Abstract
Purpose:
Characterizing and comparing speech recognition development in children with cochlear implants (CIs) is challenging because of variations in test type. This retrospective cohort study modified the Pediatric Ranked Order Speech Perception (PROSPER) scoring system to (a) longitudinally analyze the speech perception of children with CIs and (b) examine the role of age at CI activation, listening mode (i.e., unilateral or bilateral implantation), and interimplant interval.
Method:
Postimplantation speech recognition scores from 31 children with prelingual, severe-to-profound hearing loss who received CIs were analyzed (12 with unilateral CI [UniCI], 13 with sequential bilateral CIs [SEQ BiCIs], and six with simultaneous BiCIs). Data were extracted from the Massachusetts Eye and Ear Audiology database. A version of the PROSPER score was modified to integrate the varying test types by mapping raw scores from different tests into a single score. The PROSPER scores were used to construct speech recognition growth curves of the implanted ears, which were characterized by the slope of the growth phase, the time from activation to the plateau onset, and the score at the plateau.
Results:
While speech recognition improved considerably for children following implantation, the growth rates and scores at the plateau were highly variable. In first implanted ears, later implantation was associated with poorer scores at the plateau (β = −0.15, p = .01), but not growth rate. The first implanted ears of children with BiCIs had better scores at the plateau than those with UniCI (β = 0.59, p = .02). Shorter interimplant intervals in children with SEQ BiCIs promoted faster speech recognition growth of the first implanted ears.
Conclusion:
The modified PROSPER score could be used clinically to track speech recognition development in children with CIs, to assess influencing factors, and to assist in developing and evaluating patient-specific intervention strategies.
Supplemental Material:
As the most successful neural prostheses to date, cochlear implants (CIs) provide critical access to speech to children with severe-to-profound hearing loss who otherwise derive little benefit from acoustic hearing aids (HAs). Hearing the speech signal through a CI increases the chances of developing good speech perception abilities in pediatric CI listeners (e.g., Colletti et al., 2012; Dettman et al., 2016; Geers & Nicholas, 2013; Svirsky et al., 2000; Zeng, 2017). Despite successful development of speech perception in many pediatric recipients of CIs, performance varies widely among children and it is unclear who will have good or poor outcomes (e.g., Davidson et al., 2011; Dunn et al., 2014; Tomblin et al., 2005). Prior studies have either focused on final long-term speech recognition outcomes and/or speech recognition at a fixed time postimplantation or between groups of children with different age at implantation (e.g., Davidson et al., 2011; Dowell, 2002; Dunn et al., 2014; Manrique et al., 2004; Ruffin et al., 2013). A major challenge in characterizing the development of speech recognition skills in children with CIs is that the test type changes based on the age, vocabulary levels, and speech perception abilities of each child, making it difficult to compare scores across individuals (Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Trimble et al., 2008). In this study, we modified a scoring chart, the Pediatric Ranked Order Speech Perception (PROSPER), that integrates all available scores from different test types administered in our center at the Massachusetts Eye and Ear (MEE) Audiology clinic into one score, allowing us to longitudinally track speech recognition outcomes (Eskander et al., 2011; Trimble et al., 2008; Yeung et al., 2018). The PROSPER score was used in prior studies to examine speech recognition of children with CIs pre- and postimplantation/re-implantation at one time point (Eskander et al., 2011; Trimble et al., 2008; Yeung et al., 2018). We use this scoring chart to better understand how the trajectory of speech recognition development varies among children with prelingual, severe-to-profound hearing loss who underwent cochlear implantation and to examine how age at CI activation, listening mode (unilateral or bilateral CI), and interimplant interval may influence children's speech recognition development.
Longitudinal assessment of speech recognition development in children with CIs in early childhood following implantation is challenging due to rapid changes in auditory abilities with age, wide ranges of hearing and language skills, or a combination of these factors. In response to these methodological challenges, several studies have focused on developing a speech recognition hierarchy by integrating different test types based on the child's age and hearing ability, to construct standardized and uniform test batteries (e.g., Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Wang et al., 2008). The first longitudinal multicenter studies to investigate various outcomes following cochlear implantation are the Childhood Development after Cochlear Implantation (CDaCI) studies (e.g., Eisenberg, Johnson, et al., 2006). In those prospective studies, scores were measured at specific intervals aimed to comprehensively assess factors that influence spoken language; speech recognition; cognitive; behavioral, and psychosocial performance; and quality of life in young children with CIs (e.g., Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Wang et al., 2008). These studies defined an integrated hierarchical test battery for their set of speech perception tests to track children's speech and language development. Wang et al. (2008) developed a speech recognition in quiet index that integrated speech recognition scores collected through a series of hierarchical tests used to track speech recognition development over 24 months following implantation. They showed that speech recognition growth was largely variable among children with CIs over that time. A small group of children with relatively later ages at implantation showed slower postimplantation trajectories.
Building on the CDaCI studies, the Pediatric Minimum Speech Test Battery (PMSTB) working group conducted a review on a variety of speech perception measures and developed a protocol for clinical use after incorporating new speech materials such as pediatric AzBio (Uhler et al., 2017). The development of this protocol was motivated by a need for a standardized and comprehensive test battery to facilitate tracking children's performance. Together, these works demonstrated that such speech recognition hierarchies can be used to track and describe the auditory abilities of children with CIs. Results have been used to compare speech recognition development between children with CIs and their normal-hearing peers. The present work further builds on the existing framework from these works (Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Niparko et al., 2010; Wang et al., 2008; the PMSTB, Uhler et al., 2011; 2017; the Longitudinal Outcomes of Children with Hearing Impairment, Ching et al., 2013; and PROSPER scoring chart, Eskander et al., 2011; Trimble et al., 2008; Yeung et al., 2018). We developed and modified the PROSPER scoring system according to the hierarchy of clinical tests used consistently in our clinic and data set at the MEE Audiology. This study demonstrates an approach that could be employed at any clinic that uses a standard protocol.
Individual Differences in Speech Recognition Development
Prior studies based on grouping children for age at implantation or school age have shown that speech recognition typically improves over time in children with CIs (e.g., Davidson et al., 2011; Dunn et al., 2014; Holt & Svirsky, 2008; Uhler et al., 2011). However, this improvement varied widely among individual children (e.g., Arjmandi et al., 2021; Bø Wie et al., 2007; Davidson et al., 2011; Dilley et al., 2020; Dowell, 2002; Dunn et al., 2014; Eisenberg, Johnson, et al., 2006; Geers et al., 2003, 2011; Geers & Moog, 1987; Niparko et al., 2010; Pisoni et al., 2018; Waltzman et al., 2002; Wie et al., 2020). For example, Davidson et al. (2011) found that word recognition scores ranged from 0% to 90% for pediatric CI recipients in elementary school (8–9 years of age) and those in high school (15–18 years of age). While prior studies have shown how speech recognition varies among individual pediatric CI listeners at different times postimplantation, or in the long term (e.g., Davidson et al., 2011; Dowell, 2002; Dunn et al., 2014; Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Geers et al., 2011; Manrique et al., 2004), studies have yet to quantify the developmental trajectory of speech recognition in a consistent manner by integrating different test types and time points. Prior studies have also been limited by selecting a specific subset of tests to longitudinally track the performance of the children in their cohort (e.g., Dunn et al., 2014; Wie et al., 2020). These limitations are mainly due to the major challenge of quantifying performance longitudinally as children develop and require a change in test type and test materials (Eisenberg, Fink, & Niparko, 2006; Geers & Moog, 1987; Trimble et al., 2008; Uhler et al., 2017; Yeung et al., 2018). To address this challenge, a scoring chart can be developed to integrate scores from different test types into one summary score (e.g., Trimble et al., 2008; Wang et al., 2008).
Age at CI Activation and Speech Recognition Development
Several factors related to the implanted CI device (e.g., the speech processing strategy and the electrode–neuron interface; e.g., DiNino et al., 2019; Fryauf-Bertschy et al., 1997), interventions (e.g., communication mode, age at implantation; Geers et al., 2003; Holt & Svirsky, 2008), and children's personal characteristics (e.g., verbal and nonverbal IQ, cognitive skills; Geers et al., 2008; Pisoni, 2012) have been investigated to understand how they may influence speech recognition scores in children with CIs. Early age at CI activation is a factor that has been frequently reported as a significant contributor to the variability in outcomes among children (e.g., Fryauf-Bertschy et al., 1997; Holt & Svirsky, 2008; Kirk et al., 2002; Leigh et al., 2013; Niparko et al., 2010; Sharma et al., 2005; Tobey et al., 2013). In addition, among different audiologic factors such as onset of hearing loss, pre-implant residual hearing, and degree of hearing loss, age at CI can be potentially intervened (earlier rather late implantation) to improve speech and language outcomes. While some studies found a positive effect of earlier implantation on word recognition scores and the rate of speech recognition development for a group of children (e.g., Bø Wie et al., 2007; Wang et al., 2008), some reported no effect of age at implantation on the rate of the word recognition development (Holt & Svirsky, 2008). In those studies, the rate of word recognition development was defined as the amount of change in speech recognition scores using a single test over a fixed period after implantation. Dunn et al. (2014) found that by the time children were 11 and 13 years old, the positive impact of earlier implantation on speech perception had diminished such that there was no significant difference between the younger implanted (less than 2 years of age) and the older implanted group (between 2 and 3.8 years of age). However, a delay in speech recognition development in early years after implantation may lead to negative consequences of delayed spoken language acquisition (Eisenberg, Johnson, et al., 2006; Houston et al., 2020) and other developmental abilities such as social (e.g., parent–child communication, engagement, self-control) and cognitive abilities (e.g., information processing speed, attention, problem-solving, and short- and long-term memory retrieval; Eisenberg, Fink, & Niparko, 2006; Fink et al., 2007; McLaughlin, 2011; Silva et al., 1983, 1987; Uhler et al., 2017). Most previous studies grouped children based on their age at CI activation to examine its effect on speech recognition development mainly because of the challenges of using different test types and materials. Further investigation using a scoring system that integrates all available scores from different tests into a single score may provide better understanding of the effect of age at CI activation on the trajectory of speech recognition development in individual children with CIs.
Listening Mode (Unilateral or Bilateral CIs) and Speech Recognition Development
Speech recognition development is also influenced by the listening mode, whether a child received a unilateral or bilateral (sequential or simultaneous) CI(s). This is particularly important as more children are receiving bilateral CIs (BiCIs; e.g., Peters et al., 2010). Bilateral cochlear implantation has been shown to facilitate speech development (e.g., Boons et al., 2012; De Raeve et al., 2015; Jacobs et al., 2016; Lammers et al., 2014; Leigh et al., 2013; Sarant et al., 2014; 2016), to facilitate the development of binaural hearing skills such as segregation of speech from competing sounds (e.g., Galvin et al., 2007; Litovsky et al., 2006), and sound source localization (e.g., Gordon, Wong, & Papsin, 2013; Grieco-Calub & Litovsky, 2011; Litovsky et al., 2006; Zheng et al., 2015). These abilities are particularly important for developing speech recognition, as children need to understand speech and learn language in daily, challenging listening environments such as school and childcare facilities (Busch et al., 2017; Manlove et al., 2001; Shield et al., 2015). Furthermore, there is evidence that listeners with BiCIs experience less listening effort compared to those who receive unilateral CIs (e.g., Hughes & Galvin, 2013; Schnabl et al., 2021). Evidence suggests that children with BiCIs also develop better and faster expressive and receptive language skills (Boons et al., 2012; Sarant et al., 2014), a trend moderated by age at CI activation in the BiCIs group (Sarant et al., 2014).
Interestingly, bimodal listening with a CI in one ear and HA in the other could be more beneficial than BiCIs for some spoken language skills depending on the level of residual hearing on the nonimplanted ear (Davidson et al., 2019). Investigation of cortical responses to bimodal stimulation, as measured by electroencephalography, showed that balanced input during early auditory development can prevent preference for hearing through better ear and enhance bimodal hearing (Polonenko et al., 2018), suggesting that bilateral implantation and earlier implantation of the poorer ear in children with asymmetric hearing loss can help with symmetric auditory cortical processing. Studies have also showed that simultaneous bilateral CIs (SIM BiCIs) further enhanced speech perception by improving the perception of sound patterns and better development of peripheral and central auditory pathways compared to unilateral and sequential listening modes (Gordon, Jiwani, & Papsin, 2013; Kral et al., 2016). In an investigation of the combined effect of earlier implantation and simultaneous BiCIs on language development, Wie et al. (2020) found that the performance gap between early-implanted children with simultaneous BiCI and their normal-hearing peers is closed within the first 4 years after implantation, although the two groups showed differences in some aspects of language (receptive vocabulary and expressive grammar) between 4 and 6 years after implantation. Despite these additional speech perception and localization benefits offered by BiCIs, it is unclear whether the trajectory of speech recognition development is influenced by the listening mode and timing of a second CI.
Interimplant Interval and Speech Recognition Development
Different factors such as the effectiveness of sequential BiCIs (SEQ BiCIs), economic concerns due to costs of implantation, and possible surgical complications could impact the family's consideration and the appropriate timing for receiving a second implant. The positive effect of shorter interimplant intervals on speech recognition outcomes of children with SEQ BiCIs has been shown (Galvin et al., 2009; Gordon & Papsin, 2009; Jeong et al., 2018; Johnston et al., 2009; Steffens et al., 2008; Zeitler et al., 2008). For example, long interimplant interval negatively influences speech recognition outcomes and cortical plasticity (Gordon et al., 2011; Gordon & Papsin, 2009; Strøm-roum et al., 2012) and short intervals led to better language scores assessed at 3 years postimplantation (Boons et al., 2012). Prolonged interimplant intervals can also lead to asymmetric speech recognition abilities with slower development of speech recognition for the second implanted ears (Zhang et al., 2020) and an unbalanced auditory cortical organization with preference for hearing through the better ear (Polonenko et al., 2018). Illg et al. (2019) reported an effective interimplant interval of up to 4 years for children who received their first implant before 4 years old. Yet, the effects of the interval between the first and second implantation on the growth and longer-term speech recognition performance remain unknown.
This Study
In this retrospective cohort study, data from children with prelingual, severe-to-profound hearing loss, who received their first implant by age 9 years, were studied to understand the trajectory of speech recognition development postimplantation. As common to most retrospective studies, the scores were not from specific and evenly distributed intervals and test types varied depending on child's age and hearing ability. In response to these challenges, we modified a scoring scheme based on the PROSPER system to integrate scores from different test types and materials and quantified the trajectory of speech recognition development (e.g., Trimble et al., 2008; Yeung et al., 2018). This modified PROSPER score was used to explore individual differences in the rate of speech recognition development and their longer term speech recognition outcomes. We focused on whether earlier implantation related to faster speech recognition growth and better longer term speech recognition scores with the prediction that early age at activation of the first implant would lead to better speech recognition scores, but not the rate of development, as suggested by Holt and Svirsky (2008). We further examined the role of listening mode (unilateral vs. bilateral CIs) on the trajectory of speech recognition development, hypothesizing that children with BiCIs would perform better than those with unilateral CI, both in terms of how rapidly they reach peak performance and their longer term speech recognition scores. Furthermore, we explored whether shorter interimplant intervals in children with SEQ BiCIs corresponds to faster rates of speech recognition growth and better longer term speech recognition scores. Finally, we examined how patterns of speech recognition development varied between the first and the second implanted ears of children with BiCIs, and whether age at CI activation contributed to possible differences. We hypothesized that the longer term speech recognition scores would be higher for the first implanted ears due to earlier implantation and longer CI use.
Materials and Method
Data Collection
The medical records of patients at the MEE Audiology clinic were reviewed to identify children with severe to profound, sensorineural hearing loss, diagnosed within the first year of life (i.e., prelingually deafened), who underwent cochlear implantation. The data reviewed were collected between 1994 and 2015. The degree of hearing loss was determined from some combination or subset of pure-tone thresholds of 70 dB HL or greater either from behavioral assessment and/or auditory brainstem response estimates for pure tones at 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, and 8 kHz. Children were classified as having severe, severe-to-profound, or profound hearing loss at the time of diagnosis (Clark, 1981). Speech recognition scores in quiet were extracted for these children. All speech materials were presented at 65 dB HL presentation level of recorded speech from a single loudspeaker at 0° azimuth at 1 m from the patient, which was the clinical standard at our center at the time the data were collected. The test materials were administered by licensed audiologists at MEE who specialized in working with children with hearing loss. The non–test ear was either plugged with a foam earplug, masked using speech-shaped noise or the contralateral CI or HA was turned off. Only scores for individual implanted ears were analyzed in this study. For each testing session, the age at testing, type of test administered, and speech recognition score were collected. The speech recognition scores were the percentage of correct responses. The type of test used at a session varied depending on the child's age, performance, and cooperation.
MEE PROSPER Score
To address the problem of the variety of test types used for speech recognition assessment in children, we modified the hierarchical approach adopted from the PROSPER score chart (Eskander et al., 2011; Trimble et al., 2008; Yeung et al., 2018), by tailoring it to the speech recognition tests used regularly in our clinics. To create a PROSPER score chart, the individual speech perception tests are ranked in a hierarchy from easiest to the most complex and difficult (Trimble et al., 2008). The ranking is based on the criteria described by Geers and Moog (1987), including if a test is closed- or open-set and the difficulty level of the vocabulary in each test. For example, pattern perception tests that examine a child's general auditory behavior and do not require any word understanding are easier than open-set tests of speech perception and are, therefore, ranked the lowest in the PROSPER hierarchy. In addition, listeners have more difficulty discriminating between monosyllabic words than spondees and multisyllabic words (Erber, 1982). Therefore, a test that contains multisyllabic words are expected to be easier than a test with monosyllabic words, having a relatively lower rank and a lower PROSPER score. Furthermore, tests with more difficult vocabularies are more challenging, thus will have a higher ranking in the PROSPER chart. The modified PROSPER score chart was constructed based on the described hierarchy such that the easiest, least complex test corresponded with lower scores and the most difficult and complex test with higher scores. See Trimble et al. (2008) and Yeung et al. (2018) for a detailed discussion of PROSPER scores. We used our MEE PROSPER score to build and compare the trajectory of children's speech recognition scores (i.e., how quickly they improve and how well they perform after reaching a plateau in performance). We then examined the role of age at implantation, listening mode, and interimplant interval on speech recognition development in our cohort while controlling for other factors available in our database (e.g., hearing loss, etiology, and device manufacturer).
Table 1 shows the MEE PROSPER score chart for the tests used in the extracted data. The tests are ordered based on the developmental appropriateness of the test materials (Geers & Moog, 1987), using three criteria: (a) whether the test was a closed-set (e.g., Early Speech Perception (ESP; Eisenberg, Johnson, et al., 2006) or open-set (e.g., Central Institute for the Deaf W-22 lists [W22] test; Hirsh et al., 1952), (b) the level of vocabulary in each test from the easiest (ESP low verbal) to most difficult (The Northwestern University Auditory Test Number Six [NU6]; Tillman & Carhart, 1966) or The Maryland Consonant–Vowel Nucleus–Consonant (CNC) test (Lehiste & Peterson, 1959; Peterson & Lehiste, 1962; or W22), and (c) the normative age range for which each test was designed (age ≤ 4 or 4 < age ≤ 6 or 6 < age ≤ 8 or age ≥ 8 years). Note that the recommended age ranges by the test manuals are based on typical development and the administered test may not necessarily correspond to children's chronological ages when administered and may change depending on the vocabulary of the child, particularly in children with CIs who are more likely to perform poorer and more variably than their age-matched peers with typical hearing (Eisenberg, Fink, & Niparko, 2006; Svirsky et al., 2000; Wie et al., 2020; Uhler et al., 2017). Closed-set tests are developed for younger children to be appropriate for their age and speech and language skills. In closed-set tests, children are asked to choose a representation from a set of pictures or objects, which increases the chance of guessing correctly compared to open-set tests in which they are asked to repeat the word heard (e.g., Geers & Moog, 1987). The level of vocabulary and number of possible answers increases from the lowest PROSPER value for the closed-set, low verbal ESP test to the highest category that includes open-set, monosyllabic word tests often used for testing adults, such as the W22, NU6, and CNC materials. The W22, NU6, and CNC tests were considered as one category as they are all open-set tests and all contain relatively complex vocabulary. Another criterion for ranking tests in the PROSPER is the age range for which each test were designed, as they were designed for different spoken language ages. For example, the CNC test was designed for children with a spoken language age of 8 years and above, while Phonetically Balanced Word Lists–Kindergarten (Haskins, 1949) was designed for children between 5 and 8 years old. Tests were also ranked to reflect the chance-level performance of each test based on the number of items for each test.
Table 1.
Massachusetts Eye and Ear (MEE) Pediatric Ranked Order Speech Perception (PROSPER) score chart.
| MEE PROSPER score | Speech perception test | % correct | Closed-set or open-set | Vocabulary level | Normative age range a |
|---|---|---|---|---|---|
| 1 | ESP low verbal | Category 1 | C | 1 | ≤ 4 |
| 2 | ESP low verbal | Category 2 | C | 1 | ≤ 4 |
| 3 | ESP low verbal | Category 3 | C | 1 | ≤ 4 |
| 4 | ESP low verbal | Category 4 | C | 1 | ≤ 4 |
| 5 | ESP standard | Category 1 | C | 2 | ≤ 4 |
| 6 | ESP standard | Category 2 | C | 2 | ≤ 4 |
| 7 | ESP standard | Category 3 | C | 2 | ≤ 4 |
| 8 | ESP standard | Category 4 | C | 2 | ≤ 4 |
| 9 | WIPI | < 25% | C | 3 | 4–6 |
| 10 | WIPI | 25% ≤ score < 50% | C | 3 | 4–6 |
| 11 | WIPI | 50% ≤ score < 75% | C | 3 | 4–6 |
| 12 | WIPI | Score ≥ 75% | C | 3 | 4–6 |
| 13 | PBK | < 25% | O | 4 | 5–8 |
| 14 | PBK | 25% ≤ score < 50% | O | 4 | 5–8 |
| 15 | PBK | 50% ≤ score < 75% | O | 4 | 5–8 |
| 16 | PBK | Score ≥ 75% | O | 4 | 5–8 |
| 17 | W22/NU-6/CNC | < 25% | O | 5 | ≥ 8 |
| 18 | W22/NU-6/CNC | 25% ≤ score < 50% | O | 5 | ≥ 8 |
| 19 | W22/NU-6/CNC | 50% ≤ score < 75% | O | 5 | ≥ 8 |
| 20 | W22/NU-6/CNC | Score ≥ 75% | O | 5 | ≥ 8 |
Note. Early Speech Perception (ESP) category score criteria for low-verbal version and standard version. Low-verbal: It is a 4-item test. Category 1 was assigned to pattern perception test scores lower than 67%. Category 2 was assigned to pattern perception test scores higher than 67% or spondee identification test scores lower than 67%. Category 3 was assigned to spondee identification test scores higher than 67% or monosyllable identification test scores lower than 83%. Category 4 was assigned to monosyllable identification test scores higher than 83%. Standard: It is a 12-item test. Category 1 was assigned to pattern perception test scores lower than 75%. Category 2 was assigned to pattern perception test scores higher than 75% or spondee identification test scores lower than 75%. Category 3 was assigned to spondee identification test scores higher than 75% or monosyllable identification test scores lower than 50%. Category 4 was assigned to monosyllable identification test scores higher than 50%. Word Intelligibility by Picture Identification (WIPI): It is a 25-item test. The Phonetically Balanced Kindergarten Test (PBK): It is a 50-item test. The W22, NU6, and CNC are all 50-item tests. C = closed-set; O = open-set; W22 = Central Institute for the Deaf W-22 lists; NU-6 = The Northwestern University Auditory Test No. 6; CNC = The Maryland Consonant–Vowel Nucleus–Consonant test.
Note that the tests were often used at different ages than the normative ages indicated here due to variability in children's performance at different ages.
Based on these criteria, the tests were ranked from simple pattern detection of ESP low verbal to more difficult open-set word recognition tests with high vocabulary levels. We increased the resolution from the previously used PROSPER to four levels (0%–25%, 25%–50%, 50%–75%, and 75%–100%) instead of two (0%–50% and 50%–100%; Yeung et al., 2018) to achieve higher precision. The final MEE PROSPER score ranged from 1 to 20. Raw speech recognition scores for each implanted ear were mapped to its corresponding PROSPER score. For example, a raw score of 85% on the Word Identification by Picture Identification test (Ross & Lerman, 1970) was converted to a score of 12 in PROSPER.
Characterizing Speech Recognition Growth Curves
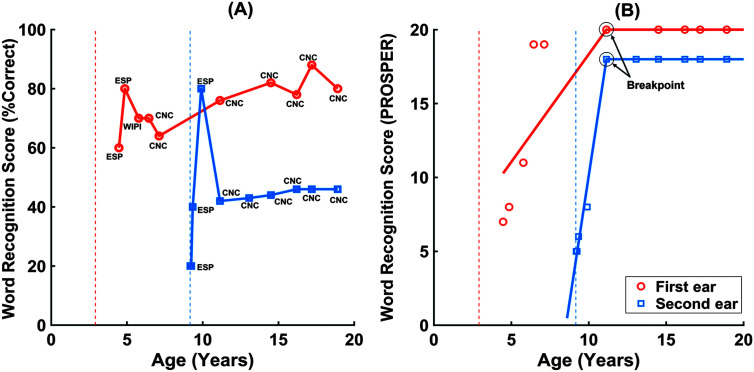
After mapping raw scores to PROSPER scores, graphical exploration of the longitudinal scores exhibited two distinct phases. Figure 1 shows an example of the raw speech recognition scores (left) and the corresponding PROSPER scores (right) over time. PROSPER scores for each ear of each child were characterized by these two phases. The growth phase was defined by an early, relatively steep phase followed by a plateau phase where scores were relatively stable. A linear, piecewise function was used to model these two distinct phases (e.g., Chou et al., 2004). The growth phase was modeled with a linear fit, and the plateau was modeled using a constant value. At least three scores after implantation were required to assess the growth phase, unless the second score was at the maximum value of 20 in PROSPER. The plateau phase was defined as the period of time where scores were stable (i.e., not significantly changing over time). One important step in a linear piecewise growth model is to identify the location of change point or breakpoint; a time point at which a trajectory transits from one phase to another (e.g., Chou et al., 2004; Dominicus et al., 2008). A breakpoint detection analysis found the point where the growth phase ended and the plateau phase began. The algorithm calculated the first derivative of the scores from the latest MEE PROSPER score and detected the positive derivative. This was done until the two consecutive time points did not change by a PROSPER score of one. The positive derivative was used to indicate a monotonically increasing trend in scores over time. The significance level for assuming that the score is constant was a change in PROSPER score of one.
Figure 1.
Example of the trajectory of speech recognition over time (subject ID 2 in Table 2) created from (A) raw speech recognition scores and (B) the corresponding Pediatric Ranked Order Speech Perception (PROSPER) scores. The vertical lines show age at activation of the first cochlear implant (CI; orange color) and the second CI (blue color). Panel B shows the estimated best fit line on the speech recognition scores for the growth phase of the first implanted (orange) and the second implanted ear (blue), as well as the average score during the plateau shown by a horizontal line in the plateau phase of the estimated growth curves. The slope of the best fit-line was used as the slope of the growth phase. The breakpoints on the speech recognition growth curves in the panel (B) are shown by the dotted circles for the first and second implanted ears. ESP = Early Speech Perception; WIPI = Word Intelligibility by Picture Identification; CNC = The Maryland Consonant–Vowel Nucleus–Consonant test.
After detecting the age at plateau onset (i.e., the breakpoint), the data were divided into two time series: the growth and plateau phases. The growth phase was modeled with a linear fit, while the plateau phase was modeled using a constant value, such that children's performance during the plateau phase is reflective of their longer term speech recognition score. This constant value was the average of scores during the plateau phase, which we call the score at the plateau hereafter. Panel B in Figure 1 shows an example of the trajectory of speech recognition over time for the first and second implanted ears of a child with SEQ BiCIs. The growth phase is described by the slope of the best-fit line (in PROSPER/years), and the time from CI activation to plateau onset (in years). The plateau phase is described by the score at the plateau (in PROSPER). These three outcome measures were studied to examine the role of age at CI activation, listening mode (UniCI or BiCI), and interimplant interval on speech recognition development. Higher slopes and shorter time from CI activation to plateau onset indicate faster speech recognition growth and higher score at the plateau implies better longer term speech recognition skills. Due to the irregularly and unequally spaced measurement times across children, which could impact the fitted regression line and the estimated slopes for the speech recognition growth curve, both the slope and the time from CI activation to the plateau onset were calculated to capture portions of variability in the growth phase that may not be reflected in one of these measures alone.
Study Sample
Children were included who had at least a severe degree of hearing loss identified prior to 1 year of age and at least three postimplantation speech recognition scores before reaching plateau performance levels. Children with progressive hearing loss and those with other cognitive, visual, or motor disorders in addition to sensorineural hearing loss were excluded from this study. For children included, English was the primary language of the children and the primary language spoken in their home. Data from 31 of the prelingually deafened children (boys = 16) met the inclusion criteria. The data were meticulously checked by three of the authors to ensure that they met these criteria. Each ear had a minimum of three scores measured over time, with at least two scores within 24 months postimplantation during the growth phase. The minimum and maximum ages of testing across children was around 3 months and 20 years postimplantation, respectively. The age of CI activation for the first implanted ears ranged from 11 months to 9 years (Mdn = 20 months, interquartile range = 15 months) with 78% of the children receiving their first implant by 3 years. Twelve children were unilaterally implanted, and 19 were bilaterally implanted. Thirteen of the 19 children with BiCIs received their implants sequentially (SEQ BiCIs), and six received them simultaneously (SIM BiCIs).
Demographic details of the patients are presented in Table 2. In addition to the data related to speech recognition scores, data were collected regarding age at CI activation, degree of hearing loss at diagnosis, etiology, revision status, listening mode (UniCI or BiCIs [SEQ or SIM]), communication mode (oral or total communication), and device manufacturer. Duration of deafness was defined as the length of time between hearing loss diagnosis and CI activation. Twenty-two patients were implanted with Advanced Bionic devices (~71%), seven with Cochlear (~22%), and two with MED-EL devices (~7%). More than 90 % of children had used oral communication. We did not specify the speech processing strategies because they changed for most children over time due to advances in CI technology. Age at HA fitting and duration of HA use are in Supplemental Material S1. Duration of HA is the time from age at HA fit to age at first CI. This study was approved by the institutional review board of the Partners Human Research Committee, Boston, Massachusetts (Protocol Number 2019P001158).
Table 2.
Demographic information of 31 children with prelingual, severe-to-profound hearing loss who underwent cochlear implantation at Massachusetts Eye and Ear audiology clinic.
| ID | Gender | Listening mode | First implanted ear/second implanted ear |
Communication mode | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ear | Age at activation | Degree of HL | Etiology | Revision | Manufacturer | ||||
| 1 | F | UniCI | R | 5.2 | Profound | Genetic | 0 | AB | TC |
| 2 | M | SEQ BiCI | R/L | 2.9/9.2 | Profound | Genetic | 0/0 | AB | OC |
| 3 | F | SEQ BiCI | L/R | 1.4/4 | Profound | Congenital | 0/0 | AB | TC |
| 4 | M | UniCI | R | 7.3 | Profound | Congenital | 0/— | Cochlear | Unknown |
| 5 | F | UniCI | L | 1.2 | Profound | Unknown | 0/— | AB | OC |
| 6 | M | SEQ BiCI | R/L | 1.7/10.2 | Profound/severe–profound | Genetic | 1/0 | AB | OC |
| 7 | F | SEQ BiCI | L/R | 2.2/5.4 | Profound/ severe–profound | Unknown | 0/0 | AB | OC |
| 8 a | F | SEQ BiCI | L/R | 5/14 | Profound | Meningitis | 0/0 | Cochlear | OC |
| 9 | F | SEQ BiCI | L/R | 1.25/2 | Profound/severe–profound | Genetic | 0/0 | Cochlear | OC |
| 10 | F | SEQ BiCI | R/L | 2/13.5 | Profound | Genetic | 0/0 | AB | OC |
| 11 | F | SIM BiCI | R/L | 1 | Profound | Unknown | 0/0 | AB | TC |
| 12 | M | UniCI | L/R | 2.67 | Profound | Genetic | 0/— | Cochlear | Unknown |
| 13 | M | SEQ BiCI | R/L | 1.5/2.5 | Profound | Unknown | 1/1 | AB | TC |
| 14 | M | UniCI | L | 2.17 | Profound | Unknown | 0/— | AB | TC |
| 15 | M | UniCI | R | 3.67 | Profound | Unknown | 1/— | AB | TC |
| 16 | F | UniCI | R | 1.83 | Severe | Genetic | 0/— | Cochlear | TC |
| 17 | M | UniCI | R | 2.08 | Severe–profound | Auditory neuropathy | 0/— | AB | TC |
| 18 | M | SEQ BiCI | L/R | 1.8/11.3 | Profound | Genetic | 0/0 | AB | OC |
| 19 a | M | SEQ BiCI | R/L | 2.5/6.6 | Profound | EVA/unknown | 0/0 | AB | OC |
| 20 | M | SIM BiCI | R/L | 1.08 | Profound | Congenital | 0/0 | MED-EL | TC |
| 21 a | M | SIM BiCI | R/L | 0.92 | Profound | Congenital | 0/0 | AB | OC |
| 22 | F | SIM BiCI | R/L | 1.75 | Profound | Auditory neuropathy | 0/0 | MED-EL | TC |
| 23 | F | SEQ BiCI | L/R | 1.08/4.75 | Profound | Unknown | 1/0 | AB | OC |
| 24 | M | SEQ BiCI | R/L | 9/15.2 | Severe–profound/severe | Congenital | 0/0 | Cochlear | Unknown |
| 25 | M | UniCI | R | 1.16 | Profound | Meningitis | 0/— | AB | Unknown |
| 26 | F | UniCI | L | 5.5 | Profound | Genetic | 0/— | AB | OC |
| 27 | F | UniCI | L | 1.58 | Severe/profound | Connexin 26 | 0/— | AB | OC |
| 28 | M | SEQ BiCI | R/L | 1.42/9.2 | Profound | Meningitis | 0/0 | AB | OC |
| 29 | F | SIM BiCI | R/L | 1 | Profound | Congenital | 0/0 | Cochlear | OC |
| 30 | M | UniCI | R | 1.5 | Profound | Genetic | 0/— | AB | Unknown |
| 31 | F | SIM BiCI | R/L | 1.25 | Profound | Genetic | 0/0 | AB | TC |
Note. The data for the first and second ears are separated by “/.” For the cells with one entry, the data were the same for the first and second implanted ears. The em dash (—) indicates that the ear was not implanted with a cochlear implant. F = female; UniCI = unilateral cochlear implantation; R = right ear; AB = Advanced Bionics; TC = total communication (a combination of spoken language and American Sign Language); M = male; SEQ BiCIs = sequential bilateral cochlear implantation; L = left ear; OC = oral communication (exclusively spoken); SIM BiCIs = simultaneous bilateral cochlear implantation; EVA = enlarged vestibular aqueduct.
Speech recognition scores for the second ear of these three children with bilateral cochlear implants were not available.
Analyses
The data were grouped into those from the first implanted ears of all children and those from the second implanted ears of children with SEQ BiCIs. Because there was no time lag and no statistically significant differences between the outcomes of ears of children with SIM BiCIs (the slope: β = 1.5, p = .28; the time between the activation and the plateau onset: β = −0.37, p = .3; score at the plateau: β = 0.04, p = .86), all ears of those six children were considered as first implanted ears. Note that patient ID was maintained as a random effect in all statistical analyses to account for the lack of independence between ears for these six children. Thirty-six ears were analyzed as the first implanted ears, including 12 implanted ears from children with UniCI, 13 first-implanted ears of children with SEQ BiCIs, and 11 ears from six children with SIM BiCIs. Data for the analysis of the second implanted ears were from 11 ears of 13 children with SEQ BiCIs. Speech recognition scores for the second ear of one child with SIM BiCIs (Child 21 in Table 2) and two children with SEQ BiCI (Children 8 and 19 in Table 2) were not available. The data extracted from the medical records were analyzed in MATLAB R2020a (The MathWorks).
Linear mixed-effects analyses were conducted using the fitlme function in MATLAB to examine the effects of the age at CI activation, the listening mode, and the interimplant interval on the three outcome measures described above. These analyses were performed for the first implanted ears, with children's IDs and the implanted ear (first or second) included as random intercepts to account for quality and/or information differences that might exist due to children- and ear-specific variations. We further used linear mixed-effects models to analyze whether these three outcome measures differed between the first and the second implanted ears of children with SEQ BiCIs. To account for the issue of small sample size, we conducted a preliminary correlational analysis to test for multicollinearity (Dormann et al., 2013). The results showed that three variables of duration of deafness, duration of HA use, and age at activation were highly correlated (|Pearson r| ≥ .9, p < .05); therefore, we used only age at CI activation and did not include the other two parameters to improve the reliability of the estimated coefficients in our linear mixed-effects analyses. We controlled for the factors of degree of hearing loss, etiology, communication mode, and device manufacturer in our statistical models. These factors were defined as categorical variables (dummy coded) and were inserted as covariates in the models.
Results
Individual Differences in Speech Recognition Development
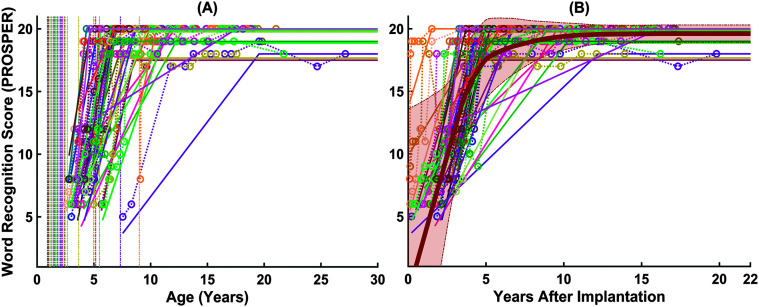
Figure 2 shows the estimated speech recognition growth curves for the first implanted ears of 31 children (i.e., 36 ears). Panel A shows the age at CI activation as the vertical lines and the wide age range among children in receiving their first implant, from 11 months to 9 years (Mdn = 20 months). Panel B shows the same curves shifted such that the x-axis is now age postactivation (age on x-axis) to demonstrate the variability in the time course of speech recognition after implantation. The average growth curve is shown as a thick, orange line with a shaded area around the average scores, representing ±1 SD. The overall pattern shows that children's PROSPER scores improved over time following their first implantation. However, as shown by the standard deviation in Figure 2B, there was a large variability among children in the trajectory of their speech recognition scores, both in terms of the growth rate and the performance at the plateau.
Figure 2.
The trajectory of speech recognition over time for the first implanted ears. Panel A shows the trajectories with the age at activations as vertical lines. Panel B shows the same trajectories shifted such that the x-axis represents age postimplantation, as well as the average growth curve shown in thickened, orange line with a shaded area around the average scores (light orange), representing ±1 SD around the average values. Data for individual ears are shown in different colors. PROSPER = Pediatric Ranked Order Speech Perception.
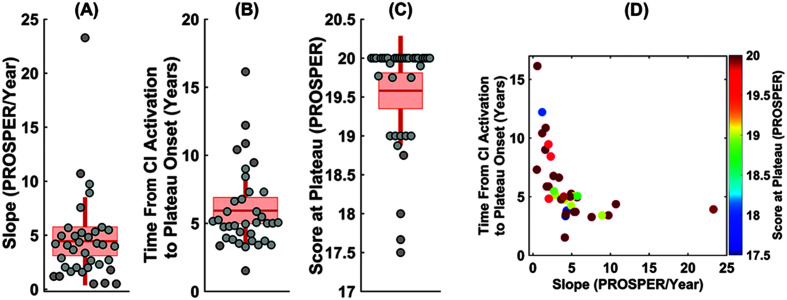
Figure 3 shows the distribution of the measures of slope (Panel A), the time from CI activation to the plateau (Panel B), and the score at the plateau (Panel C) for the first implanted ears. The descriptive statistics for these outcome measures are summarized in Table 3. Children varied widely in the rate of speech recognition growth for their first implanted ears with slopes ranging from around 0.5 to 23 PROSPER points per year (SD = ±4.11 PROSPER points per year).
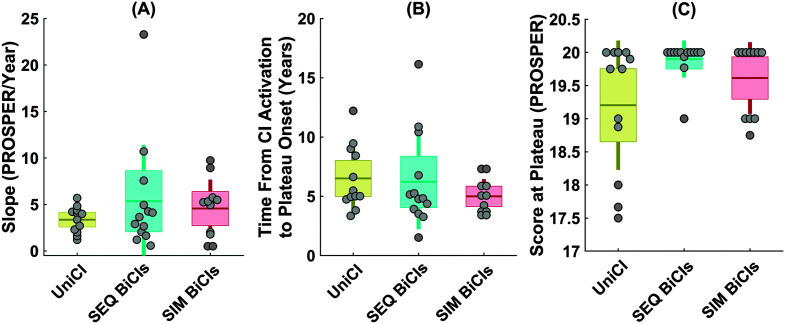
Figure 3.
Distribution of three outcome measures of (A) slope (PROSPER/Year), (B) time from activation to the plateau onset, and (C) score at the plateau calculated from the estimated speech recognition growth curves of the 31 first-implanted ears. In each panel of A, B, and C, a scatter plot shows the individual ears, while the boxplot summarizes the distribution of each outcome measure. The data points are laid over a ±1.96 standard error of the mean (95% confidence interval) in light orange and ±1 SD shown by solid vertical lines in dark orange color. The solid dark horizontal orange lines in the boxes show the mean as a measure of central tendency. Panel D shows the three outcome measures of the slope, the time from activation to the plateau onset, and the score at the plateau in a two-dimension scatter plot where the color of each data point represents the score at the plateau. PROSPER = Pediatric Ranked Order Speech Perception; CI = cochlear implant.
Table 3.
Descriptive statistics of the speech recognition growth curves of the first implanted ears.
| Measures from speech recognition growth curve | Measures of central tendency and variability |
||||||
|---|---|---|---|---|---|---|---|
| Min | Max | Range | M | SD | Mdn | IQR | |
| First implanted ears | |||||||
| Slope (PROSPER/year) | 0.51 | 23.28 | 22.78 | 4.44 | 4.1 | 4.0 | 3.33 |
| Time (years) | 1.52 | 16.14 | 14.62 | 5.94 | 2.96 | 5.0 | 3.17 |
| Score at plateau (PROSPER) | 17.5 | 20 | 2.5 | 19.52 | 0.70 | 20 | 1 |
Score map for relating prosper scores to raw scores: PROSPER score < 18 ➞ raw score < 25%; 18 ≤ PROSPER score < 19 ➞ 25% ≤ raw score < 50%; 19 ≤ PROSPER score < 20 ➞ 50% ≤ raw score < 75%; PROSPER score = 20 ➞ raw score < 75%. Note that these raw scores are from the last category of “Speech Perception Test” presented in the second column of Table 2 (i.e., “W22/NU6/CNC”). IQR = interquartile range; PROSPER = Pediatric Ranked Order Speech Perception.
On average, the speech recognition score for the first implanted ears plateaued at around 6 years after activation, while there was a wide intersubject variability ranging from 1.5 to 16 years (SD = 2.96 years). Although most children (~66%) reached a plateau by 6 years, reaching plateau performance was much longer (> 10 years) for a small group of poorer performing children (~13%; see Figure 3B). In terms of the score at the plateau, Figure 3C suggests three clusters of children, including those with relatively poorer performance (PROSPER score between 17 and 18), those with average performance (PROSPER score between 18 and 19), and good performance (PROSPER score between 19 and 20), ranging from raw scores lower than 25% to scores greater than 75% in the open-set word category of “W22/NU6/CNC” in Table 2). On average, most children developed high levels of speech recognition performance at the plateau. In fact, the scores at the plateau were higher than or equal 19 for 86% of children, however, there remains an important group of children; (~14%) whose scores at the plateau were lower than 19, corresponding to a raw score lower than 50%.
Figure 3 shows these three outcome measures in a two-dimension scatter plot (Panel D), where the color represents the score at the plateau. This figure shows that the rate of speech recognition growth (i.e., slope and/or time to plateau onset) does not predict the score at plateau. For example, the two children in the upper left corner and lower right corner of this plot were good-performing children in terms of score at the plateau (PROSPER score of 20). However, these two children had largely different rates of speech recognition development such that one developed relatively fast (slope > 20 PROSPER/year with a short time to plateau of less than 5 years) and the other had relatively slow growth (slope of less than 2 PROSPER/year with a long time to plateau of more than 15 years). There was a difference of ~12 years in the time from activation to reaching a PROSPER score of 20 for these two children, highlighting that the relationship between the slope or the time to plateau and plateau performance is not predictable from one another.
Age at CI Activation, Listening Mode, and Speech Recognition Development
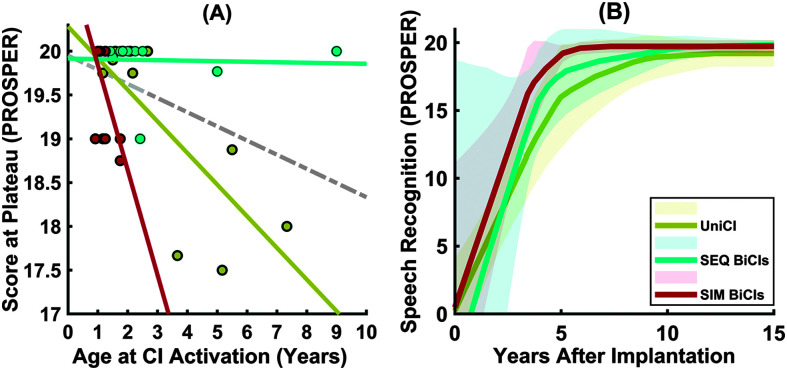
Table 4 shows the results of mixed-effects analyses examining the role of age at CI activation on the three outcome measures (slope, time to plateau onset, and score at the plateau). The results showed that age at CI activation significantly affects speech recognition at the plateau (β = −0.15, p = .01, 95% confidence interval [−0.27, −0.03]). The results did not, however, show any effect of age at CI activation on the measures of rate of speech recognition development (i.e., slope and time to plateau onset). We further examined whether there was an interaction between age at CI activation and listening mode (unilateral CI, SEQ BiCIs, or simultaneous BiCIs) on the score at the plateau. The results of the mixed-effects analysis showed a significant interaction between age at CI activation and listening mode (F = 9.98, p < .001, 95% confidence interval [0.2, 2.18]). Figure 4A plots the score at the plateau as a function of the age at CI activation for children with unilateral CI, SEQ BiCIs, and SIM BiCIs. The slopes of the regression lines were steeper for the SIM BiCIs group, r(34) = .76, p = 0.002, than UniCI, r(34) = .74, p = .005, and SEQ BiCI groups, r(34) = .046, p = .88. Children who received their first implant before 1.25 years performed well with scores at the plateau of 19 PROSPER or higher. For children activated later than 1.25 years, the score at the plateau for children with UniCI and SIM BiCI was negatively influenced by their age at CI activation (see Figure 4A). The average speech recognition growth curves for the children grouped by listening mode and shifted for age at postimplantation are shown in Figure 4B. Note the differences in the slope, the time from the activation to plateau, and final score at the plateau among the three groups.
Table 4.
Results of linear mixed-effects analyses in testing whether the age at cochlear implane (CI) activation relates to three outcome measures of the slope, the time from CI activation to the plateau onset, and the score at the plateau.
| Parameter | First implanted ears |
|||
|---|---|---|---|---|
| Estimate (β) | SE | t | p | |
| Slope | ||||
| Intercept | 5.48 | 1.14 | 4.60 | .00 |
| Age at CI activation | −0.42 | 0.38 | −1.10 | .28 |
| Degree of hearing loss | −0.03 | 0.45 | −0.32 | .34 |
| Etiology | −0.54 | 1.95 | −0.28 | .78 |
| Communication mode | −1.97 | 1.57 | −1.25 | .22 |
| Manufacturer | −1.05 | 1.60 | 0.66 | .52 |
| Time from CI activation to the plateau onset | ||||
| Intercept | 6.35 | 0.83 | 7.62 | .00 |
| Age at CI activation | −0.11 | 0.26 | −0.42 | .68 |
| Degree of hearing loss | −1.48 | 2.85 | −0.52 | .61 |
| Etiology | −0.33 | 1.18 | −0.28 | .78 |
| Communication mode | 1.15 | 1.07 | 1.57 | .29 |
| Manufacturer | 0.96 | 2.08 | 0.20 | .84 |
| Score at the plateau | ||||
| Intercept | 19.93 | 0.20 | 92.94 | .00 |
| Age at CI activation | −0.15 | 0.06 | −2.53 | .01* |
| Degree of hearing loss | −0.8 | 0.68 | 1.2 | .23 |
| Etiology | −0.14 | 0.32 | −0.25 | .65 |
| Communication mode | 0.23 | 0.26 | −0.87 | .39 |
| Manufacturer | −0.15 | 0.26 | −0.6 | .55 |
Note. Statistically significant p values are in bold font.
Significant differences are represented with p < .05.
Figure 4.
(A) Scatter plot showing the score at the plateau against the age at CI activation for the first implanted ears. The data and the corresponding fitted regression lines are shown for children with UniCI, SEQ BiCIs, and SIM BiCIs. The gray line shows the best-fit lines on all data. (B) The average speech recognition growth curves estimated for three groups of listening mode; UniCI, SEQ BiCIs, and SIM BiCIs. The data for UniCI, SEQ BiCIs, and SIM BiCIs are shown in mustard color, aqua color, and maroon color, respectively. PROSPER = Pediatric Ranked Order Speech Perception; CI = cochlear implant; UniCI = unilateral cochlear implantation; SEQ BiCI = sequential bilateral implantation; SIM BiCIs = simultaneous bilateral implantation.
Figure 5 shows the distribution of the three outcome measures for these groups of children. The results of linear mixed-effects analyses demonstrated that the three groups did not significantly differ in the age at CI activation of the first implanted ears (F = 2.4, p = .1; mean age at CI activation with ±1 SD was 2.98 ± 2 for UniCI group, 2.57 ± 2.17 for SEQ BiCIs group, and 1.17 ± 0.31 for SIM BiCIs group), duration of hearing loss (F = 1.93, p = .16; mean duration of hearing loss with ±1 SD was 2.82 ± 2.02 for UniCI group, 2.49 ± 2.23 for SEQ BiCIs group, and 1.17 ± 0.31 for SIM BiCIs group), duration of HA use (F = 0.88, p = .43; mean duration of HA use with ±1 SD was 2.27 ± 1.46 for UniCI group, 2.23 ± 2.35 for SEQ BiCIs group, and 0.46 ± 0.32 for SIM BiCIs group), and etiology (F = 2.7, p = .31). The linear mixed-effects analyses demonstrated that the score at the plateau was significantly higher for children with BiCIs (both SEQ and SIM BiCIs) than those with UniCIs (β = 0.59, p = .02, 95% confidence interval [0.1, 1.07]). Post hoc analyses showed that this difference was driven by the difference between UniCIs and either group of bilaterally implanted children (SEQ BiCIs and UniCI [β = 0.34, p = .03]; and SIM BiCIs and UniCI [β = 0.15, p = .046]), suggesting that children with BiCIs achieved better speech recognition scores at the plateau. The speech recognition growth rates, however, did not significantly differ between children with BiCIs and those with UniCI (i.e., the slope and the time to the plateau onset). Only the interaction between age at CI activation and listening mode influenced the score at the plateau (F = 9.98, p < .001, 95% confidence interval [0.2, 2.18]). No significant effect of the other variables were observed between groups of listening mode for the measures of growth and the score at the plateau.
Figure 5.
Distribution of three outcome measures of (A) slope (PROSPER/year), (B) time from activation to the plateau onset, and (C) score at the plateau, calculated from the estimated speech recognition growth curves of the 36 first implanted ears for three groups of listening mode (UniCI, SEQ BiCIs, and SIM BiCIs). In each panel, a scatter plot shows the individual first implanted ears, while the boxplot summarizes the distribution of each outcome measure. The data points are laid over a ±1.96 standard error of the mean (95% confidence interval) shown as boxes and ±1 SD shown by vertical lines. The solid horizontal lines on the boxes show the mean for each group of data as a measure of central tendency. PROSPER = Pediatric Ranked Order Speech Perception; CI = cochlear implant; UniCI = unilateral cochlear implantation; SEQ BiCI = sequential bilateral implantation; SIM BiCIs = simultaneous bilateral implantation.
Interimplant Interval and Speech Recognition Development in Children With SEQ BiCIs
Within the sequential bilateral implant group, we examined whether the time between first and second implantation (i.e., interimplant interval) affected the development of speech recognition for each ear. The results of the mixed-effects analyses showed that speech recognition scores of the first ears develop faster when the second implant is received sooner after the first (the slope: β = −1.27, p = .01, 95% confidence interval [−2.17, −0.37]; the time to the plateau: β = 0.74, p = .02, 95% confidence interval [0.1, 1.38]), but there was no effect on performance at the plateau (β = −0.005, p = .85). Speech recognition development was also highly variable for the second implanted ears (see Supplemental Material S2). Results from our statistical analyses did not show any effect of the interimplant interval on the speech recognition development of the second implanted ears (the slope: β = −0.28, p = .5; the time to the plateau: β = 0.29, p = .11; the score at plateau: β = −0.15, p =.1). A summary of the data from children with SEQ BiCIs and how they differed for the first and second implanted ears are shown in Figure 6. Figure 6A shows the growth curves, while Figure 6B–6D shows the distribution of the three outcome measures of children with SEQ BiCIs. Table 5 represents the results of the mixed-effects analyses for statistically testing these differences. These differences were statistically significant for the time to the plateau onset (β = −3.66, p = .01, 95% confidence interval [−6.55, −0.78]) and the scores at the plateau (β = −0.9, p = .004, 95% confidence interval [−1.5, −0.31]), suggesting that the second implanted ears reached a plateau faster than the first implanted ears, but the scores at the plateau were poorer for the second implanted ears. Age at CI activation was significantly associated with the difference between the first and second implanted ears for the time from the activation to the plateau (β = −0.57, p < .001, 95% confidence interval [−0.87, −0.26]) and the score at the plateau (β = −0.13, p < .001, 95% confidence interval [−0.2, −0.06]).
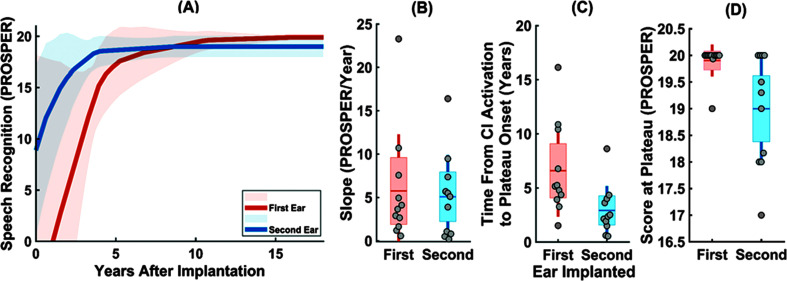
Figure 6.
(A) The average speech recognition growth curves estimated for the first implanted (orange line) and the second implanted (blue line) ears of children with SEQ BiCIs. The ±1 SD across the average curves are shown in light orange and light blue for the first and the second implanted ears, respectively. Distribution of three outcome measures of (B) the slope (in PROSPER/year), (C) the time from activation to the plateau onset (in years), and (D) the score at the plateau (in PROSPER) calculated from the estimated speech recognition growth curves of the first (orange color) and the second (blue colors) implanted ears of children with SEQ BiCIs. In each panel, a scatter plot shows the data for individual ears, while the boxplots summarize the distributions of each outcome measure for the first and second implanted ears. The data points are laid over a ±1.96 standard error of the mean (95% confidence interval) shown as boxes and ±1 SD shown by solid vertical lines. The solid horizontal, dark orange/blue lines show the mean or each group of data, respectively. PROSPER = Pediatric Ranked Order Speech Perception; CI = cochlear implant; SEQ BiCI = sequential bilateral implantation.
Table 5.
Results of the mixed-effects analyses testing whether the three outcomes of speech recognition development vary between the first and second implanted ears of children with sequential bilateral implantations.
| Parameter | Estimate (β) | SE | t | p |
|---|---|---|---|---|
| Slope | ||||
| Intercept | 5.75 | 1.65 | 3.84 | .002 |
| Ear implanted | −0.66 | 2.31 | −0.28 | .78 |
| Time between activation and plateau onset | ||||
| Intercept | 6.58 | 0.98 | 6.74 | .0000 |
| Ear implanted | −3.66 | 1.38 | −2.65 | .01* |
| Score at plateau | ||||
| Intercept | 19.90 | 0.22 | 89.62 | .000 |
| Ear implanted | −0.90 | 0.29 | −3.15 | .004** |
Note. Statistically significant p values are in bold font.
Significant differences are represented with p values < .05.
Significant differences are represented with p values < .01.
Discussion
The present work studied speech recognition development in pediatric CI listeners cared for at the MEE Audiology clinic, while addressing a major challenge in longitudinal studies that is the variety of speech perception tests used for speech recognition assessment (Eisenberg, Fink, & Niparko, 2006; Eisenberg, Johnson, et al., 2006; Trimble et al., 2008). The change in test type and materials due to testing children of different ages and developmental status has made it challenging to longitudinally quantify performance in a consistent and integrated way. Accordingly, prior studies have either focused on long-term speech recognition scores, speech recognition scores at certain time intervals, or between groups of children with different ages at implantation (e.g., Davidson et al., 2011; Dowell, 2002; Dunn et al., 2014; Manrique et al., 2004; Ruffin et al., 2013). Building on studies that used a hierarchy of speech recognition measures to prospectively track children with CIs' speech perception development over time (e.g., Eisenberg, Fink, & Niparko, 2006; Fink et al., 2007; Wang et al., 2008), in our retrospective study, we modified the PROSPER score chart to match the clinical data obtained at MEE, enabling the characterization of longitudinal speech recognition scores in our cohort. Using this MEE PROSPER score, this study aimed to understand how the growth rate and longer term speech recognition scores vary among children with prelingual, severe-to-profound hearing loss with CIs and to examine the role of various factors such as age at CI activation, listening mode (unilateral or bilateral), and interimplant interval on speech recognition development. We also investigated how the speech recognition development may differ between the first and the second implanted ears of children with SEQ BiCIs.
Individual Differences in Speech Recognition Development
The speech recognition growth curves for the first and second implanted ears demonstrated that children's speech perception abilities progressed over time after implantation, corroborating prior findings (e.g., Davidson et al., 2011; Dunn et al., 2014; Eisenberg, Johnson, et al., 2006; O'Donoghue et al., 2000; Svirsky et al., 2000). However, children's speech recognition development was largely variable both in terms of the rate of growth (as measured by the slope and the time from activation to the plateau onset) and the longer term outcome (as measured by the score at the plateau). To our knowledge, this is the first study that demonstrates these findings by longitudinally tracking speech recognition outcomes after integrating all post-implantation speech recognition scores using a tool like the PROSPER score. Although many children (~86%) achieved high scores at the plateau (PROSPER score ≥ 19), an important group of poorer performing children (~14%) were not able to reach scores higher than 19 on the PROSPER. The scores of at least 19 on the PROSPER correspond with more than 50% correct on open-set word recognition using tests designed for children 8 years or older. This group of poorer performing children then was scoring less than 50% correct on these same tests.
Another important finding was that children who had faster speech recognition development did not necessarily have better speech recognition scores at the plateau. This highlights an important consideration that speech recognition development in children with CIs could exhibit independent and different trends for the growth rate as compared to their longer term outcomes. Although the speech recognition score at the plateau is a primary outcome measure in CI recipients, slow growth of speech recognition scores could reflect delayed language development and may negatively impact other developmental domains such as social, emotional, and cognitive abilities (McLaughlin, 2011; Silva et al., 1983, 1987; Eisenberg et al., 2006; Uhler et al., 2017), as well as overall health-related quality of life (e.g., Lin et al., 2012). Therefore, children whose speech perception abilities grow faster are more likely to perform better in other aspects of development such as psychosocial and scholastic skills, which may lead to meaningful differences among children in their quality of life, as indirectly assessed by their parents' perception of quality of life (Eisenberg, Fink, & Niparko, 2006; Fink et al., 2007; Lin et al., 2012; Warner-Czyz et al., 2011). The analysis of the trajectory of the PROSPER scores could help clinicians to identify when a child has a slow growth phase and if further interventions or support are needed that could compensate for the delay.
Effects of Age at CI Activation, Listening Mode, and Interimplant Interval on Speech Recognition Development
Earlier implantation resulted in higher scores at the plateau as a measure of longer term speech recognition outcomes, consistent with previous studies (e.g., Davidson et al., 2011; Geers et al., 2003; Holt & Svirsky, 2008; Lu & Qin, 2018; O'Donoghue et al., 2000). Our results suggest that there is a decline of 0.15 PROSPER score for every 1-year delay in implantation, which could lead to a considerable difference of approximately 1 PROSPER score for a 6-year delay in implantation. This means the score at the plateaus correspond to a raw score of less than 50% versus a higher than 50% for PROSPER scores of 18 versus 19 for a 6-year delay in implantation. Such delay in implantation, if for the poorer ear, could also have negative consequences for the symmetric development of cortical auditory processing, leading to undesirable preference and reliance on the better hearing ear (Polonenko et al., 2018). Our results did not suggest an effect of age at implantation on the growth rate of speech recognition development, consistent with prior findings (Holt & Svirsky, 2008; Niparko et al., 2010; Tomblin et al., 2005). It is possible that the effect of age at implantation on speech recognition growth might become evident in studies with larger sample sizes such as the CDaCI studies and studies from the PMSTB working group (e.g., Eisenberg, Fink, & Niparko, 2006; Eisenberg, Johnson, et al., 2006; Fink et al., 2007; Uhler et al., 2017; Wang et al., 2008). It is also important to note that this study focused on the effects of age at CI activation on the overall trend in the developmental trajectories rather than its effects on speech recognition across groups of children implanted at different ages (e.g., implanting before 1 year vs. implanting between 1 and 2 years) or scores measured at a single age. Our results confirm that the age at CI activation affects speech recognition scores to the extent that older implanted children may not be able to compensate. There was an interaction between age at implantation and listening mode such that the age at implantation had a stronger effect on the score at the plateau for children with SIM BiCIs than those with UniCI and SEQ BiCIs and was also greater for children with UniCI than those with SEQ BiCIs. In fact, the score at the plateau was lower for all three groups of UniCI, SEQ BiCIs, and SIM BiCIs as the age at activation was higher with the steepest trend for listeners with SIM BiCIs. Of note, children with SEQ BiCI reached high levels of longer term speech recognition scores, regardless of the age at the first implanted ears, which might be due to experiencing a period of bimodal listening or additional factors such as residual acoustic hearing during interimplant period (Davidson et al., 2019), although the children in our cohort had a severe-to-profound degree of hearing loss, which is different from those who benefited from acoustic hearing in the Davidson et al. (2019) study.
The supportive role of bilateral implantation on speech perception and language development has been well documented (Boons et al., 2012; De Raeve et al., 2015; Galvin et al., 2007; Gordon, Wong, & Papsin, 2013; Grieco-Calub & Litovsky, 2011; Jacobs et al., 2016; Lammers et al., 2014; Leigh et al., 2013; Litovsky et al., 2006; Sarant et al., 2014, 2016; Zheng et al., 2015). Yet, there is limited information about how the growth rate and longer term speech recognition outcomes vary with different listening modes. Our results showed that children with BiCIs achieve greater longer term speech recognition scores than those with UniCI, suggesting a benefit of around 0.6 PROSPER score (β = 0.59, p = .02). Our further investigation revealed that the score at the plateau for the first implanted ears did not differ between children with SEQ BiCIs and those with SIM BiCIs, but children with both SEQ and SIM BiCIs had higher scores at the plateau than those with UniCI. Although there was no difference between groups in the rate of speech recognition development, this null effect of listening mode on the growth rate might be due to either the small sample size in our cohort or the fact that the outcome measure of speech recognition might not sufficiently capture variation in language skills or both. Therefore, it is possible that such an effect would become evident in future studies with additional outcome measures such as receptive and expressive language scores and a larger number of children. Nonetheless, the present findings reveal an advantage in bilateral implantation over unilateral implantation for longer term speech recognition of children with prelingual, severe-to-profound hearing with CIs.
Speech Recognition Development in Children With SEQ BiCIs
Our findings confirm that performance with the first implanted ears of children with SEQ BiCIs benefit from a shorter time interval between the first and second implantation. Children with prolonged interimplant interval exhibited a longer time from activation to achieving plateau performance, which could have negative consequences on the development of other skills. These results confirm prior findings on the supportive role of the shorter interval between two implantations on the development of the first implanted ears of children with SEQ BiCIs (Boons et al., 2012; Illg et al., 2019), suggesting that receiving a second implant sooner after the first implantation facilitates faster rates of the growth for the first implanted ear. In cases with asymmetric hearing loss, a shorter inter-implant interval could also promote symmetric organization of auditory cortical pathways, which could prevent preferring the implanted ear over the nonimplanted ear for hearing and facilitate balanced bilateral hearing (Polonenko et al., 2018, 2019), although potential loss of residual hearing should be considered.
The comparison of speech recognition development between the first and second implanted ears of children with SEQ BiCIs showed higher scores at the plateau for the first implanted ears. Our results show that the score at the plateau was approximately 1 PROSPER score greater for the first than second implanted ears of children with SEQ BiCIs (β = −0.9, p = .004). This difference was associated with the age at activation, underscoring the long-term effect of earlier implantation on better performance of the first implanted ear in speech recognition. However, there was a faster growth rate for the second implanted ears, which is likely due to using more advanced test materials for testing word recognition in the second implanted ears because the children were older. In fact, because of the relatively older age at the time of the second CI, speech recognition is more likely to be tested with more advanced test materials, leading to starting at higher values in the PROSPER score chart, which could confound the comparisons.
Clinical Implications
The findings have important clinical implications for better serving children with prelingual, severe-to-profound hearing loss. We demonstrated a modified scoring chart that integrates all available speech recognition scores from different test types into one score and allows for tracking speech recognition outcomes longitudinally. This scoring chart can be readily adjusted for different clinical centers and used to develop individualized rehabilitation programs tailored to the abilities of children with CIs. This tool could provide evidence for clinicians and researchers about the factors that predict speech recognition development following cochlear implantation. These findings could also assist clinicians in refining their decision-making process and setting evidence-based recommendations for optimizing the rehabilitation of prelingually deaf children who receive CIs, such as the timing of a second CI. Clinicians can use the trajectory of PROSPER scores for any child to compare them to children with normal hearing or other interventions to evaluate if the child is on track for speech perception development. To achieve this goal, this method will need to be used to develop normative data for children with normal hearing and those with hearing loss, HA devices, and CIs, allowing for the comparison of performance across groups. Our proposed system could serve as a standardized assessment protocol to allow clinicians a means to readily summarize patient's speech recognition scores over time, to facilitate continuity of care, evaluate the current rehabilitative strategies, and provide evidence-based counseling to families regarding realistic expectations for improvement of speech recognition abilities over time. After establishing this tool, future work could study speech perception development in children with progressive hearing loss and/or those with HAs. Future work can also examine how the PROSPER may also be useful in tracking performance of pediatric HA users. Overall, our results provide evidence on the trajectory of speech recognition development in prelingually deaf children with CIs, benefits of earlier implantation, bilateral implantation, and a shorter interimplant interval on the speech recognition development.
Limitations
As shared by most retrospective studies, it was not possible to control for many variables. The small number of samples in some groups, such as listening mode, limits the strength of our analyses and necessitates some caution in the interpretation and the generalization of the results. Compared to larger multicenter studies such as the CDaCI study (e.g., Eisenberg, Johnson, et al., 2006), our small sample also restricted our ability to construct benchmarks for speech recognition development of this population of pediatric CI users. Our study was also limited by challenges related to analyzing sparse longitudinal data (e.g., McKeague et al., 2011), which complicates obtaining a detailed understanding of growth patterns in speech recognition growth curves, although we employed two measures of the slope and the time from the activation to the plateau onset to enhance our characterization of the growth phase. In addition, children may vary in other aspects unrepresented in this study, such as their parents' socioeconomic status (e.g., Nittrouer et al., 2020; O'Donoghue et al., 2000), the quality of electrode–neuron interfaces (e.g., Arenberg Bierer, 2010; DiNino et al., 2019), and the properties of early linguistic environments (Arjmandi et al., 2021, 2022; Arora et al., 2020; Dilley et al., 2020) that could explain some variability in speech perception scores. For this cohort of children, this additional information was not available. Furthermore, clinical speech perception tests themselves do not completely capture the hearing abilities of children with CIs. Four of thirty-one children underwent revision cochlear implantation. The time between the removal of the initial CI and the activation of the revision CI was less than 1 month. Further prospective studies with more controlled variables, larger sample sizes, and employing the MEE PROSPER score are warranted to establish normative data for children with normal hearing and those with CIs and to explain the variance in performance and track the progress of our patients in reference to that of NH children. Furthermore, the findings of our study were based on speech recognition scores in quiet, which do not reflect performance of children with CIs in complex listening conditions such as in the presence of background noise or competing speech (Eisenberg et al., 2016). Future studies could use the same methodology to assess development of speech perception in noise and examine how different factors may contribute to the development of those skills.
Conclusions
This study used a modified PROSPER score to analyze the trajectory of speech recognition development over time in an integrated manner. Using this method, we quantified speech recognition development in children with CIs. Our study showed that age at CI activation is an important factor for longer term speech recognition in children with CIs, and that the first implanted ears of children improve more when children receive a second CI. For children with SEQ BiCIs, our results suggest that patients who receive their second implant sooner after their first to have a faster rate of growth in speech recognition, which could conceivably benefit the development of cognitive, social, and scholastic skills. The results further revealed that although speech recognition growth is faster for the second implanted ears of children with sequential bilateral CIs than their first implanted ears, the longer term speech recognition scores are better for the first implanted ears. Overall, the findings provide evidence for the clinical management of children with congenital hearing loss, in terms of the average trend and the extent of individual variability in the growth of speech recognition scores and longer term speech recognition abilities, the timing of CI activation, and listening mode.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01 DC012142 (awarded to J.G.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health. The authors would like to thank Piotr Marciniak for help with data extraction. The authors would also like to thank Lori Rakita and Charlotte Morse-Fortier for their support and thoughtful input on this work.
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01 DC012142 (awarded to J.G.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health.
References
- Arenberg Bierer, J. (2010). Probing the electrode-neuron interface with focused cochlear implant stimulation. Trends in Amplification, 14(2), 84–95. https://doi.org/10.1177/1084713810375249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjmandi, M. K. , Houston, D. , & Dilley, L. (2022). Variability in quantity and quality of early linguistic experience in children with cochlear implants: Evidence from analysis of natural auditory environments. Ear and Hearing, 43(2), 685–698. https://doi.org/10.1097/AUD.0000000000001136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjmandi, M. K. , Houston, D. , Wang, Y. , & Dilley, L. (2021). Estimating the reduced benefit of infant-directed speech in cochlear implant-related speech processing. Neuroscience Research, 171, 49–61. https://doi.org/10.1016/j.neures.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, S. , Smolen, E. R. , Wang, Y. , Hartman, M. , Howerton-fox, A. , & Rufsvold, R. (2020). Language environments and spoken language development of children with hearing loss. Journal of Deaf Studies and Deaf Education, 25(4), 457–468. https://doi.org/10.1093/deafed/enaa018 [DOI] [PubMed] [Google Scholar]
- Boons, T. , Brokx, J. P. L. , Frijns, J. H. M. , Peeraer, L. , Philips, B. , Vermeulen, A. , Wouters, J., & Van Wieringen, A. (2012). Effect of pediatric bilateral cochlear implantation on language development. Archives of Pediatrics and Adolescent Medicine, 166(1), 28–34. https://doi.org/10.1001/archpediatrics.2011.748 [DOI] [PubMed] [Google Scholar]
- Bø Wie, O. , Falkenberg, E. S. , Tvete, O. , & Tomblin, B. (2007). Children with a cochlear implant: Characteristics and determinants of speech recognition, speech-recognition growth rate, and speech production. International Journal of Audiology, 46(5), 232–243. https://doi.org/10.1080/14992020601182891 [DOI] [PubMed] [Google Scholar]
- Busch, T. , Vanpoucke, F. , & van Wieringen, A. (2017). Auditory environment across the life span of cochlear implant users: Insights from data logging. Journal of Speech, Language, and Hearing Research, 60(5), 1362–1377. https://doi.org/10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- Ching, T. Y. C. , Leigh, G. , & Dillon, H. (2013). Introduction to the longitudinal outcomes of children with hearing impairment (LOCHI) study: Background , design, sample characteristics. International Journal of Audiology, 52(Suppl. 2), S4–S9. https://doi.org/10.3109/14992027.2013.866342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, C. P. , Yang, D. , Pentz, M. A. , & Hser, Y. I. (2004). Piecewise growth curve modeling approach for longitudinal prevention study. Computational Statistics and Data Analysis, 46(2), 213–225. https://doi.org/10.1016/S0167-9473(03)00149-X [Google Scholar]
- Clark, J. G. (1981). Uses and abuses of hearing loss classification. ASHA, 23(7), 493–500. [PubMed] [Google Scholar]
- Colletti, L. , Mandalà, M. , & Colletti, V. (2012). Cochlear implants in children younger than 6 months. Otolaryngology—Head & Neck Surgery, 147(1), 139–146. https://doi.org/10.1177/0194599812441572 [DOI] [PubMed] [Google Scholar]
- Davidson, L. S. , Geers, A. E. , Blamey, P. J. , Tobey, E. , & Brenner, C. (2011). Factors contributing to speech perception scores in long-term pediatric cochlear implant users. Ear and Hearing, 32(1), 19S–26S. https://doi.org/10.1097/AUD.0b013e3181ffdb8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, L. S. , Geers, A. E. , Uchanski, R. M. , & Firszt, J. B. (2019). Effects of early acoustic hearing on speech perception and language for pediatric cochlear implant recipients. Journal of Speech, Language, and Hearing Research, 62(9), 3620–3637. https://doi.org/10.1044/2019_JSLHR-H-18-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raeve, L. , Vermeulen, A. , & Snik, A. (2015). Verbal cognition in deaf children using cochlear implants: Effect of unilateral and bilateral stimulation. Audiology and Neurotology, 20(4), 261–266. https://doi.org/10.1159/000381003 [DOI] [PubMed] [Google Scholar]
- Dettman, S. J. , Dowell, R. C. , Choo, D. , Arnott, W. , Abrahams, Y. , Davis, A. , Dornan, D. , Leigh, J. , Constantinescu, G. , Cowan, R. , & Briggs, R. J. (2016). Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology and Neurotology, 37(2), e82–e95. https://doi.org/10.1097/MAO.0000000000000915 [DOI] [PubMed] [Google Scholar]
- Dilley, L. , Lehet, M. , Wieland, E. A. , Arjmandi, M. K. , Kondaurova, M. , Wang, Y. , Reed, J. , Svirsky, M. , Houston, D. , & Bergeson, T. (2020). Individual differences in mothers' spontaneous infant-directed speech predict language attainment in children with cochlear implants. Journal of Speech, Language, and Hearing Research, 63(7), 2453–2467. https://doi.org/10.1044/2020_JSLHR-19-00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNino, M. , O'Brien, G. , Bierer, S. M. , Jahn, K. N. , & Arenberg, J. G. (2019). The estimated electrode-neuron interface in cochlear implant listeners is different for early-implanted children and late-implanted adults. Journal of the Association for Research in Otolaryngology, 20(3), 291–303. https://doi.org/10.1007/s10162-019-00716-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominicus, A. , Ripatti, S. , Pedersen, N. L. , & Palmgren, J. (2008). A random change point model for assessing variability in repeated measures of cognitive function. Statistics in Medicine, 27(27), 5786–5798. https://doi.org/10.1002/sim.3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , García Marquéz, J. R. , Gruber, B. , Lafourcade, B. , Leitão, P. J. , Münkemüller, T. , McClean, C. , Osborne, P. E. , Reineking, B. , Schröder, B. , Skidmore, A. K. , Zurell, D. , & Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36(1), 27–46, https://doi.org/10.1111/j.1600-0587.2012.07348.x [Google Scholar]
- Dowell, R. C. (2002). Speech perception in children using cochlear implants: Prediction of long-term outcomes. Cochlear Implants International, 3(1), 1–18. https://doi.org/10.1179/cim.2002.3.1.1 [DOI] [PubMed] [Google Scholar]
- Dunn, C. C. , Walker, E. A. , Oleson, J. , Kenworthy, M. , Van Voorst, T. , Tomblin, J. B. , Ji, H. , Kirk, K. , McMurray, B. , Hanson, M. , & Gantz, B. J. (2014). Longitudinal speech perception and language performance in pediatric cochlear implant users: The effect of age at implantation. Ear and Hearing, 35(2), 148–160, https://doi.org/10.1097/AUD.0b013e3182a4a8f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, L. S. , Fink, N. E. , & Niparko, J. K. (2006). Childhood development after cochlear implantation: Multicenter study examines language development. The ASHA Leader, 11(16), 5–29. https://doi.org/10.1044/leader.ftr2.11162006.5 [Google Scholar]
- Eisenberg, L. S. , Fisher, L. M. , Johnson, K. C. , Ganguly, D. H. , Grace, T. , & Niparko, J. K. (2016). Sentence recognition in quiet and noise by pediatric cochlear implant users: Relationships to spoken language. Otology & Neurotology, 37(2), e75–e81. https://doi.org/10.1097/mao.0000000000000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, L. S. , Johnson, K. C. , Martinez, A. S. , Cokely, C. G. , Tobey, E. A. , Quittner, A. L. , Fink, N. E. , Wang, N.-Y. , & Niparko, J. K. (2006). Speech recognition at 1-year follow-up in the Childhood Development after Cochlear Implantation study: Methods and preliminary findings. Audiology and Neurotology, 11(4), 259–268. https://doi.org/10.1159/000093302 [DOI] [PubMed] [Google Scholar]
- Erber, N. P. (1982). Auditory training. Alexander Graham Bell Association for the Deaf. https://doi.org/10.3109/00016485209136874 [Google Scholar]
- Eskander, A. , Gordon, K. , Kadhim, L. , Papaioannou, V. , Cushing, S. L. , James, A. L. , & Papsin, B. C. (2011). Low pediatric cochlear implant failure rate: Contributing factors in large-volume practice. Archives of Otolaryngology—Head & Neck Surgery, 137(12), 1190–1196. https://doi.org/10.1001/archoto.2011.200 [DOI] [PubMed] [Google Scholar]
- Fink, N. E. , Wang, N. Y. , Visaya, J. , Niparko, J. K. , Quittner, A. , Eisenberg, L. S. , & Tobey, E. A. (2007). Childhood development after Cochlear Implantation (CDaCI) study: Design and baseline characteristics. Cochlear Implants International, 8(2), 92–112. https://doi.org/10.1002/cii.333 [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy, H. , Tyler, R. S. , Kelsay, D. M. , Gantz, B. J. , & Woodworth, G. G. (1997). Cochlear implant use by prelingually deafened children. Journal of Speech, Language, and Hearing Research, 40(1), 183–199. https://doi.org/10.1044/jslhr.4001.183 [DOI] [PubMed] [Google Scholar]
- Galvin, K. L. , Leigh, J. R. , & Hughes, K. C. (2009). How we do it: Clinical management of the child receiving a second, bilateral cochlear implant. Cochlear Implants International, 10(2), 84–91. https://doi.org/10.1002/cii.371 [DOI] [PubMed] [Google Scholar]
- Galvin, K. L. , Mok, M. , & Dowell, R. C. (2007). Perceptual benefit and functional outcomes for children using sequential bilateral cochlear implants. Ear and Hearing, 28(4), 470–482. https://doi.org/10.1097/AUD.0b013e31806dc194 [DOI] [PubMed] [Google Scholar]
- Geers, A. , Brenner, C. , & Davidson, L. (2003). Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing, 24(1), 24S–35S. https://doi.org/10.1097/01.aud.0000051687.99218.0f [DOI] [PubMed] [Google Scholar]
- Geers, A. E. , & Moog, J. S. (1987). Predicting spoken language acquisition of profoundly hearing-impaired children. Journal of Speech and Hearing Disorders, 52(1), 84–94. https://doi.org/10.1044/jshd.5201.84 [DOI] [PubMed] [Google Scholar]
- Geers, A. E. , & Nicholas, J. G. (2013). Enduring advantages of early cochlear implantation for spoken language development. Journal of Speech, Language, and Hearing Research, 56(2), 643–655. https://doi.org/10.1044/1092-4388(2012/11-0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers, A. E. , Strube, M. J. , Tobey, E. A. , & Moog, J. S. (2011). Epilogue: Factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear and Hearing, 32(1), 84S–92S. https://doi.org/10.1097/AUD.0b013e3181ffd5b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers, A. , Tobey, E. , Moog, J. , & Brenner, C. (2008). Long-term outcomes of cochlear implantation in the preschool years: From elementary grades to high school. International Journal of Audiology, 47(Suppl. 2), S21–S30. https://doi.org/10.1080/14992020802339167 [DOI] [PubMed] [Google Scholar]
- Gordon, K. A. , Jiwani, S. , & Papsin, B. C. (2011). What is the optimal timing for bilateral cochlear implantation in children? Cochlear Implants International, 12(Suppl. 2), S14–SS8. https://doi.org/10.1179/146701011x13074645127199 [DOI] [PubMed] [Google Scholar]
- Gordon, K. A. , Jiwani, S. , & Papsin, B. C. (2013). Benefits and detriments of unilateral cochlear implant use on bilateral auditory development in children who are deaf. Frontiers in Psychology, 4, 719. https://doi.org/10.3389/fpsyg.2013.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, K. A. , & Papsin, B. C. (2009). Benefits of short interimplant delays in children receiving bilateral cochlear implants. Otology and Neurotology, 30(3), 319–331. https://doi.org/10.1097/MAO.0b013e31819a8f4c [DOI] [PubMed] [Google Scholar]
- Gordon, K. A. , Wong, D. D. E. , & Papsin, B. C. (2013). Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain, 136(5), 1609–1625. https://doi.org/10.1093/brain/awt052 [DOI] [PubMed] [Google Scholar]
- Grieco-Calub, T. M. , & Litovsky, R. Y. (2011). Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear and Hearing, 31(5), 645–656. https://doi.org/10.1097/AUD.0b013e3181e50a1d.Sound [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins, H. L. (1949). A phonetically balanced test of speech discrimination for children [Doctoral dissertation, Northwestern University] . [Google Scholar]
- Hirsh, I. J. , Davis, H. , Silverman, S. R. , Reynolds, E. G. , Eldert, E. , & Benson, R. W. (1952). Development of materials for speech audiometry. Journal of Speech and Hearing Disorders, 17(3), 321–337. https://doi.org/10.1044/jshd.1703.321 [DOI] [PubMed] [Google Scholar]
- Holt, R. F. , & Svirsky, M. A. (2008). An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing, 29(4), 492–511. https://doi.org/10.1097/AUD.0b013e31816c409f.An [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston, D. M. , Chen, C. , Monroy, C. , & Castellanos, I. (2020). How early auditory experience affects children's ability to learn spoken words. In Marschark M. & Knoors H. (Eds.), The Oxford handbook of deaf studies in learning and cognition (pp. 122–137). Oxford University Press. [Google Scholar]
- Hughes, K. C. , & Galvin, K. L. (2013). Measuring listening effort expended by adolescents and young adults with unilateral or bilateral cochlear implants or normal hearing. Cochlear Implants International, 14(3), 121–129. https://doi.org/10.1179/1754762812Y.0000000009 [DOI] [PubMed] [Google Scholar]
- Illg, A. , Sandner, C. , Büchner, A. , Lenarz, T. , Kral, A. , & Lesinski-Schiedat, A. (2019). The optimal inter-implant interval in pediatric sequential bilateral implantation. Hearing Research, 372, 80–87. https://doi.org/10.1016/j.heares.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Jacobs, E. , Langereis, M. C. , Frijns, J. H. M. , Free, R. H. , Goedegebure, A. , Smits, C. , Stokroos, R. J. , Ariens-Meijer, S. A. M. , Mylanus, E. A. M. , & Vermeulen, A. M. (2016). Benefits of simultaneous bilateral cochlear implantation on verbal reasoning skills in prelingually deaf children. Research in Developmental Disabilities, 58, 104–113. https://doi.org/10.1016/j.ridd.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Jeong, S. W. , Chung, S. H. , & Kim, L. S. (2018). P1 cortical auditory evoked potential in children with unilateral or bilateral cochlear implants; implication for the timing of second cochlear implantation. European Archives of Oto-Rhino-Laryngology, 275(7), 1759–1765. https://doi.org/10.1007/s00405-018-5021-5 [DOI] [PubMed] [Google Scholar]
- Johnston, J. C. , Durieux-Smith, A. , Angus, D. , O'Connor, A. , & Fitzpatrick, E. (2009). Bilateral paediatric cochlear implants: A critical review. International Journal of Audiology, 48(9), 601–617. https://doi.org/10.1080/14992020802665967 [DOI] [PubMed] [Google Scholar]
- Kirk, K. I. , Miyamoto, R. T. , Lento, C. L. , Ying, E. , O'Neill, T. , & Fears, B. (2002). Effects of age at implantation in young children. Annals of Otology, Rhinology & Laryngology, 111(Suppl. 5), 69–73. https://doi.org/10.1177/00034894021110s515 [DOI] [PubMed] [Google Scholar]
- Kral, A. , Kronenberger, W. G. , Pisoni, D. B. , & O'Donoghue, G. M. (2016). Neurocognitive factors in sensory restoration of early deafness: A connectome model. The Lancet Neurology, 15(6), 610–621. https://doi.org/10.1016/S1474-4422(16)00034-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers, M. J. W. , Van Der Heijden, G. J. M. G. , Pourier, V. E. C. , & Grolman, W. (2014). Bilateral cochlear implantation in children: A systematic review and best-evidence synthesis. The Laryngoscope, 124(7), 1694–1699. https://doi.org/10.1002/lary.24582 [DOI] [PubMed] [Google Scholar]
- Lehiste, I. , & Peterson, G. E. (1959). Linguistic considerations in the study of speech intelligibility. The Journal of the Acoustical Society of America, 31(3), 280–286. https://doi.org/10.1121/1.1907713 [Google Scholar]
- Leigh, J. , Dettman, S. , Dowell, R. , & Briggs, R. (2013). Communication development in children who receive a cochlear implant by 12 months of age. Otology and Neurotology, 34(3), 443–450. https://doi.org/10.1097/MAO.0b013e3182814d2c [DOI] [PubMed] [Google Scholar]
- Lin, F. R. , Niparko, J. K. , & Francis, H. W. (2012). Outcomes in cochlear implantation: Assessment of quality-of-life impact and economic evaluation of the benefits of the cochlear implant in relation to costs. In Niparko J. K. (Ed.), Cochlear implants: Principles and practices (2nd ed., pp. 229–244). Wolters Kluwer Health. [Google Scholar]
- Litovsky, R. Y. , Johnstone, P. M. , & Godar, S. P. (2006). Benefits of bilateral cochlear implants and/or hearing aids in children. International Journal of Audiology, 45(Suppl. 1), S78–S91. https://doi.org/10.1080/14992020600782956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X. , & Qin, Z. (2018). Auditory and language development in Mandarin-speaking children after cochlear implantation. International Journal of Pediatric Otorhinolaryngology, 107, 183–189. https://doi.org/10.1016/j.ijporl.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Manlove, E. E. , Frank, T. , & Vernon-Feagans, L. (2001). Why should we care about noise in classrooms and child care settings? Child and Youth Care Forum, 30(1), 55–64. https://doi.org/10.1023/A:1016663520205 [Google Scholar]
- Manrique, M. , Cervera-Paz, F. J. , Huarte, A. , & Molina, M. D. A.-A. (2004). Advantages of cochlear implantation in prelingual deaf children before 2 years of age when compared with later implantation. The Laryngoscope, 114(8), 1462–1469. https://doi.org/10.1097/00005537-200408000-00027 [DOI] [PubMed] [Google Scholar]
- McKeague, I. W. , López-Pintado, S. , Hallin, M. , & Šiman, M. (2011). Analyzing growth trajectories. Journal of Developmental Origins of Health and Disease, 2(06), 322–329. https://doi.org/10.1017/S2040174411000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, M. R. (2011). Speech and language delay in children. American Family Physician, 83(10), 1183–1188. [PubMed] [Google Scholar]
- Niparko, J. K. , Tobey, E. A. , Thal, D. J. , Eisenberg, L. S. , Wang, N.-Y. , Quittner, A. L. , & Fink, N. E. (2010). Spoken language development in children following cochlear implantation. JAMA, 303(15), 1498–1506. https://doi.org/10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer, S. , Lowenstein, J. H. , & Antonelli, J. (2020). Parental language input to children with hearing loss: Does it matter in the end? Journal of Speech, Language, and Hearing Research, 63(1), 234–258. https://doi.org/10.1044/2019_JSLHR-19-00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue, G. M. , Nikolopoulos, T. P. , & Archbold, S. M. (2000). Determinants of speech perception in children after cochlear implantation. The Lancet, 356(9228), 466–468. https://doi.org/10.1016/S0140-6736(00)02555-1 [DOI] [PubMed] [Google Scholar]
- Peters, B. R. , Wyss, J. , Aud, D. , & Manrique, M. (2010). Worldwide trends in bilateral cochlear implantation. The Laryngoscope, 120(S2), S17–S44. https://doi.org/10.1002/lary.20859 [DOI] [PubMed] [Google Scholar]
- Peterson, G. E. , & Lehiste, I. (1962). Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders, 27(1), 62–70. https://doi.org/10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- Pisoni, D. B. (2012). Cognitive factors and cochlear implants: some thoughts on perception, learning, and memory in speech perception. Ear and Hearing, 21(1), 70–78. https://doi.org/10.1097/00003446-200002000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni, D. B. , Kronenberger, W. G. , Harris, M. S. , & Moberly, A. C. (2018). Three challenges for future research on cochlear implants. World Journal of Otorhinolaryngology—Head and Neck Surgery, 3(4), 240–254. https://doi.org/10.1016/j.wjorl.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko, M. J. , Papsin, B. C. , & Gordon, K. A. (2018). Delayed access to bilateral input alters cortical organization in children with asymmetric hearing. NeuroImage: Clinical, 17, 415–425. https://doi.org/10.1016/j.nicl.2017.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko, M. J. , Papsin, B. C. , & Gordon, K. A. (2019). Cortical plasticity with bimodal hearing in children with asymmetric hearing loss. Hearing Research, 372, 88–98. https://doi.org/10.1016/j.heares.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Ross, M. , & Lerman, J. A. Y. (1970). A picture identification test for hearing-impaired children. Journal of Speech and Hearing Research, 13(1), 44–53. https://doi.org/10.1044/jshr.1301.44 [DOI] [PubMed] [Google Scholar]
- Ruffin, C. V. , Kronenberger, W. G. , Colson, B. G. , Henning, S. C. , & Pisoni, D. B. (2013). Long-term speech and language outcomes in prelingually deaf children, adolescents and young adults who received cochlear implants in childhood. Audiology and Neurotology, 18(5), 289–296. https://doi.org/10.1159/000353405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant, J. , Harris, D. , Bennet, L. , & Bant, S. (2014). Bilateral versus unilateral cochlear implants in children: A study of spoken language outcomes. Ear and Hearing, 35(4), 396–409. https://doi.org/10.1097/AUD.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant, J. Z. , Harris, D. C. , & Bennet, L. A. (2016). Academic outcomes for school-aged children with severe–profound hearing loss and early unilateral and bilateral cochlear implants. Journal of Speech, Language, and Hearing Research, 58(3), 1017–1032. https://doi.org/10.1044/2015_JSLHR-H-14-0075 [DOI] [PubMed] [Google Scholar]
- Schnabl, J. , Bumann, B. , Rehbein, M. , Müller, O. , Seidler, H. , Wolf-Magele, A. , Sprinzl, G. , Windfuhr, J. , & Weichbold, V. (2021). Höranstrengung bei Cochlea-Implantaten. HNO 63, 63(8), 546–551. https://doi.org/10.1007/s00106-015-0020-y [DOI] [PubMed] [Google Scholar]
- Sharma, A. , Dorman, M. F. , & Kral, A. (2005). The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hearing Research, 203(1–2), 134–143. https://doi.org/10.1016/j.heares.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Shield, B. , Conetta, R. , Dockrell, J. , Connolly, D. , Cox, T. , & Mydlarz, C. (2015). A survey of acoustic conditions and noise levels in secondary school classrooms in England. The Journal of the Acoustical Society of America, 137(1), 177–188. https://doi.org/10.1121/1.4904528 [DOI] [PubMed] [Google Scholar]
- Silva, P. A. , McGee, R. , & Williams, S. M. (1983). Developmental language delay from three to seven years and its significance for low intelligence and reading difficulties at age seven. Developmental Medicine & Child Neurology, 25(6), 783–793. https://doi.org/10.1111/j.1469-8749.1983.tb13847.x [DOI] [PubMed] [Google Scholar]
- Silva, P. A. , Williams, S. , & McGee, R. (1987). A longitudinal study of children with developmental language delay at age three: Later intelligence, reading and behaviour problems. Developmental Medicine & Child Neurology, 29(5), 630–640. https://doi.org/10.1111/j.1469-8749.1987.tb08505.x [DOI] [PubMed] [Google Scholar]
- Steffens, T. , Lesinski-Schiedat, A. , Strutz, J. , Aschendorff, A. , Klenzner, T. , Rühl, S. , Voss, B. , Wesarg, T. , Laszig, R. , & Lenarz, T. (2008). The benefits of sequential bilateral cochlear implantation for hearing-impaired children. Acta Oto-Laryngologica, 128(2), 164–176. https://doi.org/10.1080/00016480701411528 [DOI] [PubMed] [Google Scholar]
- Strøm-roum, H. , Laurent, C. , & Wie, O. B. (2012). Comparison of bilateral and unilateral cochlear implants in children with sequential surgery. International Journal of Pediatric Otorhinolaryngology, 76(1), 95–99. https://doi.org/10.1016/j.ijporl.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Svirsky, M. A. , Robbins, A. M. , Kirk, K. I. , Pisoni, D. B. , & Miyamoto, R. T. (2000). Language development in profoundly deaf children with cochlear implants. Psychological Science, 11(2), 153–158. https://doi.org/10.1111/1467-9280.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman, T. W. , & Carhart, R. (1966). An expanded test for speech discrimination utilizing CNC monosyllabic words: Northwestern University Auditory Test No. 6. Northwestern University Auditory Research Lab. [DOI] [PubMed]
- Tobey, E. A. , Thal, D. , Niparko, J. K. , Eisenberg, L. S. , Quittner, A. L. , & Wang, N. Y. (2013). Influence of implantation age on school-age language performance in pediatric cochlear implant users. International Journal of Audiology, 52(4), 219–229. https://doi.org/10.3109/14992027.2012.759666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin, J. B. , Barker, B. A. , Spencer, L. J. , Zhang, X. , & Gantz, B. J. (2005). The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research, 48(4), 853–867. https://doi.org/10.1044/1092-4388(2005/059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble, K. , Rosella, L. C. , Propst, E. , Gordon, K. A. , Papaioannou, V. , & Papsin, B. C. (2008). Speech perception outcome in multiply disabled children following cochlear implantation: Investigating a predictive score. Journal of the American Academy of Audiology, 19(8), 602–611. https://doi.org/10.3766/jaaa.19.8.4 [DOI] [PubMed] [Google Scholar]
- Uhler, K. , Warner-Czyz, A. , & Gifford, R. (2017). Pediatric minimum speech test battery. Journal of the American Academy of Audiology, 28(3), 232–247. https://doi.org/10.3766/jaaa.15123 [DOI] [PubMed] [Google Scholar]
- Uhler, K. , Yoshinaga-Itano, C. , Gabbard, S. A. , Rothpletz, A. M. , & Jenkins, H. (2011). Longitudinal infant speech perception in young cochlear implant users. Journal of the American Academy of Audiology, 22(3), 129–142. https://doi.org/10.3766/jaaa.22.3.2 [DOI] [PubMed] [Google Scholar]
- Waltzman, S. B. , Cohen, N. L. , Green, J. , & Roland, J. T. (2002). Long-term effects of cochlear implants in children. Otolaryngology—Head & Neck Surgery, 126(5), 505–511. https://doi.org/10.1067/mhn.2002.124472 [DOI] [PubMed] [Google Scholar]
- Wang, N. Y. , Eisenberg, L. S. , Johnson, K. C. , Fink, N. E. , Tobey, E. A. , Quittner, A. L. , Niparko, J. K. , & The CDaCI Investigative Team (2008). Tracking development of speech recognition: Longitudinal data from hierarchical assessments in the childhood development after cochlear implantation study. Otology and Neurotology, 29(2), 240–245. https://doi.org/10.1097/MAO.0b013e3181627a37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Czyz, A. D. , Loy, B. , Tobey, E. A. , Nakonezny, P. , & Roland, P. S. (2011). Health-related quality of life in children and adolescents who use cochlear implants. International Journal of Pediatric Otorhinolaryngology, 75(1), 95–105. https://doi.org/10.1016/j.ijporl.2010.10.018 [DOI] [PubMed] [Google Scholar]
- Wie, O. B. , Torkildsen, J. V. K. , Schauber, S. , Busch, T. , & Litovsky, R. (2020). Long-term language development in children with early simultaneous bilateral cochlear implants. Ear and Hearing, 41(5), 1294–1305. https://doi.org/10.1097/AUD.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, J. , Griffin, A. , Newton, S. , Kenna, M. , & Licameli, G. R. (2018). Revision cochlear implant surgery in children: Surgical and audiological outcomes. The Laryngoscope, 128(11), 2619–2624. https://doi.org/10.1002/lary.27198 [DOI] [PubMed] [Google Scholar]
- Zeitler, D. M. , Kessler, M. A. , Terushkin, V. , Roland, J. T. , Svirsky, M. A. , Lalwani, A. K. , & Waltzman, S. B. (2008). Speech perception benefits of sequential bilateral cochlear implantation in children and adults: A retrospective analysis. Otology and Neurotology, 29(3), 314–325. https://doi.org/10.1097/MAO.0b013e3181662cb5 [DOI] [PubMed] [Google Scholar]
- Zeng, F. G. (2017). Challenges in improving cochlear implant performance and accessibility. IEEE Transactions on Biomedical Engineering, 64(8), 1662–1664. https://doi.org/10.1109/TBME.2017.2718939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Wei, C. , Zhang, Y. , Zeng, Z. , Cao, K. , & Liu, Y. (2020). Sequential bilateral cochlear implantation with prolonged time intervals. Journal of Speech, Language, and Hearing Research, 63(9), 3195–3207. https://doi.org/10.1044/2020_JSLHR-20-00140 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. , Godar, S. P. , & Litovsky, R. Y. (2015). Development of sound localization strategies in children with bilateral cochlear implants. PLOS ONE, 10(8), e0135790. https://doi.org/10.5061/dryad.0m09h [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.