Abstract
Objectives:
Preterm infants are born functionally pancreatic insufficient with decreased pancreatic production of lipase and proteases. Developmental pancreatic insufficiency (PI) may contribute to reduced nutrient absorption and growth failure. We sought to determine longitudinal fecal elastase (ELA1) levels in a cohort of preterm infants and whether levels are associated with growth outcomes.
Methods:
Prospective observational study of 30 infants 24-34 weeks gestational age and birth weight ≤1250 grams fed the exclusive human milk diet, consisting of human milk with human milk-based fortifier. ELA1 was quantified by ELISA during the first two weeks of life (Early; 7.5 ± 1.8 days of life) and after attainment of full, fortified feedings (Late; 63.6 ± 24.1 days of life).
Results:
Early ELA1 levels were 192.2 ± 96.4 μg/g, and Late ELA1 levels were 268.0 ± 80.3 μg/g, 39.4% higher (p=0.01). Infants with early PI (ELA1 <200 μg/g) were more likely male and of lower gestational age, weight, length, and head circumference at birth. These variables, but not PI status, independently predicted somatic growth.
Conclusions:
Fecal ELA1 in preterm infants fed exclusive human milk diet increases with postnatal age. Although pancreatic function in preterm infants may serve as a biological contributor to early postnatal growth failure, additional studies using fecal ELA1 as a predictive biomarker for growth failure are needed in larger cohorts.
Keywords: exclusive human milk diet, growth failure, pancreatic insufficiency, fecal elastase
Introduction
Growth failure in preterm infants increases risk for morbidities and long-term metabolic derangements.1,2 Initial nutritional strategies focus on parenteral and enteral combinations3 with human milk as the recommended enteral diet.4 Human milk contains bioactive agents that support gastrointestinal development and function,4 increase gut microbial diversity,5 and decrease gastrointestinal morbidity.5
Immature pancreatic function may contribute to growth failure. Fecal elastase 1 (ELA1) is a marker of exocrine pancreatic function.6 ELA1 is a protease, first synthesized at 12 weeks and secreted at 20 weeks gestation.7 Pancreatic sufficiency (PS) defined as ELA1 >200 μg/g stool is the currently accepted measure with no established standard in preterm infants.6 ELA1 concentrations are lower in preterm compared to term infants8 and lower in meconium compared to stool.8 ELA1 increases with formula feedings and time, the individual contributions of which are difficult to differentiate.9 The ELA1 ontogeny remains poorly understood8 with only sparse data related to gestational age.10 Furthermore, a paucity of data exists regarding ELA1 in preterm infants with different feeding practices.
Growth management in pancreatic insufficiency (PI) remains challenging.11 Term newborns’ PI may be compensated by human milk enzymes.12 Whether pancreatic maturation rates are influenced by human milk feeding remains unknown. Previously, ELA1 was measured in preterm infants fed human milk with bovine fortification,13 but little is known regarding ELA1 from preterm infants fed the exclusive human milk diet, consisting of human milk fortified with donor human milk-based fortifier.14 We hypothesized that preterm infants fed the exclusive human milk diet would exhibit increasing ELA1 over time.
Methods
This is a prospective observational study of 30 preterm infants between 24-34 weeks gestational age (GA) and birth weight (BW) ≤1250 g. Study subjects were recruited from a single level IV neonatal intensive care unit at Baylor College of Medicine/Texas Children’s Hospital, Houston, Texas. Infants were enrolled from October 8, 2018, through August 8, 2019, within the first 3 days of life (DOL). Infants were excluded for >1250 g BW, concern for survival, major congenital anomalies or clinically significant congenital heart disease, intestinal perforation, Bell’s stage 2 or greater necrotizing enterocolitis, early transfer to another institution, or failure to achieve full enteral feedings by 28 DOL. During the study period, 147 infants were screened, among which 117 infants were excluded (Supplemental Digital Content Figure 1). The Institutional Review Board at Baylor College of Medicine/Texas Children’s Hospital approved this study, and informed written consent was obtained prior to enrollment in each parent’s/guardian’s preferred language.
A standard feeding protocol was followed by all clinicians in this center. Enteral feeding was initiated with unfortified human milk at 20 ml/kg/day for the first 3-5 DOL, then advanced by 20 ml/kg/day to full feeding volume of 150-160 ml/kg/day. When enteral feeding volume reached 60 ml/kg/day, fortification occurred with human milk-based fortifier providing an additional 6 kcal/oz.14 If infants displayed growth failure (<15g/kg/day weight gained averaged over 5 days)1 after attainment of full volume enteral feedings, additional human milk-based fortifier was provided.
Stool samples were collected at two time points. Early is defined as within the first two weeks of life. Late is defined as at or later than 4 weeks of life and after attainment of full, fortified enteral feedings. Late stool samples were obtained prior to 4 weeks if the infant was discharged home earlier. PI was defined as ELA1 concentrations <200 μg/g stool as the currently accepted measure with no established standard for preterm infants,9 and infants were classified as PS or PI at the Early timepoint and remained in the assigned group for study duration. Each stool sample was collected and stored at −80 C until analysis by enzyme-linked immunosorbent assay for ELA1 (Immundiagnostik AG, Bensheim, Germany).15 Results were expressed as micrograms ELA1 per gram of stool (μg/g). Stool samples were not dried prior to analysis due to the small volume fecal sample and consisted of a consecutive 24-hour period of collection, recognizing the degree of fluctuation in ELA1 between stool samples.
Maternal and infant clinical data were collected by electronic medical record review. Maternal data included age, gravida, parity, and race. Infant data included demographics, delivery information, and dietary intake. The Score for Neonatal Acute Physiology version II (SNAP-II) was used to measure severity of illness at birth.16,17 Anthropometric measures of weight, length, and head circumference (HC), growth Z scores, and individual growth velocities18 were obtained. Using the sex-specific Fenton 2013 growth curves,19 Z scores were calculated at DOL 28 and 36 weeks post menstrual age (PMA) for weight, length, and HC. Because most infants within our institution achieve full, fortified enteral feedings by DOL 21, nutritional intake data were quantified at DOL 28 when most infants achieve growth targets with minimal adjustments in feeding regimens until transition to discharge feeding regimen. Growth outcomes were measured at 36 weeks PMA to allow for direct comparison of growth to other studies in the literature, and additionally to serve as the latest time point to capture all infants prior to discharge.
Daily enteral milk preparation record sheets were obtained throughout study enrollment and included volumes of each constituent of the exclusive human milk diet. Macronutrients and energy totals were calculated from enteral milk feeding volumes with product information for each constituient.14 All human milk, both mother’s own milk and donor human milk, was assumed to provide 20 kcal/oz when unfortified, although human milk is known to have significant variation in energy and macronutrients.20 Total volume of base human milk is defined as the sum of unfortified mother’s own milk and donor human milk. Majority mother’s own milk is defined as >70% of total volume of base human milk documented for both Early and Late sample collection dates.
Normality was determined by Shapiro-Wilk test. For comparisons of normally distributed quantitative variables, an unpaired t-test was used, and the mean ± standard deviation was reported for each group (Early and Late). For the comparison of Early vs Late ELA1 concentrations, a paired t-test was used and the mean ± standard deviation was reported for each time point. If the assumption of normality was violated in either group, the Wilcoxon Rank Sum test was used for comparisons of PI versus PS and the median with interquartile range (IQR) was used to summarize group distributions. Fisher’s exact test was used to compare categorical variables, such as race, surfactant use, or prenatal steroids. A multilinear regression analysis was performed through stepwise procedures to estimate the contribution of PS to growth outcomes at 28 DOL and at 36 weeks PMA (R Version 4.2.1). To characterize the ELA1 over time for all obtained levels, we performed linear regression models of ELA1 by DOL adjusted by percent human milk and GA (Figure 2). We conducted stepwise analysis of potential confounders in two sequential categories: (1) infant characteristics: GA, BW, birth length, birth HC, male sex, small for gestational age (SGA), race, Apgar at 1 or 5 minutes, SNAP-II scores; (2) nutritional outcomes: DOL at first feeding, days to full feedings, days to regain birth weight, total parenteral nutrition (TPN) days, length of stay, percent mother’s own milk of base human milk provided, enteral volume and energy, and enteral protein, fat and carbohydrate intake (Supplemental Digital Content Table 2). If a variable was found to be significant, it was left in the model prior to adjusting for factors in the second category. We created a model leaving in variables with p value of less than 0.1 or if variables are reported as important confounders in prior studies. The final model included GA, male sex, SGA, and days to full feedings (Supplemental Digital Content Table 1). Type 1 error (alpha) of 0.05 was used to determine statistical significance between two groups, PI compared to PS or Early compared to Late.
Figure 2:
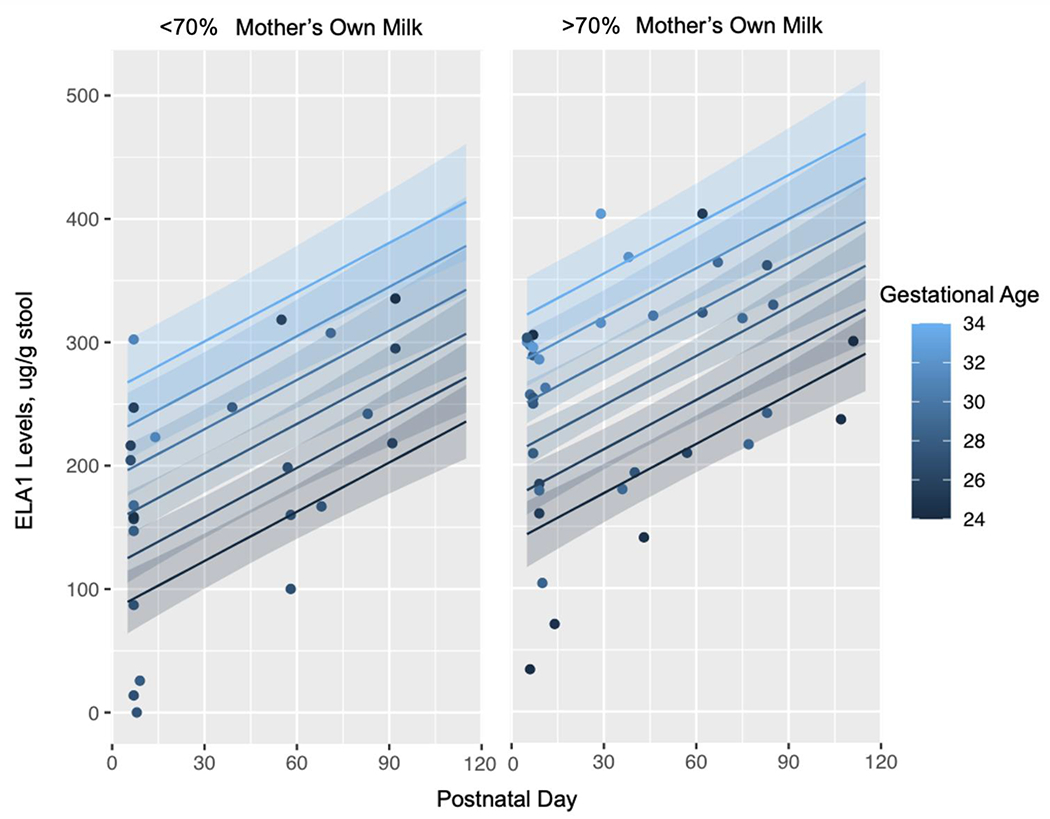
Linear Regression Models of Predicted Fecal ELA1 levels by Postnatal Day Adjusted for Majority Mother’s Own Milk (MOM) and Gestational Age.
Results
Of the 30 infants enrolled in the study, n=16 infants were PS and n=14 infants were PI at the Early time point (Table 1). Compared to infants with PS, infants with PI were younger in GA (p=0.02), had lower BW (p=0.03), were smaller in length (p=0.02) and HC (p=0.007), and were more likely male sex (p=0.004). All other characteristics were similar between the groups.
Table 1:
Birth Infant Baseline Characteristics, Nutritional Data and Growth Outcomes of Pancreatic Insufficient (PI) to Pancreatic Sufficient (PS).
| PI (N=14) | PS (N=16) | p Value | Adjusted p Value* | |
|---|---|---|---|---|
| Gestational Age (Weeks)1 | 26.8 ± 1.6 | 28.6 ± 2.3 | 0.02 | |
| Weight (g)1 | 903.6 ± 176.0 | 1052.8 ± 168.8 | 0.03 | |
| Length (cm)2 | 33.9 (33.2, 36.3) | 36.5 (35.3, 37.8) | 0.02 | |
| Head Circumference (cm)1 | 23.2 ± 1.4 | 24.9 ± 1.7 | 0.007 | |
| Male3 | 12 (86) | 5 (31) | 0.004 | |
| Small for Gestational Age3 | 1 (7) | 2 (13) | 1.0 | |
| Race3 | 0.4 | |||
| White | 6 (43) | 7 (44) | ||
| Black | 6 (43) | 9 (56) | ||
| Asian | 2 (14) | 0 (0) | ||
| Apgar at 1 Minute2 | 4 (3, 8) | 6 (4, 8) | 0.4 | |
| Apgar at 5 Minutes2 | 7.5 (6, 8) | 8 (7, 9) | 0.5 | |
| SNAP-II Scores2 | 9 (0, 25) | 2.5 (0, 22) | 0.7 | |
| Caesarean Section Delivery3 | 11 (79) | 13 (81) | 1.0 | |
| Single Birth3 | 11 (79) | 12 (75) | 1.0 | |
| Prenatal Steroids3 | 10 (71) | 14 (88) | 0.4 | |
| Surfactant3 | 6 (43) | 5 (31) | 0.7 | |
| Gestational Age at Discharge (weeks)2 | 40.1 (37.7, 44.1) | 38.9 (37.5, 41.2) | 0.6 | |
| DOL First Feeding2 | 1 (0, 1) | 1 (0.5, 1) | 1.0 | 0.8 |
| Days to Full Feedings2 | 16 (12, 17) | 10 (9.5, 11) | 0.005 | 0.4 |
| Days to Regain Birth Weight1 | 4.5 ± 4.0 | 5.9 ± 3.7 | 0.3 | 0.4 |
| Total Parenteral Nutrition (Days)2 | 15.5 (11, 18) | 9 (8, 11) | 0.03 | 0.4 |
| Length of Stay (Days)1 | 103.6 ± 28.0 | 96.0 ± 42.0 | 0.6 | 0.8 |
| MOM of Base Human Milk >70%, n (%)3,5 | 6 (43) | 13 (82) | 0.06 | 0.2 |
| Enteral Volume (ml/kg/day)2,5 | 151.6 (147.9, 161.5) | 139.5 (106.9, 163.0) | 0.3 | 0.4 |
| Enteral Energy (kcal/kg/day)2,5 | 151.1 (138.3, 163.0) | 133.6 (103.1, 157.7) | 0.2 | 0.5 |
| Enteral Protein (kcal/kg/day)2,5 | 16.1 (14.3, 17.2) | 15.8 (7.3, 18.0) | 0.7 | 0.6 |
| Enteral Fat (kcal/kg/day)2,5 | 85.4 (74.1, 93.5) | 74.9 (51.2, 87.3) | 0.2 | 0.6 |
| Enteral Carbohydrate Intake (kcal/kg/day)2,5 | 49.7 (49.0, 52.9) | 46.2 (36.3, 53.1) | 0.2 | 0.4 |
| Weight (kg)1,5 | 1.2 ± 0.3 | 1.5 ± 0.3 | 0.02 | 0.2 |
| Length (cm)1,5 | 37.0 ± 2.8 | 39.6 ± 2.7 | 0.02 | 0.4 |
| HC (cm)1,5 | 25.5 ± 2.3 | 27.3 ± 2.6 | 0.05 | 0.7 |
| Weight Velocity (g/kg/day)2,6 | 11.7 (10.4, 12.5) | 12.4 (10.7, 13.8) | 0.3 | 1.0 |
| Length Velocity (cm/week) 1,6 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.8 | 0.6 |
| HC Velocity (cm/week)2,6 | 0.8 (0.7, 0.8) | 0.8 (0.6, 0.8) | 1.0 | 1.0 |
| Change in Weight Z Score from Birth to 36 weeks PMA2 | −1.6 (−1.9, −1.2) | −1.3 (−1.8, −0.8) | 0.3 | 0.7 |
| Change in Length Z Score from Birth to 36 weeks PMA1 | −1.2 ± 0.9 | −1.00 ± 1.0 | 0.5 | 0.9 |
| Change in HC Z Score from Birth to 36 weeks PMA2 | −1.0 (−2.0, −0.6) | −1.0 (−1.5, −0.4) | 0.6 | 0.8 |
Mean ± SD, T-Test P-value;
Median (IQR), Wilcoxon Rank Sum Test P-value;
Frequency (%), Fisher’s Exact Test P-value;
Calculated using Fenton 201321;
At 28 Days of Life (DOL);
At 36 Weeks Post Menstrual Age (PMA).
DOL, Day of Life. HC, Head Circumference. PI, Pancreatic Insufficient. PS, Pancreatic Sufficient.
Model adjusted for gestational age, SGA, and male sex.
At the Early time point (7.4 ± 1.9 DOL) the mean fecal ELA1 concentration for all infants was 192.2 ± 96.4 μg/g, compared to 268.0 ± 80.3 μg/g (p=0.01) at the Late time point (63.6 ± 24.1 DOL) (Figure 1). Additionally, when comparing the DOL of samples obtained at the Early timepoint, samples in the PS infants were obtained at 6.9 ± 1.5 days, compared to PI infants obtained at 8.3 ± 2.0 days (p=0.035, data not shown). When comparing the DOL of samples were obtained at the Late timepoint, samples in the PS infants occurred at 60.6 ± 26.8 days, compared to PI infants occurred at 67.0 ± 21.1 days (p=0.480, data not shown). In a second linear regression model to characterize ELA over time for all obtained values, ELA1 by DOL was adjusted by percent human milk (majority defined as >50%) and GA and demonstrates that time, birth gestation age, and diet all directly are associated with ELA1 concentrations (Figure 2). These data confirm that most preterm infants have PI in the early postnatal period, and that PI improves with feedings and over time.
Figure 1:
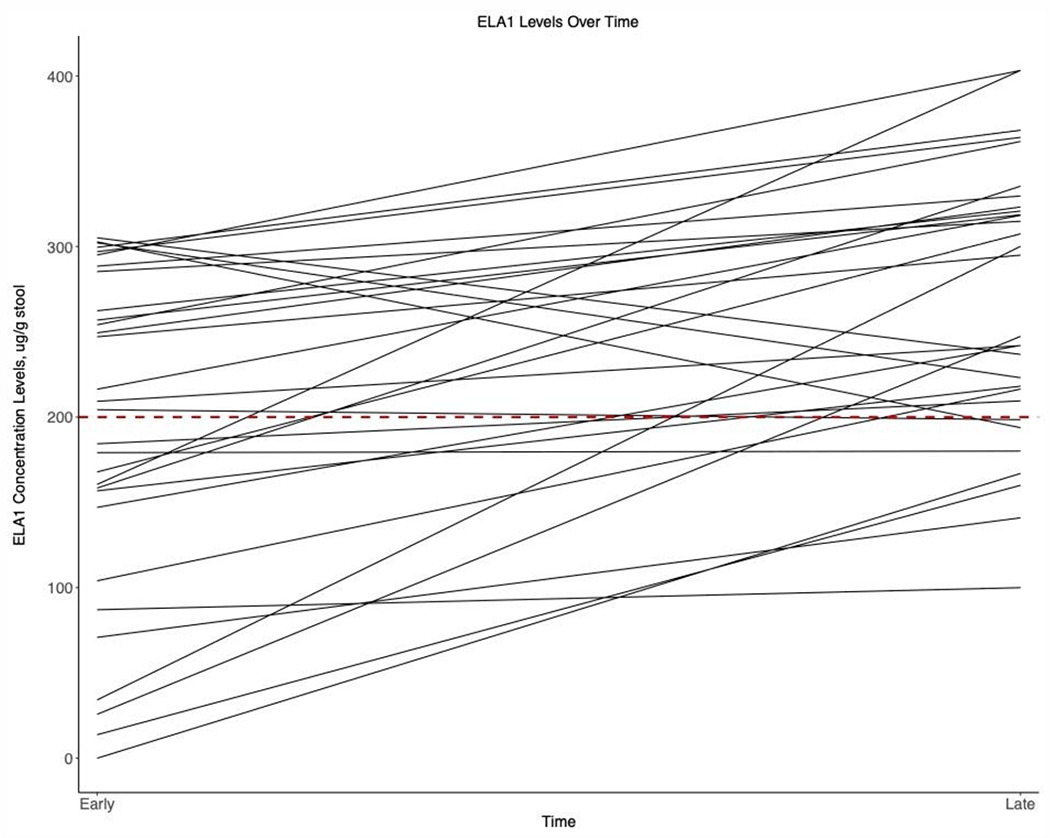
Longitudinal Fecal Elastase Levels (ELA1) at Early (~ 1 week of age) versus Late (~2 months of age) Time Points. Pancreatic Insufficiency, PI = ELA1 <200 μg/g.
Growth outcomes were compared between PI and PS groups at 36 weeks PMA (Table 1). Prior to adjusting for covariates in the model, PI infants took 6 days longer to achieve full feedings (p=0.005) and required 6 additional days of TPN (p=0.03) compared to PS infants. However, the number of days requiring TPN did not differ after adjusting the model for GA, SGA, male sex, and days to full feedings. Length of hospital stay was similar between PI and PS. Enteral nutrition data at DOL 28 were similar between PI and PS groups (Table 1). Before model adjustments at DOL 28, infants with PI were an average of 300 grams lower in weight (p=0.02), 2.6 centimeters shorter in length (p=0.02), and 1.8 centimeters smaller in HC (p=0.05) compared to PS infants. However, after model adjustments, anthropometric measures at DOL 28 were similar between PI and PS infants. Only 43% (6/14) of PI infants received majority mother’s own milk (defined as >70% mother’s own milk) compared to 82% (13/16) of PS infants receiving majority mother’s own milk (p=0.06 unadjusted, p=0.2 after adjustment). Enteral feeding volumes, total enteral energy, enteral fat, enteral carbohydrate, and enteral protein all were similar in PI and PS infants with and without model adjustments.
The final multilinear regression model was conducted to adjust for GA, SGA, male sex, and days to full feedings for preterm infants according to pancreatic function (Supplemental Digital Content Table 1). The complete model with all variables tested is provided (Supplemental Digital Content, Table 2). For each of the growth outcomes, the multilinear regression model demonstrated that PI (or PS) status did not predict somatic growth in this cohort (Supplemental Digital Content Table 1). Weight, length, and HC at 28 DOL correlated strongly with GA (p <0.001) and SGA (p<0.001). Unsurprisingly, time to full feedings was 1.8 days less for each additional week of GA (p=0.03, Supplemental Digital Content, Table 1). Days to full feedings correlated with each growth measurement at 28 DOL, including weight (p=0.07), length (p<0.001), and HC (p=0.03). Growth velocities at 28 DOL were not strongly correlated with PS. Change in Z score at 36 weeks PMA also showed weak correlation coefficients with respect to PS (R2 <0.5). At 36 weeks PMA change in weight Z score (R2=0.2) showed weak association with GA (p=0.04), length (R2=0.4) with GA (p=0.001) and SGA (p=0.03), and HC (R2=0.3) with GA (p=0.009) and male sex (p=0.03). GA and SGA remained significant consistently throughout the model. Taken together, these results indicate that PI status did not predict somatic growth with model adjustments in this cohort.
Among 14 infants who were classified as PI at the Early time point, 5 infants remained PI at the Late time point (Persistent PI). Comparing Persistent PI infants to PS infants at the Late time point (n=23) did not identify any differentiating clinical characteristics or growth outcomes (Table 2).
Table 2:
Comparison of infants classified as Persistent PI (n=5) and Late PS (n=23). Persistent PI classified as PI at Early time point and remaining PI at Late time point.
| Persistent PI (N=5) |
Late PS (N=23) |
p value | |
|---|---|---|---|
| Gestational Age (weeks) 1 | 27.0 ± 1.5 | 28.0 ± 2.4 | 0.4 |
| Birth Weight (g) 1 | 961.0 ± 188.2 | 983.3 ± 195.6 | 0.8 |
| Birth Length (cm) 1 | 35.8 ± 1.8 | 35.1 ± 2.6 | 0.6 |
| Birth HC (cm) 1 | 23.1 ± 1.1 | 24.4 ± 1.8 | 0.2 |
| Male Sex, n (%) 2 | 4 (80%) | 12 (52%) | 0.4 |
| Gestational Age at Discharge (weeks) 1 | 39.6 ± 5.1 | 40.8 ± 3.7 | 0.6 |
| Length of Stay (days) 1 | 87.8 ± 32.6 | 104.2 ± 37.2 | 0.4 |
| DOL of First Feeding 1 | 0.8 ± 0.4 | 1.3 ± 1.8 | 0.5 |
| Days to Full Feedings 1 | 14.4 ± 2.4 | 14.7 ± 8.4 | 0.9 |
| Days to Regain Birth Weight 1 | 5.2 ± 4.6 | 5.0 ± 3.8 | 0.9 |
| Total Days of Parenteral Nutrition 1 | 15.8 ± 4.0 | 14.3 ± 8.1 | 0.7 |
| Weight Velocity Birth to 36 Weeks PMA 1 | 11.9 ± 1.9 | 11.6 ± 3.1 | 0.9 |
| Length Velocity Birth to 36 Weeks PMA 1 | 0.9 ± 0.05 | 0.9 ± 0.3 | 0.9 |
| HC Velocity Birth to 36 Weeks PMA 1 | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.07 |
| Enteral Feeding Volume (ml/kg/day) 1 | 139.9 ± 26.7 | 129.7 ± 50.6 | 0.7 |
| Enteral Feeding Energy Intake (kcal/kg/day) 1 | 133.0 ± 40.9 | 123.8 ± 52.7 | 0.7 |
| Average Enteral Carbohydrate Intake (g/kg/day) 1 | 11.5 ± 2.4 | 10.6 ± 4.2 | 0.7 |
| Average Enteral Protein Intake (g/kg/day) 1 | 3.6 ± 1.6 | 3.2 ± 1.6 | 0.7 |
| Average Enteral Fat Intake (g/kg/day) 1 | 8.1 ± 2.8 | 7.6 ± 3.4 | 0.8 |
| Weight at DOL 28 (grams) 1 | 1282 ± 234 | 1365 ± 345 | 0.6 |
| Length at DOL 28 (cm) 1 | 38.3 ± 2.2 | 38.3 ± 3.3 | 1.0 |
| HC at DOL 28 (cm) 1 | 26.2 ± 1.4 | 26.6 ± 2.9 | 0.8 |
Mean ± SD, T-Test P-value;
Frequency (%), Fisher’s Exact Test P-value.
DOL, Day of Life. HC, Head Circumference. PI, Pancreatic Insufficient. PS, Pancreatic Sufficient.
Discussion
To our knowledge, this is the first study to assess pancreatic function with longitudinal fecal ELA1 measurements, and the first to determine relationships between fecal ELA1 and growth outcomes, in preterm infants fed the exclusive human milk diet. We found that fecal ELA1 improved by a mean of 75.8 μg/g over the first nine weeks of life in preterm infants fed the exclusive human milk diet, which is consistent with previous studies of preterm infants fed formula9 and human milk with bovine-based fortifier.13 Expecting mature digestion when developmentally unable may lead to maldigestion and interfere with growth and tolerance, and increased energy needs.
Exocrine PI leads to maldigestion, malabsorption of macro- and micronutrients, and poor growth.11 Our data confirm that PI infants are smaller in weight, length, and HC at birth, and more likely to be a younger GA than PS infants. Similar growth parameters between PI and PS infants were observed at 36 weeks PMA. However, because PI infants in our study had non-statistically significant higher needs of energy, carbohydrate, protein, and fat compared to PS preterm infants, we speculate that this increase in nutritional delivery minimized growth differences. This is important because poor growth of preterm infants results in neurodevelopmental impairment with cognitive and motor delay.1 Earlier awareness of the potential for growth failure also may help prevent morbidities such as chronic lung disease, intraventricular hemorrhage, and long-term neurodevelopmental impairment.1–3 Interestingly, PI infants in our study were more likely to be male. We are unaware of any prior studies that report an association between sex and pancreatic status in infants. In adults, no sex differences with respect to PI have been reported.21 Further investigations are needed to determine how ELA1 levels correlate with sex, nutrient digestion and absorption in the small bowel, and with subsequent growth.
Pancreatic status of most preterm infants is known to improve longitudinally from birth in the first two weeks of life,22 but PI does persist in some infants.13 In our study, PI infants at the Early time point who remained PI at the Late time point (Persistently PI) demonstrated similar clinical characteristics and growth outcomes compared to PI infants at the Early time point who became PS at the Late time point. This finding might be due to the small sample size of Persistent PI infants, or it might reflect our inability to clinically determine at-risk infants and the need for a biologically relevant biomarker of pancreatic development and function.
We cannot exclude the possibility that specific components of the preterm infants’ diets may contribute to PS. Münch et al found among preterm infants receiving a diet of human milk with bovine fortifier, the PI infants exhibited slower weight gain compared to those with PS fecal ELA1 quantities.13 In this prior study, mother’s own milk was preferred over donor human milk, but volume of mother’s own milk was not quantified.13 In our study, there were more infants receiving majority (>70%) mother’s own milk who were defined as PS, 13/16 (82%), compared to only 6/14 (43%) of PI infants. This finding suggests a possible association of mother’s own milk to fecal ELA1 concentrations that could be due to earlier exposure to human milk elastase or to augmentation of endogenous pancreatic production. Our study proposes that another added benefit of mother’s own milk is to provide direct transfer of amylase and lipase found in human milk and to compensate for PI. It is possible any PI in infants may be compensated for by human milk enzymes, as well as lingual lipase, which may contribute to nutrient digestion and absorption not recognized with ELA1 measurements. This unmeasurable contribution of human milk enzymes on the nutritional outcomes is not reflected in ELA1 levels, and this may account for a lack of significant differences in nutritional outcomes despite differences in ELA1 concentrations, and differences noted in prior studies.23 Although it may be difficult given that human milk is now usual nutritional care, future studies could study the impact of human milk elastase by comparing formula fed versus human milk fed infants.
Our study has several limitations. The observational study design and single site limited our ability to generalize the data to larger populations, and our small infant sample size potentially limited our ability to identify differences between PI and PS infants that may be influenced by diet. Understanding the limitations of ELA1 as a marker to identify PI, our study attempted to limit temporal and consistency variation among stool samples by obtaining a continuous 24-hour period of stool collection, recognizing that in the Late sample there were variations in collection time by 24 days among infants. In particular, the small number of SGA infants limited our ability to assess fecal ELA1 as a marker of growth failure. Nonetheless, this study confirms that the exclusive human milk diet can support the development of PS, similar to other diets examined previously.
Conclusion
This is the first study to establish longitudinal fecal ELA1 levels in preterm infants fed an exclusive human milk diet. Preterm infants have early, functional PI that improves over time and with exclusive human milk diet feedings. Larger studies are needed to investigate whether fecal ELA1 levels are a reliable indicator of pancreatic function in preterm infants and whether earlier identification of infants at high risk might help mitigate growth failure.
Supplementary Material
Summary Box.
What is known:
Preterm infants are born with functionally pancreatic insufficiency (PI)
PI measured by fecal elastase 1 (ELA1) in preterm infants improves over time with human milk fortified with bovine-based fortifier compared to only formula.
PI is associated with growth failure in term infants and children.
What is new:
Fecal ELA1 increases over time and with feedings of human milk fortified with human milk-based fortifier in preterm infants.
Fecal ELA1 warrants exploration as a potential biomarker for growth failure in preterm infants.
Acknowledgements
The authors thank the nursing staff of Texas Children’s Hospital neonatal intensive care unit and the parents of our preterm infants who were critical in ensuring successful completion of this study. The authors would also like to thank Pam Gordon, RN for her invaluable assistance with sample collection and processing. This project would have not been possible without the funding provided by the Evangelina “Evie” Whitlock Neonatology Training Grant and Clinical Research Center at Texas Children’s Hospital, for which the authors are sincerely grateful.
Funding
The Evangelina “Evie” Whitlock Neonatology Training Grant, Clinical Research Center at Texas Children’s Hospital; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K08 DK113114 to GP.
Conflicts of Interest
Amy B. Hair, MD previously received research support from Prolacta Bioscience® and Fresenius Kabi. Camilia R. Martin, MD, MS is a consultant with Mead Johnson Nutrition. Dr. Martin is an advisory board member to LUCA Biologics, Plakous Therapeutics, LactaLogics, Inc., Vitara Biomedical, Inc., and previously with Prolacta Biosciences. Dr. Martin received past and current funding from Shire, Mead Johnson Nutrition, Abbott Nutrition, Massachusetts Life Sciences Center, Charles H. Hood Foundation, and NIH (NIDDK and NICHD). All other authors declare no conflict of interest. No funding was received from these affiliations for this study.
Footnotes
Disclaimers: None.
Contributor Information
Lindsay F. Holzapfel, Department of Pediatrics, Division of Neonatology, University of Texas at Houston Health Science Center, Houston, TX; Department of Pediatrics, Section of Neonatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Amy B. Hair, Department of Pediatrics, Section of Neonatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Geoffrey A. Preidis, Division of Gastroenterology, Hepatology & Nutrition, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX.
Tripti Halder, Division of Gastroenterology, Hepatology & Nutrition, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX.
Heeju Yang, Department of Pediatrics, Section of Neonatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Jana P. Unger, Department of Pediatrics, Section of Neonatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX; Clinical Nutrition Services, Texas Children’s Hospital, Houston, TX.
Steven Freedman, Department of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; Division of Translational Research, Beth Israel Deaconess Medical Center, Boston, MA.
Camilia R. Martin, Division of Neonatology, Weill Cornell Medicine, New York, NY.
References
- 1.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368 [DOI] [PubMed] [Google Scholar]
- 2.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107(1). doi: 10.1542/peds.107.1.e1 [DOI] [PubMed] [Google Scholar]
- 3.Belfort MB, Ehrenkranz RA. Neurodevelopmental outcomes and nutritional strategies in very low birth weight infants. Semin Fetal Neonatal Med. 2017;22(1):42–48. doi: 10.1016/j.siny.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3). doi: 10.1542/peds.2011-3552 [DOI] [Google Scholar]
- 5.Ford SL, Lohmann P, Preidis GA, et al. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother’s own milk compared with donor breast milk. Am J Clin Nutr. 2019;109(4):1088–1097. doi: 10.1093/ajcn/nqz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüth S, Teyssen S, Forssmann K, Kölbel C, Krummenauer F, Singer MV. Fecal elastase-1 determination: “Gold standard” of indirect pancreatic function tests? Scand J Gastroenterol. 2001;36(10):1092–1099. doi: 10.1080/003655201750422729 [DOI] [PubMed] [Google Scholar]
- 7.McClean P, Weaver LT. Ontogeny of human pancreatic exocrine function. Arch Dis Child. 1993;68(1 SPEC NO):62–65. doi: 10.1136/adc.68.1_Spec_No.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kori M, Maayan-Metzger A, Shamir R, Sirota L, Dinari G. Faecal elastase 1 levels in premature and full term infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):106–108. doi: 10.1136/fn.88.2.f106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campeotto F, Kapel N, Kalach N, et al. Low levels of pancreatic elastase 1 in stools of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86(3):198–199. doi: 10.1136/fn.86.3.f198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissler K, Von Katte I, Huebner A, Henker J. Pancreatic elastase 1 in feces of preterm and term infants. J Pediatr Gastroenterol Nutr. 2001;33(1):28–31. doi: 10.1097/00005176-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 11.Sankararaman S, Schindler T, Sferra TJ. Management of Exocrine Pancreatic Insufficiency in Children. Nutr Clin Pract. 2019;34(S1):S27–S42. doi: 10.1002/ncp.10388 [DOI] [PubMed] [Google Scholar]
- 12.Berkow SE, Hamosh P, York CM, Mehta NR, Hamosh M. Lipases In Human Milk: Effect Of Gestational Age And Length Of Lactation On Enzyme Activity. J Am Coll Nutr. 1989;8(2):143–150. doi: 10.1080/07315724.1989.10720289 [DOI] [PubMed] [Google Scholar]
- 13.Münch A, Garten L, Bührer C. Protracted maturation of pancreatic-specific elastase 1 excretion in preterm infants of extremely low gestational age. J Pediatr Gastroenterol Nutr. 2013;56(5):532–536. doi: 10.1097/MPG.0b013e31827fb091 [DOI] [PubMed] [Google Scholar]
- 14.Products N, Prolacta F. Nutrition Information 100%.; 2019. https://www.prolacta.com/Data/Sites/14/media/box.com/mkt-0627-rev-0_prolacta-product-nutrition-brochure-us_v18.pdf.
- 15.Ag I, Tel B. Pankreatische Elastase Pancreatic Elastase. 2013. [Google Scholar]
- 16.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: A Physiologic Severity Index for Neonatal Intensive Care. Pediatrics. 1993;91(3). [PubMed] [Google Scholar]
- 17.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608 [DOI] [PubMed] [Google Scholar]
- 18.Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol. 2009;29(9):618–622. doi: 10.1038/jp.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13(1). doi: 10.1186/1471-2431-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14(1):1–14. doi: 10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tod J, Fine D. Fecal elastase: A useful test for pancreatic insufficiency? Dig Dis Sci. 2010;55(10):2709–2711. doi: 10.1007/s10620-010-1409-9 [DOI] [PubMed] [Google Scholar]
- 22.Nissler K, Von Katte I, Huebner A, Henker J. Pancreatic elastase 1 in feces of preterm and term infants. J Pediatr Gastroenterol Nutr. 2001;33(1):28–31. doi: 10.1097/00005176-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 23.Münch A, Bührer C, Longardt AC. Digestive enzyme replacement relieves growth failure in preterm infants with poor exocrine pancreatic function: a retrospective case series. Eur J Pediatr. 2021. doi: 10.1007/s00431-021-04069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


