Abstract
Cholera is an acute diarrheal disease that is caused by the gram-negative bacterium Vibrio cholerae. The low efficacy of currently available killed-whole-cell vaccines and the reactinogenicity coupled with potential reversion of live vaccines have thus far precluded widespread vaccination for the control of cholera. Recent studies on the molecular nature of the virulence components that contribute to V. cholerae pathogenesis have provided insights into possible approaches for the development of a defined subunit cholera vaccine. Genetic analysis has demonstrated that the toxin-coregulated pilus (TCP) is the major factor that contributes to colonization of the human intestine by V. cholerae. In addition, polyclonal and several monoclonal antibodies directed against TCP have been shown to provide passive immunity to disease in the infant mouse cholera model. In the present study, synthetic peptides corresponding to portions of the C-terminal disulfide region of TcpA pilin were formulated with polymer adjuvants currently in clinical trials and used to actively immunize adult female CD-1 mice. The experimental vaccine formulations elicited high levels of antigen-specific immunoglobulin G (IgG), including a broad spectrum of subclasses (IgG1, IgG2a, IgG2b, and IgG3), and lower levels of IgA. Infant mice born to the immunized mothers showed 100% protection against a 50% lethal dose (1 LD50) challenge and 50% protection against a 10-LD50 challenge with virulent strain O395. These results indicate that specific regions of TcpA, including those delineated by the peptides used in this study, have the potential to be incorporated into an effective defined subunit vaccine for cholera.
Infection by Vibrio cholerae remains a major cause of morbidity and mortality throughout the world (2). Adequate supplies of uncontaminated food and water coupled with proper sanitary measures, if supported by the appropriate infrastructure, would control cholera, but, unfortunately, this is still not feasible for many affected countries. An alternate means to control cholera is an effective economical cholera vaccine that would mitigate the spread of cholera and stabilize areas of endemicity.
Currently, the following two types of cholera vaccines are approved and in general use for humans: (i) a killed-whole-cell formulation representing both biotypes and serotypes representative of serogroup O1 (35) that is combined with the purified cholera toxin (CT-B) subunit and (ii) a genetically engineered, live-attenuated V. cholerae vaccine (e.g., CVD 103-HgR, O1, classical biotype, Inaba serotype) (17). Multiple doses of the killed-whole-cell vaccine afforded 50% protection in field trials. The most important target population for a cholera vaccine, young children, was even less well protected by this vaccine (less than 25%) (6). A single dose of the live-attenuated vaccine used in clinical trials of North American adults provided >90% protection against the virulent homologous strain and 65 to 80% protection against virulent El Tor biotype, Inaba serotype strains (18, 37, 38). Administration of the CVD 103-HgR vaccine in a large-scale field trial in an area of endemicity of Indonesia did not show any correlation between vaccination and increased protection (12). Another new oral vaccine strain CVD 111, which is a live-attenuated El Tor biotype, Ogawa serotype strain, provided 80% protection in adult volunteers (36). This vaccine is being evaluated together with CVD 103-HgR to determine if the combination can provide further protection against both biotypes in a single dose (40).
Despite the potential of live vaccine strains, two problems are related to their use. First, the live-attenuated strains cause side effects such as mild diarrhea, abdominal cramps, and low fever. Second, because these live strains are attenuated by deleting the ctx genes that are carried on a bacteriophage, there is concern that infection of vaccine strains by ctx-carrying phage may lead to reversion to virulence if these vaccines are used in regions where cholera is endemic (15).
An alternative approach to cholera vaccine design is the development of a subunit vaccine. Research during the last decade has provided new insights into the molecular mechanisms of V. cholerae pathogenesis. Prominent among these is the identification and characterization of the major colonization factor, toxin-coregulated pilus (TCP) (13, 16, 28, 31, 33, 34, 39, 41). TCP and its antigens are obvious targets for testing for inclusion in a subunit cholera vaccine. Of note, there is very little TCP detectable in the commercially available killed-whole-cell cholera vaccines (31), perhaps due to certain culture conditions that need to be optimized for TCP expression.
TCP is composed of a homopolymer of TcpA pilin, which is a 20.5-kDa pilin subunit (41). Rabbit polyclonal antibodies directed against TCP provide 100% protection against a challenge with 100 times the 50% lethal dose (100 LD50) in the infant mouse cholera model (31). Various levels of protection in the infant mouse cholera model were achieved by passive administration of monoclonal antibodies (MAbs) raised against TCP. All the MAbs recognized TcpA, but the most protective MAbs mapped to the C-terminal region of the pilin (32, 33). Synthetic peptides TcpA4, TcpA5, and TcpA6 represent contiguous overlapping peptides corresponding to the carboxyl disulfide bond region of the TcpA pilin. Peptides 5 and 6 were recognized by protective MAbs. In other studies, rabbit antibodies raised against TcpA peptides 4 and 6 were found to be the most protective while antibodies to peptide 5 afforded some protection (34).
One of the problems of utilizing a peptide-based antigen is that, because of their small size, peptides are not likely to elicit a robust stimulation of the immune system. The inclusion of the appropriate adjuvant in vaccine formulations can overcome this problem (9). Polydi(carboxylatophenoxy) phosphazene (PCPP) (Avant Immunotherapeutics, Inc., Needham, Mass.) is a water-based ionically cross-linkable polymer adjuvant that has been used in human clinical trials. PCPP promotes sustained antigen release while retaining antigenic integrity (25, 26). Another promising adjuvant for human use is the nonionic block copolymer mixture of polyoxyethylene (POE) and polyoxypropylene (POP) (Vaxcel, Inc., Norcross, Ga.). By varying both the molecular weight and the proportions of hydrophilic and hydrophobic components of the POP and POE molecules, the formulations can be designed to achieve differential levels and specificities of adjuvant activity (21, 44). One of these copolymer mixtures, termed CRL-1005, has been used to augment the immune response of mice and rhesus monkeys to a commercially available human influenza vaccine (42, 43).
To date none of these adjuvants have been tested for their efficacy in enhancing immune responses directed against small synthetic peptides. In the present study, TcpA peptides 4 and 6 were formulated with either PCPP or CRL-1005 and used to immunize adult CD-1 mice. The immune responses to peptides alone and to the peptide-adjuvant mixture were assessed. The efficacy of each combination was then determined by challenging infant mice born to vaccinated and nonvaccinated adults.
MATERIALS AND METHODS
Peptide antigens and challenge strain.
Peptides corresponding to portions of TcpA from classical biotype strain O395 were synthesized on an Applied Biosystems model 430A synthesizer using the N-hydroxybenzotriazole esters of tert-butoxycarbonyl amino acids in an N-methylpyrrolidone solvent coupling system as described by the manufacturer. Peptides 4 and 6 correspond to amino acid residues 145 to 168 and 174 to 199, respectively, of TcpA pilin (33). PCPP adjuvant was kindly provided by Avant Immunotherapeutics, Inc. The POE and POP copolymers (CRL-1005) were kindly provided by Vaxcel, Inc. V. cholerae O395 (classical, Ogawa) was used for challenge studies after growth in Luria-Bertani broth with a starting pH of 6.5 at 30°C for 18 h with aeration.
Experimental vaccination protocol.
Female CD-1 mice, 6 to 8 weeks of age, were immunized in groups of five for each experiment. For all experimental and control formulations, mice were immunized three times subcutaneously at 4-week intervals. Experimental groups received formulations containing 100 μg of TcpA peptide/dose emulsified in adjuvant. The negative-control groups received phosphate-buffered saline (PBS) or adjuvant alone. For PCPP formulations, aqueous suspensions of TcpA peptides were freshly mixed with 100 μg of adjuvant/dose. The vaccine formulations containing TcpA peptide and the Vaxcel copolymer adjuvant were made by emulsifying 1 volume of aqueous antigen with 1 volume of oil phase copolymer adjuvant. Four weeks after each immunization, sera were collected for antibody analysis. Four to 6 weeks after the third immunization, female CD-1 mice were boosted intraperitoneally and mated with male CD-1 mice. Newborn suckling pups (4 to 5 days old) from the immunized or control mothers were used for challenge studies, with each challenge group containing 8 to 12 neonatal mice.
Measurement and characterization of immune responses.
Murine antibody responses were measured by enzyme-linked immunosorbent assay (ELISA). TcpA peptide antigens were diluted in PBS to a concentration of 5 μg/ml and passively adsorbed to the wells of Immulon-2 assay plates (Dynex Technologies, Inc., Chantilly, Va.) by incubation of 100 μl/well for 18 h at 4°C. Sera from individual mice were diluted in PBS containing 4% (wt/vol) bovine serum albumin using a log10 titration scheme. For immunoglobulin G (IgG) subclass analysis, sera were serially diluted fivefold starting with a 1:10 dilution. Antibody binding to the test antigen was detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse Igs (IgG and IgA; Sigma, St. Louis, Mo.) and HRP-conjugated goat anti-IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3), obtained from Southern Biotechnology Associates, Inc. (Birmingham, Ala.). HRP activity was measured using the colorimetric substrate 3′,3′,5′,5′-tetramethylbenzidine (Sigma), and the absorbance was read at 450 nm by a 96-well microplate reader. Serum samples from individual mice were tested in triplicate to determine the means and standard deviations (SD). Endpoint titers were defined as the highest dilution of serum that yielded an absorbance value of at least twice that of the background. The levels of mucosal secretory IgA were approximated by measuring intestinal samples using the method described by Dickinson and Clements (10). The small intestine from duodenum to ileal-cecal junction was excised and homogenized with PBS. Samples were centrifuged, and the supernatants were used in ELISA.
Determination of immunization efficacy using the infant mouse model.
Neonatal mice (4 to 5 days old) were removed from mothers and orally challenged with 1 LD50 (5 × 105 CFU) or 10 LD50 (5 × 106 CFU) of virulent V. cholerae O395 (41). Animals were monitored for 48 h. After 48 h, any surviving animals were euthanized and sera were collected for antibody analysis.
Statistical analyses.
Antibody titers were scored as endpoint titers for a particular serum sample for individual mice in all groups. The log10 titers were used to generate means and SD that were analyzed by analysis of variance (ANOVA) and Tukey's posttest analysis using Prizm software (Graph Pad Software, San Diego, Calif.). The survival curves were also generated using Prizm software. Survival fractions were calculated using the Kaplan-Meier method. To compare survival curves, the log rank test was used to determine if there was a linear trend for the survival curves. Statistical comparisons were based on the null hypothesis that the treatment did not change the slope of the curves. P values of >0.05 are not considered significant.
RESULTS
Enhanced immunogenicity of TcpA synthetic peptides in the presence of copolymer adjuvants.
Previously, rabbit polyclonal antibodies were raised against individual TCP peptides TcpA4 and TcpA6 coupled to keyhole limpet hemocyanin and emulsified in Freund's complete adjuvant (31, 32). Anti-TcpA4 sera (titer of 1:3,200) were shown to be very effective at providing passive immunity against cholera infection in the infant mouse cholera model, while the sera specific for TcpA6 (1:3,200) were somewhat less effective.
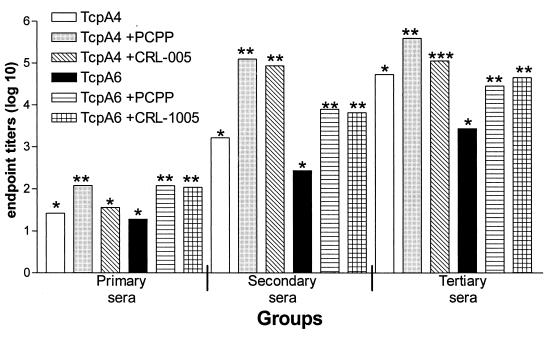
To determine if it was possible to raise protective antibody responses against the TcpA4 and TcpA6 peptides delivered with adjuvants approved for human vaccine trials, the peptides without carrier protein (keyhole limpet hemocyanin) were combined with the PCPP or CRL-1005 polymer adjuvant. The formulations were used to immunize adult female CD-1 mice subcutaneously three times at 4-week intervals. Serum anti-TcpA4 or anti-TcpA6 titers resulting from each immunization were measured by ELISA using the corresponding TcpA peptides as the test antigens (Fig. 1).
FIG. 1.
Measurement of mouse serum antibodies to TcpA4 and TcpA6 peptides by ELISA. Female CD-1 mice were immunized with TcpA peptides alone and TcpA peptides formulated with PCPP or CRL-1005 copolymer adjuvants. All mice were immunized three times at 4-week intervals. Serum samples were taken 4 weeks after each immunization. The data shown represent the log10 means of the endpoint titers. The data were evaluated by ANOVA and Tukey multiple means comparison test. Those means which are significantly different within a group comparison (e.g., for the tertiary-serum comparison, TcpA4 alone [∗] versus TcpA4 plus PCPP [∗∗] or TcpA4 plus CRL-1005 [∗∗∗]) have different numbers of asterisks above the bar. Immunization of mice with PBS or either adjuvant alone did not induce anti-TcpA peptide titers (data not shown).
The initial titers of the first immunization were low regardless of the group assessed. With respect to TcpA4, only the TcpA4-plus-PCPP response in the primary sera was significantly higher than that in sera from mice that received peptide alone. In contrast, both adjuvants significantly enhanced the anti-TcpA6 responses in the primary sera. The titers measured after inoculation of PBS or adjuvant alone were similar. Adjuvant alone did not induce anti-TcpA4 or anti-TcpA6 titers (data not shown).
Mice that had received either peptide formulated with either adjuvant had significantly higher anti-TcpA peptide response to the corresponding peptide after the second immunization than mice that received peptide antigens alone. There were 63-fold (anti-TcpA4) and 14.4-fold (anti-TcpA6) increases in the titer between the primary and secondary immunizations with TcpA peptide alone. This is in contrast to approximately a 3-log-unit increase in titer for anti-TcpA4 sera if either adjuvant was included. Similarly, the use of adjuvants for TcpA6 increased the titers for the secondary sera over those for the primary sera (TcpA6 plus PCPP, 66-fold; TcpA6 plus CRL-1005, 60.2-fold). The immunoenhancing effect of adjuvant is higher (four- to eightfold) for immunization with TcpA4 than for immunization with TcpA6.
After three immunizations with TcpA4 or TcpA6 alone, anti-TcpA titers were always lower than the titers for mice that received TcpA peptides and adjuvant. Immunization with peptides alone caused a greater increase in the tertiary-serum titers than immunization with peptides and either adjuvant. The increases in anti-TcpA peptide titers for mice immunized with peptides only were 32.7-fold and 104.5-fold for TcpA4 and TcpA6, respectively. This is in contrast to only a 4.3-fold average increase in titers for the secondary and tertiary sera of mice immunized with either peptide and either adjuvant, suggesting that the titers were maximized earlier if adjuvant was used. In all comparisons, for peptides alone versus peptides plus either adjuvant, the adjuvant significantly increased the anti-TcpA peptide response except for the primary response to TcpA4 plus CRL-1005.
Characterization of the Ig profile induced by vaccination with the peptides alone and in combination with the polymer adjuvants.
To characterize the nature of the immune responses induced by the peptide-adjuvant formulations, the anti-TcpA peptide titers of IgG subclasses and of IgA (serum and secretory IgA [sIgA]) were determined from mice after the third immunization with the TcpA4 and TcpA6 peptides alone or peptides in combination with the PCPP or CRL-1005 adjuvant (Table 1). This analysis revealed that the vaccine formulations induced a broad spectrum of IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3), with IgG1 and IgG2a being predominant.
TABLE 1.
Antibody isotypes and subclasses induced by TcpA peptides and vaccine formulations containing polymer adjuvantsa
| Antigen | Mean antibody titers for adult female CD-1 micec ± SD for:
|
|||||
|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | Serum IgA | sIgAb | |
| TcpA4 | 200 ± 87∗ | 337 ± 42∗ | 10 ± 0.01∗ | 50 ± 1.2∗ | 10 ± 0.01∗ | 50 ± 17∗ |
| TcpA4 + PCPP | 4,167 ± 160∗∗ (20.8) | 1,167 ± 44∗∗ (3.5) | 83 ± 29∗∗ (8.3) | 417 ± 74∗ (8.3) | 100 ± 0.1∗ (10.0) | 542 ± 72∗∗ (16.8) |
| TcpA4 + CRL-1005 | 2,333 ± 16∗∗ (11.7) | 917 ± 90∗ (2.7) | 37 ± 12∗ (3.7) | 200 ± 64∗ (4.0) | 83 ± 9.7∗∗ (8.3) | 417 ± 24∗∗ (8.3) |
| TcpA6 | 112 ± 10∗ | 50 ± 0.01∗ | 10 ± 0.01∗ | 37 ± 9.6∗ | 10 ± 0.01∗ | ND |
| TcpA6 + PCPP | 2,833 ± 108∗∗ (25.3) | 283 ± 18∗∗ (5.7) | 23 ± 6∗ (2.3) | 150 ± 87∗ (4.1) | 108 ± 25∗∗ (10.8) | ND |
| TcpA6 + CRL-1005 | 2,500 ± 175∗∗ (22.3) | 1,667 ± 28† (33.3) | 50 ± 0.01∗∗ (5.0) | 100 ± 0.01∗ (2.7) | 200 ± 48∗∗ (20.0) | ND |
Antibody responses of adult female CD-1 mice were measured by ELISA. All mice were immunized three times at 4-week intervals. Serum samples were taken 4 weeks after the last immunization. Mean titers were determined from log10 endpoint titers for each experimental group. ND, not determined.
sIgA, IgA from intestinal tissue.
The symbols after the values for each group indicate Tukey's multiple means comparisons of the ANOVA results for that group. The comparisons are peptide to peptide plus PCPP, peptide to peptide plus CRL-1005, and peptide plus PCPP to peptide plus CRL-1005. Symbols that are the same indicate the mean titer comparisons within that group have a P value of >0.05; those that are different have a P value of <0.05 and thus the titers are significantly different. Numbers in parentheses are the fold increases of the anti-TcpA peptide titers of mice immunized with peptides and adjuvant compared to those for mice immunized with TcpA4 or TcpA6 peptide alone.
Surprisingly, the degree of titer enhancement of various subclass responses was dependent on the specific peptide-adjuvant combination. For TcpA4, the PCPP adjuvant formulation increased all IgG subclasses as well as IgA, serum or secretory, to a greater extent (average of 11.3-fold versus an average of 6.5-fold) than the CRL-1005 adjuvant. For TcpA6, CRL-1005 increased the response on average 16.6-fold while PCPP increased the response on average 9.6-fold. The most remarkable effects of the adjuvants were consistently seen in the IgG1 (average 20-fold increase) and IgA (average 12.4-fold increase) responses. The other IgG subclasses increased on average 7-fold (range, 2.3-fold to 33.3-fold).
Statistical analysis of individual subclass and isotype titers for anti-TcpA4 or anti-TcpA6 revealed a pattern similar to that presented in Fig. 1. The major difference in the data presented in Table 1 versus that in Fig. 1 is that the analysis of the IgG2b and IgG3 subclass responses revealed that in general they did not differ from that induced by peptides alone. This was true except in two cases: (i) TcpA4 plus PCPP, for which the response was different from that for peptides alone, and (ii) TcpA6 plus CRL-1005, for which the response was different from that for TcpA6 alone. As a whole the IgG1, IgG2a, and IgA responses with peptides plus either adjuvant were significantly higher than those with peptides alone.
Induction of protective host immunity by TcpA peptide vaccine formulations.
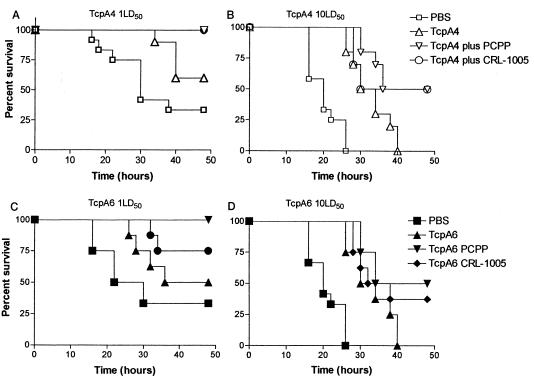
Since the acquisition of passive immunity in young children from mother's milk is one of the goals of cholera vaccine development, we used a modification of the infant mouse protection assay based on the types of methods used in rotavirus vaccine development, whereby mothers are immunized and protective antibodies are acquired by placental transfer or in milk (5, 7, 23, 24, 29). The use of this method not only assesses the ability of the immunization regimen to select a VDJ, VJ solution for the specific antibodies but also assesses the efficacy of inducing a functional antibody that is delivered via natural means, which is not the case in the typical infant mouse cholera protection assay. In this study, after the third immunization, females were mated and pups were born on average 35 days postimmunization. Newborn CD-1 suckling pups were challenged with either 1 LD50 (5 × 105 CFU) or 10 LD50 (5 × 106 CFU) of virulent V. cholerae O395, and the survival rate was monitored for 48 h (Fig. 2). Statistical comparisons of the survival curves for selected treatment groups are shown in Table 2. For vaccine formulations that included TcpA4, the results with the polymer adjuvants were nearly identical. All the neonatal mice from mothers immunized with TcpA4 plus polymer adjuvant survived the 1-LD50 challenge (Fig. 2A). This difference was statistically significant (P = 0.0017 for PBS versus TcpA4 plus PCPP; P = 0.0017 for PBS versus TcpA4 plus CRL-1005) in comparison with that for the neonatal mice born to nonimmunized CD-1 mothers given PBS (Table 2). The difference in protection afforded by the adjuvants was not significant. Neonatal mice from mothers immunized with TcpA4 peptide alone were partially protected (60%) from 1 LD50, and the time until death was increased, but the protection was not significantly different (Fig. 2A and Table 2). The greater protection (1 LD50) for neonatal mice born to mothers immunized with TcpA4 plus adjuvant than for those immunized with TcpA4 alone was statistically significant (P = 0.0297 for TcpA4 versus TcpA4 plus PCPP; P = 0.0297 for TcpA4 versus TcpA4 plus CRL-1005).
FIG. 2.
Protection from O395 challenge of neonatal CD-1 mice born to mothers immunized with TcpA4, TcpA4 plus PCPP, TcpA4 plus CRL-1005, TcpA6, TcpA6 plus PCPP, or TcpA6 plus CRL-1005. Five-day-old neonatal mice born to mothers immunized with TcpA4, TcpA4 plus PCPP, or TcpA4 plus CRL-1005 adjuvant (10 to 14 neonatal mice from each experimental group for TcpA4) were challenged with 1 LD50 (A) or 10 LD50 (B) of virulent V. cholerae O395. For TcpA6-immunized groups, eight neonatal mice were challenged with 1 LD50 (C) or 10 LD50 (D) of virulent V. cholerae O395. The survival rates were monitored every 2 h for 48 h. The data shown represent the combined results of two independent experiments.
TABLE 2.
Correlation of anti-TcpA-peptide titers with protection for this study and others
| Challenge method/dosea | Anti-TCP titer (% protection) | Anti-TcpA4 titer (% protection) | Anti-TcpA6 titer (% protection) | Reference or study |
|---|---|---|---|---|
| In vitro studies | ||||
| In vitro mix/500 LD50 | NDb | 1:3,200 (89) | 1:3,200 (70) | 32 |
| In vitro mix/500 LD50 | 1:79,400 (100) | ND | ND | 29 |
| In vitro mix/500 LD50 | 1:32,000 (87.5) | ND | ND | 29 |
| In vitro study | ||||
| In vivo mix/1 LD50 | 1:200 (60) | ND | This study | |
| In vivo mix/10 LD50 | 1:200 (0) | ND | This study | |
| In vivo mix/1 LD50 | ND | 1:112 (50) | This study | |
| In vivo mix/10 LD50 | ND | 1:112 (0) | This study | |
| In vivo mix/1 LD50 | 1:2,333 (100) | ND | This study | |
| In vivo mix/10 LD50 | 1:2,333 (50) | ND | This study | |
| In vivo mix/1 LD50 | 1:4,167 (100) | ND | This study | |
| In vivo mix/10 LD50 | 1:4,167 (50) | ND | This study | |
| In vivo mix/1 LD50 | ND | 1:2,500 (75) | This study | |
| In vivo mix/10 LD50 | ND | 1:2,500 (33) | This study | |
| In vivo mix/1 LD50 | ND | 1:2,833 (100) | This study | |
| In vivo mix/10 LD50 | ND | 1:2,833 (50) | This study |
In vitro mix, infant mouse assay in which viable pathogenic V. cholerae is mixed 1:1 with antisera (usually undiluted) and then the mixture is introduced intragastrically to neonatal mice through gavage; in vivo mix, method utilized in the present study, in which 6- to 8-week-old female mice undergo an immunization regimen and the efficacy is tested by challenging their infants with V. cholerae at 1 LD50 or 10 LD50.
ND, not determined.
When neonatal mice were challenged with 10 LD50 of V. cholerae O395, 50% of neonatal mice from mothers immunized with TcpA4 plus adjuvant survived, compared to no surviving pups from PBS-treated mothers (P < 0.0001 for PBS versus TcpA4 plus PCPP; P < 0.0001 for PBS versus TcpA4 plus CRL-1005) (Fig. 2B). All neonatal mice from mothers immunized with TcpA4 peptide only succumbed after 40 h, although the time until death was increased in comparison with that for neonatal mice born to PBS-treated mothers (P < 0.0001 for PBS versus TcpA4). The infants that survived (immunized with TcpA4 plus adjuvant) had specific anti-TcpA4 IgG antibody titers ranging from 1:1,000 to 1:10,000 and detectable serum IgA titers ranging from 1:10 to 1:50 (data not shown). The survival of mice given TcpA4 alone was significantly different from that of mice given the peptide plus adjuvant if the adjuvant was PCPP (P = 0.0149) but not if it was CRL-1005 (P = 0.0618). The levels of protection afforded by either adjuvant with TcpA4 were not significantly different (P = 0.6640). For TcpA6 formulations, there was a greater difference in the efficacy of the two polymer adjuvants than was seen for TcpA4 formulations. Infant mice born to mothers immunized with TcpA6 plus PCPP adjuvant were 100% protected (P = 0.0045 for PBS versus TcpA6 plus PCPP) against a 1-LD50 challenge (Fig. 2C) and 50% protected (P < 0.0001 for PBS versus TcpA6 plus PCPP) against a 10-LD50 challenge (Fig. 2D). Infant mice born to mothers immunized with TcpA6 and copolymer CRL-1005 showed a lower (75%), but still statistically significant (P = 0.0394 for PBS versus TcpA6 plus CRL-1005), protection rate against a 1-LD50 challenge (Fig. 2C). The protection afforded infant mice vaccinated with the TcpA6-plus-CRL-1005 formulation against the 10-LD50 challenge was also statistically significant (P < 0.0001 for PBS versus TcpA6 plus CRL-1005), but only 33% of the mice survived the challenge (Fig. 2D). The use of TcpA6 peptide alone to vaccinate produced some protection at 1 LD50, but the protection was not significantly different from that for mothers that received PBS (P = 0.2562 for PBS versus TcpA6). However, the protection afforded by TcpA6 was significant (P = 0.002 for PBS versus TcpA6) with a 10-LD50 challenge dose. If the protection afforded by the TcpA6 peptide alone and the protection afforded by the peptide emulsified in either PCPP or CRL-1005 are compared, it is obvious that the inclusion of the adjuvant is effective against 1 LD50 (P = 0.0250 for TcpA6 versus TcpA6 plus PCPP, and P = 0.0250 for TcpA6 versus TcpA6 plus CRL-1005) but not against 10 LD50 (P = 0.5260 for TcpA6 versus TcpA6 plus PCPP, and P = 0.2223 for TcpA6 versus TcpA6 plus CRL-1005). At either challenge level, the difference in protection by either adjuvant was not significant (for 1 LD50 P = 0.7720, and for 10 LD50 P = 0.4998).
Interestingly, and similar to the results noted for TcpA4 plus adjuvant, sera of pups that survived infection from each group had specific IgG antibody titers in the range of 1:1,000 to 1:10,000. They also had detectable serum IgA antibody titers of 1:10 to 1:50.
Titer, antibody source, and LD50 influence survival.
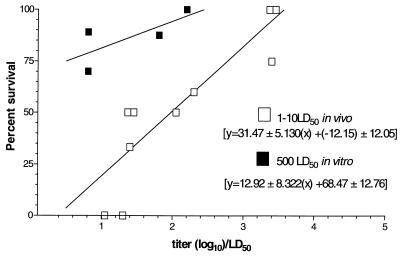
We correlated the serologic titer of the IgG1 responses to peptides alone or preparations of peptides with the two adjuvants to the percent protection provided in the modified infant mouse challenge assay (Fig. 3). We chose to focus on IgG1 as it is representative of the isotypes (IgG1 and IgA) that completely correlate with protection. We developed a model that relates the mean log10 titer of the mice for a particular treatment group/LD50 of bacteria given plotted against the percent protection. Retrospective data (Table 3) for percent protection following in vitro mixing of antibody and bacteria were also analyzed and compared to the trend of the line for antibody mixed with bacteria in vivo. There is a direct correlation of anti-TcpA IgG1 titers and protection. The slopes are significantly different from each other (in vivo, 31.47; in vitro, 12.92) and indicate that, if bacteria are mixed with antibodies in vitro, the protection does not fall off as quickly as it does if they are mixed in vivo. For example, if the ratio of the log10 titer is 2 to 1, for the in vivo mixture the protection decreases from 50.4 to 19.0%. The decrease for an in vitro mixture is a change of only about 13% (94.3 to 81.4%).
FIG. 3.
Correlation of the percent protection in the neonatal mouse assay with anti-TcpA titer/LD50. Data from this study and from others presented in Table 2 were used to generate curves that show the relationship between anti-TcpA titer and LD50 challenge dose and the percent protection provided by an antibody mixed with bacteria in vitro or obtained passively in vivo. The in vitro studies used 500 LD50, while the in vivo curve based on data from this study combined data for pups challenged with either 1 LD50 or 10 LD50. The curves were generated using the Prizm program, whereby the percent protection was plotted against log10 transformed titer/LD50. The equations for each curve are representative of the standard equation for a line, y = (slope ± SDx + y intercept ± SD).
TABLE 3.
Tabulation of log rank comparisons indicating the statistical significance of various treatment regimens on protection in the infant mouse challenge assaya
| Peptide, dose, and comparison | P | Significance |
|---|---|---|
| TcpA4 | ||
| 1 LD50 | ||
| PBS vs TcpA4 | 0.0679 | No |
| PBS vs TcpA4/PCPP | 0.0017 | Yes |
| PBS vs TcpA4/CRL-1005 | 0.0017 | Yes |
| TcpA4 vs TcpA4/PCPP | 0.0297 | Yes |
| TcpA4 vs TcpA4/CRL-1005 | 0.0297 | Yes |
| TcpA4/PCCP vs TcpA4/CRL-1005 | 0.9999 | No |
| 10 LD50 | ||
| PBS vs TcpA4 | <0.0001 | Yes |
| PBS vs TcpA4/PCPP | <0.0001 | Yes |
| PBS vs TcpA4/CRL-1005 | <0.0001 | Yes |
| TcpA4 vs TcpA4/PCPP | 0.0159 | Yes |
| TcpA4 vs TcpA4/CRL-1005 | 0.0618 | No |
| TcpA4/PCPP vs TcpA4/CRL-1005 | 0.6640 | No |
| TcpA6 | ||
| 1 LD50 | ||
| PBS vs TcpA6 | 0.2560 | No |
| PBS vs TcpA6/PCPP | 0.0045 | Yes |
| PBS vs TcpA6/CRL-1005 | 0.0394 | Yes |
| TcpA6 vs TcpA6/PCPP | 0.0250 | Yes |
| TcpA6 vs TcpA6/CRL-1005 | 0.7772 | No |
| TcpA6/PCCP vs TcpA6/CRL-1005 | 0.1435 | No |
| 10 LD50 | ||
| PBS vs TcpA6 | 0.0020 | Yes |
| PBS vs TcpA6/PCPP | <0.0001 | Yes |
| PBS vs TcpA6/CRL-1005 | <0.0001 | Yes |
| TcpA6 vs TcpA6/PCPP | 0.5260 | No |
| TcpA6 vs TcpA6/CRL-1005 | 0.2223 | No |
| TcpA6/PCpP vs TcpA6/CRL-1005 | 0.4988 | No |
To compare survival curves, the Prizm software was used to perform the log rank test. Statistical comparisons were based on the null hypothesis that the treatment did not change the slope of the curves. Infant mice were challenged with either 1 LD50 or 10 LD50 and monitored for 48 h to determine time of death (Fig. 2). Individual comparisons were made as indicated; P values of >0.05 are not considered significant.
DISCUSSION
In this report, we show that TcpA peptide antigens formulated with newly developed adjuvants intended for use in humans can be used to immunize adult female CD-1 mice, which can then provide passive immunity to their neonatal offspring. Vaccine formulations containing TcpA4 or TcpA6 peptides emulsified in PCPP or CRL-1005 adjuvants augmented antibody responses beyond those achieved for either peptide alone, which in turn corresponded to greater protection against V. cholerae challenge. The amount of peptide used in these studies, 100 μg, is on the high end of the immunogenic dose in mice. Smaller amounts of TcpA peptides (e.g., 25 μg) are immunogenic even if given in only two immunizations (W. F. Wade, personal observation). However, the dose and immunization schedule and route of administration of TcpA peptides in humans remain to be determined, and the amount of antigen will likely be different depending on the route and method chosen to deliver the peptides.
The finding that the vaccine formulations containing the TcpA4 peptide were more protective than those containing TcpA6 is consistent with previous passive immunization studies (34). Importantly, female mice immunized with TcpA peptides in adjuvant were able to transfer an antibody that was protective to suckling pups challenged with virulent V. cholerae O1. It is not known if the protective antibody in our present report was present in milk, transferred through the placenta, or both (1, 4). In humans, IgG has been shown to bind to placental Fc receptors, which facilitate its transport into the fetal circulation (14). Alternatively, there is IgG among other isotypes present in human breast milk. The extension of the adjuvants described in this study and TcpA peptides into humans could result in protective immunity due to IgG acquired by several means. The identity of the protective isotype that is most effective for protection against cholera is an open question. Historically, sIgA, which is usually present in mucosal secretions, has been considered the likely protective isotype against cholera. In support of the potential role of other isotypes, recent evidence indicates that IgG as well as IgM can enter the gut and that the entry of IgG may be enhanced in the local area where there is infection (3). The selective IgA deficiency is one of the most common immunodeficiencies, with 0.5% of the population having no or very low sIgA. There are no published reports of increased incidence of cholera or increased severity of disease in individuals of that phenotype. These patients clearly respond to vaccination with cholera vaccines with increased IgG (22). More data need to be acquired based on the responses of humans to determine the contribution of single or multiple isotypes to affording protection from cholera.
The formulations of TcpA peptides with PCPP or copolymer CRL-1005 adjuvants induced a broad spectrum of IgG subclasses. The highest titers were predominantly for the IgG1 and IgG2a subtypes, suggesting activation of both Th1 and Th2 type T helper cells. This is consistent with the PCPP-formulated influenza vaccine, which induced all IgG subclasses (14, 26). Copolymer CRL-1005 also activates both Th1 and Th2 type responses (43). While the TcpA-specific IgG induced by the vaccine formulations could contribute to the protection observed for the infant mice in the present study, a more accepted mechanism of protection involves mucosal IgA. Studies have shown that both Th1 and Th2 type cytokines can stimulate a mucosal immune response (8, 11, 19, 20).
Although the anti-TcpA peptide IgA titers induced by these experimental vaccine formulations were not as high as the IgG titers, they may contribute to the immune protection seen in the infant mouse challenge. Clearly there were significant increases in sIgA that paralleled the IgG1 increase. Thus, this adjuvant-peptide combination could be an important vaccine component that would generate a protective antibody titer in both the human intestine and breast milk. Since the protective antigens of cholera have not been conclusively defined, it is difficult to assess why people are protected after surviving infection or being immunized with the current whole-cell vaccines. Clearly, antilipopolysaccharide (anti-LPS) titers increase and the specific anti-LPS antibodies in the IgG and IgA compartments as well as in the IgM compartment can be measured. Work in our laboratory suggests that certain somatic mutations need to be acquired by the antibodies for anti-Ogawa immunity directed at the terminal LPS sugar (A. Chernyak et al., submitted for publication). However, since TcpA is not a strong immunogen in natural infections, it is not known how anti-TcpA antibodies mature upon repeated exposure to V. cholerae. The elements of what make a protective anticholera antiserum are usually associated with anti-LPS antibodies rather than anti-TcpA antibodies. This, however, does not preclude TcpA from being used as an immunogen with the types of adjuvants we described here. We expect that vaccination with TcpA would result in TcpA serologic responses that would also mature with the affinity and specificity that would be protective for subsequent exposure to V. cholerae.
The means by which an anti-TcpA antibody is combined with or is available along with the infectious inoculum is an important issue to understand. The standard infant mouse model mixes the antibody and bacteria in vitro, which is a very efficient means of linking the antibody to target epitopes. In the modification of the infant challenge assay that we report here, the antibody has to transfer to the infant mouse naturally and find the target epitope in the context of the gut milieu. Thus, the amounts of antibodies (titer) needed for protection for the two assays are likely to be different. A similar model to evaluate the efficacy of vaccine preparation has been utilized in studies of rotavirus. Rotavirus infection of infants causes disease by replication of the virus in mature epithelial cells of the small intestine. Like V. cholerae, rotavirus will not colonize the intestines in adult animals (27). Most animal studies with respect to rotavirus vaccine development have been performed by immunizing adult female mice and determining the efficacy by challenge of infant mice (5, 7, 23, 24, 28). To our knowledge this is the first study to show the efficacy of evaluating a maternally transferred mouse antibody for its role in protection against cholera. This is particularly relevant as infants are often cited as the population most at risk and are the targeted group for improved vaccines. Our new method of challenge will allow us to assess vaccine formulations in a setting that has direct relevance to human health applications.
Our results show that TcpA peptides can be combined with either PCPP or copolymer adjuvant CRL-1005 for use in inducing protective immunity to cholera infection. The V. cholerae TcpA antigen formulated with polymer adjuvants could have utility for human use. A subunit or peptide vaccine would be safer and less reactinogenic than a live cholera vaccine. Subunit vaccines can also be rigorously standardized during the manufacturing process, eliminating lot-to-lot variance that is problematic with attenuated vaccines. TCP is an obvious choice for this type of vaccine as it is a member of the type 4 pili, which contribute to the colonization capability of many species of gram-negative bacteria, such as Neisseria gonorrhoeae, Pseudomonas aeruginosa, and pathogenic strains of Escherichia coli (30).
Clearly, a data set that indicates that whole V. cholerae does not have to be used to induce antibodies that can protect against cholera is developing. The challenge is to find the right formulation (antigens and adjuvants) and the most effective method to deliver the vaccines that promote the concentration, affinity, and fine specificity of the protective antibodies that protect adults, children, and infants.
ACKNOWLEDGMENT
We express our sincere thanks to David Beattie of Avant Immunotherapeutics, Inc., and Mark Newman of Vaxcel, Inc., for providing PCPP and CRL-1005 adjuvants, respectively.
This work was supported by NIH grants to R.K.T. (AI 25096) and W.F.W. (AI 47373) and also a Dartmouth Hitchcock Foundation grant to J.-Y.W.
REFERENCES
- 1.Bell S C, Billington W D. Humoral immune responses in murine pregnancy. III. Relationship between anti-paternal alloantibody levels in maternal serum, placenta and fetus. J Reprod Immunol. 1983;5:299–310. doi: 10.1016/0165-0378(83)90256-5. [DOI] [PubMed] [Google Scholar]
- 2.Bern C, Martine J, deZoysa I, Glass R I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull W H O. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- 3.Bovet J-P, Fiscchett V A. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun. 1999;67:2687–2691. doi: 10.1128/iai.67.6.2687-2691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castilla J, Sola I, Pintado B, Sanchez-Morgando J M, Enjuanes L. Lactogenic immunity in transgenic mice producing recombinant antibodies neutralizing coronavirus. Adv Exp Med Biol. 1998;440:675–686. doi: 10.1007/978-1-4615-5331-1_87. [DOI] [PubMed] [Google Scholar]
- 5.Clark J F, Offit P A, Ellis R W, Eiden J J, Krah D, Shaw A R, Pichichero M, Treanor J J, Borian F E, Bell L M, Plotkin S A. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174(Suppl. 1):S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 6.Clemens J D, Sack D A, Harris J R, van Loon F, Chakraborty J, Ahmed F, Rao M R, Khan M R, Yunus M D, Huda N, Stanton B F, Kay B A, Walter S, Eeckels R, Svennerholm A-M, Holmgren J. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 7.Coffin S E, Moser C A, Cohen S, Clark H F, Offit P A. Immunologic correlates of protection against rotavirus challenge after intramuscular immunization of mice. J Virol. 1997;71:7851–7857. doi: 10.1128/jvi.71.10.7851-7856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin S E, Clark S L, Bos N A, Brubaker J O, Offit P A. Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J Immunol. 1999;163:3064–3070. [PubMed] [Google Scholar]
- 9.Del Giudice G, Pizza M, Rappuoli R. Molecular basis of vaccination. Mol Aspects Med. 1998;19:1–70. doi: 10.1016/s0098-2997(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2742. [PubMed] [Google Scholar]
- 12.Fournier J M, Villeneuve S. Cholera update and vaccination problems. Med Trop. 1998;8(Suppl. 2):32–35. [PubMed] [Google Scholar]
- 13.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferis R. Structure-function relationships of IgG subclasses. In: Shakib F, editor. The human IgG subclasses: molecular analysis of structure, function and regulation. Oxford, United Kingdom: Pergamon Press; 1990. pp. 93–108. [Google Scholar]
- 15.Kimsey H H, Waldor M K. CTXφ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci USA. 1998;95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirn T J, Lafferty M J, Sandoe C M P, Taylor R K. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol Microbiol. 2000;35:896–910. doi: 10.1046/j.1365-2958.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- 17.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;ii:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 18.Levine M M, Kaper J B. Live oral vaccines against cholera: an update. Vaccine. 1993;11:207–212. doi: 10.1016/0264-410x(93)90019-t. [DOI] [PubMed] [Google Scholar]
- 19.Lillard J W, McGhee J R. Adjuvants or live delivery systems for the characterization of mucosal T helper subset responses. Res Immunol. 1997;148:520–527. doi: 10.1016/s0923-2494(98)80145-4. [DOI] [PubMed] [Google Scholar]
- 20.McGhee J R, Fujihashi K, Xu-Amano J, Jackson R J, Elson C O, Beagley K W, Kiyono H. New perspectives in mucosal immunity with emphasis on vaccine development. Semin Hematol. 1993;30(Suppl. 4):3–14. [PubMed] [Google Scholar]
- 21.Newman M J, Todd C W, Lee E M, Balusubramanian M, Didier P J, Katz J M. Increasing the immunogenicity of a trivalent influenza virus vaccine with adjuvant-active nonionic block copolymers for potential use in the elderly. Mech Ageing Dev. 1997;93:189–203. doi: 10.1016/s0047-6374(96)01811-8. [DOI] [PubMed] [Google Scholar]
- 22.Nilssen D E, Friman V, Theman K, Bjorkander J, Kilander A, Holmgren J, Hanson L A, Brandtzaeg P. B-cell activation in duodenal mucosa after oral cholera vaccination in IgA deficient subjects with or without IgG subclass deficiency. Scand J Immunol. 1993;38:201–208. doi: 10.1111/j.1365-3083.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 23.Offit P A, Clark H F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985;54:58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Offit P A, Dudzik K I. Noninfectious rotavirus (strain RRV) induces an immune response in mice which protects against rotavirus challenge. J Clin Microbiol. 1989;27:885–888. doi: 10.1128/jcm.27.5.885-888.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne L G. Trivalent influenza vaccine. Vaccine. 1998;16:92–98. doi: 10.1016/s0264-410x(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 26.Payne L G, Jenkins S A, Andrianov A, Roberts B E. Water-soluble phosphazene polymers for parenteral and mucosal vaccine delivery. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 473–492. [DOI] [PubMed] [Google Scholar]
- 27.Ramig R F. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb Pathog. 1988;4:189–202. doi: 10.1016/0882-4010(88)90069-1. [DOI] [PubMed] [Google Scholar]
- 28.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan J F, Smith C C, Manak M M. Prevention of rotavirus-induced diarrhea in neonatal mice born to dams immunized with empty capsids of simian rotavirus SA-11. J Infect Dis. 1984;149:434–438. doi: 10.1093/infdis/149.3.434. [DOI] [PubMed] [Google Scholar]
- 30.Strom M S, Lory S. Structure and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 31.Sun D, Mekalanos J J, Taylor R K. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J Infect Dis. 1990;161:1231–1236. doi: 10.1093/infdis/161.6.1231. [DOI] [PubMed] [Google Scholar]
- 32.Sun D, Tillman D M, Marion T N, Taylor R K. Production and characterization of monoclonal antibodies to the toxin coregulated pilus (TCP) of Vibrio cholerae that protect against experimental cholera in infant mice. Serodiagn Immunother Infect Dis. 1990;4:73–81. [Google Scholar]
- 33.Sun D, Seyer J M, Kovari I, Sumrada R A, Taylor R K. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect Immun. 1991;59:114–118. doi: 10.1128/iai.59.1.114-118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D, Lafferty M J, Peek J A, Taylor R K. Domains within the Vibrio cholerae toxin coregulated pilin subunit that mediate bacterial colonization. Gene. 1997;192:79–85. doi: 10.1016/s0378-1119(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm A M, Holmgren J. Oral combined B-subunit-whole cell cholera vaccine. In: Holmgren J, Lindberg A, Mollby R, editors. Development of vaccines and drugs against diarrhea. Proceedings of the 11th Nobel Conference. Lund, Sweden: Studentlitteratur; 1985. pp. 33–43. [Google Scholar]
- 36.Tacket C O, Kotloff K L, Losonsky G, Nataro J P, Michalski J, Kaper J B, Edelman R, Levine M M. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am J Trop Med Hyg. 1997;56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 37.Tacket C O, Cohen M B, Wasserman S S, Losonsky G, Livio S, Kotloff K, Edelman R, Kaper J B, Cryz S J, Giannela R A, Schiff G, Levine M M. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect Immun. 1999;67:6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket C O, Losonsky G, Nataro J P, Cryz S J, Edelman R, Kaper J B, Levine M M. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1992;16:837–841. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- 39.Tacket C O, Taylor R K, Losonsky G, Lim Y, Nataro J P, Kaper J B, Levine M M. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66:692–695. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor D N, Tacket C O, Losonsky G, Castro O, Gutierrez J, Meza R, Nataro J P, Kaper J B, Wasserman S S, Edelman R, Levine M M, Cryz S J. Evaluation of a bivalent (CVD 103–HgR/CVD 111) live oral cholera vaccine in adult volunteers from the United States and Peru. Infect Immun. 1997;65:3852–3856. doi: 10.1128/iai.65.9.3852-3856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. The use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todd C W, Balusubramanian M, Shah H, Henk W G, Younger L E, Newman M J. Systematic development of a block copolymer adjuvant for trivalent influenza virus vaccine. Dev Biol Stand. 1998;92:341–351. [PubMed] [Google Scholar]
- 43.Todd C W, Pozzi L A, Guarnaccia J R, Balasubramanian M, Henk W G, Younger L E, Newman M J. Development of an adjuvant-active nonionic block copolymer for use in oil-free subunit vaccine formulations. Vaccine. 1997;14:564–570. doi: 10.1016/s0264-410x(97)00209-0. [DOI] [PubMed] [Google Scholar]
- 44.Triozzi P L, Stevens V C, Aldrich W, Powell J, Todd C W, Newman M J. Effects of a β-human chorionic gonadotropin subunit immunogen administered in aqueous solution with a novel nonionic block copolymer adjuvant in patients with advanced cancer. Clin Cancer Res. 1997;3:2355–2362. [PubMed] [Google Scholar]