Keywords: blood flow, endothelial, heart failure with preserved ejection fraction, microvascular, nitric oxide
Abstract
There is accumulating evidence for both peripheral vascular dysfunction and impaired functional capacity in patients with heart failure with a preserved ejection fraction (HFpEF). Although derangements in the l-arginine-nitric oxide (l-Arg-NO) pathway are likely to contribute to these aspects of HFpEF pathophysiology, the impact of increased NO substrate on vascular health and physical capacity has not been evaluated in this patient population. Thus, using a single-arm study design, we evaluated the impact of enteral l-citrulline (l-Cit, 6 g/day for 7 days), a precursor for l-Arg biosynthesis, on vascular function [flow-mediated dilation (FMD), reactive hyperemia (RH), and passive limb movement (PLM)], functional capacity [6-min walk test (6MWT)], and biomarkers of l-Arg-NO signaling in 14 patients with HFpEF (n = 14, 4 M/10 F, 70 ± 10 yr, EF: 66 ± 7%). Compared with baseline (0d), 7 days of l-Cit administration improved FMD (0d: 2.5 ± 1.6%, 7d: 4.5 ± 2.9%), RH (0d: 468 ± 167 mL, 7d: 577 ± 199 mL), PLM blood flow area-under-the-curve (0d: 139 ± 130 mL, 7d: 198 ± 115 mL), and 6MWT distance (0d: 377 ± 27 m, 7d: 397 ± 27 m) (P < 0.05). An increase in plasma l-Cit (0d: 42 ± 11 µM/L, 7d: 369 ± 201 µM/L), l-Arg (0d: 65 ± 8 µM/L, 7d: 257 ± 25 µM/L), and the ratio of l-Arg to asymmetric dimethylarginine (ADMA) (0d: 136 ± 13 AU, 7d: 481 ± 49 AU) (P < 0.05) was also observed. Though preliminary in nature, these functional and biomarker assessments demonstrate a potential benefit of l-Cit administration in patients with HFpEF, findings that provide new insight into the mechanisms that govern vascular and physical dysfunction in this patient group.
NEW & NOTEWORTHY The current investigation has demonstrated that l-Cit administration may improve brachial artery endothelium-dependent vasodilation, upper and lower limb microvascular function, and physical capacity in patients with HFpEF, highlighting the potential therapeutic potential of interventions targeting the l-Arg-NO signaling cascade to improve outcomes in this patient group.
INTRODUCTION
Indeed, there is now accumulating evidence for peripheral vascular dysfunction in heart failure with preserved ejection fraction (HFpEF), including impaired endothelium-dependent vasodilation (1) and microvascular dysfunction in both the upper (2) and lower (3) limbs, suggesting a disease-related decrement in nitric oxide (NO) signaling in the peripheral circulation that may represent a novel therapeutic target in this patient group. However, in randomized clinical trials, administration of organic (NEAT-HFpEF) (4) and inorganic (INDIE-HFpEF) (5) nitrate have proven largely unsuccessful in improving outcomes in patients with HFpEF, suggesting that simply providing NO precursor through the nonenzymatic nitrate-nitrite-NO pathway may not represent an effective strategy for improving NO bioavailability in this patient group. Interestingly, the potential benefit of specific targeting of endogenous, enzymatic NO signaling has not been well studied in patients with HFpEF.
Canonical NO signaling relies on several successive events to produce NO, including enzymatic coupling of endothelial NO synthase (eNOS) to the cofactor tetrahydrobiopterin (BH4) and proper substrate metabolism of l-arginine (l-Arg). Although l-Arg acts as the sole substrate for endogenous NO synthesis, it also serves as a substrate for arginase, the final enzyme of the urea cycle. Thus, therapeutic approaches seeking to improve NO signaling through enteral l-Arg are hindered by presystemic (intestinal) and systemic (hepatic) metabolism that significantly decrease bioavailability for synthesis of NO (6). In contrast, l-citrulline (l-Cit), an α-amino acid that is catabolized to l-Arg, has emerged as a more effective approach for increasing NO substrate (7). Indeed, there is accumulating evidence supporting the efficacy of l-Cit administration to improve vascular health in a variety of populations, including individuals with obesity (8) and patients with coronary artery disease (CAD) (9) and vasospastic angina (10). Although there is initial evidence for a beneficial effect of oral l-Cit administration on endothelial function in patients with HFpEF (11), this previous work used digital photoplethysmography for the determination of endothelial function, a methodology that is less robust compared with ultrasonography. Furthermore, the efficacy of l-Cit administration to improve peripheral vascular function at both macro- and microvascular levels has not been comprehensively evaluated.
Thus, using an array of functional and biomarker assessments, the purpose of this study was to comprehensively evaluate the efficacy of l-Cit administration on peripheral vascular and physical function in patients with HFpEF. We hypothesized that 7 days of l-Cit administration (6 g/day) would improve both conduit artery endothelium-dependent vasodilation, as determined by brachial artery flow-mediated dilation (FMD), as well as microvascular function in the upper and lower limbs, as assessed by reactive hyperemia (RH) and passive limb movement (PLM), respectively. We also hypothesized that 6-min walk test (6MWT) distance, a standardized metric of functional capacity, would increase following l-Cit administration. Finally, we hypothesized that plasma biomarkers of l-Arg-NO signaling [ l-Arg, l-Cit, and the ratio of l-Arg to asymmetric dimethylarginine (ADMA)] would all increase after l-Cit administration.
METHODS
Patients
All patients with HFpEF (NYHA Class II–III) were recruited from the HF clinic at the University of Utah and the Salt Lake City Veterans Affairs Medical Center (VAMC). Inclusion criteria for patients with HFpEF were consistent with the TOPCAT trial (12), which uses the following: 1) HF defined by the presence of ≥1 symptom at the time of screening (paroxysmal nocturnal dyspnea, orthopnea, and dyspnea on exertion) and 1 sign (edema, elevation in jugular venous distention) in the previous 12 mo; 2) left ventricular ejection fraction (LVEF) ≥45%; 3) controlled systolic blood pressure; and 4) either ≥1 hospitalization in the previous 12 mo for which HF was a major component of hospitalization, or B-type natriuretic peptide (BNP) ≥100 pg/mL, in the previous 60 days. Exclusion criteria for patients with HFpEF included significant valvular heart disease, acute atrial fibrillation, or a body mass index (BMI) >45 kg/m2. All participants were nonsmokers and were stable on guideline-directed pharmacotherapy. No patients were prescribed nitrate medications. All procedures were approved by the University of Utah and the Salt Lake City VAMC Institutional Review Boards in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before study participation. Studies were performed at the Veterans Affairs Salt Lake City Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory.
Experimental Protocol
All participants visited the laboratory for a preliminary study day that included familiarization with all procedures. Patients reported to the laboratory in the morning and were studied at similar times for all visits. All participants were 8-h postprandial except for two individuals who required food with their morning medication regimen and were, therefore, 4-h postprandial. All participants abstained from exercise for 24 h before testing. Upon arrival, patients provided a venous blood sample, and seated arterial blood pressure was determined. Patients then moved to a supine position and rested for 20 min, after which FMD testing was performed in accordance with current guidelines (13). The brachial artery was insonated approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle. Baseline measurements of brachial artery diameter and intensity-weighted mean blood velocity (Vmean) were recorded for 1 min, after which a blood pressure cuff, placed on the upper arm proximal to the elbow and distal to the Doppler probe measurement site, was inflated to a suprasystolic pressure (250–260 mmHg) for 5 min. The cuff was then rapidly deflated, and brachial artery diameter and Vmean were measured continuously for 2 min. To maintain continuity between exams, investigators confirmed a similar distance between the antecubital fossa and the ultrasound probe/occlusion cuff on each study visit.
After FMD testing, patients underwent PLM procedure in the upright, seated position using current guidelines (14). The common femoral artery was insonated ∼3 cm proximal to the femoral artery bifurcation. Baseline measurements of common femoral artery diameter and Vmean were recorded for 1 min, after which a member of the research team moved the knee joint through 90° range of motion, flexion-extension, at 1 Hz continuously for 1 min. Finally, functional capacity was evaluated using the 6MWT, conducted following a standardized procedure (15, 16). At a self-guided walking speed, patients walked, unaccompanied, through an obstacle-free hallway with time remaining being announced every minute. During the test, a measuring wheel and tally counter were used to record the total distance walked and steps taken, respectively, to determine stride length as meters per step.
l-Citrulline
After 0 day assessments, all patients consumed l-Cit twice daily (3 g/dose) (NOW Foods, Inc.) for 7 days, and returned to the laboratory on day 7 (7 d) for follow-up assessments. The final 3-g dose of l-Cit was taken in the morning, at least 2 h before returning to the laboratory. The rationale for this regimen is based on previous work that identified the efficacy of this dose and duration to increase plasma l-Cit concentration (7) and to significantly improve blood pressure, V̇o2 kinetics, and exercise performance (17). No medications were withheld on study days, and prescribed pharmacotherapy was unchanged across the 7-day l-Cit treatment period.
Interrater Reliability
A subset of patients (n = 5) returned to the laboratory within 6 mo of completing the l-Cit protocol for two additional study visits to evaluate interrater reliability of FMD testing across time (days 0 and 10) in the absence of l-Cit treatment.
Measurements and Analyses
Blood velocity and vessel diameter were determined using an ultrasound Doppler system (GE Medical Systems, Milwaukee, WI) operating in duplex mode. Blood velocity was collected at a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2–25 kHz). Sample volume was optimized in relation to vessel diameter and centered within the vessel. Vessel diameter was obtained during end diastole (corresponding to each R wave documented by the simultaneous ECG signal) using the same transducer at an imaging frequency ranging from 9–14 MHz. An angle of insonation of 60° (18) was achieved for all measurements.
Blood flow was calculated with the formula: blood flow (mL·min−1) = [Vmean × π (vessel diameter/2) 2 × 60]. Shear rate (SR) was calculated as: SR (s−1) = 8 × Vmean/arterial diameter. For both shear rate and blood flow, cumulative area-under-the-curve values were integrated with the trapezoidal rule and calculated as follows: Σ{yi[x(i + 1)−xi]+(1/2)[y(i + 1)−yi][x(i + 1)−xi]}. Leg vascular conductance was calculated with the formula: conductance = leg blood flow (mL/min)/mean arterial pressure (mmHg). Brachial artery vasodilation was determined offline from end-diastolic, ECG R-wave-triggered images, collected from the ultrasound Doppler system, using automated edge-detection software (Medical Imaging Applications, Coralville, IA), as described in detail elsewhere (19). FMD was quantified as the maximal change in brachial artery diameter after cuff release, and FMD normalized to SR was calculated by dividing FMD by the cumulative SR AUC at the time of peak brachial artery vasodilation. Reactive hyperemia was quantified as cumulative BA blood flow for 2 min (area-under-the-curve) after cuff occlusion. For PLM, leg blood flow was analyzed second-by-second and smoothed using a 3-s rolling average. The leg blood flow and vascular conductance responses during PLM were quantified as 1) peak, 2) ΔPeak (the difference between baseline and peak response), and 3) the AUC above baseline, assessed for 60 s. Arterial blood pressure (ABP) was determined noninvasively using photoplethysmography (Finometer, Finapres Medical Systems BV, Amsterdam, The Netherlands), and mean arterial pressure (MAP) was calculated as MAP (mmHg) = diastolic arterial pressure + (pulse pressure ×0.33). Heart rate was monitored from a standard three-lead electrocardiogram. A single investigator performed all analyses, blinded to condition.
Blood Biomarker Analyses
Blood was sampled from the antecubital vein, and plasma and serum samples were stored at −80°C for subsequent analyses. l-Cit (Immundiagnostik, no. K6600), l-Arg (Immundiagnostik, no. KR7733), and ADMA (Immundiagnostik, no. K7828) were assessed using enzyme-linked immunosorbent assays according to manufacturer’s instructions.
Echocardiography
Results from a recent (<6 mo before the experimental study visit) clinical echocardiogram were obtained via chart review.
Statistical Analyses
Statistics were performed using commercially available software (SigmaStat 3.13; Systat Software, Point Richmond, CA). Paired Student’s t tests and two-way repeated-measure analyses were performed, Shapiro–Wilk normality test confirmed normal distribution, and the Bonferroni test was used for post hoc analysis when a significant main effect was found. The Pearson correlation coefficient test was used to assess relationships between changes in 6MWT distance and changes in plasma biomarkers and FMD, and to determine interclass reliability for FMD testing. Sample size analyses were also performed for the primary outcomes (FMD, RH, and PLM). All data are expressed as mean ± SD. Significance was established at P < 0.05 for all tests.
RESULTS
Patient Characteristics
Anthropometric data and clinical biomarkers are presented in Table 1, and disease characteristics and medications are documented in Table 2. Supine systolic (0 d: 122 ± 19, 7 d: 121 ± 21 mmHg, P = 0.80), diastolic (0 d: 67 ± 10, 7 d: 68 ± 8 mmHg, P = 0.64), and mean arterial (0 d: 85 ± 11, 7 d: 86 ± 11 mmHg, P = 0.81) blood pressure did not change after 7-day l-Cit. Clinical echocardiographic characteristics are recorded in Table 3.
Table 1.
Characteristics and clinical biomarkers
| Anthropometric Data | Value | Range |
|---|---|---|
| Sex, M/F | 4/10 | |
| Age, yr | 70 ± 10 | 55–86 |
| Height, cm | 167 ± 10 | 152–188 |
| Weight, kg | 94 ± 16 | 74–132 |
| BMI, kg/m2 | 33 ± 4 | 27–40 |
| Supine heart rate, beats/min | 64 ± 8 | 53–82 |
| Supine mean arterial pressure, mmHg | 85 ± 11 | 66–108 |
Data are means ± SD. BMI, body mass index.
Table 2.
Disease characteristics and medications
| Disease Characteristics | n, % |
|---|---|
| NYHA class II | 10, 71 |
| NYHA class III | 4, 29 |
| Diabetes | 2, 14 |
| COPD | 0, 0 |
| CAD | 2, 14 |
| Hypertension | 10, 71 |
| Hyperlipidemia | 4, 29 |
| Medications | |
| β-Blockers | 7, 50 |
| ACEi | 4, 29 |
| ARB | 4, 29 |
| Loop diuretics | 10, 71 |
| Aldosterone antagonists | 11, 79 |
| Statin | 8, 57 |
| Ca2+ channel blocker | 7, 50 |
| Mean Rx types taken, n | 3.7 ± 1.1 |
| Patients taking Rx, % | 100 |
Data are means ± SD or count (%) and included n = 14 (4 M/10 F). ARB; angiotensin-receptor blockers; ACEi, angiotensin-converting enzyme inhibitors; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Table 3.
Echocardiogram characteristics
| HFpEF | Normal Range | |
|---|---|---|
| Ejection fraction, % | 67 ± 6 | ≥55 |
| LV IVSD, cm | 1.1 ± 0.1 | 0.6–1.1 |
| LV PWD, cm | 1.0 ± 0.1 | 0.6–0.9 |
| LV ID diastole, cm | 4.6 ± 0.5 | 3.9–5.3 |
| LV ID systole, cm | 2.8 ± 0.4 | 2.0–4.0 |
| Peak E wave, cm/s | 97 ± 27 | ≤ 50 |
| Peak A wave, cm/s | 81 ± 21 | 12–36 |
| E/A ratio | 1.4 ± 1.3 | 0.6–1.32 |
| E′ lateral wall, m/s | 8.7 ± 5 | 13–28 |
| E/E′ lateral ratio | 15.1 ± 8.2 | ≤8 |
| Mitral E-wave deceleration time, ms | 197 ± 46 | 142–258 |
Data are means ± SD (n = 14, 4 M/10 F). A wave, peak velocity of late transmitral flow; E wave, peak velocity of early diastolic transmitral flow; E′, peak velocity of early diastolic mitral annular motion; ID, internal dimension; IVSD, interventricular septum thickness at end-diastole; LV, left ventricle; PWD, posterior wall thickness.
Flow-Mediated Dilation
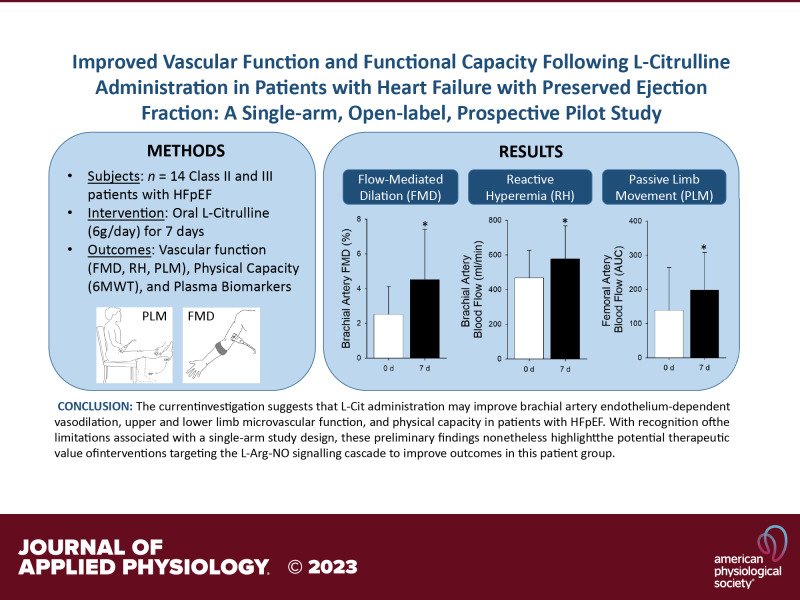
Brachial artery FMD responses are illustrated in Fig. 1. l-Cit administration had no effect on baseline brachial artery diameter (0 d: 4.44 ± 0.79 mm, 7 d: 4.33 ± 0.57 mm, P = 0.68), resting brachial artery blood flow (0 d: 97 ± 31 mL/min, 7 d: 111 ± 55 mL/min, P = 0.16), or resting brachial artery vascular conductance (0 d: 1.17 ± 0.48 mL/min/mmHg, 7 d: 1.34 ± 0.76 mL/min/mmHg, P = 0.11). The change in brachial artery diameter, expressed as percent change, was greater after 7 days l-Cit (0 d: 2.5 ± 1.6%, 7 d: 4.5 ± 2.9%, P = 0.003, power = 0.91) (Fig. 1A). Based on this effect size (1.99 ± 2.1), a minimum of 11 participants were required for the desired power of >0.8 with α = 0.05. The absolute change in brachial artery diameter was also significantly greater after l-Cit (0 d: 0.11 ± 0.07 mm, 7 d: 0.19 ± 0.12 mm, P = 0.01). When performed in accordance with published guidelines (13), an intersession coefficient of variation for FMD testing of ≈16% has been reported (20). Viewed on an individual basis, the increase in percent FMD after l-Cit treatment in the present study exceeded 16% in 11 of 14 participants (range −86 to +493% change). When FMD was normalized for SR, 7-day l-Cit administration increased both %FMD/SR (0 d: 0.07 ± 0.06 AU, 7 d: 0.12 ± 0.05 AU, P = 0.012, power = 0.80) (Fig. 1B). Based on this effect size (0.054 ± 0.06), a minimum of 12 participants were required for the desired power of >0.8 with α = 0.05. The absolute change in diameter/SR was also significantly increased after l-Cit (0 d: 3.3 ± 3.3 AU, 7 d: 5.9 ± 3.6 AU, P = 0.02). The time to peak diameter (0 d: 55 ± 27 s, 7 d: 48 ± 29 s, P = 0.4) and the sum of SR at peak diameter (0 d: 4.3 ± 1.8 AU, 7 d: 3.7 ± 2.2 AU, P = 0.43) were both unchanged by l-Cit administration.
Figure 1.
A: brachial artery flow-mediated dilation (FMD) expressed as percent change before (white bar) and after (black bar) 7-day l-citrulline administration. Gray lines indicate individual responses in females (solid lines, n = 10) and males (dashed lines, n = 4). Values are presented as means ± SD. Paired Student’s t test was performed. *Significant difference between 0 and 7 days, P = 0.003. B: brachial artery FMD normalized for shear rate (SR) area-under-the-curve before (white bar) and after (black bar) 7-day l-citrulline administration. Gray lines indicate individual responses in females (solid lines, n = 10) and males (dashed lines, n = 4). Values are presented as means ± SD. Paired Student’s t test was performed. *Significant difference between 0 and 7 days, P = 0.012.
Brachial Artery Reactive Hyperemia
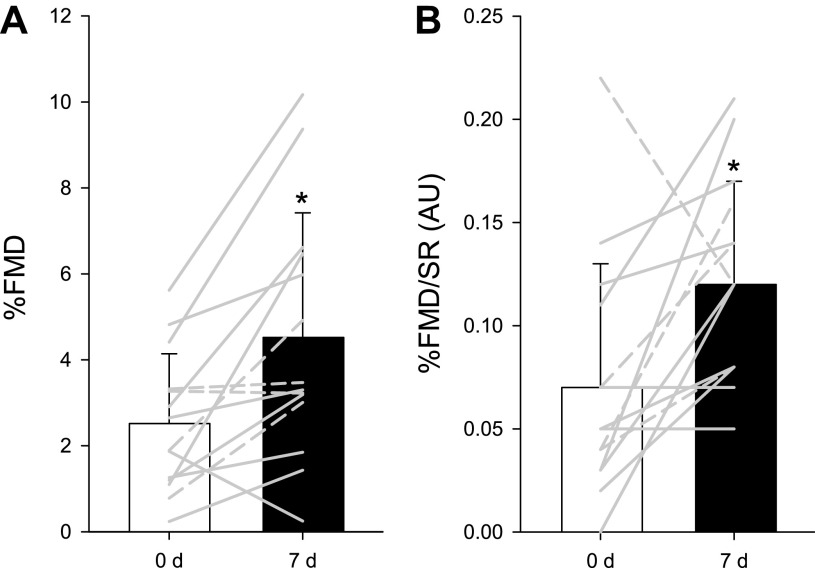
Brachial artery RH increased following 7-day l-Cit, whether viewed as brachial artery blood flow after cuff release (Fig. 2A) or AUC (0 d: 468 ± 167 mL, 7 d: 577 ± 199 mL, P = 0.004, power = 0.95) (Fig. 2B). Based on this effect size (109 ± 105), a minimum of 10 participants were required for the desired power of >0.8 with α = 0.05.
Figure 2.
A: brachial artery postocclusion reactive hyperemia (RH) before (white circles) and after (black circles) 7-day l-citrulline administration. Values are presented as means ± SD. Paired Student’s t test was performed. Two-way repeated-measure analyses were performed, with the Bonferroni test used for post hoc analysis when a significant main effect was found. *Significant difference between 0 and 7 days, P < 0.05. B: brachial artery blood flow area-under-the-curve (AUC) before (white bar) and after (black bar) 7-day l-citrulline administration. Gray lines indicate individual responses in females (solid lines, n = 10) and males (dashed lines, n = 4). Values are presented as means ± SD. Paired Student’s t test was performed. *Significant difference between 0 and 7 days, P = 0.002.
Passive Limb Movement
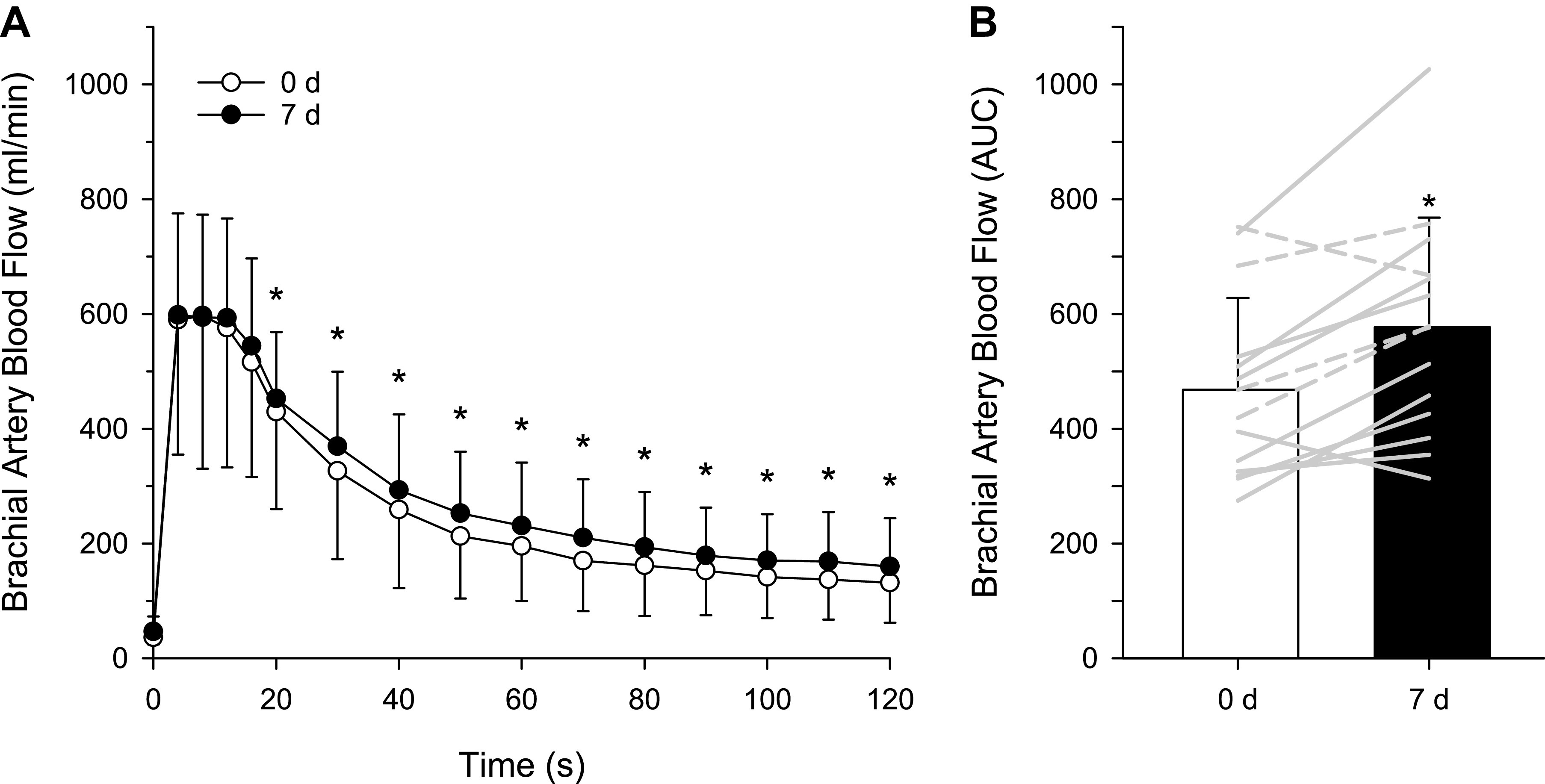
No significant changes were observed as a consequence of 7-day l-Cit treatment for baseline femoral artery blood flow (0 d: 447 ± 151 mL/min, 7 d: 502 ± 236 mL/min, P = 0.29), maximal femoral artery blood flow during PLM (0 d: 836 ± 316 mL/min, 7 d: 951 ± 403 mL/min, P = 0.06), or the change in blood flow from baseline to maximal blood flow response to PLM (0 d: 359 ± 216 mL/min, 7 d: 499 ± 199 mL/min, P = 0.11). In contrast, the AUC for the blood flow response increased following 7-day l-Cit administration (0 d: 139 ± 130 mL, 7 d: 198 ± 115 mL, P = 0.02, power = 0.8) (Fig. 3B). Based on this effect size (58 ± 72), a minimum of 14 participants were required for the desired power of >0.8 with α = 0.05. l-Cit treatment did not change baseline leg vascular conductance (0 d: 5.2 ± 2.1, 7 d: 5.7 ± 3.2 mL/min/mmHg, P = 0.19), maximal leg vascular conductance during PLM (0 d: 9.9 ± 4.3, 7 d: 10.9 ± 5.5 mL/min/mmHg, P = 0.08), or the change in leg vascular conductance from baseline to maximal response to PLM (0 d: 4.7 ± 2.7, 7 d: 5.2 ± 2.5 mL/min/mmHg, P = 0.13). The AUC for the leg vascular conductance response tended to increase following 7-day l-Cit administration (0 d: 139 ± 130 mL, 7 d: 198 ± 115 mL, P = 0.06) (Fig. 3D).
Figure 3.
A: femoral artery blood flow response to passive limb movement (PLM) responses before (white circles) and after (black circles) 7-d l-citrulline administration. Values are presented as means ± SD. Two-way repeated-measure analyses were performed, with the Bonferroni test used for post hoc analysis when a significant main effect was found. B: femoral artery blood flow area-under-the-curve (AUC) before (white bar) and after (black bar) 7-d l-citrulline administration. Gray lines indicate individual responses in females (solid lines, n = 10) and males (dashed lines, n = 4). Values are presented as means ± SD. Paired Student’s t test was performed. *Significant difference between 0 and 7 days, P = 0.011. C: femoral vascular conductance response to passive limb movement (PLM) responses before (white circles) and after (black circles) 7-d l-citrulline administration. Values are presented as means ± SD. Two-way repeated-measure analyses were performed, with the Bonferroni test used for post hoc analysis when a significant main effect was found. D: femoral vascular conductance area-under-the-curve (AUC) before (white bar) and after (black bar) 7-d l-citrulline administration. Gray lines indicate individual responses in females (solid lines, n = 10) and males (dashed lines, n = 4). Values are presented as means ± SD. Paired Student’s t test was performed.
6-Min Walk Test
The 6MWT was completed in a subset of patients (n = 10). Four patients did not complete the 6MWT due to concerns regarding the risk of exertional angina. 6MWT distance improved following 7-day l-Cit administration (0 d: 377 ± 27 m, 7 d: 397 ± 27 m, P = 0.049). The total number of steps taken was unchanged following l-Cit (0 d: 660 ± 34 steps, 7 d: 667 ± 26 steps, P = 0.78), though number of steps was numerically greater after treatment in 7 of 10 patients. Similarly, the group average for stride length was not different between days 0 and 7 (0 d: 0.57 ± 0.02 m/steps, 7 d: 0.59 ± 0.02 m/steps, P = 0.30), likely owing to individual variation within the group (i.e., n = 5 increased and n = 5 decreased following treatment). No significant correlations were observed between the change in 6MWT distance and the change in percent FMD (r = 0.17, P = 0.63) or between the change in 6MWT distance and the change in plasma l-Cit (r = 0.07, P = 0.845) following treatment.
Circulating Biomarkers
Plasma was collected for determination of l-Cit, l-Arg, ADMA, and the ratio of l-Arg to ADMA (n = 11). No blood samples were obtained in three participants due to unsuccessful phlebotomy encounters. All biomarkers increased with 7-day l-Cit and are reported in Table 4.
Table 4.
Circulating plasma biomarkers
| 0 Day | Range | 7 Day | Range | |
|---|---|---|---|---|
| l-Citrulline, µM/L | 41.6 ± 11.3 | 21–60 | 368.7 ± 200.5* | 28–682 |
| l -Arginine, µM/L | 65.1 ± 7.8 | 35–126 | 256.7 ± 24.5* | 132–379 |
| ADMA, µM/L | 0.48 ± 0.03 | 0.32–0.66 | 0.54 ± 0.04* | 0.36–0.73 |
| l-Arg:ADMA | 136 ± 13 | 73–226 | 481 ± 49* | 358–898 |
Data are means ± SD (n = 11, 4 M/7 F). ADMA, asymmetric dimethylarginine. Paired Student’s t test was performed. *Significant difference between 0 and 7 days, P < 0.05.
FMD Test-Retest Reliability
In a subset of patients with HFpEF (n = 5) who underwent additional serial FMD testing, good day-to-day reproducibility [Pearson correlation (r) = 0.87] was observed for percent FMD across time.
DISCUSSION
The purpose of this pilot study was to investigate the impact of augmented NO substrate, achieved through enteral l-Cit administration, on peripheral vascular function, physical capacity, and biomarkers of l-Arg-NO signaling in patients with HFpEF. After a 7-day regimen of l-Cit, a marked improvement of brachial artery FMD was observed, supporting the efficacy of this intervention to improve endothelium-dependent vasodilation in the macrovasculature of this patient group. Importantly, indices of microvascular reactivity increased in vascular beds of both the arm and leg after l-Cit, which together with the l-Cit-induced changes in FMD suggest that increased NO substrate may favorably affect peripheral vascular function at multiple levels of the arterial tree. A significant increase in 6MWT distance was also observed following l-Cit, demonstrating a promising improvement in physical capacity. These physiological and functional assessments were accompanied by increases in circulating l-Arg, l-Cit, and l-Arg:ADMA after 7-d l-Cit, confirming NO substrate absorption and suggesting an increase in NO bioavailability as a consequence of treatment. Taken together, these preliminary findings support the effectiveness of short-term l-Cit administration to stimulate l-Arg-NO signaling, which may improve both peripheral vascular function and physical capacity in patients with HFpEF, providing new evidence that these aspects of HFpEF pathophysiology may be remediable through selective targeting of the enzymatic NO pathway in this patient group.
FMD and l-Citrulline in HFpEF
Brachial artery vasodilation after a period of blood flow occlusion represents a noninvasive index of conduit artery endothelium-dependent vasodilation, which has been used in both healthy and diseased populations (13, 21) and is predictive of future cardiovascular events (22). In one of the largest studies to date evaluating vascular function in patients with HFpEF, Haykowsky et al. (23) reported no difference in FMD between patients and age-matched controls, with similar responses reported in the lower limb (24). However, more recent work has reported lower FMD values in HFpEF compared with healthy age-matched (25) or control subjects with hypertension (26, 27). Our group previously provided evidence of decreased FMD in HFpEF compared with healthy age-matched controls, though this decrement was no longer evident after the FMD response was normalized for the shear stimulus (2). Unfortunately, significant differences in both methodology (occlusion time period, consideration of shear stimulus) and choice of control group (healthy, age-matched vs. comorbidity-matched) preclude consensus on the degree to which vascular dysfunction is present in HFpEF. However, the majority of studies to date collectively indicate some decrement in conduit artery endothelial function in patients with HFpEF (1). Although the current study did not include a control group, baseline (0 d) FMD (2.5 ± 1.6%) represents the low end of published values for patients with HFpEF and is well below the 7% cut-off value that was recently identified as a representative of “normal” endothelial function (28). The apparent vascular dysfunction identified in these former and current studies, therefore, raises the question of whether peripheral vasculature may be a modifiable aspect of HFpEF pathophysiology.
Studies examining the effects of l-Cit among various healthy and diseased populations have been largely inconclusive concerning the benefit of this intervention on vascular health. No improvement in peripheral vascular function was observed in patients with HFrEF when assessments were performed within 1 h of l-Cit consumption (29), suggesting longer administration of l-Cit and/or assessments in a population with more severe deficits in peripheral vascular function may be needed. Indeed, while 1 wk of l-Cit administration failed to increase brachial artery FMD in young, healthy individuals (7), a 15-day supplement was sufficient to improve FMD in patients with coronary artery disease (9) and in vasospastic angina after 4 and 8 wk of l-Cit treatment (10). To our knowledge, only one study to date has evaluated the impact of l-Cit in patients with HFpEF. In this study, Orea-Tejeda et al. (11) reported an improvement in endothelial function, as determined by finger photoplethysmography, in patients with HFpEF after 2 mo of l-Cit administration. Although this prior work provides important information concerning the efficacy of l-Cit to favorably affect digital microvascular hyperemia, the role of NO in RH is uncertain (30), leaving some questions as to whether the observed improvements were the consequence of a change in l-Arg-NO signaling. The current investigation thus extends these initial findings in patients with HFpEF, providing new evidence concerning the efficacy of l-Cit to improve FMD in this patient group in as little as 7 days.
The observed increase in FMD (Fig. 1A) following 7-day l-Cit administration in patients with HFpEF is clinically meaningful, as every 1% increase in brachial artery FMD may represent up to a 13% decrease in cardiovascular events risk (31). Furthermore, when normalized to the shear stimulus (Fig. 1B), the FMD response was nearly doubled after l-Cit administration in patients with HFpEF. These marked improvements in conduit artery endothelium-dependent vasodilation complement recent findings from our group that identified the efficacy of acute antioxidant administration to improve brachial artery FMD in patients with HFpEF (32), providing additional evidence for vascular adaptability in this patient population. Together, these previous and current data support the efficacy of nonpharmacological interventions targeting the l-Arg-NO pathway to improve endothelium-dependent vasodilation in HFpEF.
Microvascular Function and l-Citrulline in HFpEF
There is increasing evidence for the presence of disease-related systemic microvascular dysfunction in patients with HFpEF that contributes to both the etiology and progression of the disease (33). The distinction between macro- and microvascular function is particularly important given that the vascular beds of the microcirculation are the primary point of blood flow regulation, constantly adapting to systemic and local signaling from the vascular space and surrounding tissue (34). Brachial artery RH quantifies the overall increase in blood flow after arterial occlusion, providing an index of arm microvascular function that is inversely related to cardiovascular disease risk (35). RH is also predictive of future cardiovascular events in healthy and diseased populations (36) and therefore is viewed as a clinically relevant vascular assessment. Our group has previously identified a marked ∼30% reduction in brachial artery RH in patients with HFpEF compared with healthy, age-matched controls (2), while others have shown a similar reduction in RH index in patients with HFpEF compared with controls that independently correlated with future cardiovascular events (37). Remarkably, the current investigation observed a ∼25% improvement in brachial artery RH following 7-day l-Cit administration (Fig. 3), which could be interpreted as a near restoration in microvascular function when compared with our previous results (38). These findings are in agreement with previous work identifying the efficacy of more prolonged l-Cit administration (5 g/day for 2 mo) on RH in patients with HFpEF (11), providing new evidence that significant improvements in upper limb microvascular function may be achieved in as little as 7 days of treatment.
To explore the potential impact of improved l-Arg-NO signaling microvascular function in a locomotor muscle group, we also used the PLM test to determine vascular reactivity in the lower limb. Though this assessment is less widely studied than brachial artery RH, our group has used this methodology extensively over the past decade to evaluate lower limb microvascular function in healthy (39–43), elderly (44–46), and diseased (47–49) populations, including HFpEF (3). Importantly, we have previously reported inhibition of nitric oxide synthase (NOS) via intra-arterial infusion of l-NMMA reduces the hyperemia and vasodilation associated with PLM by almost 80%, suggesting that NO plays an essential role in this response (40). PLM is unique in that this test directly interrogates the leg, which plays a major role in locomotion and exercise capacity, thus providing a very specific assessment of the challenges faced by patients with HFpEF. In response to 7d l-Cit administration, a clear improvement in femoral artery blood flow and vascular conductance AUC was observed (Fig. 3, B and D). To our knowledge, this is the first evidence of “plasticity” of microvascular function in the lower limb of this patient group. Together, the l-Cit-mediated improvements in both upper and lower limb microvascular reactivity highlight the capacity of enhanced NO substrate to improve this aspect of HFpEF pathophysiology. Further studies are warranted to determine whether new pharmacological approaches targeting the NO pathway [i.e., soluble guanylate cyclase activators/stimulators, sodium-glucose cotransporter-2 (SGLT2) inhibitors, or angiotensin receptor neprilysin inhibitors (ARNIs)] can leverage the apparent plasticity in microvascular function to improve outcomes in patients with HFpEF.
Functional Capacity and l-Citrulline in HFpEF
The 6MWT is a well-tolerated and highly reproducible assessment that has been widely used to quantify overall functional capacity and exercise tolerance in patients with heart failure (38, 50). In the present study, a modest, but statistically significant increase in 6MWT distance (+20 m, a ∼5% improvement) was observed following 7-day l-Cit administration, suggesting a favorable change in physical capacity after treatment. However, it should be noted that the improvement in 6MWT following l-Cit was not significantly correlated with changes in either plasma l-Cit concentration or percent FMD, suggesting that caution is warranted in interpreting the mechanisms responsible for improved physical capacity in this patient group. This potential beneficial effect following administration of NO substrate is similar to prior work from Rector et al. (51) that identified an ∼8% increase in 6MWT distance in patients with HFrEF after 6 wk of oral l-Arg supplementation. No studies to date have evaluated the impact of NO substrate (l-Arg or l-Cit) administration on physical capacity in HFpEF. However, clinical trials examining the efficacy of drugs targeting specific points in the NO pathway have been largely negative. Indeed, studies administering isosorbide mononitrate (4), soluble guanylate cyclase stimulators (52), and phosphodiesterase-5 inhibitors (53) have all failed to identify a favorable effect on 6MWT distance in this patient population. In this light, the improvement in 6MWT distance in the present study may suggest that upstream targeting of enzymatic l-Arg-NO signaling is a uniquely effective strategy to improve physical capacity in patients with HFpEF, a concept that should be explored further using a traditional, randomized trial design.
Inflammation, Oxidative Stress, and NO Bioavailability in HFpEF
Although the unifying concept of inflammation as a key feature of HFpEF pathophysiology was introduced by Paulus et al. (54) only a few years ago, the axis of inflammation, oxidative stress, and vascular function has been well-described in both health and disease. Specifically, inflammation is known to diminish cellular antioxidant capacity (55) and increase the production of O2-centered free radicals such as superoxide (O2−), which readily bind with NO to form the free radical peroxynitrite (ONOO−). This “scavenging” process reduces the amount of NO available to provoke smooth muscle cell relaxation, resulting in an impairment in NO-dependent vasodilation. Though this aspect of free radical biology has been studied extensively, the importance of disease-related changes in NO substrate bioavailability and subsequent NO synthesis is less well understood.
With respect to substrate bioavailability, we observed a ninefold increase in plasma l-Cit after 7-day treatment, providing clear evidence for the effective absorption of this l-Arg precursor. Likewise, a marked increase in plasma l-Arg was also evident, supporting the presence of increased NO substrate due to 7-day l-Cit administration. However, the effectiveness of increased substrate on NO synthesis is dictated to a large degree by the enzymatic conversion of l-Arg to NO via NOS, a process that is governed by many cofactors and extrinsic influences. Chief among these regulators of l-Arg-NO signaling is asymmetric dimethylarginine (ADMA), an analog of l-Arg that acts as a competitive inhibitor and uncoupler of NOS. Increases in ADMA, presumably through an increase in oxidative stress (56), appear to be present in a variety of cardiovascular diseases (57–63) and are elevated up to 12-fold in various stages of chronic HF (64, 65). Thus, in the case of interventions manipulating NO substrate, the ratio between l-Arg and ADMA provides an important index of NO bioavailability. In the present investigation, the l-Arg to ADMA ratio improved nearly fourfold after l-Cit administration. Together with the FMD and PLM responses, assessments that are both predominantly NO-mediated (40, 66), the l-Cit-mediated increase in these biomarkers lends additional mechanistic support for the efficacy of enteral 7-day l-Cit administration to improve l-Arg-NO signaling in patients with HFpEF.
Perspectives
Given the link between disease-related changes in inflammation, oxidative stress, and NO bioavailability, it is not surprising that agents targeting the NO pathway have become an area of intense interest for the potential treatment of patients with HFpEF (67). Initial investigations evaluating infused (68) and enteral (69, 70) nitrate administration provoked promising improvements in exercise capacity, though subsequent clinical trials have failed to identify a favorable effect of exogenous NO on aerobic capacity, daily activity levels, or quality of life (5). Although there are likely many potential explanations for the equivocal findings in these studies, one of the most likely causes is the somewhat narrow focus on administering a NO precursor through the nonenzymatic, nitrate-nitrite-NO pathway. Thus, strategies such as NO substrate administration, an approach that seeks to improve upon disease-related impairments in endogenous, enzymatic NO signaling, may represent a new opportunity for targeting vascular dysfunction and impaired physical capacity in patients with HFpEF. Indeed, 7-day administration of l-Cit (6 g/day) has been demonstrated to improve exercise time to exhaustion in young, healthy adults (17), providing proof of concept that may be translated to diseased populations. Further investigations are certainly warranted to determine whether the beneficial effects of l-Cit observed in the present study translate to improvements and exercise capacity and clinical status in this patient group.
Experimental Considerations
We enrolled patients with HFpEF on optimized pharmacotherapy, and no medications were withheld on experimental days. Thus, we cannot exclude the possibility that existing drug therapies may have influenced measurements of peripheral vascular function and physical capacity. We also recognize the limitations associated with the use of a single-arm, open-label, prospective study design. This is an efficient approach for evaluating the efficacy of an intervention using a small number of patients and repeated measurements for pre-/posttreatment comparisons (71). Though not uncommon in clinical studies seeking to evaluate the efficacy of an intervention before large-scale trials (72, 73), we acknowledge that the lack of a control group and blinding of participants may allow for potential introduction of conscious and unconscious bias. It is also recognized that findings regarding the efficacy of l-Cit to improve vascular outcomes may not be specific to patients with HFpEF, and thus further studies are needed to examine the potential value of this intervention in other patient populations. Diet was not controlled during the treatment period, and we therefore cannot exclude the possibility that consumption of nitrate-enriched foods may have impacted plasma ADMA values. It should also be noted that administering l-Cit on day 7 of the experimental visit precludes the determination of acute versus chronic effects of treatment. Although we did not collect physical activity data in the present study, all patients were NYHA Class II or III and suffered from significant dyspnea upon exertion, and thus there is a high likelihood that all enrolled patients were sedentary apart from activities associated with daily living. Finally, we recognize that the observed improvement in 6MWT distance could be due, at least in part, to the presence of a “learning effect” as a consequence of performing multiple tests across time (74).
Conclusion
The current investigation suggests that l-Cit administration may improve brachial artery endothelium-dependent vasodilation, upper and lower limb microvascular function, and physical capacity in patients with HFpEF. With the recognition of the limitations associated with a single-arm study design, these preliminary findings nonetheless highlight the potential therapeutic value of interventions targeting the -Arg-NO signaling cascade to improve outcomes in this patient group.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was funded in part by the National Institutes of Health (T32 HL139451, to K.B.), the US Department of Veterans Affairs (RX001311 and CX002152 to D.W.W.; IK2RX003670, to K.B.), and the American Heart Association (18POST33960192, to K.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.R., K.B., J.K.A., and D.W.W. conceived and designed research; S.M.R., K.B., J.K.A., and D.W.W. performed experiments; S.M.R., K.B., J.K.A., J.Z., and D.W.W. analyzed data; S.M.R., K.B., J.K.A., J.Z., J.B.W., J.J.R., and D.W.W. interpreted results of experiments; S.M.R. and D.W.W. prepared figures; S.M.R. and D.W.W. drafted manuscript; S.M.R., K.B., J.K.A., J.Z., J.B.W., J.J.R., and D.W.W. edited and revised manuscript; S.M.R., K.B., J.K.A., J.Z., J.B.W., J.J.R., and D.W.W. approved final version of manuscript.
REFERENCES
- 1. Ambrosino P, Papa A, Buonauro A, Mosella M, Calcaterra I, Spedicato GA, Maniscalco M, Di Minno MND. Clinical assessment of endothelial function in heart failure with preserved ejection fraction: a meta-analysis with meta-regressions. Eur J Clin Invest 51: e13552, 2021. doi: 10.1111/eci.13552. [DOI] [PubMed] [Google Scholar]
- 2. Lee JF, Barrett-O'Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 102: 278–284, 2016. doi: 10.1136/heartjnl-2015-308403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Francisco MA, Lee JF, Barrett-O'Keefe Z, Groot HJ, Ratchford SM, Bunsawat K, Alpenglow JK, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Locomotor muscle microvascular dysfunction in heart failure with preserved ejection fraction. Hypertension 78: 1750–1759, 2021. doi: 10.1161/HYPERTENSIONAHA.121.17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WHW, McNulty SE, Velazquez EJ, Shah MR, Braunwald E; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 373: 2314–2324, 2015. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM; National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 320: 1764–1773, 2018. doi: 10.1001/jama.2018.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris SM Jr. Enzymes of arginine metabolism. J Nutr 134: 2743S–2747S, 2004. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- 7. Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Boger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 65: 51–59, 2008. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Figueroa A, Alvarez-Alvarado S, Jaime SJ, Kalfon R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br J Nutr 116: 279–285, 2016. doi: 10.1017/S0007114516001811. [DOI] [PubMed] [Google Scholar]
- 9. Safi M, Mahjoob MP, Nateghi S, Khaheshi I, Akbarzadeh MA, Naderian M. The assessment of short-term effect of l-Citrulline on endothelial function via FMD to NMD ratio in known CAD patients: a randomized, cross-over clinical trial (Clinical trial number: NCT02638727). Rom J Intern Med 55: 23–27, 2017. doi: 10.1515/rjim-2016-0045. [DOI] [PubMed] [Google Scholar]
- 10. Morita M, Sakurada M, Watanabe F, Yamasaki T, Doi H, Ezaki H, Morishita K, Miyakex T. Effects of oral L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol Endocr Metab Agents Med Chem 13: 214–220, 2013. doi: 10.2174/18715222113139990008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orea-Tejeda A, Orozco-Gutierrez JJ, Castillo-Martinez L, Keirns-Davies C, Montano-Hernandez P, Vazquez-Diaz O, Valdespino-Trejo A, Infante O, Martinez-Memije R. The effect of L-arginine and citrulline on endothelial function in patients in heart failure with preserved ejection fraction. Cardiol J 17: 464–470, 2010. [PubMed] [Google Scholar]
- 12. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 162: 966–972.e10, 2011. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gifford JR, Richardson RS. CORP: ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JGF. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J 26: 1742–1751, 2005. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 16. Beltran P, Palau P, Dominguez E, Faraudo M, Nunez E, Guri O, Mollar A, Sanchis J, Bayes-Genis A, Nunez J. Sacubitril/valsartan and short-term changes in the 6-minute walk test: a pilot study. Int J Cardiol 252: 136–139, 2018. doi: 10.1016/j.ijcard.2017.10.074. [DOI] [PubMed] [Google Scholar]
- 17. Bailey SJ, Blackwell JR, Lord T, Vanhatalo A, Winyard PG, Jones AM. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J Appl Physiol (1985) 119: 385–395, 2015. doi: 10.1152/japplphysiol.00192.2014. [DOI] [PubMed] [Google Scholar]
- 18. Logason K, Barlin T, Jonsson ML, Bostrom A, Hardemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50-69% and 70-99% carotid artery stenosis. Eur J Vasc Endovasc Surg 21: 311–313, 2001. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 19. Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghiadoni L, Faita F, Salvetti M, Cordiano C, Biggi A, Puato M, Di Monaco A, De Siati L, Volpe M, Ambrosio G, Gemignani V, Muiesan ML, Taddei S, Lanza GA, Cosentino F. Assessment of flow-mediated dilation reproducibility: a nationwide multicenter study. J Hypertens 30: 1399–1405, 2012. doi: 10.1097/HJH.0b013e328353f222. [DOI] [PubMed] [Google Scholar]
- 21. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 22. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/circulationaha.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci 68: 161–167, 2013. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol 292: H1427–H1434, 2007. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 25. Kishimoto S, Kajikawa M, Maruhashi T, Iwamoto Y, Matsumoto T, Iwamoto A, Oda N, Matsui S, Hidaka T, Kihara Y, Chayama K, Goto C, Aibara Y, Nakashima A, Noma K, Higashi Y. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol 231: 181–187, 2017. doi: 10.1016/j.ijcard.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 26. Marechaux S, Samson R, van Belle E, Breyne J, de Monte J, Dedrie C, Chebai N, Menet A, Banfi C, Bouabdallaoui N, Le Jemtel TH, Ennezat P-V. Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. J Card Fail 22: 3–11, 2016. doi: 10.1016/j.cardfail.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 27. Farrero M, Blanco I, Batlle M, Santiago E, Cardona M, Vidal B, Castel MA, Sitges M, Barbera JA, Perez-Villa F. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 7: 791–798, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000942. [DOI] [PubMed] [Google Scholar]
- 28. Maruhashi T, Kajikawa M, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, , et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc 9: e013915, 2020. doi: 10.1161/JAHA.119.013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim I-Y, Schutzler SE, Schrader A, Spencer HJ, Azhar G, Deutz NE, Wolfe RR. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am J Physiol Endocrinol Metab 309: E915–E924, 2015. doi: 10.1152/ajpendo.00339.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 32. Ratchford SM, Clifton HL, Gifford JR, LaSalle DT, Thurston TS, Bunsawat K, Alpenglow JK, Richardson RS, Wright JB, Ryan JJ, Wray DW. Impact of acute antioxidant administration on inflammation and vascular function in heart failure with preserved ejection fraction. Am J Physiol Regul Integr Comp Physiol 317: R607–R614, 2019. doi: 10.1152/ajpregu.00184.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weerts J, Mourmans SGJ, Barandiaran Aizpurua A, Schroen BLM, Knackstedt C, Eringa E, Houben AJHM, van Empel VPM. The role of systemic microvascular dysfunction in heart failure with preserved ejection fraction. Biomolecules 12: 278, 2022. doi: 10.3390/biom12020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segal SS. Integration and modulation of intercellular signaling underlying blood flow control. J Vasc Res 52: 136–157, 2015. doi: 10.1159/000439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res 83: 960–965, 1998. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- 36. Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/atvbaha.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 60: 1778–1786, 2012. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 38. Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail 2: 549–555, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 39. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Venturelli M, Layec G, Trinity J, Hart CR, Broxterman RM, Richardson RS. Single passive leg movement-induced hyperemia: a simple vascular function assessment without a chronotropic response. J Appl Physiol (1985) 122: 28–37, 2017. doi: 10.1152/japplphysiol.00806.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. doi: 10.1152/ajpheart.00656.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Walter Wray D, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010. doi: 10.1152/ajpheart.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Witman MAH, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson AD, Rossman MJ, Witman MA, Barrett-O'Keefe Z, Groot HJ, Garten RS, Richardson RS. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol (1985) 120: 991–999, 2016. doi: 10.1152/japplphysiol.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis 13: 175394471987008, 2019. doi: 10.1177/1753944719870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation 93: 2135–2141, 1996. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 52. Thakker RA, Elbadawi A, Albaeni A, Perez C, Hasan SM, Murrieta JI, Almustafa A, Duarte A, Berbarie RF, Chatila KF, Khalife W. Outcomes with sGC therapy in patients with HFpEF: a meta-analysis of prior trials. Curr Probl Cardiol 47: 100924, 2021. doi: 10.1016/j.cpcardiol.2021.100924. [DOI] [PubMed] [Google Scholar]
- 53. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 55. Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 3: 73–80, 2009. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 56. Rochette L, Lorin J, Zeller M, Guilland J-C, Lorgis L, Cottin Y, Vergely C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther 140: 239–257, 2013. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 57. Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98: 1842–1847, 1998. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 58. Lundman P, Eriksson MJ, Stuhlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 38: 111–116, 2001. doi: 10.1016/s0735-1097(01)01318-3. [DOI] [PubMed] [Google Scholar]
- 59. Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode-Boeger SM, Kruszelnicka-Kwiatkowska O, Kokot F, Dubiel JS, Froelich JC. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol 33: 652–658, 1999. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 60. Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. Plasma levels of asymmetrical dimethyl-L-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol 37: 489–492, 2001. doi: 10.1097/00005344-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 61. Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, Stuehlinger M, Tsao PS. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol 88: 1201–1203, 2001. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 62. Boger RH, Bode-Boger SM, Thiele W, Junker W, Alexander K, Frolich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 95: 2068–2074, 1997. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 63. Boger RH, Bode-Boger SM, Thiele W, Creutzig A, Alexander K, Frölich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol 32: 1336–1344, 1998. doi: 10.1016/s0735-1097(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 64. Kielstein JT, Boger RH, Bode-Boger SM, Schaffer J, Barbey M, Koch KM, Frölich JC. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol 10: 594–600, 1999. doi: 10.1681/asn.v103594. [DOI] [PubMed] [Google Scholar]
- 65. Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Boger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358: 2113–2117, 2001. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 66. Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 63: 376–382, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 67. Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr, Roessig L, Stasch J-P, Solomon SD, Paulus WJ, Butler J. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc 2: e000536, 2013. doi: 10.1161/JAHA.113.000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 66: 1672–1682, 2015. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 69. Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131: 371–380, 2015. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 4: 428–437, 2016. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Evans SR. Clinical trial structures. J Exp Stroke Transl Med 3: 8–18, 2010. doi: 10.6030/1939-067x-3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sciascia S, Apra F, Baffa A, Baldovino S, Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, Converso M, Cozzi M, Crosasso P, De Iaco F, Di Perri G, Eandi M, Fenoglio R, Giusti M, Imperiale D, Imperiale G, Livigni S, Manno E, Massara C, Milone V, Natale G, Navarra M, Oddone V, Osella S, Piccioni P, Radin M, Roccatella D, Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol 38: 529–532, 2020. [PubMed] [Google Scholar]
- 73. Zeng Y, Moscicki A-B, Sahasrabuddhe VV, Garcia F, Woo H, Hsu CH, Szabo E, Dimond E, Vanzzini S, Mondragon A, Butler V, DeRose H, Chow H-HS. A prospective, single-arm, open-label, non-randomized, phase IIa trial of a nonavalent prophylactic HPV vaccine to assess immunogenicity of a prime and deferred-booster dosing schedule among 9-11 year-old girls and boys - clinical protocol. BMC Cancer 19: 290, 2019. doi: 10.1186/s12885-019-5444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu G, Sanderson B, Bittner V. The 6-minute walk test: how important is the learning effect? Am Heart J 146: 129–133, 2003. doi: 10.1016/S0002-8703(03)00119-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.