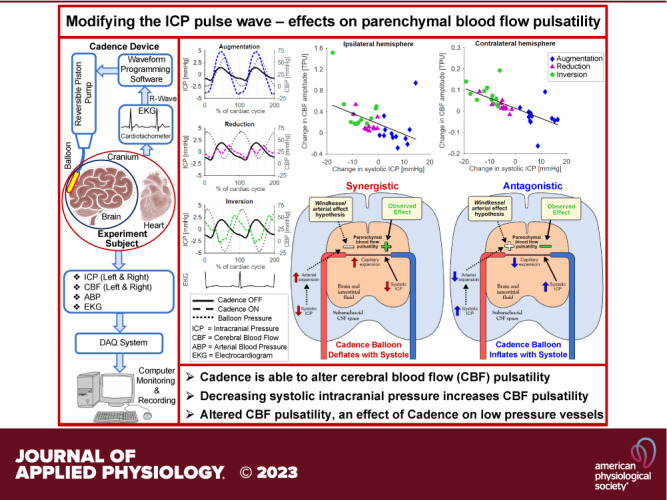
Keywords: cardiac-gated inflatable device, cerebral blood flow, experimental model, intracranial pressure, intracranial pulsatility
Abstract
Pulsation of the cerebral blood flow (CBF) produces intercranial pressure (ICP) waves. The aim of this study is to determine whether externally modifying ICP pulsatility alters parenchymal blood flow pulsatility. A cardiac-gated inflatable device was inserted in the lateral epidural space of 12 anesthetized canines (canis familiaris) and used to cause reduction, inversion, and augmentation of the ICP pulse. CBF in each hemisphere was measured using laser Doppler velocimetry. A significant increase in both mean CBF and its amplitude was observed for reduction as well as inversion of the ICP pulse, with larger changes observed for the inversion protocol. Significant increases in the mean CBF were also observed ipsilaterally for the augmentation protocol together with indications of reduced CBF amplitude contralaterally. External alteration of the ICP pulse thus caused significant changes in parenchymal blood flow pulsatility. The inverse relationship between the ICP and CBF amplitude suggests that the changes did not occur via modification of the intracranial Windkessel mechanism. Thus, the effects likely occurred in the low-pressure vessels, i.e., capillaries and/or venules, rather than the high-pressure arteries. Future MRI studies are however required to map and quantify the effects on global cerebral blood flow.
NEW & NOTEWORTHY This study demonstrated that external modification of ICP pulsatility, using a cardiac-gated inflatable device implanted epidurally in canines, alters brain tissue blood flow pulsatility. Specifically, decreasing systolic ICP increased blood flow pulsatility in brain tissue. The results suggest that the altered CBF pulsatility is unlikely to depend on modification of the Windkessel effect on the feeding arterial system but was rather an effect directly on tissue and the lower pressure distal vessels.
INTRODUCTION
The heart beats millions of times per year, supplying blood to the brain, and other organs, but also exposing the cerebral vasculature and parenchyma to repeated pulsatile stress. In organs throughout the body, this flow pulsatility is buffered by vascular elasticity, i.e., arteries expand to store blood in systole and then release more blood during diastole, which is called the Windkessel effect (1). However, the brain is considered to be uniquely vulnerable to flow pulsatility due to its high blood flow and its confining rigid skull (2). With aging, the stiffening of the systemic and cerebral vascular tree that occurs largely as a result of atherosclerotic processes results in increased pulsatile stress on the cerebral vascular bed (3). It has been hypothesized that this can cause damage to the microvasculature, called “pulse wave encephalopathy” (4), and that this contributes to a decline of neurological functions (3–6). Altered pressure/flow pulsatility of blood or cerebrospinal fluid (CSF) has been implicated in several neurodegenerative conditions, e.g., small vessel disease, mild cognitive impairment, dementia, and normal pressure hydrocephalus (3–10). Cerebral arterial pulsations have also been suggested as a potential driving force for the clearance of metabolites from the brain via the glymphatic system (11).
In the unique confines of the rigid skull, pulsed influx of blood drives an intercranial pressure (ICP) pulsation in the CSF that may, in turn, reflect back to affect cerebral blood flow. Understanding the mechanisms controlling the intracranial pressure pulsatility and experimental manipulation of its magnitude or phase may allow for understanding its potential effect on cerebral blood flow pulsatility and have relevance for understanding the effects of aging and neurodegenerative conditions. This group has previously demonstrated that the ICP pulse wave can be experimentally manipulated, in terms of both amplitude (AMP) and waveform, by active modification of the intracranial volume at the cardiac frequency (12). This was achieved using Cadence, a cardiac-gated device that inflates and deflates a small intracranially implanted balloon. In a previous study, this group used Cadence in a canine model with three different balloon volume variation protocols, designed to modify the ICP pulse wave in three different ways: augmentation (AUG), reduction (RED), or inversion (INV; phase shift) of the ICP pulse wave (13). The study demonstrated that ICP pulse wave modification caused increases in mean cerebral (parenchymal) blood flow (CBF), but did not examine the pulsatile aspect of CBF.
In the present study, it is hypothesized that these ICP pulse wave modifications affect the pulsatility of the parenchymal blood flow. Such an effect could occur either by a modification of the Windkessel effect, i.e., altered damping of the pulsatile arterial flow, or alternatively through a direct effect on the tissue through an altered pressure on the microvasculature and venous systems. The aim of this study is to determine if externally modifying ICP pulsatility with Cadence alters parenchymal blood flow pulsatility in accordance with either of these potential mechanisms.
MATERIALS AND METHODS
The Cadence System
The goal was to vary the volume of a micro-balloon in the cranial epidural space of canines in synchrony with the cardiac cycle, in an effort to modify the ICP pulse wave. This was achieved using a state-of-the-art system [the Cadence system; US patent nos. 8,956,379 and 9,011,378, more details are available in the previous publications (12, 13)] that consists of a positive displacement pump driven by a stepper motor in response to the R-wave, obtained from ECG, and operator inputs. The operator-specified parameters were the time delay between the R-wave of the ECG and initiation of deflation/inflation (in addition to the inherent minimum delay of the system), volume of deflation/inflation, deflation/inflation duration, and reinflation/deflation duration. The system could thus be programmed for each individual canine model to adjust the timing of the maximum deflation/inflation of the balloon to match the systolic peak and to deflate/inflate to a degree that produced an appropriate response in the systolic ICP level.
Balloon Protocols
Based on pilot testing of the Cadence system, three types of balloon protocols were defined, aimed to produce reduction, inversion, or augmentation of the ICP pulse wave (12, 13). The reduction (RED) and inversion (INV) protocols both entailed deflation of the balloon during systole, from a baseline with an inflated balloon, followed by reinflation. The difference between the two protocols lies in the degree of deflation. INV entailed a larger deflation and was meant to reduce the systolic ICP below the diastolic level, inverting the normal pulse wave. RED, however, was meant to maintain a systolic increase in ICP but with a reduced amplitude. The augmentation protocol (AUG) was antagonistic to the cardiac cycle, inflating the balloon at systole, as the intracranial arteries are expanding, to augment the normal ICP pulse wave. Each protocol was run for 2 min at a time, preceded by a 2-min (minimum) baseline measurement. The 2 min off-on sequence was repeated three times, which formed one set of protocol measurements. Each protocol measurement set was run four times for each balloon protocol, in a semirandomized order (augmentation was never run between inversion and reduction, as the baseline volume of the balloon had to be altered, followed by a wait period to allow the ICP to equilibrate). This led to a total of 12 measurements of 2 min with each balloon protocol and 12 corresponding baseline measurements. The appropriate delay, volume variation, and inflation/deflation duration for each protocol were experimentally determined for each canine, before the start of the randomized measurements.
Measurements and Experimental Setup
The measurement protocol is summarized herein and has previously been described in detail (13). Male random source hounds (canis familiaris), considered to be normal, healthy animals without known neurological or systemic disease/condition, were obtained from licensed suppliers (Marshall Farms and Hodgins Kennel) and quarantined for a minimum of 7 days before study entry. The canines were aged ∼8–9 mo (i.e., young adult) and weighed ∼25–30 kg. All animals were maintained in the Cleveland Clinic Foundation’s (CCF) fully accredited Animal Care Facility under the guidelines of the Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and are reported in compliance with the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments). Twelve animals had the full measurement protocol and dataset required for the analysis of this study (1 animal was excluded due to no time-resolved data available for analysis).
The Cadence balloons (and catheters) as well as probes for measurement of ICP and parenchymal blood flow were implanted under sterile conditions. The balloons were placed in the cranial epidural space of each canine. Anesthesia was induced using sodium pentothal (20 mg/kg iv) before the animal was intubated and placed on a ventilator. Isoflurane gas (1.0–1.5%) was administered to maintain a steady plane of anesthesia throughout the study. Presurgical medications included Dilantin (5 mg/kg iv) to prevent postoperative seizures, dexamethasone (0.25–1.0 mg/kg iv) to reduce inflammation, glycopyrrolate (0.01 mg/kg iv) to reduce respiratory secretions, and Gentamicin (3 mg/kg iv) and Cefazolin (1 g sc) to prevent infection. To monitor arterial blood pressure (ABP), the femoral artery was catheterized. Blood pH and blood gases (pH = 7.4, Pco2 = 35–45 mmHg, Po2 = 95–100) were also monitored via the femoral artery and maintained mechanically by controlling the ventilation volume and frequency. Body temperature was maintained with a thermal blanket (37 ± 3°C) and a saline solution (Ringer’s lactate) was infused intravenously at a rate of 25 mL/h throughout the surgical session.
The Cadence balloons were custom-made inelastic polyether-polyurethane balloon catheters having a fixed maximum volume of 0.5 mL and dimensions were ∼55 × 5 × 2 mm (length × width × thickness) (NT Medical, Wilmington, MA). Implantation was performed under sterile conditions with the canines secured in a stereotactic head frame in prone position. A single sagittal incision was made at the midline, and skin, fascia, and the temporalis muscle were retracted to expose the skull and an 8-mm burr hole was made by twist drill above the dorsolateral convexity of the parietal-occipital cortex. The balloon was inserted into the epidural space above the Sylvian sulcus along a posterior-to-anterior plane, and the epidural space was then filled with saline to remove air, after which the skull was repaired with acrylic paste to prevent direct communication between the atmosphere and intracranial space. The balloon was connected to the closed-air pump of the Cadence system and kept in place throughout the study unless balloon leakage necessitated replacement.
An additional burr hole was manually drilled into the skull overlying the dorsolateral parietal cortex of each hemisphere, and the dura opened to implant an ICP probe (ispilateral to the balloon: Camino, #110-4BT, Integra Lifesciences, Inc.; contralateral: Codman Microsensor, Johnson & Johnson) and a laser Doppler CBF probe (ABLPHN20, Transonic Systems, Inc., Ithaca, NY) directly in brain parenchyma of each hemisphere (∼1–2 cm below the cortical surface). The burr holes were sealed, securing the probes in place, using bone wax.
The animals were euthanized upon conclusion of the experiments. Euthanasia was carried out in accordance with the American Veterinary Medical Association panel on Euthanasia. The animals were deeply anesthetized with sodium pentobarbital (100 mg/kg iv) in combination with inhaled Isoflorane (5%) and perfused via left atrial access port or bilateral catheterization of the carotid arteries with 0.1 M phosphate-buffered saline (PBS) solution (0.9%, 15 min) followed by 4.0% paraformaldehyde (PFA) in 0.1 M PBS (30 min). A bolus injection of Beuthanasia (200 mg/kg iv) was given as overdose. Pain was managed with Butorphanol (0.2 mg/kg iv), or Buprenex (0.01–0.03 mg/kg iv) The brains of the animals were removed immediately following euthanasia and then postfixed for 48 h in 4% PFA for the study of gross pathology, frozen sectioning, and routine histology (H&E, cresyl violet). Selected blocks of brain tissue were embedded in paraffin for long-term storage and subsequent use for routine immunohistochemical analyses.
Data Processing
The ICP, CBF, and ABP signals were recorded and monitored in real-time via a data acquisition system (PowerLab version 5.3.2, ADInstruments Pty Ltd, Dunedin, New Zealand) (sampling rate 200 Hz). The saved data files were manually tagged in the corresponding signal analysis software (LabChart 7, ADInstruments Pty Ltd, Dunedin, New Zealand) with the timepoints when the Cadence system was turned on and off, for the repeated measurements with the different protocols. The data were then exported to MATLAB (RRID:SCR_001622; MathWorks, Natick, MA) for further analysis.
The last 60 s of each measurement with the Cadence system active was used for analysis, and the last 60 s before each activation of the system were used as paired baseline measurements. The data were filtered to isolate the cardiac pulsations using MATLAB’s digital zero-phase filtering algorithm [5th order Butterworth filters, low-pass cutoff: 20 Hz (ICP and CBF only), high pass cutoff: 1 Hz (ICP, CBF, ABP)]. The filtered data were used for the detection of systolic and diastolic timepoints in each cardiac cycle, pulse amplitude calculation (max – min), and ICP waveform averaging. Mean values and the magnitudes of systolic and diastolic ICP was determined from unfiltered data.
Each cardiac cycle was considered to begin with the R-tag of the ECG, which was detected by a threshold algorithm. In segments with disturbances in the ECG data, the systolic peaks in the ABP signal were detected instead and adjusted using the mean time delay between R-tag and systolic ABP (from the other segments). The end-diastolic ICP timepoint was determined as the minimum of the first half of each cardiac cycle in the baseline measurements; the average value for each canine was then used as the diastolic timepoint for the measurements where the Cadence system was active. The systolic phase was defined as half of the cardiac cycle, starting after the diastolic timepoint. The systolic ICP was defined as the maximum (baseline and augmentation measurements) or the minimum (inversion measurements) during the systolic phase. For reduction measurements, the systolic ICP was defined as the minimum of the second half of the systolic phase (to avoid detecting a low ICP value at the beginning of the systolic ICP rise). The measured parameters were averaged for each canine, producing one mean value and one corresponding baseline value for each parameter and balloon protocol. Individual cardiac cycles where the Cadence system misfired (i.e., no inflation/deflation of the balloon) were automatically filtered out based on the balloon pressure. Measurement segments where more than 25% of the cardiac cycles were filtered out were excluded completely; in these cases, the corresponding segment where the Cadence system was off was excluded as well. Similarly, pairs of data segments were excluded if the system was mistakenly fired during the “off” segment. For some segments there was a loss of signal in the laser Doppler measurement, these segments were excluded from the analysis of laser Doppler results, along with the corresponding segment where the system was on/off (i.e., always a pair of segments). In all cases, a minimum of eight pairs of segments were analyzed for each protocol (out of 12).
Waveform Averaging
To illustrate changes in the waveforms of ICP and CBF, average waveforms were constructed by resampling the filtered data of each cardiac cycle to 100 data points, corresponding to one (bias corrected) data sample at every 100th of a cardiac cycle duration. For each measurement segment, these sets of data points were then averaged to produce a mean waveform consisting of 100 values. To avoid distortion of the waveforms, cardiac cycles that differed more than 10% from the median cardiac cycle duration of the measurement segment in question were excluded. This averaging procedure was repeated for each canine (excluding segments that differed >10% in cardiac cycle duration) and then for the group, producing one average waveform with the system active and one with the system turned off, for each balloon protocol. Segments where small changes in the balloon waveform had been made during the experiment (to adjust to a changing heart rate) were excluded from averaging.
Statistics
Paired comparisons between each balloon protocol and the corresponding baseline measurements were made with two-tailed Wilcoxon signed rank tests (one pair of values per canine and protocol). Correlations between changes in the investigated parameters were assessed with Pearson’s linear correlation coefficient or when controlling for the influence of another parameter, Pearson’s linear partial correlation coefficient. Statistical significance was considered for P < 0.05.
RESULTS
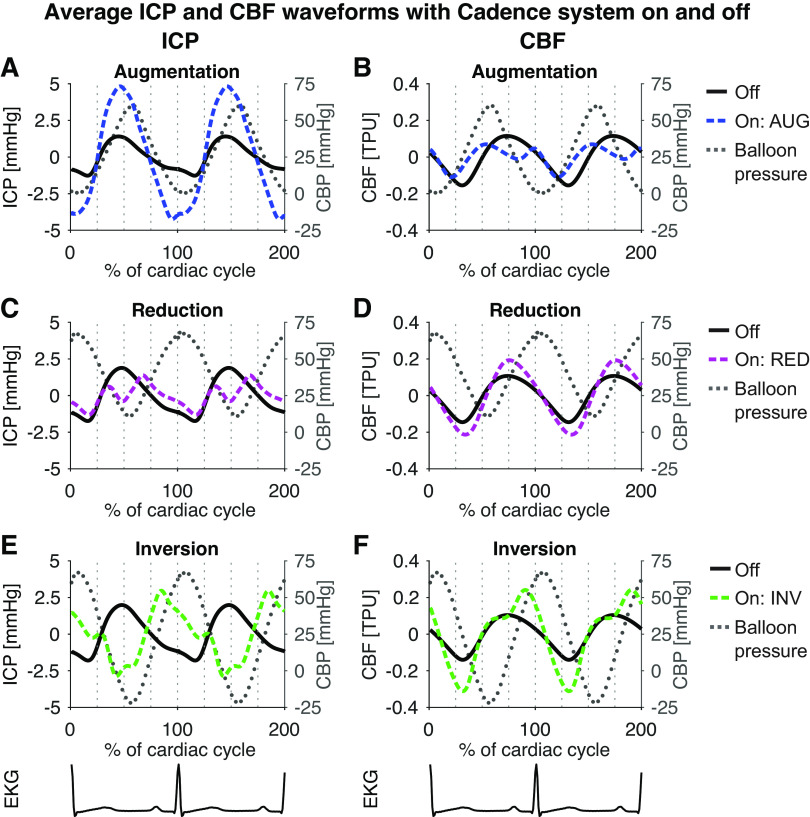
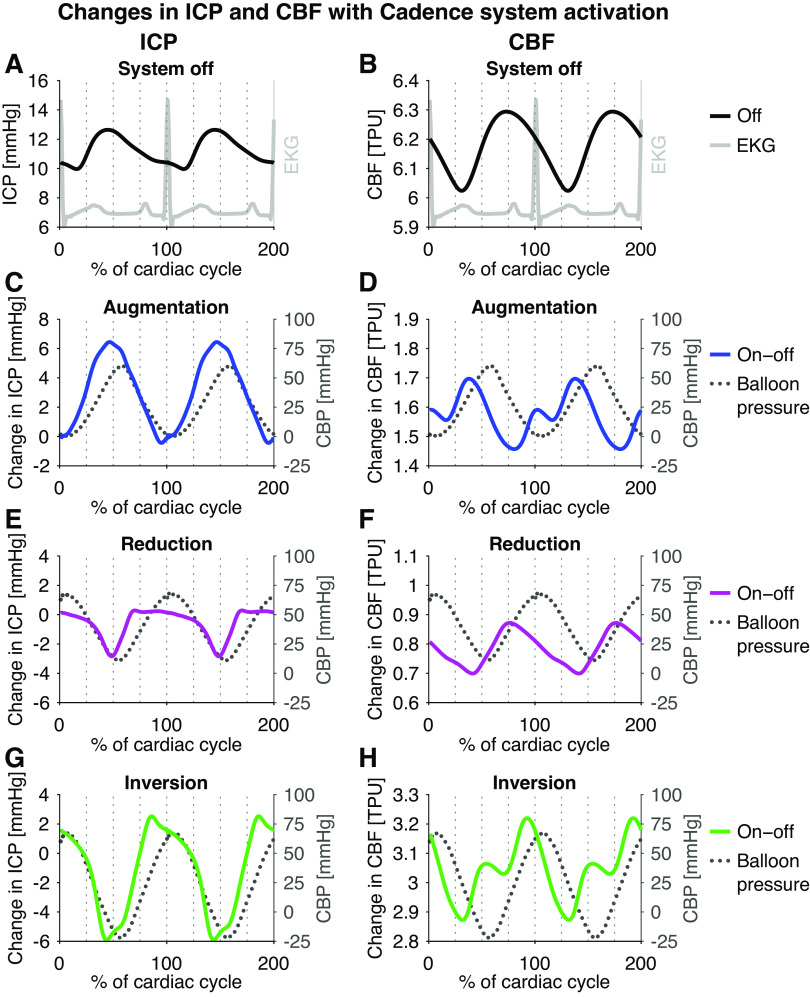
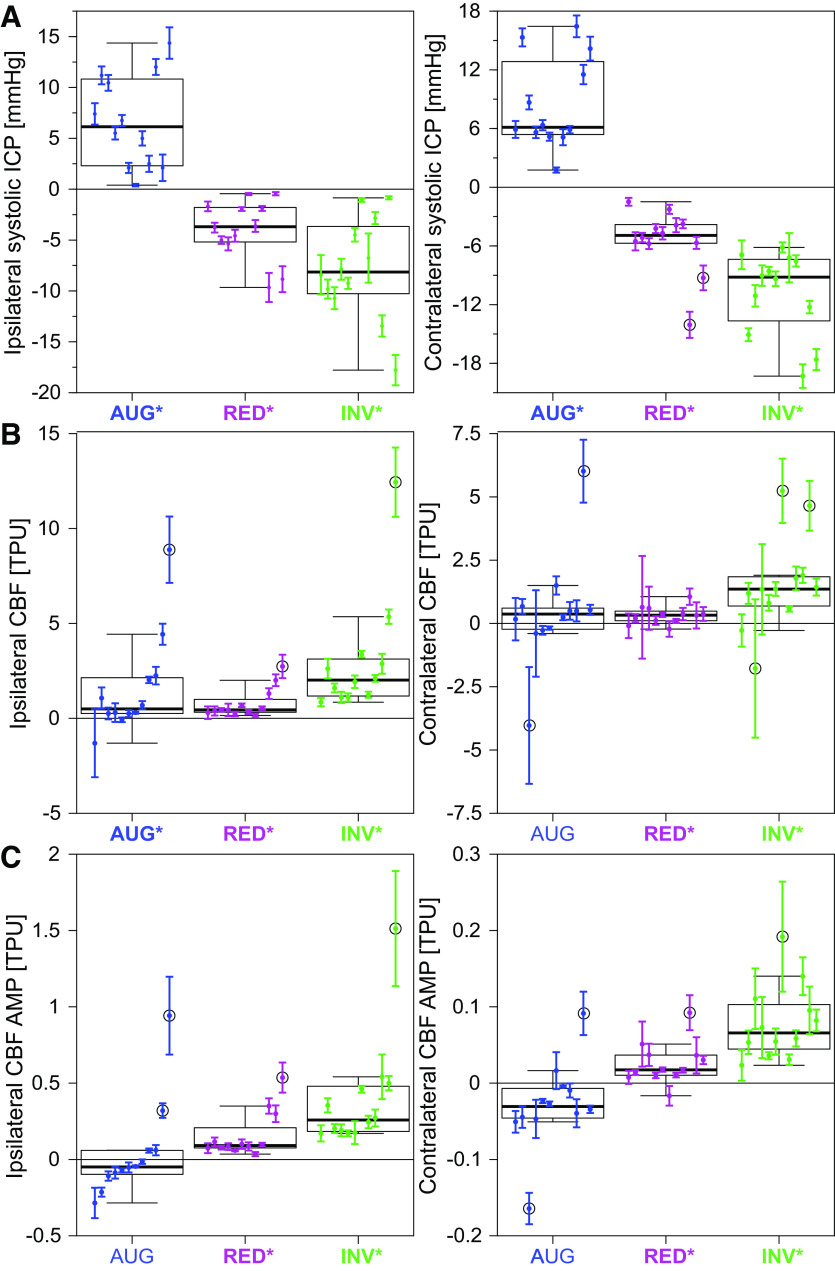
Figure 1 shows the averaged waveforms of ICP and CBF for the baseline and the three balloon protocols, whereas Fig. 2 shows the changes in the average waveform for each protocol, compared to the corresponding baseline measurements (ipsilateral hemisphere). The waveforms and changes on the contralateral side were similar in shape, though the amplitude/magnitude was somewhat larger for ICP, and smaller for CBF. The quantified changes in the investigated ICP and CBF parameters are illustrated in box plots in Fig. 3. All changes were statistically significant (P < 0.02) except the following: AUG: CBF AMP and contralateral mean CBF; RED: ipsilateral ICP AMP, contralateral diastolic ICP; INV: ipsilateral diastolic ICP. Although the absolute changes in CBF AMP were small in magnitude compared with the changes in mean CBF, the changes for the INV and RED protocols were still statistically significant and the P value for the contralateral change for the AUG protocol was P = 0.052. In relation to the unaltered amplitude, the changes were also of significant size with median changes of −18% (AUG), +34% (RED), and +127% (INV) on the ipsilateral side, and −21% (AUG), +12% (RED), and +53% (INV) on the contralateral side. Intra-animal variability across repeated measurements (means ± SD) is presented for changes in systolic ICP, mean CBF, and CBF AMP in Fig. 4.
Figure 1.
Averaged ICP (left) and CBF waveforms (right) for the baseline measurements and the three balloon protocols: augmentation (blue, A and B), reduction (magenta, C and D), and inversion (green, E and F). For easier comparison of the original and altered waveforms, the graphs show mean corrected waveforms. Cadence balloon pressure (CBP) is shown as grey dotted lines. The augmentation protocol augmented the typical ICP pulse wave (A), while suppressing the peak of the CBF signal (B). Conversely, the reduction protocol suppressed the systolic peak in ICP (C), while enhancing the CBF pulse wave (D). The inversion protocol inverted the systolic part of the ICP pulse wave (E), enhancing the CBF pulse wave even more than the reduction protocol (F). Interestingly, the onset of systolic increase in ICP occurred slightly earlier with the augmentation protocol, and this behavior was reflected in the CBF pulse wave. AUG, augmentation balloon protocol; CBF, cerebral (parenchymal) blood flow; ICP, intracranial pressure; INV, inversion balloon protocol; RED, reduction balloon.
Figure 2.
Changes in the average waveform of ICP (left) and CBF (right) resulting from Cadence system activation: EKG (grey) and baseline waveforms (A and B), waveform change with augmentation (C and D, blue), reduction (E and F, magenta), and inversion (G and H, green). Balloon pressure is shown as grey dotted lines. For the inversion and augmentation protocol, two peaks/valleys in the CBF change occurred, while for the reduction protocol only one peak/valley was evident. Though individual data is not shown, it was noted that the double peak was not seen for all canines. CBF, cerebral (parenchymal) blood flow; ICP, intracranial pressure.
Figure 3.
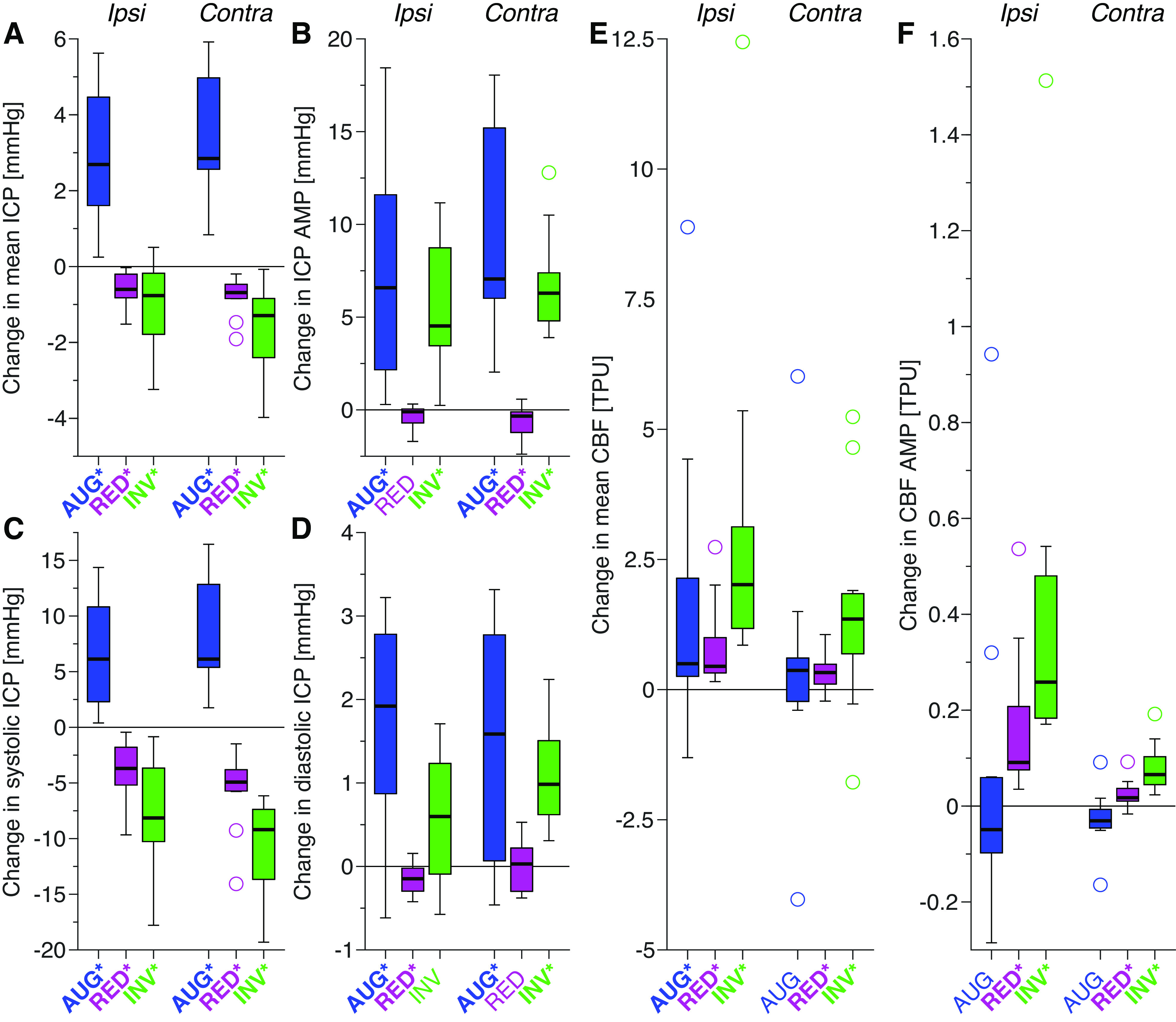
Box plots of the changes in ICP and CBF parameters for the different balloon protocols: augmentation (blue), reduction (magenta), and inversion (green). The analyzed parameters are mean ICP (A), ICP AMP (B), systolic ICP (C), diastolic ICP (D), mean CBF (E), and CBF AMP (F). Statistically significant changes (P < 0.05) are represented using bold text and * in the x-axis labels (the P value of contralateral CBF AMP for AUG was P = 0.052). The boxplots show the median (line), first and third quartile (box), range (whiskers), and statistical outliers (circles). AMP, amplitude; AUG, augmentation balloon protocol; CBF, cerebral (parenchymal) blood flow; contra, contralateral; CSF, cerebrospinal fluid; ICP, intracranial pressure; INV, inversion balloon protocol; ipsi, ipsilateral; RED, reduction balloon protocol; TPU, tissue perfusion units.
Figure 4.
Intra-animal variability across the repeated measurements (means ± SD: dots and error bars, 8–12 repetitions) for changes in systolic ICP (top, A), mean CBF (middle, B), and CBF AMP (bottom, C). The data for each hemisphere (ispi-/contralateral) and balloon protocol (augmentation: blue, reduction: magenta, inversion: green) are presented with the individual animals in the same order (left to right according to ipsilateral CBF AMP, AUG). Overlayed are the corresponding box plots for the group, showing median (line), first and third quartile (box), and range (whiskers); statistical outliers are circled. Statistically significant changes on the group level (P < 0.05) are represented using bold text and * in the x-axis labels. Note that the scale of the y-axis is different for each panel. ABP, arterial blood pressure; AMP, amplitude; AUG, augmentation balloon protocol; CBF, cerebral (parenchymal) blood flow; contra, contralateral; CSF, cerebrospinal fluid; ICP, intracranial pressure; INV, inversion balloon protocol; ipsi, ipsilateral; RED, reduction balloon protocol; SD, standard deviation; TPU, tissue perfusion units.
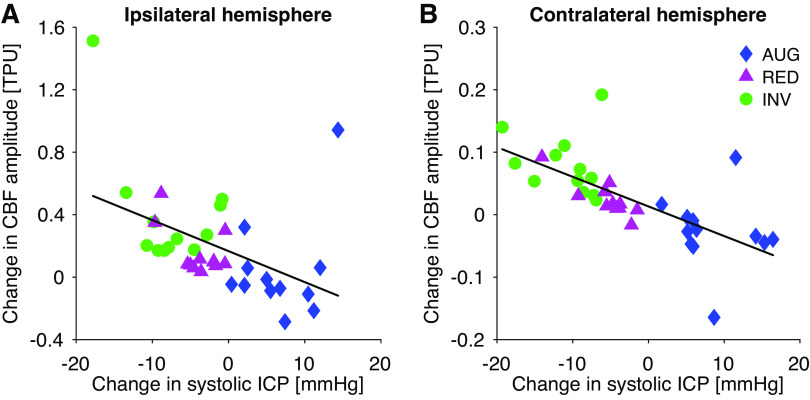
Change in CBF amplitude showed a statistically significant negative correlation to change in systolic ICP in the same hemisphere (ipsilateral to balloon: R = −0.45, P < 0.01; contralateral: R = −0.69, P < 0.01) when all protocols were analyzed together (Fig. 5). Change in CBF AMP also showed a significant negative correlation to change in mean ICP (ipsilateral: R = −0.39, P = 0.02; contralateral: R = −0.67, P < 0.01). There was no significant correlation to change in diastolic ICP or ICP pulse amplitude. Changes in mean CBF and CBF AMP correlated significantly in each hemisphere (ipsi-/contra-lateral: R = 0.93/0.70, P < 0.01). Accounting for this relationship when calculating the correlation between changes in systolic ICP and changes in CBF AMP (partial correlation) demonstrated that these parameters correlated independently of changes in mean CBF (ipsilateral: R = −0.80, P < 0.01; contralateral: R = −0.81, P < 0.01). There was a significant increase in the ABP AMP for the AUG and INV protocols (AUG: 0.7 ± 1.1 mmHg, P = 0.02; INV: 1.8 ± 1.6 mmHg, P < 0.01), but not for RED (0.4 ± 0.5 mmHg, P = 0.11). The changes in ABP AMP did not correlate significantly to the changes in CBF AMP (P > 0.2).
Figure 5.
Illustration of the correlation between changes in systolic ICP and CBF amplitude for the hemisphere ipsilateral (left, A; R2 = 0.20) and contralateral (right, B; R2 = 0.47) to the Cadence balloon. Each symbol and color illustrate measurements from a different type of protocol: inversion (INV; green circles), reduction (RED; magenta triangles), and augmentation (AUG; blue diamonds). AUG, augmentation balloon protocol; CBF, cerebral (parenchymal) blood flow; ICP, intracranial pressure; INV, inversion balloon protocol; RED, reduction balloon protocol; TPU, tissue perfusion units.
DISCUSSION
This study investigated how three different types of ICP pulse wave modifications (RED, INV, and AUG), achieved with the oscillating balloon method of the Cadence system, affected parenchymal blood flow pulsatility. There was an increase in the CBF pulsatility for the RED and INV protocols, whereas AUG resulted in a tendency toward reduced CBF amplitude. This implies that, at least with experimental manipulation, the ICP pulse wave can play an active part in governing the pulsatility of parenchymal blood flow.
As demonstrated in our previous study, for mean and systolic ICP, there was a pattern across protocols: the largest increase was produced by the augmentation protocol, the intermediate effect by the reduction protocol, and the largest decrease by the inversion protocol (Figs. 1, 2, and 3). This is expected by the very design of the experiment. The present work demonstrated for the first time, to the best of our knowledge, that this pattern was reversed for the CBF pulsatility, where the smallest change (potentially a reduction) was seen for AUG, and the largest increase was seen with INV. This behavior was further evidenced by the negative correlation between the changes in systolic ICP and CBF AMP (Fig. 5). Such a pattern was however not evident for the mean CBF, where the RED seemed to show the smallest magnitude of the increase, though with more consistency than the AUG protocol, potentially reflecting the pattern shown by the ICP AMP changes. Altogether, this suggests that the ICP pulse wave modification specifically affected the pulsatility of the blood flow through the parenchyma and the ICP pulse wave can play an active part in dampening or enhancing blood flow pulsatility in the brain. Overall, the volume of blood flowing through the parenchyma was increased for the cardiac cycle as a whole, not just during part of the cardiac cycle, given that the mean CBF increased more than the CBF AMP (Fig. 3).
Effect on the CBF Waveform
Changes in the average CBF waveform resulting from Cadence activation (Figs. 2 and 3) suggest that, at least for INV and AUG protocols, there was a tendency for the peak of the CBF waveform to shift towards the inflation part of the balloon cycle, i.e., an earlier peak for AUG and a later peak for the INV protocol (Fig. 1). The timing of the modified CBF peak (Fig. 1) indicates that the CBF waveform change occurred in response to the ICP pulse wave modification, as the CBF peaks occurred closer to peak ICP rather than peak balloon pressures, though the exact relative timing of these signals cannot be confirmed. This is, however, also supported by the inverse correlation between the changes in systolic ICP and the changes in CBF pulse amplitude (Fig. 3). Interestingly, the earlier occurrence of systolic onset in ICP with the AUG protocol was reflected in the CBF, further indicating the ICP pulse wave affected the CBF pulsatility.
Modulation of the ICP by the Cadence system can be based on the Windkessel mechanism and the Monro-Kellie doctrine. During systole, elastic arteries expand and essentially store some of the blood volume pumped from the heart and this volume then flows downstream during diastole (1). This Windkessel mechanism leads to reduced pulsatility of blood flow distally from the heart, as it approaches the capillaries. When intracranial arteries expand, they take up an increased portion of the finite intracranial space, and the Monro-Kellie doctrine then states that the volume of CSF and venous blood must equally decrease (as the brain is incompressible) (14). This shift of volumes corresponds to an increase in the ICP, which can be measured as ICP pulsatility at the cardiac frequency. When the Cadence balloon is inflated and deflated in synergy with the cardiac activity, the intracranial volume in effect increases as the arteries expand, and thus the ICP does not increase as it normally should. Working antagonistically to the cardiac cycle can instead mimic a larger relative systolic arterial expansion, thus enhancing the systolic increase in ICP. The exact mechanism of how the Cadence alters CBF pulsatility cannot be confirmed, but the inverse relationship between systolic ICP and CBF amplitude strongly suggests that it is not by facilitating or hindering the Windkessel mechanism (Fig. 6). If a deflation of the balloon and decreased systolic ICP facilitated arterial expansion during systole, the synergistic protocols would have resulted in reduced CBF pulsatility rather than increased since the dampening effect of the Windkessel mechanism would then be enhanced. Given the much higher pressures in the arteries, it is perhaps to be expected that the observed ICP changes are not large enough to enhance or interfere with the arterial systolic expansion. The likely alternative is then that the effect occurs at the low-pressure distal vessels/veins or directly on the capillaries. One potential explanation is that capillary expansion was facilitated (synergistic) or hindered (antagonistic) by the modulated ICP pulse wave, increasing or decreasing the peripheral resistance and thus affecting the perfusion pressure. However, this mechanism is not necessarily reflected in the altered CBF waveforms. These effects could potentially be explained by the balloon acting in a pump-like fashion, i.e., the ICP wave caused by balloon inflation may be pushing some blood from the intracranial arterioles into the capillaries. However, this pressure wave should also compress the cerebral veins, which could impede outflow from the capillaries on the venous side, potentially contradicting this explanation unless cerebral venous outflow concurrently increases, analog to the concept of how ICP pulsatility normally corresponds to the pulsatility of cerebral venous outflow (2).
Figure 6.
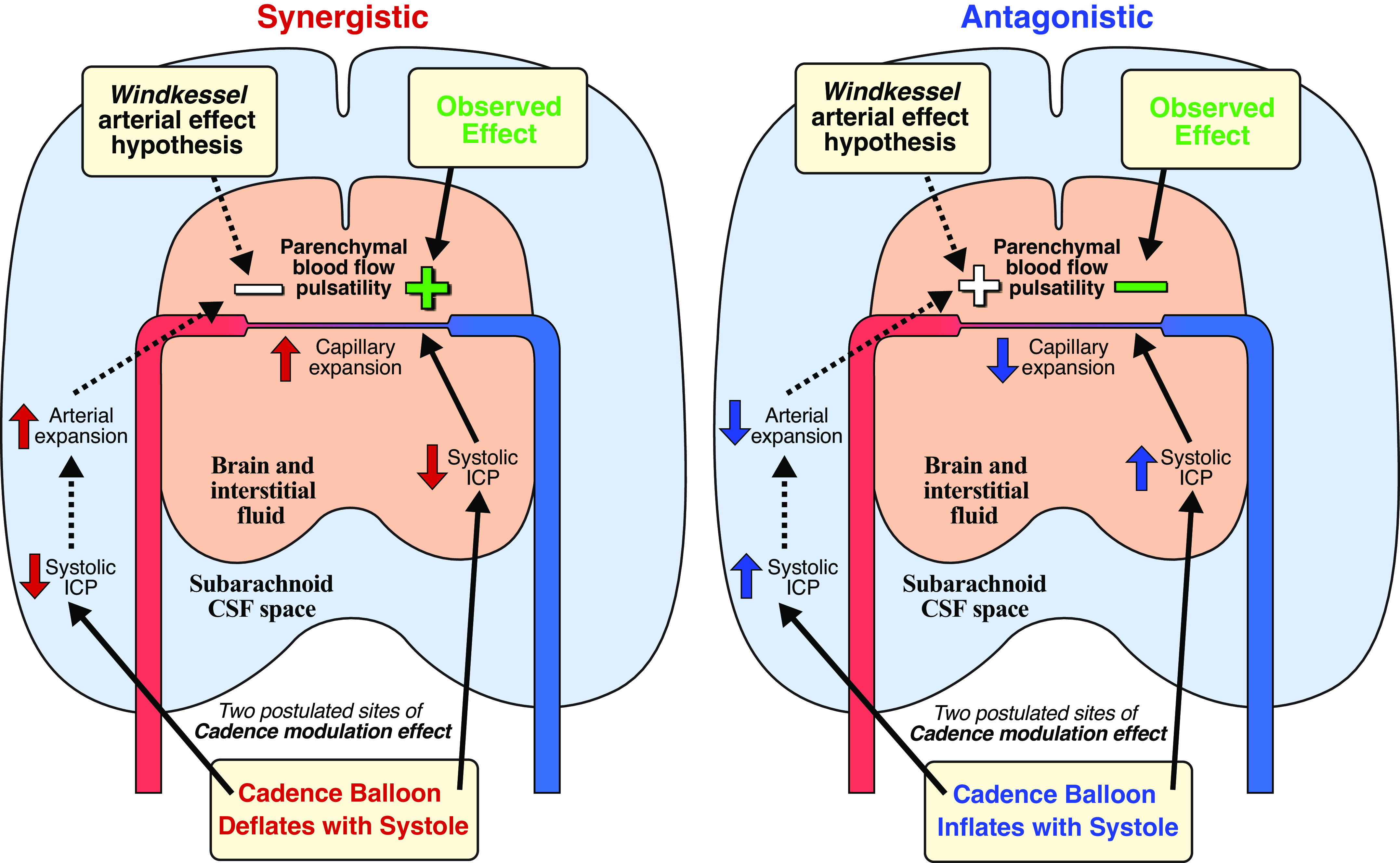
Illustration of the two hypothesized mechanisms by which the Cadence device may affect the pulsatility of parenchymal blood flow. One potential mechanism was by facilitating or hindering the arterial Windkessel mechanism, but this was not supported by the data. Thus, it is hypothesized that the relevant effect occurred more locally in the tissue, e.g., by facilitating or hindering capillary expansion. Left: synergistic inflation protocol. Right: antagonistic inflation protocol. CSF, cerebrospinal fluid; ICP, intracranial pressure.
As laser Doppler sensors are based on the measurement of the movement of red blood cells, they are also sensitive to the movement of the sensor in relation to the tissue and microvessels. Thus, it is important to take into account whether this affected the results, given that the inflation/deflation of the balloon may have induced tissue motion. Using flow phantoms, it has been shown that whether tissue motion is linear or pulsatile, the potential artifact registers as increased perfusion (15). Importantly, the mean CBF effects of this study were corroborated with thermal diffusion measurements in a previous study (13) by the authors, supporting that this is not a motion artifact. Comparable results for mean CBF were also found in a similar study by another group, using a corresponding device in swine (16). In the present study, the AUG and INV protocol caused similar but phase-shifted effects on the CBF signal, leading to opposite effects on the CBF amplitude even though the motion of the balloon was the same except for the direction of movement. This would also argue against a motion artifact, however, in an in vivo situation, given the unknown relative directions of the microvessels in the measurement volume, it is difficult to predict how pulsatile tissue motion due to the balloon may have interacted with the pulsatile flow in these vessels. The magnitude of the CBF pulsatility effect was smaller in the contralateral hemisphere, further from the source of the potential motion artifact, but the same pattern of effects was seen for the different protocols and there was also less variability across individual canines. In a post hoc analysis, the authors analyzed the correlation between the degree of balloon volume change, as the parameter most representative of balloon-induced motion, and the CBF AMP. This correlation was not significant (ipsi-/contralateral R = 0.22/0.21, P > 0.19). With the direction of motion taken into account (setting the volume change of the AUG protocol as negative), there was a correlation to CBF AMP (ipsi-/contralateral R = 0.39/0.65, P< 0.05). However, the correlation between volume change and systolic ICP was stronger (ipsi-/contralateral R = −0.75/−0.86, P < 0.01) and using partial correlation to correct for this relationship reduced the correlation between volume change and CBF AMP to nonsignificant levels (ipsi-/contralateral R = 0.09/0.16, P = 0.62/0.37). This suggests that balloon motion did not have a major direct effect on CBF amplitude, which therefore implies that there was indeed increased pulsatility at the parenchymal level when the Cadence system was run with the synergistic protocols and that there was a true trend toward decreased pulsatility with the antagonistic protocol.
Measurement Aspects and Limitations
The laser Doppler sensors measure perfusion in tissue perfusion units (TPU), which are proportional to mL/min/100 g tissue but cannot be translated to absolute numbers without in vivo calibration. Therefore, the measurements were only used to study changes in CBF with activation of the Cadence system.
With regards to the comparison of waveform alterations, the exact relative timing between changes in ICP, balloon pressure, and CBF within the cardiac cycle cannot be verified. Although the effects in the CBF signal could be observed within the first cardiac cycle after Cadence activation, the occurrence of any smaller time delay (∼ms) between the CBF and pressure signals, due to the different measurement techniques, is unknown.
Disregarding motion from the balloon, it may not be possible to fully differentiate between flow pulsatility and pulsatile motion of the vessel walls when using laser Doppler. The results of the present study could thus reflect an increase in the pulsatile expansion of the microvessels, without a corresponding increased pulsatility of the flow inside these vessels. Both possibilities amount to increased transmission of pulsatility into the parenchyma and, as both increased flow and volume pulsatility imply that the arteriolar/capillary pulse pressure was elevated, that the tissue was exposed to increased pulsatile stress. However, in the context of glymphatic theory, where pulsatile wall movements may play an important role in driving flow and therefore brain clearance (11), clarifying this distinction could be a relevant aspect to explore in future studies.
Another relevant aspect of the blood flow measurement method used is the very localized measurement area of the sensors (∼1 mm3). The sensors were placed in the same area in both hemispheres, and in all canines, and thus the results might be suspected to not be valid for the global parenchyma. With results seen in both hemispheres, though smaller in the contralateral one, it seems clear that CBF effects may indeed extend to the entire brain in terms of distance to the balloon, but there may also be anatomical and/or physiological factors affecting which areas of the brain may be more or less responsive to the Cadence modulation.
Implications for Pathophysiology and Future Research
The results of this study indicate that the pulsatility of parenchymal blood flow can be affected via ICP waveform modification. This is of importance for future research, as several hypotheses have been put forward regarding the potential contribution of intracranial pulsatile stress to cognitive aging and multiple forms of dementia (4, 9, 10). It has been suggested that over time pulsatile stresses on capillaries and tissues may be damaging, and that increasing cardiovascular pulsatility with age may contribute to neurological deterioration in the aging process (3, 5, 6, 8). A method such as the Cadence system, which allows for manipulation of the ICP and CBF pulsatility in an in vivo setting, may thus help to advance the understanding of the pathophysiology and development of dementia and cognitive decline.
Recently, there has also been a lot of interest in the link between cognitive function and the glymphatic system, which is potentially an important clearance pathway for the brain (17, 18). Intracranial vascular pulsatility has been suggested as a driving force for glymphatic flow and, accordingly, reduced cerebrovascular pulsatility is a potential mechanism for cognitive decline via reduced clearance of e.g., amyloid-ß (11, 17, 19). In the context of cardiopulmonary bypass, artificial hearts, and left ventricular assist devices, flow pulsatility has been investigated with regards to the effect on cerebral perfusion, with studies showing either an increased perfusion level with the pulsatile flow (20, 21) or no difference between pulsatile and continuous flow (22). It is however important to note that in these cases the change between pulsatile and continuous flow was produced at the cardiac level, altering the pulsatility of inflow to the intracranial space. Many studies on the link between cognition and intracranial pulsatility have also focused on measurements indicating the pulsatile input to the brain, such as pulse wave velocity or pulsatility in the larger arteries (5, 6, 10). The results, however, indicate that there might also be an interaction between the pulsatility of ICP and parenchymal blood flow, i.e., a more “local” effect. This suggests that not only the incoming arterial pulse wave but also the “reflected” wave within the rigid cranium, is of importance to capillary wall movement and pulsatile stress that the microvasculature and tissue are exposed to. More research is needed to establish what level of pulsatility is required to maintain normal brain clearance and perfusion or, conversely, to produce damage to the intracranial microvasculature due to excess pulsatile stress. A device such as Cadence may be a way to elucidate these issues in an experimental setting.
Conclusions
This study demonstrates that alteration of the ICP pulsatility alters brain tissue blood flow pulsatility. Specifically, across the experimental ICP pulse wave manipulations performed, decreasing systolic ICP increased blood flow pulsatility in brain tissue. Based on this inverse relationship, it can be implied that the altered CBF pulsatility is unlikely to depend on modification of the Windkessel effect on the feeding arterial system, but was rather an effect directly on tissue and the lower pressure distal vessels. The ability of the Cadence system to modify the ICP pulse wave, and thereby CBF pulsatility, opens up for further studies where it may be used as an adaptable experimental model for investigating pathophysiology hypotheses connecting altered intracranial pulsatility to neurological conditions.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was funded by an National Institutes of Health (NIH) grant awarded to Dr. Luciano (No. NS060916-01A2) and a Cleveland Clinic Innovations Grant for device development pilot testing also awarded to Dr. Luciano.
DISCLOSURES
M.G.L. and S.M.D. are listed as inventors on two patents regarding the device presented in this paper, but do not have any conflict of interest otherwise. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.M.D. and M.G.L. conceived and designed research; S.M.D., J.Y., and M.G.L. performed experiments; S.Q., S.T. and F.L. analyzed data; S.Q., D.B., and M.G.L. interpreted results of experiments; S.Q. prepared figures; S.Q., D.B., and M.G.L. drafted manuscript; S.Q., S.M.D., D.B., S.T., F.L., J.Y., and M.G.L. edited and revised manuscript; S.Q., S.M.D., D.B., S.T., F.L., J.Y., and M.G.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Anthony Shawan (Mechanical Prototype Core), Barry Kuban (Electronics Core), and Ji-Feng Chen (Polymer Core, Medical Device Solutions, Cleveland Clinic) for help with the Cadence system and balloon construction, as well as Prashant Rawat, Tim Moran, and Jeff Arnold from CSF Therapeutics Inc., for engineering (P.R.) and administrative (T.M. and J.A.) help and consultation. The authors also acknowledge Serge El-Khoury for contributions to data collection.
REFERENCES
- 1. Westerhof N, Lankhaar J-W, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput 47: 131–141, 2009. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 2. Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8: 5, 2011. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateman GA. Pulse wave encephalopathy: a spectrum hypothesis incorporating Alzheimer’s disease, vascular dementia and normal pressure hydrocephalus. Med Hypotheses 62: 182–187, 2004. doi: 10.1016/S0306-9877(03)00330-X. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell GF, Van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility-Reykjavik Study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorin-Trescases N, de Montgolfier O, Pinçon A, Raignault A, Caland L, Labbé P, Thorin XE. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 314: H1214–H1224, 2018. doi: 10.1152/ajpheart.00637.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 27: 145–165, 2004. doi: 10.1007/s10143-004-0326-9. [DOI] [PubMed] [Google Scholar]
- 8. Wåhlin A, Nyberg L. At the heart of cognitive functioning in aging. Trends Cogn Sci 23: 717–720, 2019. doi: 10.1016/j.tics.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 9. De Montgolfier O, Thorin-Trescases N, Thorin E. Pathological continuum from the rise in pulse pressure to impaired neurovascular coupling and cognitive decline. Am J Hypertens 33: 375–390, 2020. doi: 10.1093/ajh/hpaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y, Thrippleton MJ, Marshall I, Wardlaw JM. Intracranial pulsatility in patients with cerebral small vessel disease: a systematic review. Clin Sci (Lond) 132: 157–171, 2018. doi: 10.1042/CS20171280. [DOI] [PubMed] [Google Scholar]
- 11. Iliff JJ, Wang M, Zeppenfeld DMM, Venkataraman A, Plog BAA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33: 18190–18199, 2013. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luciano MG, Dombrowski SM, Qvarlander S, El-Khoury S, Yang J, Thyagaraj S, Loth F. Novel method for dynamic control of intracranial pressure. J Neurosurg 126: 1629–1640, 2017. doi: 10.3171/2016.4.JNS152457. [DOI] [PubMed] [Google Scholar]
- 13. Luciano MG, Dombrowski SM, El-Khoury S, Yang J, Thyagaraj S, Qvarlander S, Khalid S, Suk I, Manbachi A, Loth F. Epidural oscillating cardiac-gated intracranial implant modulates CBF. Neurosurgery 87: 1299–1310, 2020. doi: 10.1093/neuros/nyaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56: 1746–1748, 2001. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 15. Karlsson MGD, Wårdell K. Polarized laser Doppler perfusion imaging—reduction of movement-induced artifacts. J Biomed Opt 10: 064002, 2005. doi: 10.1117/1.2120467. [DOI] [PubMed] [Google Scholar]
- 16. Doron O, Or T, Battino L, Rosenthal G, Barnea O. Cerebral blood flow augmentation using a cardiac-gated intracranial pulsating balloon pump in a swine model of elevated ICP. J Neurosurg 132: 1606–1615, 2019. doi: 10.3171/2019.1.JNS182864. [DOI] [PubMed] [Google Scholar]
- 17. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science 370: 50–56, 2020. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ündar A. Myths and truths of pulsatile and nonpulsatile perfusion during acute and chronic cardiac support. Artif Organs 28: 439–443, 2004. doi: 10.1111/j.1525-1594.2004.00086.x. [DOI] [PubMed] [Google Scholar]
- 21. Guan Y, Karkhanis T, Wang S, Rider A, Koenig SC, Slaughter MS, El Banayosy A, Ündar A. Physiologic benefits of pulsatile perfusion during mechanical circulatory support for the treatment of acute and chronic heart failure in adults. Artif Organs 34: 529–536, 2010. doi: 10.1111/j.1525-1594.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- 22. Sezai A, Shiono M, Orime Y, Nakata K, Hata M, Iida M, Kashiwazaki S, Kinoshita J, Nemoto M, Koujima T, Furuichi M, Eda K, Hirose H, Yoshino T, Saitoh A, Taniguchi Y, Sezai Y. Major organ function under mechanical support: comparative studies of pulsatile and nonpulsatile circulation. Artif Organs 23: 280–285, 1999. doi: 10.1046/j.1525-1594.1999.06318.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.