 4 (2.67) | ||
| Arm | 0 (0.00) | 2 (1.33) | ||
| Abdomen | 0 (0.00) | 2 (1.33) | ||
| Shoulder | 0 (0.00) | 2 (1.33) | ||
| Armpit | 0 (0.00) | 2 (1.33) | ||
| Buttocks and perineum | 0 (0.00) | 2 (1.33) | ||
| Back | 0 (0.00) | 1 (0.67) | ||
| Diameter, cm, M (P25, P75) | 4.00 (1.88, 5.80) | 2.00 (1.00, 4.00) | 2.711 | 0.007 |
| Thickness, cm, M (P25, P75) | 3.40 (1.05, 3.88) | 2.00 (0.78, 4.55) | 0.285 | 0.776 |
| Duration, months, M (P25, P75) | 5 (3.5, 11.25) | 18 (6, 42) | 2.073 | 0.038 |
| Regional lymph node metastasis, Freq (%) | 1 (5.26) | 8 (5.33) | 0.000 | 1.000 |
| Carcinoma history, Freq (%) | 4 (21.05) | 7 (4.67) | 4.992 | 0.025 |
| Regional non-carcinoma lesions, Freq (%) | 4 (21.05) | 22 (14.67) | 0.152 | 0.697 |
M, Median.
Table 3.
Analysis of independent risk factors for TLC recurrence.
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Duration | 0.925 | 0.831–1.029 | 0.151 |
| Diameter | 1.048 | 0.960–1.144 | 0.295 |
| Carcinoma history | 5.448 | 1.428–20.778 | 0.013 |
Discussion
Starting from three clinical cases, we conducted a comprehensive statistical analysis of TLC, which will help us in the diagnosis and treatment of TLC. From the perspective of susceptible population, the majority of patients with TLC are men aged 60–80 and women over 80. The specific reason for this age distribution difference is unclear, probably owing to the inconsistent lifestyles of men and women, in that women generally pay more attention to sun protection. It is worth noting that patients as young as 9 years old also have the possibility of TLC, especially when they have genetic disorders, local trauma, or other skin lesions. Moreover, radiotherapy for previous tumors may lead to the occurrence of TLC. Given the progressive increase in the incidence of TLC recently, dermatologists should be alert to the possibility of TLC when encountering adnexal skin tumors, especially those located on head and neck.
TLC has diverse and, sometimes, confusing clinical manifestations and occasionally may not be correctly diagnosed (64). Partly because of the advanced age, some patients’ TLC was accompanied by a variety of other skin diseases from actinic keratosis to BCC, suggesting that doctors should fully consider all possibilities when making diagnosis. As a rare adnexal carcinoma, TLC was not generally considered as an aggressive tumor. However, the longer duration of TLC generally represents a larger diameter, which, in turn, represents a deeper infiltration and, in some cases, manifested as locally aggressive or metastasis to regional lymph nodes (51). In cases with longer course of disease, dermatologist should explore the draining lymph nodes, and the resection margins should be surely free of tumor cells and carefully observed for infiltration.
Nowadays, the differential diagnosis of TLC from other diseases has mainly relied on HE staining. PAS staining was also commonly employed. However, we cannot rely on PAS staining for making the diagnosis of TLC. It can also be positive in clear-cell SCC or clear-cell BCC because it highlights glycogen (65, 66). Special stains can be additionally suggestive clues when differential diagnosis is difficult.
Compared with TLC, clear-cell SCC lacks trichilemmal keratinization, lobular proliferation, peripheral palisading, and pushing border. Moreover, clear-cell BCC lacks keratinization of outer root sheath. Balloon cell melanoma was positive for S-100 and HMB-45 staining, and the melanin pigment was positive to the Masson-Fontana reaction. Hidradenocarcinoma has ductal differential with CEA and EMA staining. Sebaceous carcinoma may present with multiple intracytoplasmic lipid-rich vacuoles indenting the nucleus, with positive adipophilin. Malignant proliferating trichilemmal tumor is synonymous with proliferating trichilemmal cystic carcinoma and has cystic structure and intercellular bridge. Metastatic clear-cell adenocarcinomas of the viscera were generally renal-derived or ovarian-derived. Renal-derived clear-cell carcinoma was commonly positive for CD10, PAX8, and Vimentin (67). In addition, ovarian-derived clear-cell carcinoma was positive for HNF1-β and Napsin A (68, 69).
After statistical analysis of all cases, we propose that the positivity of Pan CK, CK15, Ki-67, p63, p53, and CK1 and the negativity of S-100, CEA, HMB-45, Vimentin, MelanA, and SMA may be constructive for the diagnosis of TLC. Furthermore, the positive expression of TLC on CK1, CK10, CK14, and CK17 indicates that TLC differentiated toward follicular infundibulum, which may have implications for expanding the use of follicular infundibulum–like cells as a diagnostic clue for TLC. Indicating the differentiation from the outer root sheath, CD34 is a valuable biomarker for the diagnosis of TLC with relatively a high positive rate. As for the expression of EMA in TLC, there is still no definite conclusion. EMA was considered to be positive in TLC in some dermatology textbooks, like Textbook of Dermatopathology by Prof. Raymond L. Barnhill. However, McKee’s Pathology of the Skin by Prof. Eduardo Calonje et al., China Clinical Dermatology by Prof. Bian Zhao, and Practical Dermatopathology by Prof. Tianwen Gao et al. hold the view that EMA is usually negative in TLC. On the basis of the statistical results of all published TLC cases, in total, the positive rate of EMA was 50% and thus may not be meaningful for TLC’s differential diagnosis (70). It should be noted that the pathological criteria for diagnosing TLC varied among different authors. Meanwhile, given the similar cellular origin and pathological phenotype between TLC and other skin tumors like SCC, some dermatologists and pathologists also hold the view that TLC may be clear-cell SCC, a subtype of SCC but not a special disease (66, 71). In addition, some doctors concluded that the tumor is very rare if it exists at all and may be over diagnosed by its adherents. Further conclusions urgently need more and larger pathological studies to summarize the unique pathological characteristics of TLC different from other skin tumors.
As most cases reported, TLC was managed with complete surgical resection (72). MMS has been proven to be an effective treatment for TLC, but it is still necessary to ensure that the surgical margins are free of tumor cells for it is not foolproof. For nodules larger than 5 cm in diameter, radiotherapy or chemotherapy can also be considered. For patients with extra carcinoma history, special care should be taken during excision and follow-up to avoid recurrence. We recommend patients to have follow-up between 6 and 12 months. During the period, patients should be advised to pay attention to sun protection and return for further consultation with new lesions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Fourth Medical Center of PLA general hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YY conceptualized the study and designed the experiments. JS and LZ wrote the manuscript. JS, MX, SL, and RC collected and analyzed the data. YL carried out the pathological staining required for article revision.
Acknowledgments
We are grateful to the patients for participation in this study.
Abbreviations
TLC, trichilemmal carcinoma; BCC, basal cell carcinoma; SCC, squamous cell carcinoma; HE, hematoxylin-eosin; PAS, periodic acid–Schiff; MRI, magnetic resonance imaging; CT, computed tomography; XP, xeroderma pigmentosum; MMS, Mohs micrographic surgery.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Headington JT. Tumors of the hair follicle. A review Am J Pathol (1976) 85(2):479–514. [PMC free article] [PubMed] [Google Scholar]
- 2. Headington JT. Tricholemmal carcinoma. J Cutan Pathol (1992) 19(2):83–4. doi: 10.1111/j.1600-0560.1992.tb01347.x [DOI] [PubMed] [Google Scholar]
- 3. Jia Q, Yuan Y, Mao D, Wen G, Chen X. Trichilemmal carcinoma of the scalp in a young female: A case report. Clinical cosmetic investigational Dermatol (2022) 15:139–43. doi: 10.2147/CCID.S349797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhargava S, Feliciani C, Longo R, Dominici MM. Two-stage ear reconstruction with a retroauricular skin flap after excision of trichilemmal carcinoma. J cutaneous aesthetic Surg (2020) 13(2):149–51. doi: 10.4103/JCAS.JCAS11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Billingsley EM, Davidowski TA, Maloney ME. Trichilemmal carcinoma. J Am Acad Dermatol (1997) 36(1):107–9. doi: 10.1016/S0190-9622(97)70339-6 [DOI] [PubMed] [Google Scholar]
- 6. Misago N, Tanaka T, Kohda H. Trichilemmal carcinoma occurring in a lesion of solar keratosis. J Dermatol (1993) 20(6):358–64. doi: 10.1111/j.1346-8138.1993.tb01298.x [DOI] [PubMed] [Google Scholar]
- 7. Oyama N, Kaneko F. Trichilemmal carcinoma arising in seborrheic keratosis: a case report and published work review. J Dermatol (2008) 35(12):782–5. doi: 10.1111/j.1346-8138.2008.00569.x [DOI] [PubMed] [Google Scholar]
- 8. Chun SH, Kim BY, Park JH, Kim IH, Ryu HJ. Simultaneous presentation of trichilemmal carcinoma and syringocystadenoma papilliferum within a nevus sebaceous. Ann Dermatol (2018) 30(3):368–70. doi: 10.5021/ad.2018.30.3.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abed K, Stopa Z, Siewert-Gutowska M. Primary cutaneous anaplastic large-cell lymphoma: A case report. Medicine (2018) 97(4):e9645. doi: 10.1097/MD.0000000000009645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basak K, Basak PY. In situ carcinoma in a hybrid cyst: a case report. J Cutan Pathol (2017) 44(2):189–92. doi: 10.1111/cup.12852 [DOI] [PubMed] [Google Scholar]
- 11. Jardim MML, Souza BCE, Fraga RC, Fraga RC. Rare desmoplastic trichilemmoma associated with sebaceous nevus. Bras Dermatol (2017) 92(6):836–7. doi: 10.1590/abd1806-4841.20176540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takata M, Rehman I, Rees JL. A trichilemmal carcinoma arising from a proliferating trichilemmal cyst: the loss of the wild-type p53 is a critical event in malignant transformation. Hum Pathol (1998) 29(2):193–5. doi: 10.1016/S0046-8177(98)90234-9 [DOI] [PubMed] [Google Scholar]
- 13. Fronek L, Brahs A, Farsi M, Miller R. A rare case of trichilemmal carcinoma: Histology and management. J Clin Aesthet Dermatol (2021) 14(6):25–30. [PMC free article] [PubMed] [Google Scholar]
- 14. Putri CA, Mudhar HS, Meeney A, Tan JHY. Eyelid skin trichilemmoma and underlying local malignancy: is an aggressive treatment necessary? BMJ Open Ophthalmol (2020) 5(1):e000513. doi: 10.1136/bmjophth-2020-000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamiya H, Kitajima Y, Ban M. Bowen's disease with invasive adnexal carcinoma: the pluripotential nature of bowen's disease cells. J Dermatol (2006) 33(12):858–64. doi: 10.1111/j.1346-8138.2006.00196.x [DOI] [PubMed] [Google Scholar]
- 16. Misago N, Toda S, Nakao T. Focus of tricholemmal differentiation (tricholemmal carcinoma) within bowen's disease/carcinoma. J Dermatol (2016) 43(4):439–42. doi: 10.1111/1346-8138.13098 [DOI] [PubMed] [Google Scholar]
- 17. Gaitskell K, Nassar S, Ibrahim H. Merkel cell carcinoma with divergent differentiation. Clin Exp Dermatol (2020) 45(3):327–32. doi: 10.1111/ced.14110 [DOI] [PubMed] [Google Scholar]
- 18. Sofianos C, Chauke NY, Grubnik A. Metastatic trichilemmal carcinoma in a patient with breast cancer. BMJ Case Rep (2016) 2016:bcr2016217661. doi: 10.1136/bcr-2016-217661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laochumroonvorapong P, Kokta V, Quan MB. Trichilemmal carcinoma in an African American. Dermatologic Surg (2002) 28(3):284–6. doi: 10.1046/j.1524-4725.2002.01128.x [DOI] [PubMed] [Google Scholar]
- 20. Dailey JR, Helm KF, Goldberg SH. Tricholemmal carcinoma of the eyelid. Am J Ophthalmol (1993) 115(1):118–9. doi: 10.1016/S0002-9394(14)73540-8 [DOI] [PubMed] [Google Scholar]
- 21. Zattra E, Guarda Nardini L, Salmaso R, Piaserico S. Trichilemmal carcinoma of the lower eyelid in a oculocutaneous albino patient. Giornale italiano di dermatologia e venereologia (2015) 150(1):131–3. [PubMed] [Google Scholar]
- 22. Roismann M, Freitas RR, Ribeiro LC, Montenegro MF, Biasi LJ, Jung JE. Trichilemmal carcinoma: case report. Bras Dermatol (2011) 86(5):991–4. doi: 10.1590/S0365-05962011000500019 [DOI] [PubMed] [Google Scholar]
- 23. Garrett AB, Azmi FH, Ogburia KS. Trichilemmal carcinoma: a rare cutaneous malignancy: a report of two cases. Dermatologic Surg (2004) 30(1):113–5. doi: 10.1111/j.1524-4725.2004.30019.x [DOI] [PubMed] [Google Scholar]
- 24. Swanson PE, Marrogi AJ, Williams DJ, Cherwitz DL, Wick MR. Tricholemmal carcinoma: clinicopathologic study of 10 cases. J Cutan Pathol (1992) 19(2):100–9. doi: 10.1111/j.1600-0560.1992.tb01350.x [DOI] [PubMed] [Google Scholar]
- 25. Cribier B, Lipsker D, Grosshans E. Eccrine porocarcinoma, tricholemmal carcinoma and multiple squamous cell carcinomas in a single patient. Eur J Dermatol EJD (1999) 9(6):483–6. [PubMed] [Google Scholar]
- 26. Kim UG, Kook DB, Kim TH, Kim CH. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch craniofacial Surg (2017) 18(1):50–3. doi: 10.7181/acfs.2017.18.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Wang L, Gao T, Lian S. Recurrent trichilemmal carcinoma with a large cutaneous horn formation. Ann Saudi Med (2012) 32(6):1–2. doi: 10.5144/0256-4947.2012.01.7.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ha JH, Lee C, Lee KS, Pak CS, Sun CH, Koh Y, et al. The molecular pathogenesis of trichilemmal carcinoma. BMC Cancer (2020) 20(1):516. doi: 10.1186/s12885-020-07009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dboush HG, Al-Doud MA, Shannaq RY, Abudarweesh IS, Jabali EH, Alabbadi AS. Trichilemmal carcinoma of the axilla with regional lymph nodes metastasis: A case report. Int J Surg Case Rep (2021) 81:105760. doi: 10.1016/j.ijscr.2021.105760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko T, Tada H, Hatoko M, Muramatsu T, Shirai T. Trichilemmal carcinoma developing in a burn scar: a report of two cases. J Dermatol (1996) 23(7):463–8. [PubMed] [Google Scholar]
- 31. Chan KO, Lim IJ, Baladas HG, Tan WT. Multiple tumour presentation of trichilemmal carcinoma. Br J Plast Surg (1999) 52(8):665–7. doi: 10.1054/bjps.1999.3180 [DOI] [PubMed] [Google Scholar]
- 32. Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the united states. JAMA Dermatol (2017) 153(3):296–303. doi: 10.1001/jamadermatol.2016.4920 [DOI] [PubMed] [Google Scholar]
- 33. Hiramatsu K, Sasaki K, Matsuda M, Hashimoto M, Eguchi T, Tomikawa S, et al. A case of trichilemmal carcinoma with distant metastases in a kidney transplantation patient. Transplant Proc (2015) 47(1):155–7. doi: 10.1016/j.transproceed.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 34. Garrett AB, Scott KA. Trichilemmal carcinoma: a case report of a rare skin cancer occurring in a renal transplant patient. Transplantation (2003) 76(7):1131. doi: 10.1097/01.TP.0000074317.77586.BA [DOI] [PubMed] [Google Scholar]
- 35. Kanitakis J, Euvrard S, Sebbag L, Claudy A. Trichilemmal carcinoma of the skin mimicking a keloid in a heart transplant recipient. J Heart Lung Transplant (2007) 26(6):649–51. doi: 10.1016/j.healun.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 36. Wong TY, Suster S. Tricholemmal carcinoma. a clinicopathologic study of 13 cases. Am J dermatopathol (1994) 16(5):463–73. doi: 10.1097/00000372-199410000-00001 [DOI] [PubMed] [Google Scholar]
- 37. Berman H, Shimshak S, Reimer D, Brigham T, Hedges MS, Degesys C, et al. Skin cancer in solid organ transplant recipients: A review for the nondermatologist. Mayo Clin Proc (2022) 97(12):2355–68. doi: 10.1016/j.mayocp.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 38. Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science (2018) 359(6375):555–9. doi: 10.1126/science.aao4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krakowski AC, Hafeez F, Westheim A, Pan EY, Wilson M. Advanced basal cell carcinoma: What dermatologists need to know about diagnosis. J Am Acad Dermatol (2022) 86(6S):S1–S13. doi: 10.1016/j.jaad.2022.03.023 [DOI] [PubMed] [Google Scholar]
- 40. Mane DR, Kale AD, Hallikerimath S, Angadi P, Kotrashetti V. Trichilemmal carcinoma associated with xeroderma pigmentosa: report of a rare case. J Oral Sci (2010) 52(3):505–7. doi: 10.2334/josnusd.52.505 [DOI] [PubMed] [Google Scholar]
- 41. Reis JP, Tellechea O, Cunha MF, Baptista AP. Trichilemmal carcinoma: review of 8 cases. J Cutan Pathol (1993) 20(1):44–9. doi: 10.1111/j.1600-0560.1993.tb01248.x [DOI] [PubMed] [Google Scholar]
- 42. Hastak K, Adimoolam S, Trinklein ND, Myers RM, Ford JM. Identification of a functional In vivo p53 response element in the coding sequence of the xeroderma pigmentosum group c gene. Genes Cancer (2012) 3(2):131–40. doi: 10.1177/1947601912456288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer (2018) 18(2):89–102. doi: 10.1038/nrc.2017.109 [DOI] [PubMed] [Google Scholar]
- 44. Chai MK, Tenzel P, Iacob C, Jordan A, Reddy HS. Eyelid trichilemmal carcinoma. Saudi J Ophthalmol (2017) 31(3):183–5. doi: 10.1016/j.sjopt.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee JH, Shin YW, Oh YH, Lee YJ. Trichilemmal carcinoma of the upper eyelid: a case report. Korean J Ophthalmol (2009) 23(4):301–5. doi: 10.3341/kjo.2009.23.4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yi HS, Sym SJ, Park J, Cho EK, Ha SY, Shin DB, et al. Recurrent and metastatic trichilemmal carcinoma of the skin over the thigh: a case report. Cancer Res Treat (2010) 42(3):176–9. doi: 10.4143/crt.2010.42.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang L, Lin Z, Wu H, Ou S. Corneal perforation caused by eyelid margin trichilemmal carcinoma: A case report and review of literature. Front Med (2022) 9:896393. doi: 10.3389/fmed.2022.896393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Short E, O'Shea A, Mukkanna K, Patel G, Docjinov S, May K. Case report: A rapidly growing cyst on the scalp. F1000Res (2019) 8:779. doi: 10.12688/f1000research.19157.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Onishi M, Takahashi K, Maeda F, Akasaka T. A case of basal cell carcinoma with outer hair follicle sheath differentiation. Case Rep Dermatol (2015) 7(3):352–7. doi: 10.1159/000442704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rongioletti F, Ferreli C, Atzori L, Smoller BR, Faa G, Kutzner H, et al. A previously undescribed variant of cutaneous clear-cell squamous cell carcinoma with psammomatous calcification and intratumoral giant cell granulomas. J Cutan Pathol (2021) 48(1):106–9. doi: 10.1111/cup.13847 [DOI] [PubMed] [Google Scholar]
- 51. Maya-Rico AM, Jaramillo-Pulgarín C, Londoño-García Á, Peña-Zúñiga B. Locally aggressive trichilemmal carcinoma. Bras Dermatol (2018) 93(4):579–81. doi: 10.1590/abd1806-4841.20187461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeh I, Bastian BC. Melanoma pathology: new approaches and classification. Br J Dermatol (2021) 185(2):282–93. doi: 10.1111/bjd.20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee JS, Park HS, Yoon HS, Chung JH, Cho S. CD34 stromal expression is inversely proportional to smooth muscle actin expression and extent of morphea. J Eur Acad Dermatol Venereol (2018) 32(12):2208–16. doi: 10.1111/jdv.15120 [DOI] [PubMed] [Google Scholar]
- 54. Pozo L, Diaz-Cano SJ. Trichilemmal carcinoma with neuroendocrine differentiation. Clin Exp Dermatol (2008) 33(2):128–31. doi: 10.1111/j.1365-2230.2007.02570.x [DOI] [PubMed] [Google Scholar]
- 55. Cardoso JC, Alves F, Carreira IM, Tellechea O. Basal cell carcinomas after radiotherapy show more frequent follicular differentiation than tumors from sun-exposed areas: Immunohistochemical study with a special focus on infundibulocystic basal cell carcinoma. Am J dermatopathol (2022) 44(12):879–85. doi: 10.1097/DAD.0000000000002321 [DOI] [PubMed] [Google Scholar]
- 56. Gulati HK, Deshmukh SD, Anand M, Morale V, Pande DP, Jadhav SE. Low-grade malignant proliferating pilar tumor simulating a squamous-cell carcinoma in an elderly female: a case report and immunohistochemical study. Int J Trichol (2011) 3(2):98–101. doi: 10.4103/0974-7753.90818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drucker AM, Adam GP, Rofeberg V, Gazula A, Smith B, Moustafa F, et al. Treatments of primary basal cell carcinoma of the skin: A systematic review and network meta-analysis. Ann Intern Med (2018) 169(7):456–66. doi: 10.7326/M18-0678 [DOI] [PubMed] [Google Scholar]
- 58. Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ (2013) 347:f6153. doi: 10.1136/bmj.f6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Allee JE, Cotsarelis G, Solky B, Cook JL. Multiply recurrent trichilemmal carcinoma with perineural invasion and cytokeratin 17 positivity. Dermatologic Surg (2003) 29(8):886–9. doi: 10.1046/j.1524-4725.2003.29241.x [DOI] [PubMed] [Google Scholar]
- 60. Xie Y, Wang L, Wang T. Huge trichilemmal carcinoma with metastasis presenting with two distinct histological morphologies: A case report. Front Oncol (2021) 11:681197. doi: 10.3389/fonc.2021.681197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jo JH, Ko HC, Jang HS, Kim MB, Oh CK, Kwon KS. Infiltrative trichilemmal carcinoma treated with 5% imiquimod cream. Dermatologic Surg (2005) 31(8 Pt 1):973–6. doi: 10.1097/00042728-200508000-00016 [DOI] [PubMed] [Google Scholar]
- 62. Agarwal A, Agarwal P, Anand A, Sagar M, Bhalla S, Agrawal M, et al. Nodular hidradenocarcinoma, trichelemmal carcinoma and squamous cell carcinoma with clear cell changes: Pitfalls of biopsy diagnosis of skin and adnexal tumours. Clin Pathol (Thousand Oaks Ventura County Calif) (2021) 14:2632010X211033840. doi: 10.1177/2632010X211033840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feng Z, Zhu HG, Wang LZ, Zheng JW, Chen WT, Zhang Z, et al. Tricholemmal carcinoma of the head and neck region: A report of 15 cases. Oncol Lett (2014) 7(2):423–6. doi: 10.3892/ol.2013.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kannan K, Mahesh KC, Manickam N, Ramdas A. Trichilemmal carcinoma of scalp masquerading as squamous cell carcinoma: A report of a rare case with histogenesis and literature review. Int J Trichol (2020) 12(2):82–5. doi: 10.4103/ijt.ijt_12_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carr RA, Taibjee SM, Sanders DSA. Basaloid skin tumours: Basal cell carcinoma. Curr Diagn Pathol (2007) 13(4):252–72. doi: 10.1016/j.cdip.2007.05.005 [DOI] [Google Scholar]
- 66. Dalton MAJSR, LeBoit PE. Squamous cell carcinoma with clear cells: How often is there evidence of tricholemmal differentiation? Am J dermatopathol (2008) 30(4):333–9. doi: 10.1097/DAD.0b013e31816c3fa4 [DOI] [PubMed] [Google Scholar]
- 67. Farcaş M, Gatalica Z, Trpkov K, Swensen J, Zhou M, Alaghehbandan R, et al. Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: next-generation sequencing multi-institutional study of 19 cases. Mod Pathol (2022) 35(3):344–51. doi: 10.1038/s41379-021-00923-6 [DOI] [PubMed] [Google Scholar]
- 68. Yamashita Y, Nagasaka T, Naiki-Ito A, Sato S, Suzuki S, Toyokuni S, et al. Napsin a is a specific marker for ovarian clear cell adenocarcinoma. Modern Pathol (2015) 28(1):111–7. doi: 10.1038/modpathol.2014.61 [DOI] [PubMed] [Google Scholar]
- 69. Chandra S, Srinivasan S, Batra J. Hepatocyte nuclear factor 1 beta: A perspective in cancer. Cancer Med (2021) 10(5):1791–804. doi: 10.1002/cam4.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ricci C, Dika E, Di Nanni DD, Zannetti G, Lambertini M, Corti B. Could EMA and cytokeratin 7 be useful in distinguishing tricholemmal carcinoma from clear-cell squamous cell carcinoma? a case series from our department and a brief review of the literature. Acta histochemica (2019) 121(6):765–7. doi: 10.1016/j.acthis.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 71. Kuo T. Clear cell carcinoma of the skin. a variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am J Surg Pathol (1980) 4(6):573–83. doi: 10.1097/00000478-198012000-00008 [DOI] [PubMed] [Google Scholar]
- 72. Xu B, Wang T, Liao Z. Surgical treatment of trichilemmal carcinoma. World J Oncol (2018) 9(5-6):141–4. doi: 10.14740/wjon1143w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.