Abstract
Epidemiological data suggest that physical activity protects against severe COVID-19 and improves clinical outcomes, but how exercise augments the SARS-CoV-2 viral immune response has yet to be elucidated. Here we determine the antigen-specific CD4 and CD8 T-cell and humoral immunity to exercise in non-vaccinated individuals with natural immunity to SARS CoV-2, using whole-blood SARS-CoV-2 peptide stimulation assays, IFN-γ ELISPOT assays, 8-color flow cytometry, deep T-cell receptor (TCR) β sequencing, and anti-RBD-1 neutralizing antibody serology. We found that acute exercise reliably mobilized (∼2.5-fold increase) highly functional SARS-CoV-2-specific T-cells to the blood compartment in those with natural immunity to the virus. The mobilized cells reacted with spike protein (including alpha (α) and delta (δ)-variants), membrane, and nucleocapsid peptides in those previously infected but not in controls. Both groups reliably mobilized T-cells reacting with Epstein-Barr viral peptides. Exercise mobilized SARS-CoV-2 specific T-cells maintained broad TCR-β diversity with no impact on CDR3 length or V and J family gene usage. Exercise predominantly mobilized MHC I restricted (i.e. CD8+) SARS-CoV-2 specific T-cells that recognized ORF1ab, surface, ORF7b, nucleocapsid, and membrane proteins. SARS-CoV-2 neutralizing antibodies were transiently elevated ∼1.5-fold during exercise after infection. In conclusion, we provide novel data on a potential mechanism by which exercise could increase SARS-CoV-2 immunosurveillance via the mobilization and redistribution of antigen-specific CD8 T-cells and neutralizing antibodies. Further research is needed to define the tissue specific disease protective effects of exercise as SARS-CoV-2 continues to evolve, as well as the impact of COVID-19 vaccination on this response.
Keywords: Exercise immunology, VSTs, SARS Specific T-cells, TCR Sequencing, Physical activity, Epstein-barr virus
Highlights
-
•
Exercise reliably mobilizes MHC I restricted SARS-CoV-2 specific T-cells.
-
•
Exercise mobilized SARS-CoV-2 T-cells recognized all major regions of the virus.
-
•
Exercise mobilized SARS-CoV-2 T-cells responded to mutations in the Spike protein.
-
•
Broad TCR-β diversity is maintained in exercise mobilized SARS-CoV-2 T-cells.
-
•
Exercise transiently elevates anti-RBD-1 neutralizing antibodies to SARS CoV-2.
1. Introduction
As the world enters the third year of the coronavirus disease of 2019 (COVID-19) pandemic, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than ∼500 million people worldwide resulting in over 6 million deaths (Dong et al., 2020). In the US, the Centers for Disease Control (CDC) estimates almost 75% of children and 60% of adults, have been infected with SARS-CoV-2 (Clarke et al., 2022). Fortunately the rapid dissemination of more than 11.1 billion vaccine doses worldwide has significantly reduced the mortality rate (Dong et al., 2020). However, the evolution of the virus (e.g. Omicron variant) has increased infections among adults who have previously been vaccinated, suggesting SARS-CoV2 is an endemic virus and COVID-19 is a disease that must be controlled (Clarke et al., 2022).
Physical activity is an important modulator of COVID-19 disease severity, as those who are physically active have less acute morbidity, decreased mortality, fewer hospitalizations, and less severe symptoms (Cummins et al., 2021; Sallis et al., 2021). Indeed, the CDC now lists physical inactivity as an underlying condition for increased risk of severe illness from COVID-19 (Centers for Disease Control and Prevention, 2022). However, the mechanisms by which exercise is capable of reducing disease severity and potentially ameliorating symptoms of COVID-19 have yet to be determined.
Moderate-to-high physical activity has been associated with improved upper respiratory tract infection incidence, duration, and severity and evidence suggests that exercise can protect the host from multiple types of viral infections and reactivation of latent herpesviruses (Nieman et al., 2011; Fondell et al., 2011; Duggal et al., 2019; Martin et al., 2009). A hypothesized mechanism underlining the protective effect of physical activity against many respiratory viral infections is the increased immunosurveillance that accompanies the frequent mobilization and redistribution of antigen-specific T-cells. We have purported that the ongoing exchange of virus-specific T-cells (VSTs) and NK-cells between the blood and tissues, coupled with an increased transportation of neutralizing antibodies, increases immune surveillance against SARS-CoV-2 and other pathogens in those who are physically active (Duggal et al., 2019; Simpson and Katsanis, 2020). We showed in an individual recently recovered from SARS-CoV-2 infection that a single exercise bout elicits a robust mobilization of functional SARS-CoV-2 specific T-cells at a magnitude that was greater than the T-cell response against other viruses, including, cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus (AdV), and influenza (Baker et al., 2021). The mobilized T-cells had broad specificity to the SARS-CoV-2 genome as determined by deep TCR-β sequencing. Furthermore, SARS-CoV-2 neutralizing antibodies were elevated during exercise, likely due to increased lymphatic flow. While the observations made in this case study indicate that a single exercise bout can mobilize and redistribute SARS-CoV-2 T-cells and serum neutralizing antibodies, it was imperative to confirm these findings in a larger cohort.
Here we compared SARS CoV-2-specific cellular and humoral immune response to exercise in non-vaccinated individuals with and without natural SARS-CoV-2 immunity. We report that SARS CoV-2 infected individuals reliably mobilize SARS-CoV-2 VSTs and neutralizing antibodies in response to a single exercise bout. The mobilized cells reacted to peptides derived from wild-type as well mutated Alpha (α) and Delta (δ)-variants of the spike protein without affecting antigen specificity, CDR3 length, or V and J family gene usage. The exercise mobilized T-cells were largely MHC I restricted (i.e. CD8+ T-cells) and reactive to ORF1ab, surface, ORF7b, nucleocapsid, and membrane proteins. These findings suggest that a bout of exercise may increase T-cell and humoral immune surveillance against SARS-CoV-2 in previously infected participants.
2. Methods
2.1. Study participants and procedures
A total of 16 healthy participants (6 females and 10 males) aged 19–43 years of age participated in this study (Table 1). Eight participants were seropositive to SARS-CoV-2 and eight SARS CoV-2 naïve participants served as controls. All participants were classified as ‘low risk’ for maximal exercise testing in accordance with the American College of Sports Medicine (ACSM)/American Heart Association (AHA) criteria and were physically active (physical activity rating >4) (Jackson et al., 1990). Participants were excluded if they had symptoms of infection (including COVID-19) within the preceding 6-weeks, were currently using or had used tobacco products within the preceding 6 months, consumed high amounts of alcohol (>2 drinks per day), regularly used any immune-modulating medication within the last 6-months, were pregnant, or had received a COVID-19 vaccine. All participants were surveyed about past diagnosis or symptoms of SARS CoV-2 infection and a blood sample was collected to confirm their serostatus against SARS CoV-2 and CMV using commercially available ELISA kits (SARS-CoV-2 Spike S1 Human IgG; BioLegend, San Diego, CA & CMV IgG, IBL America, Minneapolis, MN). Participants arrived at the laboratory between 06:00 and 10:00 after an overnight fast and were required to abstain from caffeine consumption and vigorous exercise for at least 24-h prior to each laboratory visit. All exercise tests occurred between November 2020 and July 2021 and infected participants COVID-19 diagnosis were between September 2020 and March 2021. Importantly, none of the infected participants developed severe adverse events from infection (e.g. hospitalization). All participants provided written informed consent and all procedures were performed in accordance with the ethical guidelines provided by the Belmont Report. The Institutional Review Board (IRB) of the University of Arizona granted ethical approval (No. 2102477676) and the trial was registered at www.clinicaltrials.gov (NCT05019456).
Table 1.
Participant demographic data. Statistical difference from non-infected (control) group indicated by * (p < 0.05).
| Non-Infected (n = 8) | Infected (n = 8) | p-value | |
|---|---|---|---|
| Female (N(%)) | 3 (37.5) | 3 (37.5) | >0.999 |
| Age (yrs) | 32.6 ± 7.1 | 34.6 ± 7.6 | 0.597 |
| Height (cm) | 172.9 ± 11.9 | 173.0 ± 9.0 | 0.993 |
| Weight (kg) | 68.3 ± 10.1 | 70.6 ± 14.2 | 0.711 |
| Resting HR (bpm) | 70.5 ± 7.7 | 73.5 ± 12.5 | 0.573 |
| Resting Systolic Blood Pressure (mmHg) | 114.4 ± 6.6 | 115 ± 11.0 | 0.892 |
| Resting Diastolic Blood Pressure (mmHg) | 75.3 ± 5.8 | 77.1 ± 7.3 | 0.579 |
| PAR Score (1–7) | 5.9 ± 0.8 | 5.5 ± 1.8 | 0.597 |
| Predicted VO2max (mL/kg/min) | 44.9 ± 7.14 | 33.6 ± 11.2 * | 0.033 |
| Peak Power (watts) | 214.4 ± 43.0 | 187.5 ± 56.6 | 0.302 |
| Spike IgG (ug/mL) | 0.4 ± 0.31 | 6.24 ± 3 * | <0.001 |
| CMV Serostatus (N (%)) | 4 (50) | 3 (37.5) | >0.999 |
| EBV Serostatus (N (%)) | 7 (87.5) | 8 (100) | >0.999 |
2.2. Submaximal exercise testing
All participants completed a submaximal graded exercise test on a stationary cycling ergometer (Velotron, Quarq Technology, San Diego, CA) with heart rate and respiratory gas exchange recorded continuously using a metabolic cart (Quark CPET, COSMED, Pabona di Albona Laziale, Italy). Participants completed a 5-min warm-up at 50 W (W) and were asked to maintain a consistence cadence (>60 rpm) for the duration of the test. After the warmup, resistance was increased by 15W every minute until heart rate reached 85% of age-predicted max (220 – age) at which point the test was terminated. Predicted VO2max was determined using Wasserman equation within the metabolic cart software and rating of perceived exertion (RPE; Modified Borg Scale) was recorded during the final 15 s of each 1-min stage (Batatinha et al., 1985).
2.3. Main exercise trial
The main exercise trial was performed 1–3 weeks after completing the submaximal exercise test. Prior to exercise, an intravenous (IV)-catheter (BD Nexiva™ Closed IV Catheter System, BD vacutainers™, Franklin Lakes, NJ, USA) was inserted into an antecubital vein and blood was collected in K2EDTA (EDTA), lithium heparin (LH) or Silica Clot Activator, Polymer Gel, Silicone-Coated Interior SST™ (BD vacutainer™, Franklin Lakes, NJ, USA) vacutainer® tubes. After each blood draw, the IV-catheter was flushed with 10 mL isotonic saline (BD PosiFlush, Franklin Lakes, NJ, USA) and immediately before blood collection, a 2 mL “waste” tube was drawn to remove residual saline. After baseline blood sample collection (rest), participants were asked to complete 5-min warmup at 50W before starting a 20 min graded exercise trial. The trial consisted of 5-min stages at power outputs corresponding to 50%, 60%, 70%, and 80% of their individual predicted VO2max. Participants were asked to maintain a similar cadence (>60 rpm) to the submaximal exercise test, and RPE were recorded during the final 15 s of each stage. Further blood samples were collected during the 60% and 80% stages, and 1 h after exercise cessation.
2.4. Blood processing & flow cytometry
Serum samples collected from each exercise time point were analyzed for SARS-CoV-2 neutralizing antibodies (nAb) (Cayman Chemical, Michigan, USA), using commercially available semi-quantitative ELISA kits. All controls required in the manufactures kit, blank, non-specific-biding, maximum binding, SARS-CoV-2 nAb positive, and SARS-CoV-2 nAb negative control were included in the analysis. Anti-RBD-1 concentrations (ng/mL) were determined by comparison to the SARS-CoV-2 neutralizing antibody standard curve, which exhibits a high level of parallelism to positive samples.
Blood collected in lithium heparin tubes were used for whole blood peptide stimulation assays and the isolation of peripheral blood mononuclear cells (PBMCs) using standard density gradient centrifugation procedures. PBMCs were used for IFN-γ ELISPOTs and isolation of genomic DNA for TCR-β sequencing. EDTA whole blood from each exercise time point were stained with combinations of the following directly conjugated antibodies, CD8-VioBlue, CD14-VioGreen, CD3-VioGreen, CD3-FITC, CD4-FITC, CD4-PE, CD62L-PE, CD20-PerCP, PD-1-PerCP, CD45RA-PerCPVio770, CD45-APC, and CD56-APCVio770 (Miltenyi Biotec Inc., Germany); and analyzed by 8-color flow cytometer (MACSQuant 10; Miltenyi Biotec Inc., Germany). The percentage of all CD45+ lymphocytes expressing the surface markers of interest were multiplied by the total lymphocyte count (determined using a volumetric flow cytometry-based whole blood assay) to enumerate each lymphocyte subtype per unit of whole blood (Graff et al., 2018).
2.5. Whole blood peptide stimulation
As previously described (Baker et al., 2021), LH whole blood (1 mL) was stimulated with 120uL (supplemented with 2 mg/mL glucose) of saline (1st negative control), PHA (10 μg/mL; Sigma Aldrich; positive control), Actin PepMix (1 μg/mL; JPT Peptide Technologies, Germany; 2nd negative control), EBV consensus PepMix (10 μg/mL; Miltenyi) or the SARS-CoV-2 spike (S) PepMix, membrane (M) PepMix, and nucleocapsid (N) PepMix, individually and pooled, each overlapping by 11 amino acids (10 μg/mL; Miltenyi). After 24-h incubation at 37 °C, plasma was removed and stored at −80°C until analysis. Interferon-gamma (IFN-γ) concentration was then determined using a commercially available ELISA kit (R&D Systems, MN, USA).
2.6. Enumeration of SARS-CoV-2 specific T-cells by IFN-γ ELISPOT
The number of functional IFN-γ producing SARS-CoV-2 specific T-cells in response to virus-specific peptide stimulation were determined by enzyme-linked immunospot (ELISPOT) assays (ELISpot Pro:Human IFN-γ (HRP); Mabtech AB, Sweden) as previously described (Keller et al., 2020). Briefly, frozen PBMCs (100,000 cells/well) were thawed and stimulated with media only (1st negative control), (1 μg/mL), actin (2nd negative control), 1 μg/mL anti-CD3 mAb (positive control), or 2 μg/mL each of the individual SARS-CoV-2 (JPT) PepMix in separate wells (performed in triplicate) in a precoated IFN-γ (mAb-1-D1K) plate overnight. The number of IFN-γ spot-forming-cells (SFCs) were enumerated by Zellnet Consulting (NJ, USA) and, for the PBMCs collected on Day 0, adjusted for the total blood T-cell count (SFC/ml). The media control SFC was subtracted from the experimental peptide mix SFC. All control data is depicted in Supplemental Fig. 1.
2.7. TCR-β sequencing
The DNeasy Blood Extraction Kit (Qiagen, USA) was used to extract genomic DNA from PBMCs collected at rest, during-exercise (80% intensity only), and 1h post-exercise from six of the Infected participants. TCR-β deep sequencing was performed by Adaptive Biotechnologies (Seattle, WA, USA) and TCR-β rearrangements specific to SARS-CoV-2 were determined using the immunoSEQ T-MAP COVID ImmuneCODE database (Adaptive Biotechnologies, USA). The number of SARS-CoV-2 productive rearrangement templates were adjusted total blood T-cell count (SARS-CoV-2 Clones/mL). Major histocompatibility complex (MHC) class I (i.e. CD8+ T-cells) and MHC II (i.e. CD4+ T-cells) restricted clones of each SARS-CoV-2 productive rearrangement were determined by uploading TCR- β deep sequencing data into VDJ database (https://vdjdb.cdr3.net/) (Bagaev et al., 2019). Only SARS CoV-2 specific T-cell clones with a confidence threshold score of 3 (very high confidence) or 2 (high confidence) were included in this analysis.
2.8. Statistical analysis
All data are presented as means ± standard deviation unless otherwise noted. All statistical analyses were completed using Graphpad Prism 9.0 or SPSS 10. Independent t-tests and Fisher’s exact test were used to detect differences in participant demographics between the infected and non-infected groups. Two-way Repeated Measures-ANOVA (RMANOVA) were used to analyze phenotypic and SARS-CoV-2 whole blood peptide stimulation data, with Bonferonni post hoc test, to determine exercise differences between groups. The model included main effects of time (rest, 60%, 80%, and 1h-post) and status (infected vs. non-infected), and an interaction (time x status) effect. Main effects of time were also determined within each group (infected and non-infected). Univariate RMANOVA were used to detect exercise time (rest, 60%, 80%, and 1h-post) differences between IFN-γ release, SFC/mL, and TCR-β sequencing data within the infected participants. TCR-β sequencing data included only three exercise time-points (rest, 80%, and 1h-post). Significance was accepted at p < 0.05.
3. Results
3.1. Immune cell mobilization kinetics in response to exercise are not majorly affected by SARS-CoV-2 infection
Exercise-induced changes in the number of monocytes, lymphocytes, CD3+ T-cells, CD4+ T-cells, CD8+ T-cells, ‘double-negative’ (DN) T-cells, NK-cells, B-Cells, and differentiated subsets (naïve; CM: central memory; EM: effector memory; EMRA: CD45RA + EM) of T-cells are shown in Table 2. Two-way RMANOVA’s revealed no status or interaction effects observed for any of the immune cell subsets between the infected and non-infected participants. Additionally, post-hoc analysis of the univariate RMANOVAs revealed most immune cell subsets were significantly mobilized with exercise regardless of immunity status, however, only PD-1+ CD8+ T-cells were not significantly mobilized with exercise in infected participants (p > 0.05). Conversely, PD-1+ NK-cells were significantly mobilized with exercise in the infected participants (p = 0.009) but not the non-infected controls (p > 0.05; Table 2).
Table 2.
The total number (cells/μL) of monocytes, lymphocytes, CD3+ T-cells, CD4+ T-cells, CD8+ T-cells, ‘double-negative’ (DN) T-cells, NK-cells, B-Cells, and subsets (naïve; CM: central memory; EM: effector memory; EMRA: EM CD45RA+) of T-cells found in peripheral blood before (Rest), during (60% and 80%), and 1 h after (1h Post) each exercise trial for the infected and non-infected participants. Data are mean ± SD. Main effects of time (Rest, 60%, 80%, 1h post), status (non-infected versus infected) and time × status interactions are shown. Statistical differences, within each status, from Rest, 60%, and 1h Post exercise trials are indicated by *, #, and $ respectively, p < 0.05.
| Rest | 60% | 80% | 1h Post | Time F (p-value) |
Status F (p-value) |
Interaction F (p-value) |
|
|---|---|---|---|---|---|---|---|
| Monocytes | |||||||
| Non-Infected | 315.8 ± 100.9 | 462.4 ± 161.4 *$ | 570.2 ± 181.2 *#$ | 332.5 ± 134.4 | 46.95 (<0.001) | 0.396 (0.539) | 1.076 (0.369) |
| Infected | 320.5 ± 84.9 | 414.5 ± 80.4 *$ | 501.3 ± 112.4 *#$ | 308.3 ± 53.2 | |||
| Lymphocytes | |||||||
| Non-Infected | 1584.6 ± 411.9 | 2253.2 ± 679.8 *$ | 2995.1 ± 787.8 *#$ | 1423.9 ± 634.1 | 80.68 (<0.001) | 0.156 (0.699) | 0.834 (0.483) |
| Infected | 1899.5 ± 549.2 | 2328.1 ± 508.4 *$ | 3004.9 ± 821.4 *#$ | 1464.9 ± 394.3 * | |||
| CD3+T-cells | |||||||
| Non-Infected | 1134.4 ± 333.9 | 1478.6 ± 472.8 *$ | 1814.6 ± 488.0 *#$ | 1060.6 ± 492.0 | 38.70 (<0.001) | 0.131 (0.723) | 0.747 (0.530) |
| Infected | 1339.8 ± 403.8 | 1549.7 ± 431.6 $ | 1829.6 ± 621.6 *$ | 1065.2 ± 252.3 | |||
| CD4+T-cells | |||||||
| Non-Infected | 599.4 ± 246.9 | 714.9 ± 311.7 | 812.0 ± 352.5 *$ | 616.9 ± 344.2 | 21.36 (<0.001) | 2.054 (0.174) | 2.360 (0.085) |
| Infected | 826.8 ± 212.4 | 919.4 ± 212.5 $ | 1040.7 ± 253.4 *$ | 675.7 ± 127.8 * | |||
| Naïve CD4+T-cells | |||||||
| Non-Infected | 225.8 ± 130.5 | 252.5 ± 157.6 | 287.6 ± 191.1 | 195.0 ± 139.3 | 21.56 (<0.001) | 0.915 (0.355) | 1.527 (0.221) |
| Infected | 311.3 ± 129.3 | 315.6 ± 107.6 | 354.5 ± 81.2 | 220.5 ± 65.6 | |||
| CM CD4+T-cells | |||||||
| Non-Infected | 143.0 ± 78.3 | 173.4 ± 93.8 $ | 192.9 ± 120.4 *$ | 147.3 ± 111.6 | 15.00 (<0.001) | 0.139 (0.715) | 1.195 (0.323) |
| Infected | 172.9 ± 70.0 | 196.0 ± 75.6 $ | 207.4 ± 84.4 *$ | 144.9 ± 65.9 * | |||
| EM CD4+T-cells | |||||||
| Non-Infected | 186.4 ± 116.7 | 237.9 ± 144.7 | 277.7 ± 166.4 *$ | 218.1 ± 142.7 | 15.57 (<0.001) | 0.585 (0.457) | 1.888 (0.146) |
| Infected | 245.3 ± 67.2 | 283.5 ± 86.0 $ | 342.0 ± 95.6 *$ | 214.0 ± 37.4 | |||
| EMRA CD4+T-cells | |||||||
| Non-Infected | 46.8 ± 50.5 | 54.2 ± 51.9 | 70.1 ± 68.9 * | 60.7 ± 62.9 | 3.984 (0.014) | 1.980 (0.181) | 1.532 (0.220) |
| Infected | 102.8 ± 97.1 | 119.5 ± 109.1 $ | 159.9 ± 147.5 *$ | 96.2 ± 111.1 | |||
| PD-1+CD4+T-cells | |||||||
| Non-Infected | 116.7 ± 134.9 | 135.1 ± 161.3 $ | 140.2 ± 160.4 $ | 64.3 ± 67.6 * | 7.280 (<0.001) | 0.466 (0.506) | 1.568 (0.213) |
| Infected | 77.0 ± 52.0 | 85.9 ± 52.5 | 95.7 ± 54.1 | 63.6 ± 42.2 | |||
| CD8+T-cells | |||||||
| Non-Infected | 407.7 ± 153.0 | 576.1 ± 272.1 *$ | 720.9 ± 333.5 *$ | 353.7 ± 142.9 | 26.08 (<0.001) | 0.152 (0.703) | 0.712 (0.551) |
| Infected | 424.7 ± 173.1 | 516.9 ± 197.4 $ | 626.8 ± 292.6 *$ | 333.8 ± 127.7 | |||
| Naïve CD8+T-cells | |||||||
| Non-Infected | 103.7 ± 34.9 | 123.7 ± 51.4 | 151.2 ± 68.2 *$ | 98.6 ± 53.8 | 12.37 (<0.001) | 0.093 (0.765) | 1.114 (0.354) |
| Infected | 109.2 ± 79.0 | 112.0 ± 70.3 | 128.9 ± 59.7 $ | 91.4 ± 63.6 | |||
| CM CD8+T-cells | |||||||
| Non-Infected | 34.8 ± 20.9 | 45.9 ± 24.8 | 53.5 ± 28.2 *$ | 34.5 ± 21.1 | 18.83 (<0.001) | 1.562 (0.232) | 0.465 (0.708) |
| Infected | 60.0 ± 39.1 | 68.2 ± 48.1 $ | 75.3 ± 47.1 *$ | 52.4 ± 43.0 | |||
| EM CD8+T-cells | |||||||
| Non-Infected | 167.8 ± 109.9 | 242.8 ± 175.8 $ | 304.2 ± 212.5 *$ | 139.9 ± 90.2 | 26.38 (<0.001) | 0.021 (0.888) | 0.229 (0.876) |
| Infected | 176.5 ± 82.8 | 229.9 ± 105.5 $ | 281.9 ± 133.2 *$ | 131.4 ± 65.9 | |||
| EMRA CD8+T-cells | |||||||
| Non-Infected | 102.5 ± 85.2 | 165.3 ± 149.0 | 220.2 ± 192.4 *$ | 82.6 ± 76.9 | 11.87 (<0.001) | 0.545 (0.473) | 0.641 (0.593) |
| Infected | 81.8 ± 94.1 | 104.2 ± 109.7 | 154.2 ± 174.5 *$ | 58.6 ± 76.1 | |||
| PD-1+CD8+T-cells | |||||||
| Non-Infected | 24.1 ± 32.7 | 36.1 ± 54.9 | 45.5 ± 69.0 *$ | 19.4 ± 30.1 | 5.670 (0.002) | 0.765 (0.397) | 0.794 (0.504) |
| Infected | 15.3 ± 12.4 | 15.4 ± 10.4 | 25.0 ± 21.9 | 10.0 ± 6.0 | |||
| DN T-cells | |||||||
| Non-Infected | 119.5 ± 82.8 | 173.1 ± 122.3 | 264.2 ± 251.3 *$ | 82.9 ± 50.6 * | 10.04 (<0.001) | 2.82 (0.115) | 1.41 (0.255) |
| Infected | 71.8 ± 33.8 | 92.9 ± 39.9 | 129.5 ± 77.9 *$ | 42.7 ± 15.2 * | |||
| Naïve DN T-cells | |||||||
| Non-Infected | 8.2 ± 8.4 | 11.7 ± 14.6 | 13.9 ± 16.5 $ | 6.6 ± 4.8 | 5.17 (0.004) | 1.40 (0.257) | 0.664 (0.579) |
| Infected | 5.0 ± 2.0 | 5.6 ± 2.3 | 7.6 ± 4.7 | 3.6 ± 1.1 | |||
| CM DN T-cells | |||||||
| Non-Infected | 43.5 ± 25.1 | 42.1 ± 23.6 | 41.6 ± 23.2 | 46.7 ± 22.7 | 2.56 (0.067) | 0.817 (0.381) | 0.604 (0.616) |
| Infected | 52.1 ± 12.3 | 52.2 ± 14.3 | 50.9 ± 17.3 | 53.2 ± 11.6 | |||
| EM DN T-cells | |||||||
| Non-Infected | 46.7 ± 45.1 | 64.6 ± 63.2 $ | 84.2 ± 84.6 *$ | 37.5 ± 35.5 | 14.43 (<0.001) | 0.551 (0.470) | 0.396 (0.756) |
| Infected | 37.5 ± 22.7 | 47.2 ± 25.1 | 60.7 ± 38.5 *$ | 23.3 ± 11.5 | |||
| EMRA DN T-cells | |||||||
| Non-Infected | 40.7 ± 72.4 | 62.6 ± 109.9 | 126.9 ± 260.9 | 23.4 ± 32.4 | 2.43 (0.078) | 1.16 (0.301) | 0.794 (0.504) |
| Infected | 12.5 ± 14.2 | 17.3 ± 22.2 | 35.0 ± 58.0 | 6.3 ± 5.8 | |||
| PD-1+DN T-cells | |||||||
| Non-Infected | 4.4 ± 5.1 | 4.7 ± 5.6 | 4.4 ± 5.4 | 3.7 ± 3.9 | 1.72 (0.178) | 1.09 (0.313) | 0.888 (0.455) |
| Infected | 2.5 ± 1.4 | 2.8 ± 1.8 | 3.4 ± 2.1 | 2.8 ± 1.6 | |||
| B-Cells | |||||||
| Non-Infected | 123.6 ± 46.6 | 170.6 ± 67.1 * | 218.5 ± 87.6 *#$ | 132.9 ± 48.0 | 21.61 (<0.001) | 0.931 (0.351) | 0.966 (0.418) |
| Infected | 183.0 ± 121.8 | 223.1 ± 120.9 $ | 242.6 ± 123.4 *$ | 167.0 ± 86.1 | |||
| NK-Cells | |||||||
| Non-Infected | 264.0 ± 105.7 | 499.3 ± 206.2 *$ | 802.3 ± 335.4 *#$ | 177.6 ± 67.5 | 63.13 (<0.001) | 0.319 (0.581) | 0.148 (0.931) |
| Infected | 338.9 ± 116.6 | 509.4 ± 125.6 $ | 843.7 ± 301.6 *$ | 223.8 ± 99.7 | |||
| PD-1+NK-Cells | |||||||
| Non-Infected | 2.1 ± 1.7 | 3.7 ± 3.4 | 5.0 ± 3.9 | 1.5 ± 1.2 | 8.301 (0.002) | 0.258 (0.619) | 0.581 (0.631) |
| Infected | 2.8 ± 3.9 | 4.2 ± 6.8 | 7.6 ± 9.6 *$ | 1.9 ± 1.9 | |||
3.2. Acute exercise mobilizes cross reactive SARS-CoV-2 specific T-cells and elevates neutralizing antibodies in infected participants
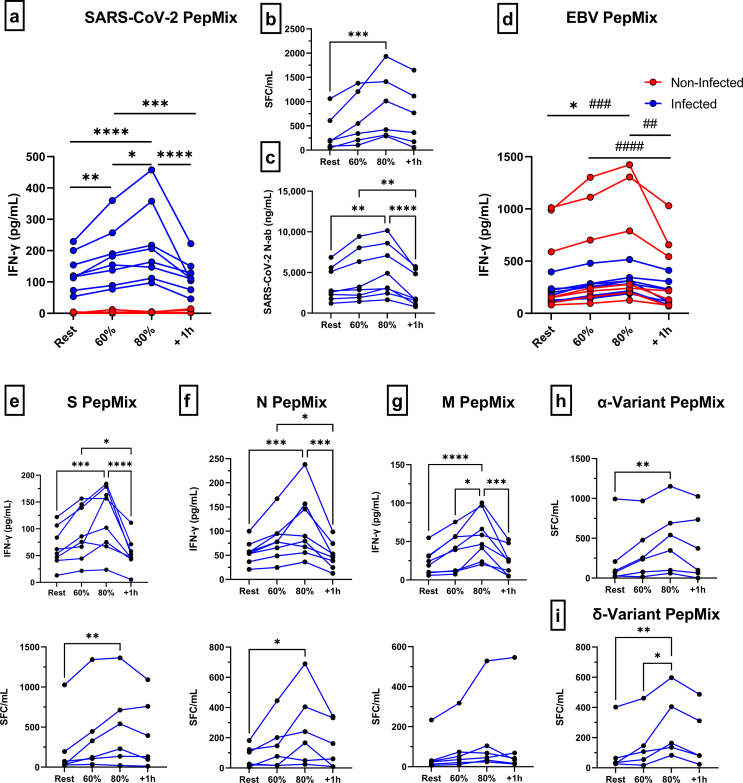
The effects of an acute bout of exercise on the mobilization of SARS-CoV-2 specific T-cells in infected and non-infected participants are depicted in Fig. 1 and Supplemental Fig. 1 (individual plots). Only SARS-CoV-2 infected participants generated an IFN-γ response after whole blood peptide stimulation with a pooled SARS CoV-2 PepMix (Fig. 1a). Significantly more IFN-γ was released by infected participants at rest (p < 0.0001), 60% (p < 0.0001), 80% (p < 0.0001), and 1h-post (p = 0.002) compared to non-infected controls. The IFN-γ response after whole blood peptide stimulation with a EBV consensus PepMix was similar between SARS-CoV-2 infected participants and non-infected controls (p > 0.05) (Fig. 1d). Since non-infected participants did not respond to SARS-CoV-2 whole blood peptide stimulation, and previous studies report that SARS-CoV-2 T-cells are rarely detectable in naïve individuals (Keller et al., 2020; Grifoni et al., 2020), we completed the remaining SARS CoV-2 specific T-cell experiments in infected participants only.
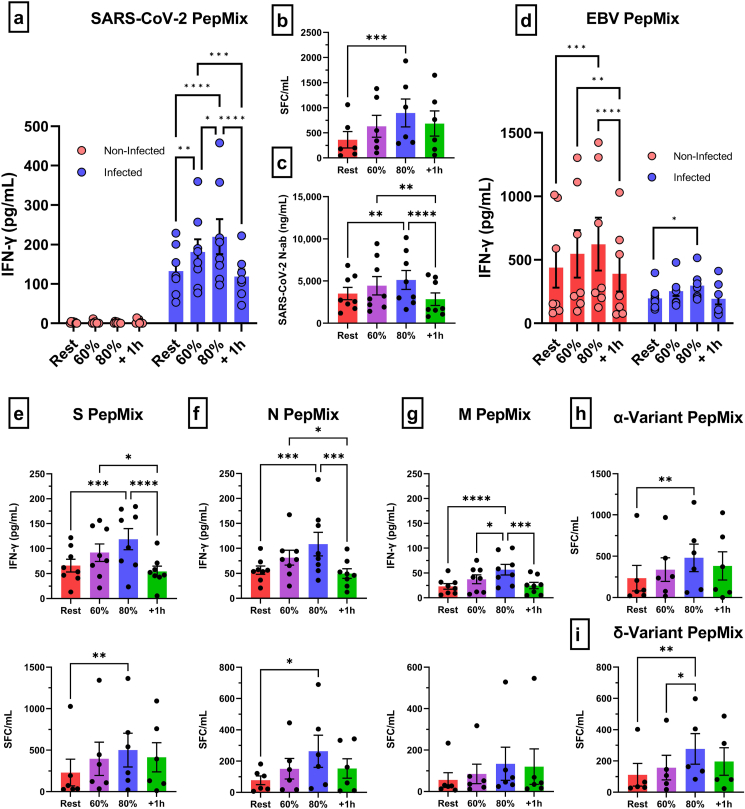
Fig. 1.
The effect of an acute exercise bout on the mobilization of SARS-CoV-2 specific T-cells and neutralizing antibodies. (a) IFN-γ response of SARS-CoV-2 specific T-cells (S/M/N PepMix combined) to exercise in infected and non-infected participants as measured by whole blood peptide stimulation and (b) PBMC ELISPOT (infected only). (c) Anti-RBD-1 neutralizing antibody responses to exercise in infected participants. (d) IFN-γ response of EBV specific T-cells (EBV consensus PepMix) to exercise in infected and non-infected participants as measured by whole blood peptide stimulation. IFN-γ response of SARS-CoV-2 T-cells, specific to (e) S, (f) N, and (g) M PepMix individually, to exercise as measured by whole blood peptide stimulation and PBMC ELISPOT. IFN-γ response of SARS-CoV-2 T-cells, specific to (h) α- and (i) δ-variant PepMix individually, to exercise as measured by PBMC ELISPOT. Data are mean ± SEM. Significance is indicated by * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). SFC = Spot Forming Cells; PBMC = Peripheral Blood Mononuclear Cells; S = Spike; N = Nucelocapsid; M = Membrane.
Specifically, we found that acute exercise increased the whole blood IFN-γ response in an intensity dependent manner (Fig. 1a). SARS-CoV-2 PepMix stimulation of blood collected during the 80% intensity elicited the strongest IFN-γ response, resulting in a 1.6-fold, 1.2 fold, and 1.9-fold change compared to rest (p < 0.0001), 60% intensity (p = 0.0491), and 1h-post (p < 0.0001) stimulated blood, respectively. The IFN-γ response was also 1.3-fold and 1.5-fold higher at the 60% intensity compared rest (p = 0.0096) and 1h-post (p = 0.0006). Moreover, IFN-γ ELISPOT assays corroborated our IFN-γ whole blood peptide stimulation results, (Fig. 1b), although, a significant increase (2.5-fold) in IFN-γ SFC/mL was only observed at the 80% intensity when compared to rest (p = 0.0009). A similar intensity dependent exercise response was observed with SARS-CoV-2 specific neutralizing antibodies, with the 80% intensity eliciting a significant increase compared to rest (p = 0.0004) and 1h-post (p < 0.0001) (Fig. 1c). The IFN-γ response to whole blood peptide stimulation was increased after exercise for T-cells recognizing all major regions of the virus, S protein (p < 0.05), N protein (p < 0.05), and M protein (p < 0.05) (Fig. 1e/f/g). Additionally, IFN-γ release was similar by T-cells recognizing the S and N protein (p > 0.05) but significantly greater than T-cell recognizing the M protein at rest (S: p < 0.0001 & N: p < 0.0001), 60% (S: p < 0.0001 & N: p < 0.0001), 80% (S: p < 0.0001 & N: p < 0.0001), and 1h-post (S: p = 0.0001 & N: p = 0.0012) (Fig. 1e/f/g). These results were partially corroborated by IFN-γ ELISPOT, as an exercise effect was observed for T-cells recognizing S protein (p = 0.0085) and N protein (p = 0.0123), but not M protein (p > 0.05), and significantly more T-cells recognized the S protein compared to M (p = 0.0021) and N protein (p = 0.0068) at the 80% intensity (Fig. 1e/f/g). Importantly, T-cells recognizing SARS-CoV-2 variants of the Spike protein, α(p = 0.065) and δ(p < 0.0054), were mobilized with exercise resulting in an increased IFN-γ response at the 80% intensity (Fig. 1h/i). Collectively, these results indicate that an acute bout of exercise transiently elevates serum neutralizing antibodies and mobilizes SARS-CoV-2 specific T-cells that recognize all major regions of the virus as well as evolving escape variants.
3.3. Exercise mobilized SARS-CoV-2 specific T-cells maintain broad TCR-β diversity
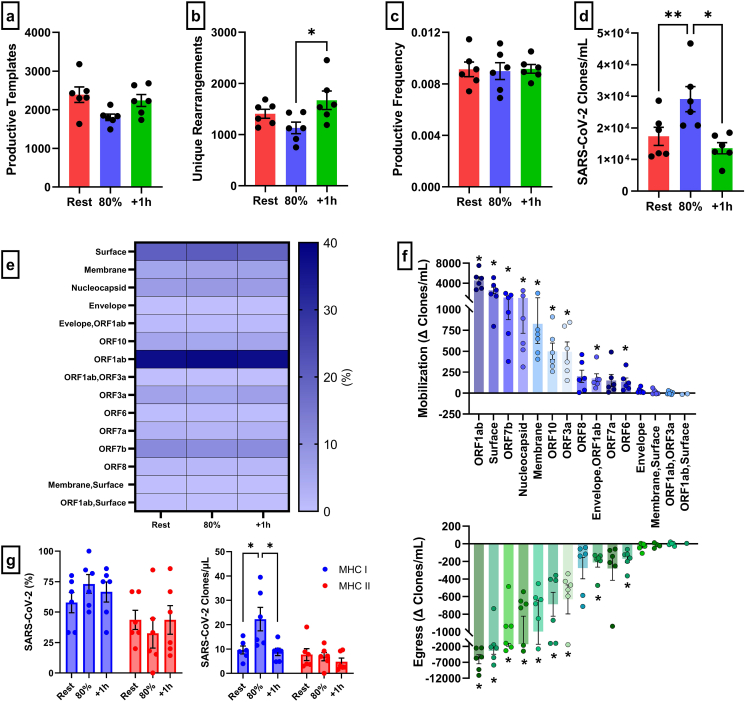
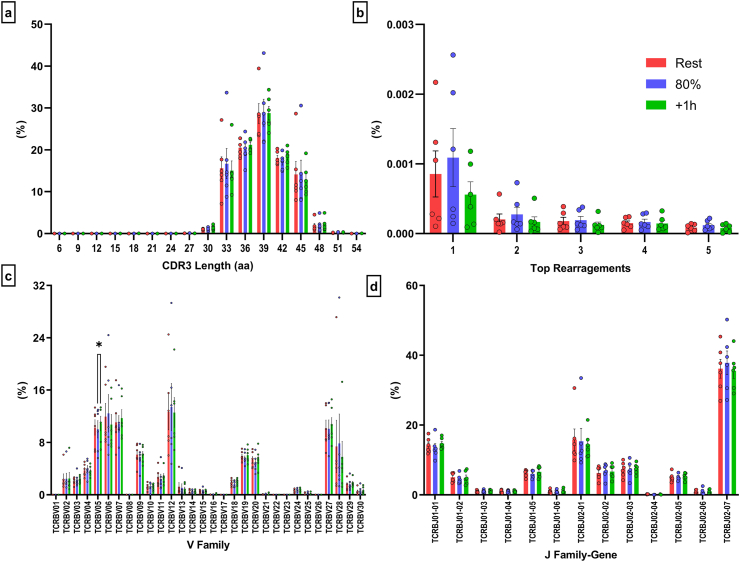
Using next generation deep TCR-β sequencing and immunoSEQ T-MAP COVID ImmuneCODE database, we were able to determine the effects of exercise on TCR-β diversity within SARS-CoV-2 T-cells. Exercise did not alter the total number of SARS-CoV-2 productive templates (p > 0.05) or the proportion of SARS-CoV-2 templates within the T-cell repertoire (p > 0.05) (Fig. 2a/c). However, the number of unique SARS-CoV-2 rearrangements were higher 1h-post compared to the 80% intensity (p = 0.0453) (Fig. 2b). After adjusting for the number of T-cells within 1 mL of blood, exercise significantly mobilized T-cell clones specific to SARS-CoV-2 (Fig. 2d), corroborating our whole blood peptide stimulation and IFN-γ ELISPOT assays. Specifically, exercise mobilized SARS-CoV-2 clones were 1.7-fold and 2.1-fold greater compared to rest (p = 0.0013) and 1h-post (p = 0.0169), respectively. Importantly, exercise mobilized SARS-CoV-2 cells maintained a broad antigen diversity as the percentage of cells recognizing the major regions and ORFs of the virus were not affected by exercise (Fig. 2e). Exercise elicited a significant mobilization (80% - Rest) and egress (1h-post – 80%) of SARS-CoV-2 T-cell clones specific to ORF1ab (p = 0.002 & p = 0.008), S (p = 0.011 & p = 0.029), ORF7b (p = 0.005 & p = 0.019), N (p = 0.026 & p = 0.040), M (p = 0.017 & p = 0.038), ORF10 (p = 0.004 & p = 0.004), ORF3a (p = 0.008 & p = 0.13), Envelope/ORF1ab (p = 0.022 & p = 0.010), and ORF6 (p = 0.031 & p = 0.010) (Fig. 2f). Expectedly, there was a preferential mobilization of MHC class I (i.e. CD8+ T-cells) restricted SARS-CoV-2 T-cells with exercise but not MHC class II (i.e. CD4+ T-cells) restricted SARS-CoV-2 T-cells (Fig. 2g). The CDR3 length (Fig. 3a) of the TCRs expressed by SARS-CoV-2 specific clones and the top 5 amino acid rearrangements, as a percentage of SARS-COV-2 productive clones were not affected by exercise (Fig. 3b). A similar response was observed within family-gene segments that comprise the CDR3 regions, V-Family and J-Family (Fig. 3c/d). However, within V-Family, the frequency of TCRBV05 was significantly higher 1h-post compared to exercise (p = 0.0227) (Fig. 3c). Together, these results indicate that exercise mobilizes diverse SARS-CoV-2 clones with similar antigen specificity to those found in resting blood.
Fig. 2.
The effect of exercise on the TCR-β diversity and MHC class of SARS-CoV-2 specific T-cells. SARS-CoV-2 specific TCR-diversity metrics, (a) total number of productive templates, (b) unique rearrangements, (c) productive frequency, and (d) number of SARS-CoV-2 productive rearrangement templates adjusted for number of T-cells within 1mL of blood (SARS-CoV-2 clones/mL), in response to exercise. (e) The proportion of SARS-CoV-2 clones that recognize each major region and ORF of the virus in response to exercise. (f) The absolute change of SARS-CoV-2 clones mobilized (80%-Rest) and egressed (+1h-80%) with exercise. (g) The proportion and number of SARS-CoV-2 MHC class (MHC I and II) restriction in response to exercise. Data are mean ± SEM. Significance is indicated by * (p < 0.05); ** (p < 0.01). MHC = Major Histocompatibility Complex; ORF = Open Reading Frame.
Fig. 3.
Exercise has minimal effects on additional TCR-β diversity metrics among the SARS-CoV-2 specific T-cell repertoire (a) The proportion of unique CDR3 amino acid (aa) sequences with different nucleotide lengths, (b) the top 5 aa rearrangements, and the relative usage of (c) V-Family and (d) J Family-Gene among SARS-CoV-2 specific T-cells in response to exercise. Data are mean ± SEM. Significance is indicated by * (p < 0.05).
4. Discussion
Physical inactivity is one of the few modifiable risk factors that is associated with risk of severe COVID-19 disease (Sallis et al., 2021). While it has yet to be experimentally shown, a purported mechanism by which physical activity may enhance immune surveillance against SARS-CoV-2 is due to the frequent mobilization and redistribution of SARS-CoV-2 VSTs and increased lymphatic distribution of neutralizing antibodies (Simpson and Katsanis, 2020). Here we show that a single bout of cycling exercise reliably mobilizes SARS-CoV-2 VSTs and neutralizing antibodies in previously infected individuals. Exercise-mobilized SARS-CoV-2 VSTs were reactive against peptides derived from all major structural proteins, as well as those derived from αand δ-variants of the Spike protein, while retaining their antigen specificity and diversity (CDR3 length and family-gene segments).
Acute exercise is known to mobilize T-cells recognizing viruses such as CMV, EBV, and AdV in a catecholamine and β2 adrenergic receptor activation dependent manner (Kunz et al., 2018, 2020; Spielmann et al., 2014, 2016). Extending our previous findings from a single participant (Baker et al., 2021), we show here, in a larger cohort of naturally infected participants, that exercise reliably mobilizes cross-reactive SARS-CoV-2 VSTs in an exercise-intensity dependent manner. The greatest IFN-γ response was observed for T-cells recognizing the S and N protein, followed by M structural proteins. IFN-γ ELISPOTs revealed that exercise increased SARS-CoV-2 VSTs (adjusted/mL of whole blood), specifically those recognizing the S protein and N structural protein, indicating that the greater whole blood IFN-γ response to SARS-CoV-2 peptides observed during exercise is due to mobilization of SARS-CoV-2 VSTs in blood. Additionally, deep TCR-β sequencing corroborated these findings, further revealing that exercise provoked a preferential mobilization of MHC class I (i.e. CD8+ T-cells) restricted SARS-CoV-2 clones. Importantly, exercise mobilized SARS-CoV-2 VSTs maintained broad antigen specificity across all regions of the virus, although VSTs specific to ORF1ab, S, ORF7b, N, and M proteins, in addition to being most abundant in resting blood, were mobilized with exercise. It has been suggested that immune cells mobilized with exercise are recruited from spleen, the lymph nodes, and the gastrointestinal tract (Pedersen and Hoffman-Goetz, 2000). As with T-cells specific to other viruses, SARS-CoV-2 VSTs, specifically those recognizing ORF1ab, surface, ORF7b, nucleocapsid, membrane, ORF10, ORF3a, ORF6, and Enevelope/ORF1ab, egressed the blood compartment after exercise cessation (Kunz et al., 2018, 2020; Spielmann et al., 2014). It has been purported that these egressing cells migrate to tissues (e.g. lungs and intestines) and subsequently re-enter the blood via the lymphatic system to potentially enhance immune surveillance (Walsh et al., 2011; Kruger et al., 2008). While TCR-β sequencing is an effective technique to characterize the SARS-CoV-2 T-cell repertoire it does not capture functional information, therefore future studies should determine the functional status of these exercise responsive clones (Gittelman et al., 2022).
SARS-CoV-2 is now considered an endemic human virus and variants have and will continue to emerge resulting in periods of increased infectivity and transmission causing continuous concern for human immunity (Clarke et al., 2022). We did not confirm if the participants in this study were infected with the original wild type SARS-CoV-2 or a specific variant, although all infections occurred before the peak of α-variant transmission (March 2021) and the initial cases of the δ-variant (April/May2021) in the United States. Importantly, we found that exercise increased the number of SARS-CoV-2 VSTs responsive to these variants, indicating that exercise might also improve immunosurveillance against evolving SARS-CoV-2 escape variants. The mobilization of T-cells reacting with peptides derived from the α- and δ-variants is likely due to T-cell cross reactivity or conserved peptide epitopes (Kedzierska and Thomas, 2022). For instance, previously infected individuals are protected against reinfection (∼90%) of the δ-variant, with immunity lasting for at least 13 months (Kim et al., 2022; Altarawneh et al., 2022). While early SARS-CoV-2 variants, α, Beta, Gamma, and δ, developed mutations mainly within the spike protein, the Omicron-variant developed mutations in the four major structural proteins, S, M, N, and Envelope (Tian et al., 2021, 2022; Harvey et al., 2021). These mutations within the Omicron variant reduced or abolished the neutralization and binding of Class 1–4 neutralizing antibodies (Ju et al., 2022) and weakened the effector and memory T-cell recognition of spike protein in about 20% of the infected and vaccinated population (Naranbhai et al., 2022), resulting in SARS-CoV-2 immune evasion and increased reinfection risk in previously infected individuals (Altarawneh et al., 2022). Further work is required to determine if the observed changes in the immune response to SARS CoV-2 with every exercise bout would offer protection against evolving variants and COVID-19 disease.
SARS-CoV-2 neutralizing antibodies mediate viral protection by disruption of virion binding to receptors, blockade of virus uptake, and prevention viral genome uncoating (Dogan et al., 2021). Here we report, that an acute bout of exercise transiently elevates the circulating levels of SARS-CoV-2 neutralizing antibodies. This is likely due to increased lymphatic flow with exercise (Nehlsen-Cannarella et al., 1991), which we purport could increase the transportation of neutralizing antibodies to enhance immune surveillance and facilitate virus clearance. While SARS-CoV-2 neutralizing antibodies are still detectable after 6 months, the anti-receptor binding-domain (RBD) function begins to decline, and the emergence of escape variants has reduced the efficacy infection-elicited antibodies (Cao et al., 2022; L'Huillier et al., 2021). Therefore, vaccines (including boosters) have been used to efficiently elevate SARS-CoV-2 neutralizing antibodies in naïve and previously infected individuals with the aim of stopping infection in almost all cases. We recently observed that vaccination in a previously infected individual was associated with 1.5-fold increase in neutralizing antibodies, but blunted the exercise induced elevations in neutralizing antibodies, most likely due to a ‘ceiling effect’ induced by the vaccine (Baker et al., 2021). It is possible, however, that exercise may provide longer lasting serological protection in both naturally infected and/or vaccinated individuals as antibodies start to wane, but this remains to be determined. A recent study showed that 90-min of acute exercise immediately after vaccination increases anti-RBD IgG responses, up to one weeks post second vaccine dose, in naïve and previously-infected individuals (Hallam et al., 2022). Influenza-based vaccine studies suggest exercise can enhance the vaccine response, as older individuals who regularly exercise developed more antibodies after influenza vaccination compared to their sedentary counterparts (Woods et al., 2009; Kohut et al., 2004; Smith et al., 1985; Schuler et al., 2003). Moreover, individuals who participated in an acute bout of exercise before or after influenza vaccination developed ∼1.5-fold more immunoglobulins than non-exercising counterparts (Hallam et al., 2022; Edwards and Booy, 2013; Edwards et al., 2007), although the immunoenhancing effects of acute exercise on vaccine responsiveness are not consistently reported (Long et al., 2012; Elzayat et al., 2021; Campbell et al., 2010).
There are a few important caveats in this study. First, initial recruitment of the SARS-CoV-2 cohort was determined by self-reported history of a positive PCR or antibody test, or clinical symptoms of SARS-CoV-2 infection and then confirmed during enrollment by detectable spike IgG levels. Unfortunately, only half of the participants provided an approximate date of infection as the other half were asymptomatic and discovered previous infection through antibody testing. Therefore, infected participants SARS-CoV-2 T-cell and anti-RBD-1 nAb response were measured as early as 46 days and potentially as long as 9 months after infection, which could explain the variation in immune responses and TCR-β diversity (Gittelman et al., 2022; Dan et al., 2021). Fortunately, strong SARS-CoV-2 T-cell and anti-RBD-1 nAb responses were detected in all of our participants but we were unable to correlate these immune responses with time since infection incidence. Second, the participants recruited in this study were younger than the population who typically display severe clinical symptoms. However, the exercise-induced mobilization of SARS-CoV-2 T-cells and nAbs was observed across a wide age range (19–43 years) and previous studies have shown CMV VSTs from older adults remain exercise responsive (Spielmann et al., 2014). Future studies should extend these findings to the older adult and elderly populations. Third, a significant decrease in the predicted VO2max was observed among the infected cohort (33.6 ± 11.2 mL/kg/min) compared to the non-infected cohort (44.9 ± 7.14 mL/kg/min), in spite of similar physical activity levels between the two cohorts. The reduced aerobic capacity is likely due to recent SARS-CoV-2 infection as decreases in VO2max have been observed in young adults and elite athletes, 1–2 months after symptomatic SARS-CoV-2 infection (Parpa and Michaelides, 2022; Crameri et al., 2020).
In conclusion, this is the first study to show that a single exercise bout mobilizes MHC I restricted SARS-CoV-2 VSTs with broad specificity across all regions of the virus and transiently elevates neutralizing antibodies in previously infected non-vaccinated individuals. While the mechanism by which physical activity can improve viral control remain to be determined, these findings do support the hypothesis that each exercise bout may enhance immune surveillance and viral clearance through the mobilization and redistribution of viral specific T-cells and neutralizing antibodies. Future experimental studies should expand these findings to vaccinated individuals and determine the potential impact of chronic exercise on the persistence of SARS-CoV-2 VSTs and neutralizing antibodies, and resulting long-term immunity to SARS CoV-2 and its evolving variants.
Declaration of competing interest
All authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100600.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplemental Fig. 1.
ELISPOT results for the negative (media and actin) and positive (PHA) controls.
Supplemental Fig. 2.
Individual effects of an acute exercise bout on the mobilization of SARS-CoV-2 specific T-cells and neutralizing antibodies. (a) IFN-γ response of SARS-CoV-2 specific T-cells (S/M/N PepMix combined) to exercise in infected and non-infected controls by whole blood peptide stimulation and (b) ELISPOT (infected only). (c)Anti-RBD-1 neutralizing antibody responses to exercise in infected participants. (d) IFN-γ response of EBV specific T-cells (EBV consensus PepMix) to exercise in infected and non-infected controls by whole blood peptide stimulation. IFN-γ response, specific to (e) S, (f) N and (g) M PepMix individually, to exercise as measured by whole blood peptide stimulation and ELISPOT. IFN-γ response, specific to (h) α and (i) δ-variant PepMix individually, to exercise as measured by ELISPOT. Significant differences within the non-infected and infected cohort are indicated by * and #, respectively. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). SFC = Spot Forming Cells; PBMC = Peripheral Blood Mononuclear Cells; S = Spike; N = Nucelocapsid; M = Membrane
Data availability
Data will be made available on request.
References
- Altarawneh H.N., et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaev D.V., et al. VDJdb in 2019: database extension, new analysis infrastructure and a T-cell receptor motif compendium. Nucleic Acids Res. 2019;48(D1):D1057–D1062. doi: 10.1093/nar/gkz874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F.L., et al. Acute exercise increases immune responses to SARS CoV-2 in a previously infected man. Brain Behav. Immun.Health. 2021;18:100343. doi: 10.1016/j.bbih.2021.100343. 100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batatinha H., et al. Recent COVID-19 vaccination has minimal effects on the physiological responses to graded exercise in physically active healthy people. J. Appl. Physiol. 1985;132(2):275–282. doi: 10.1152/japplphysiol.00629.2021. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.P., et al. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav. Immun. 2010;24(2):236–242. doi: 10.1016/j.bbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Cao Y., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2022. COVID-19 People of Any Age with Underlying Medical Conditions; p. 2022.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available from: [PubMed] [Google Scholar]
- Clarke K.E.N., et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, september 2021-february 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71(17):606–608. doi: 10.15585/mmwr.mm7117e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri G.A.G., et al. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland. Euro Surveill. 2020;25(36) doi: 10.2807/1560-7917.ES.2020.25.36.2001542. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins L., et al. Factors associated with COVID-19 related hospitalisation, critical care admission and mortality using linked primary and secondary care data. Influenza Respir Viruses. 2021;15(5):577–588. doi: 10.1111/irv.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;(6529):371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan M., et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun. Biol. 2021;4(1):129. doi: 10.1038/s42003-021-01649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal N.A., et al. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019;19(9):563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Booy R. Effects of exercise on vaccine-induced immune responses. Hum. Vaccines Immunother. 2013;9(4):907–910. doi: 10.4161/hv.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.M., et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 2007;21(2):209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- Elzayat M.T., et al. No effect of acute eccentric resistance exercise on immune responses to influenza vaccination in older adults: a randomized control trial. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.713183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell E., et al. Physical activity, stress, and self-reported upper respiratory tract infection. Med. Sci. Sports Exerc. 2011;43(2):272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- Gittelman R.M., et al. Longitudinal analysis of T cell receptor repertoires reveals shared patterns of antigen-specific response to SARS-CoV-2 infection. JCI Insight. 2022;7(10) doi: 10.1172/jci.insight.151849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff R.M., et al. β(2)-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Grifoni A., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam J., et al. Exercise after influenza or COVID-19 vaccination increases serum antibody without an increase in side effects. Brain Behav. Immun. 2022;102:1–10. doi: 10.1016/j.bbi.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.S., et al. Prediction of functional aerobic capacity without exercise testing. Med. Sci. Sports Exerc. 1990;22(6):863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- Ju B., et al. Immune escape by SARS-CoV-2 Omicron variant and structural basis of its effective neutralization by a broad neutralizing human antibody VacW-209. Cell Res. 2022;32(5):491–494. doi: 10.1038/s41422-022-00638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K., Thomas P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022;3(3) doi: 10.1016/j.xcrm.2022.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.D., et al. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood. 2020;136(25):2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., et al. Duration of severe acute respiratory syndrome coronavirus 2 natural immunity and protection against the delta variant: a retrospective cohort study. Clin. Infect. Dis. 2022;75(1):e185–e190. doi: 10.1093/cid/ciab999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut M.L., et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22(17–18):2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Kruger K., et al. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 2008;22(3):324–338. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kunz H.E., et al. A single exercise bout augments adenovirus-specific T-cell mobilization and function. Physiol. Behav. 2018;194:56–65. doi: 10.1016/j.physbeh.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Kunz H.E., et al. The effects of β(1) and β(1+2) adrenergic receptor blockade on the exercise-induced mobilization and ex vivo expansion of virus-specific T cells: implications for cellular therapy and the anti-viral immune effects of exercise. Cell Stress Chaperones. 2020;25(6):993–1012. doi: 10.1007/s12192-020-01136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Huillier A.G., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin. Microbiol. Infect. 2021;27(5):784.e1–784.e8. doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.E., et al. Vaccination response following aerobic exercise: can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations? Brain Behav. Immun. 2012;26(4):680–687. doi: 10.1016/j.bbi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc. Sport Sci. Rev. 2009;37(4):157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V., et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022;185(6):1041–1051.e6. doi: 10.1016/j.cell.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen-Cannarella S.L., et al. The effects of acute moderate exercise on lymphocyte function and serum immunoglobulin levels. Int. J. Sports Med. 1991;12(4):391–398. doi: 10.1055/s-2007-1024700. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., et al. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 2011;45(12):987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- Parpa K., Michaelides M. Aerobic capacity of professional soccer players before and after COVID-19 infection. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-16031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80(3):1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Sallis R., et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br. J. Sports Med. 2021;55(19):1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- Schuler P.B., Leblanc P.A., Marzilli T.S. Effect of physical activity on the production of specific antibody in response to the 1998-99 influenza virus vaccine in older adults. J. Sports Med. Phys. Fit. 2003;43(3):404. [PubMed] [Google Scholar]
- Simpson R.J., Katsanis E. The immunological case for staying active during the COVID-19 pandemic. Brain Behav. Immun. 2020;87:6–7. doi: 10.1016/j.bbi.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.P., Kennedy S.L., Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J. Appl. Physiol. 1985;97(2):491–498. doi: 10.1152/japplphysiol.01404.2003. 2004. [DOI] [PubMed] [Google Scholar]
- Spielmann G., et al. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav. Immun. 2014;39:142–151. doi: 10.1016/j.bbi.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Spielmann G., et al. A single exercise bout enhances the manufacture of viral-specific T-cells from healthy donors: implications for allogeneic adoptive transfer immunotherapy. Sci. Rep. 2016;6 doi: 10.1038/srep25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., et al. The global epidemic of the SARS-CoV-2 delta variant, key spike mutations and immune escape. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.751778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., et al. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022;94(6):2376–2383. doi: 10.1002/jmv.27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N.P., et al. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Woods J.A., et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J. Am. Geriatr. Soc. 2009;57(12):2183–2191. doi: 10.1111/j.1532-5415.2009.02563.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.