In this issue of the Canadian Journal of Cardiology, Zuin et al.1 completed a systematic review and meta-analysis to assess the risk of incident acute myocarditis (AM) in novel coronavirus disease 2019 (COVID-19) survivors within 1 year following their index infection. Data from 4 studies reporting cases of AM in the post-acute phase of infection (4-12 months) were identified in searches of Medline and Scopus (up to September 1, 2022). Mean age, sex, preexisting comorbid conditions, including presence of hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, heart failure, and stroke, and length of follow-up were recorded. Each study compared their patients recovered from COVID-19 with contemporary cohorts without a confirmed COVID-19 infection. More than 20 million patients were followed over a mean follow-up period of 9.5 months. COVID-19 infection was identified in 1.25 million patients. During the follow-up period, the authors report that AM occurred in 0.21 (95% CI 0.13-0.42) of 1000 patients who survived their COVID-19 infection, compared with 0.09 (95% CI 0.07-012) of 1000 control subjects. The results show an increased risk of incident AM (hazard ratio 5.16, 95% CI 3.87-6.89; P < 0.0001; I 2 = 7.9%) in previously infected COVID 19 patients 1 year after index infection. Though AM has been well reported in the acute infective period and after mRNA therapy, this manuscript highlights the development of AM in patients recovered from COVID-19.
COVID-19 Infection–Related Myocarditis
First identified in Wuhan, China, in December 2019, COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to mild-moderate respiratory symptoms in a majority of cases. Multiple series have documented the variety of cardiac complications that can manifest during the acute infection phase, including acute cardiac injury seen with elevated troponins, acute coronary syndrome, heart failure with reduced ejection fraction, pericardial effusion, and, most rarely, AM. Definite or probable AM during the acute phase of illness has been estimated to occur at a rate of 2.4 per 1000 hospitalisations.2 , 3 Early recognition is of importance given the younger population it tends to afflict (< 40 years) and the fulminant presentation with need for intensive care in 70% of cases. In 39% of patients, inotropic/vasopressor support or temporary mechanical circulatory support is required.3 Prognosis is favourable in those with isolated AM, compared with those with concomitant respiratory involvement, where mortality has been reported to be 15%.3
It is well established that AM is a consequence of a “trigger,” which includes infectious (viral, bacterial, or fungal) or immune disturbance (autoimmunity or hypersensitivity), leading to a cascade of secondary autoimmunity.4 The pathophysiology in acute COVID-19 infection is likely related to both viral and host immune factors, but the exact mechanisms are not yet clear. Proposed viral-mediated mechanisms include direct myocardial injury via angiotensin-converting enzyme 2 (ACE2) receptors, which may indirectly cause myocardial inflammation, cytokine storm mediated by immune dysregulation, and finally, ongoing hypoxia from oxygen supply-demand mismatch.5, 6, 7, 8 Direct myocardial injury by SARS-Cov-2 has been thought to occur by the virus binding on the spike protein of the ACE2 receptor. Entry into the cell triggers decreased myocardial ACE2 protein expression and causes macrophage infiltration and myocardial damage.5 In a subset of individuals, immune dysregulation occurs following COVID-19 infection, leading to a rise in proinflammatory cytokines such as interleukins 2, 6, and 7, tumour necrosis factor alpha, C-X-C motif cytokine 10, and C motif ligand 2. The rapid rise in circulating cytokines leads to activation of T lymphocytes that cause myocardial injury resulting in a profound hyperinflammatory reaction.6 , 7 Though gene sequences of the SARS-CoV-2 have been found in endomyocardial biopsy specimens, there is a paucity of data that supports that the virus can cause direct cardiomyocyte death.9
COVID-19 Vaccine–Related Myocarditis
With the development of mRNA therapy for SARS-CoV-2, cases of AM have been reported as a rare complication, with a reported rate of 12.6 cases per million of second mRNA dose administered. Afflicted individuals are predominately < 40 years of age and present with a variety of symptoms, including chest pain, dyspnea, and palpitations, usually within 1 week after mRNA administration.10 The inclusion of modified mRNA within the vaccine, which serves as a delivery medium to transport the viral spike glycoprotein of SARS-CoV-2 into cells is believed to be the “trigger.” Despite nucleoside modifications of the mRNA, which is meant to reduce its immunogenicity, a subset of the general population with a genetic predisposition develops an aberrant response causing an up-regulation of the innate and acquired immune system that leads to circulation of heart-reactive antibodies.11 Because the clinical trajectory of those who develop COVID-19 mRNA–related myocarditis is often mild and self-limiting,12, 13, 14, 15, 16, 17 clear guidelines from public health authorities had recommended the therapy in all age groups.10
Incident Myocarditis After COVID-19 Infection
What is of interest in the meta-analysis by Zuin et al. is the development of AM outside of the acute phase of illness or after an mRNA dose. As the pandemic has evolved, it is well recognised that these two previously discussed scenarios are quite rare. What is unclear from a mechanistic point of view is the “trigger” that causes the immune system to become “re”-activated in the post-acute phase of illness in incident AM. Given what is known about COVID-19 infection and the host immune response, it could be surmised that incident AM may occur in an individual with a predisposed immunogenetic background or a history of autoimmune disease, with a “trigger” that reactivates the immune system and its subsequent hyperinflammatory response.
Although one could postulate that a COVID-19 mRNA dose may been the “trigger” for an AM presentation after COVID-19 recovery, 2 of the included studies would suggest otherwise. Wang et al.18 evaluated non vaccinated patients with COVID-19 infection, and found the rate of AM was higher than those without previous infection (HR 4.406 [2.89-6.720]. And Xie et al.19 found that the incidence of AM in patients recovered from COVID-19 was higher than in those without previous infection (HR 5.38, 95% CI 3.8-7.6). To eliminate a potential COVID-19 mRNA treatment exposure as a potential “trigger” for AM, they completed 2 different analyses. Incident rates for AM were still higher even when the authors censored patients at time of receiving first dose of any COVID-19 mRNA treatment (HR 5.31, 95% CI 3.75-7.53) and after adjustment for vaccination as a time varying covariate (HR 5.34, 95% CI 3.76-7.6), compared with those without previous COVID-19 infection..
An alternative hypothesis that has been proposed concerns whether incidence AM is a result of a persistent chronic inflammatory response that results from persistent viral load in myocardial cells.20 As the cases of recovered COVID-19 patients rise, it has become apparent that some patients have persistent symptoms well beyond infection onset. In some series, 20% of patients report symptoms beyond 4 weeks and 10% have ongoing symptoms beyond 12 weeks.21 Termed “post–COVID-19 syndrome” by the World Health Organisation, ongoing cardiac, respiratory, and neurologic symptoms beyond 3 months without an alternative diagnosis22 has now become a common diagnosis seen in the offices of subspecialty providers. It is not apparent if incident myocarditis is part of the post–COVID-19 syndrome, as suggested in one review,23 or is a consequence of a second inciting agent or “trigger” in an immune-susceptible individual after symptom resolution. Further studies are needed to fully understand the pathophysiology of this “rare” event, as well as the clinical outcomes in this group of patients.
Future Directions
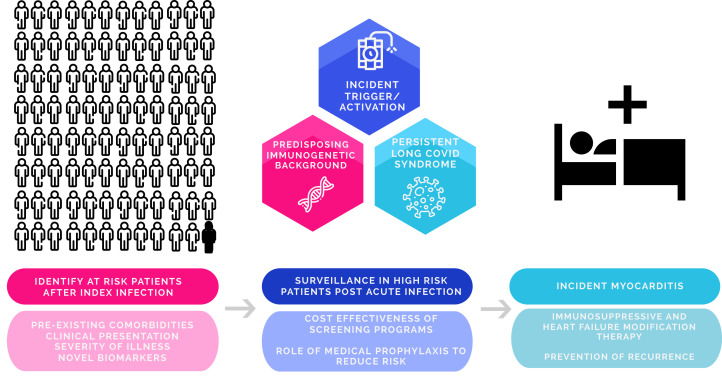
Along with the limitations already described within this study, one major limitation is the lack of further clinical information about the patients who develop incident AM. Given the unknown patient profile for this at-risk population, it remains challenging to know how we should best manage recovered patients. Clinicians might wonder if identification and subsequent risk assessment can be undertaken at time of index infection. Can providers identify these patients at risk up front and follow them more closely clinically? If so, how? Are there clinical parameters that could predict those at higher risk? Are there novel biomarkers which can be used? Does the risk vary with COVID-19 variant? In those that are at higher risk, how does one monitor them, and for how long after their infection and how frequently? If screening is done, which diagnostic tools should be used? The latter is of utmost importance given the lack of data supporting routine cardiac testing after COVID-19 infection and the utility as well as cost benefit of widespread screening in an event that is infrequent. In addition, in those deemed to be at higher risk, are there prophylactic medications that can reduce inflammatory burden and risk? If so, how long do they need to be given? In those who develop incident AM, how best do we treat them aside from vasopressors/inotropes in the critical care setting along with foundational therapy for heart function? Which immunosuppressive therapies do we use and for how long do the need to be given? (Fig. 1).
Figure 1.
Potential areas for future research in incident acute myocarditis (AM) based on stage of illness (index illness, after recovery from acute illness, and at time of AM). Figure created with the use of Wepik.com.
What is expected as we continue to gain experience with this disease is further understanding of the projected large proportion of patients with residual cardiac symptoms, including those with incident AM. The epidemiologic findings presented in this study presents yet another “rare” myocarditis event associated with COVID-19 infection. Prospective registries such as CV COVID-19 Registry Investigators and the American Heart Association’s COVID-19 CVD Registry powered by Get With The Guidelines24 , 25 as well as case series will be valuable in our understanding of the clinical outcomes of these patients. Perhaps, it is still too early to sound the alarm bells.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See article by Zuin et al., pages 839-844 of this issue.
See page 847 for disclosure information.
References
- 1.Zuin M., Rigatelli G., Bilato C., et al. One-year risk of myocarditis after COVID-19 Infection: a systematic review and meta-analysis. Can J Cardiol. 2023;39:839–844. doi: 10.1016/j.cjca.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esfandiarei M., McManus B.M. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol Mech Dis. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 3.Ammirati E., Lupi L., Palazzini M., et al. Prevalence, characteristics and outcomes of COVID-19–associated acute myocarditis. Circulation. 2022;145:1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper L.T. Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudit G.Y., Kassiri Z., Jiang C., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2022 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 8.Basu-Ray I, Almaddah Nk, Adeboye A, et al. Cardiac Manifestations Of Coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2023[Updated 2023 Jan 9]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK556152/. Accessed December 2022. [PubMed]
- 9.Lindner D., Fitzek A., Brauninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caso F., Costa L., Ruscitti P., et al. Could SARS–coronavirus 2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimm Rev. 2020;19 doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammirati E., Cavalotti C., Milazzo A., et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-CoV-2 infection. Int J Cardiol Heart Vasc. 2021;34 doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista G.J., Pena O.P., Bonilla Fernandez J.A., et al. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed) 2021;74:812–814. doi: 10.1016/j.rec.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mclean K., Johnson T. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med. 2021;28:918–921. doi: 10.1111/acem.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Angelo T., Cattafi A., Carerj M.L., et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthukumar A., Narasimhan M., Li Q.Z., et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Wang C., Wang S., Wei J.C.C. Long term cardiovascular outcomes in COVID-19 survivors among non vaccinat3d population: a retrospective cohort study from the TriNetX US collaborative networks. EClinical Medicine. 2022;53 doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 21.Michelen M., Manoharan L., Elkheir N., et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. WHO Clinical Case Definition Working Group on Post–COVID-19 Condition. A clinical case definition of post–COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raman B., Bluemke D.A., Luscher T., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arevalos V., Ortega-Paz L., Fernandez-Rodriguez D., et al. Long-term effects of coronavirus disease 2019 on the cardiovascular system, CV COVID registry: a structured summary of a study protocol. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heather M.A., Rutan C., Willams J.H., et al. American Heart Association COVID-19 CVD Registry powered by Get With The Guidelines. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006967. [DOI] [PMC free article] [PubMed] [Google Scholar]