Abstract
Background
The Medicare-enrolled population is heterogeneous across race, ethnicity, age, dual eligibility, and a breadth of chronic health, mental and behavioral health, and disability-related conditions, which may be differentially impacted by the COVID-19 pandemic.
Objective
To quantify changes in all-cause mortality prior-to and in the first year of the COVID-19 pandemic across Medicare's different sociodemographic and health-condition subpopulations.
Methods
This observational, population-based study used stratified bivariate regression to investigate Medicare fee-for-service subpopulation differences in pre-pandemic (i.e., 2019 versus 2016) and pandemic-related (2020 versus 2019) changes in all-cause mortality.
Results
All-cause mortality in the combined Medicare-Advantage (i.e., managed care) and fee-for-service beneficiary population improved by a relative 1% in the ten years that preceded the COVID-19 pandemic, but then escalated by a relative 15.9% in 2020, the pandemic's first year. However, a closer look at Medicare's fee-for-service subpopulations reveals critical differences. All-cause mortality had actually been worsening prior to the pandemic among most psychiatric and disability-related condition groups, all race and ethnicity groups except White Non-Hispanic, and Medicare-Medicaid dual-eligible (i.e., low-income) beneficiaries. Many of these groups then experienced all-cause mortality spikes in 2020 that were over twice that of the overall Medicare fee-for-service population. Of all 61 chronic health conditions studied, beneficiaries with schizophrenia were the most adversely affected, with all-cause mortality increasing 38.4% between 2019 and 2020.
Conclusion
This analysis reveals subpopulation differences in all-cause mortality trends, both prior to and in year-one of the COVID-19 pandemic, indicating that the events of 2020 exacerbated preexisting health-related inequities.
Keywords: All-cause mortality, Chronic conditions, COVID, Disparity, Schizophrenia, Mental health
1. Introduction
The risk of severe illness due to Coronavirus Disease 2019 (COVID-19), an infectious disease caused by the Sudden Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), varies by a number of chronic conditions, however there is a dearth of information on its impact on individuals with lower-prevalence psychiatric and disability-related conditions [[1], [2], [3], [4], [5], [6]]. With Medicare eligibility based on being age 65 or older or having a qualifying disability or end-stage renal disease, the Medicare-enrolled population represents a broad diversity of clinical and sociodemographic profiles. Given this, in addition to the known risks associated with advancing age [[4], [5], [6]], it is important to understand the extent to which the COVID-19 pandemic may have impacted Medicare's different subpopulations.
The concept of “excess mortality” (i.e., “number of deaths exceeding a given norm for the given time and place”) [7] has been used to examine the impact of the large-scale health events in populations (e.g., the 1840 influenza outbreak [8], the 1918 influenza pandemic [9], and more recently, the COVID-19 pandemic [4,6,[10], [11], [12]]). Researchers estimated that the COVID-19 pandemic has been directly or indirectly associated with over 500,000 excess deaths from all causes in the U.S. during the pandemic's first 10 months [6]. This all-cause mortality measure is critical to understanding the true toll of this virus on human life in a population as it is able to capture both direct and indirect effects of the COVID-19 pandemic. Additionally, it is unaffected by evolving disease definitions, asymptomatic cases, testing-uptake variability, and variable cause-of-death reporting, including health conditions exacerbated by SARS-CoV-2 infection, and even politics [[13], [14], [15], [16], [17]]. All-cause mortality also encompasses deaths among persons who never contracted SARS-CoV-2, including the potential downstream fatal effects of delayed or foregone medical care resulting from early pandemic mitigation measures [[18], [19], [20], [21]], and pandemic-associated stress and exacerbated psychiatric issues [[22], [23], [24], [25], [26], [27], [28], [29]].
In addition to understanding all-cause mortality in the Medicare population, it is critical to bring to light any differences across Medicare's many diverse subpopulations, especially given that differences in mortality have been found between Medicare beneficiary subgroups even before the COVID-19 outbreak [30], coupled with the consistent finding that people of color and lower-income individuals in the general U.S. population have been disproportionally affected by the pandemic [12,[31], [32], [33], [34], [35]]. In contrast, there have been evolving and sometimes conflicting findings on the extent to which different health conditions place people at greater or lesser risk of COVID-19-related adverse outcomes [[2], [3], [4], [5], [6]]. Our study addresses these research gaps by quantifying the magnitude and direction of changes in all-cause mortality in the first year of pandemic across Medicare's multifarious clinical and sociodemographic subpopulations, even the less populous ones, while also putting these changes in the context of pre-existing trends.
2. Methods
2.1. Data sources and study population
This population-based, observational study of changes in all-cause mortality used beneficiary-level enrollment and claims data from the Centers for Medicare & Medicaid Services' Chronic Conditions Data Warehouse (CCW) [36]. The first study component was a broader view of all Medicare-enrolled individuals, including both managed care (i.e., Medicare Advantage) and fee-for-service (Parts A and B), conducted to contextualize the second fee-for-service-only study component. In this first component, we calculated all-cause mortality trends over a long pre-pandemic period (2009–2019) plus the pandemic's first year (2020). The second component was a more focused comparative analysis of Medicare's fee-for-service subpopulations, as permitted by available data. Our aim was to identify and quantify how Medicare beneficiaries, across different sociodemographic characteristics and health diagnoses, may have been differentially affected by the COVID-19 pandemic. The health-conditions feature necessitated limiting the second analytic component to all individuals enrolled exclusively in Medicare fee-for-service Parts A and B because CCW condition category algorithms do not include managed-care encounter data [37]. For comparability, we also applied this constraint to the sociodemographic subpopulation analyses. We analyzed all-cause mortality changes between 2019 and 2020, and for context, between 2016 and 2019, with a shorter timespan chosen than for the first broader-view study component in order to enhance the temporal relevancy of subpopulation comparisons.
2.2. Measures
We calculated all-cause mortality as the percentage of enrolled beneficiaries meeting our study criteria who died from any cause in a given year. Death information was sourced from the Medicare date-of-death field, populated from Social Security Administration (SSA) records [38].
The comparative analyses of the second analytic component employed the following sociodemographic variables: age (categorically redefined, based on age as of January 1 of a given year); a singular, algorithm-enhanced CCW race and Hispanic-ethnicity variable [39,40]; sex; residential area (from ZIP codes translated into Rural Urban Commuting Area codes [41]); and dual enrollment in Medicaid, hierarchically redefined as a three-level annual variable (i.e., any full-benefit dual enrollment; no full-benefit and any partial-benefit dual enrollment; or no dual enrollment).
The second-component comparative analyses included 61 health-condition categories (Table 1 ). Of the 61 condition categories, 60 were pre-defined CCW variables [42], and one (“non-schizophrenic psychotic disorders”) was constructed from the non-overlapping portion of two overlapping pre-defined CCW variables (i.e., “schizophrenia” and “schizophrenia and other psychotic disorders”). CCW condition-category variables are Medicare fee-for-service claims-based indicators, available on every year of data since 1999, and constructed from algorithms of diagnosis and procedure codes, with lookback periods of one or two years, except for Alzheimer's disease (three years) and sickle cell disease (five years) [36,43]. Note that a beneficiary may be counted within more than one condition category (e.g., someone defined by the algorithms as having both obesity and diabetes would be counted under both categories). We created beneficiary-level, binary condition variables for “ever” versus “never” having met the CCW-defined criteria for each condition category, as of a given year.
Table 2.
Relative Differences in All-Cause Mortality, by Chronic Condition, within the Study Population of Beneficiaries Enrolled in Medicare Fee-For-Service (FFS) Parts A and B: 2019 vs. 2016 and 2020 vs. 2019.
| Health Condition Categories⁎ | Group | Change in all-cause mortality (2019 vs 2016) |
Change in all-cause mortality (2020 vs 2019) |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Total FFS Parts A and B Enrolled Study Population | 0.990 (0.989, 0.991) | 1.159 (1.156, 1.620) | |
| Acquired Hypothyroidism (Hypothyroid) | Age-related | 0.991 (0.990, 0.992) | 1.172 (1.168, 1.177) |
| ADHD and Conduct Disorders (ADHD) | Mental/behavioral | 1.015 (1.009, 1.020) | 1.263 (1.246, 1.280) |
| Alcohol Use Disorders (Alcohol) | Mental/behavioral | 1.002 (0.999, 1.005) | 1.161 (1.152, 1.170) |
| Alzheimer's Disease (Alzheimer's) | Disability-related | 1.008 (1.006, 1.009) | 1.320 (1.313, 1.327) |
| Alzheimer's Disease & Other Dementias (Dementia) | Disability-related | 0.998 (0.997, 0.999) | 1.241 (1.237, 1.246) |
| Anemia | Age-related | 0.995 (0.994, 0.996) | 1.172 (1.169, 1.175) |
| Anxiety Disorders (Anxiety) | Mental/behavioral | 0.983 (0.982, 0.984) | 1.166 (1.162, 1.171) |
| Asthma | Age-related | 0.975 (0.974, 0.977) | 1.150 (1.144, 1.156) |
| Atrial Fibrillation (AF) | Age-related | 0.987 (0.985, 0.988) | 1.123 (1.118, 1.128) |
| Autism Spectrum Disorders (Autism) | Disability-related | 1.007 (0.988, 1.026) | 1.307 (1.245, 1.373) |
| Benign Prostatic Hyperplasia (BPH) | Age-related | 0.992 (0.990, 0.993) | 1.178 (1.172, 1.183) |
| Bipolar Disorder (Bipolar) | Mental/behavioral | 1.034 (1.031, 1.037) | 1.295 (1.285, 1.305) |
| Blindness and Visual Impairment (Vision Loss) | Disability-related | 0.993 (0.989, 0.997) | 1.231 (1.215, 1.246) |
| Breast Cancer (Breast Ca) | Age-related | 0.981 (0.978, 0.984) | 1.110 (1.100, 1.120) |
| Cerebral Palsy (CP) | Disability-related | 1.013 (1.002, 1.024) | 1.301 (1.262, 1.341) |
| Chronic Kidney Disease (CKD) | Age-related | 0.964 (0.964, 0.965) | 1.164 (1.160, 1.167) |
| Chronic Obstructive Pulmonary Disease (COPD) | Other | 0.977 (0.976, 0.979) | 1.157 (1.153, 1.161) |
| Chronic Pain/Fatigue, Fibromyalgia (Chronic Pain) | Age-related | 0.997 (0.994, 1.001) | 1.106 (1.095, 1.117) |
| Colorectal Cancer (CR Ca) | Age-related | 0.994 (0.993, 0.995) | 1.163 (1.159, 1.167) |
| Cystic Fibrosis and Related (CF) | Disability-related | 1.033 (1.029, 1.037) | 1.219 (1.206, 1.232) |
| Deafness & Hearing Impairment (Hearing Loss) | Disability-related | 0.987 (0.986, 0.989) | 1.187 (1.181, 1.193) |
| Developmental Delays (DD) | Disability-related | 1.007 (0.994, 1.020) | 1.337 (1.291, 1.385) |
| Diabetes (DM) | Age-related | 1.000 (0.999, 1.001) | 1.209 (1.205, 1.213) |
| Endometrial Cancer (Uterine Ca) | Age-related | 0.977 (0.971, 0.983) | 1.104 (1.084, 1.125) |
| Epilepsy | Disability-related | 1.002 (1.000, 1.005) | 1.221 (1.211, 1.230) |
| Eye Cataract (Cataract) | Age-related | 0.989 (0.988, 0.990) | 1.171 (1.168, 1.175) |
| Glaucoma | Age-related | 0.995 (0.993, 0.996) | 1.191 (1.185, 1.196) |
| Heart Failure (HF) | Age-related | 0.996 (0.995, 0.997) | 1.165 (1.161, 1.169) |
| Hip/Pelvic Fracture (Hip Fx) | Age-related | 0.995 (0.993, 0.997) | 1.186 (1.178, 1.195) |
| HIV/AIDS | Other | 1.011 (0.999, 1.022) | 1.184 (1.144, 1.225) |
| Hyperlipidemia (Lipids) | Age-related | 0.993 (0.992, 0.994) | 1.169 (1.166, 1.172) |
| Hypertension (HTN) | Age-related | 0.992 (0.991, 0.993) | 1.171 (1.168, 1.174) |
| Intellectual Disabilities and Related (Intell. Disab.) | Disability-related | 1.019 (1.013, 1.026) | 1.312 (1.289, 1.336) |
| Ischemic Heart Disease (IHD) | Age-related | 0.998 (0.997, 0.998) | 1.173 (1.170, 1.176) |
| Learning Disabilities (Learning Disab.) | Disability-related | 0.997 (0.988, 1.006) | 1.285 (1.256, 1.314) |
| Leukemias and Lymphomas (L & L) | Age-related | 0.977 (0.973, 0.980) | 1.088 (1.076, 1.099) |
| Liver Disease, Cirrhosis & Related (Non-Hepatitis) (Liver (Non-hepatitis)) | Age-related | 0.975 (0.973, 0.977) | 1.120 (1.115, 1.126) |
| Lung Cancer (Lung Ca) | Age-related | 0.958 (0.956, 0.961) | 1.022 (1.013, 1.032) |
| Major Depressive Disorder (Depression) | Mental/behavioral | 0.989 (0.988, 0.990) | 1.187 (1.184, 1.191) |
| Migraine and Other Chronic Headache (Headache) | Other | 1.020 (1.016, 1.023) | 1.174 (1.163, 1.184) |
| Mobility Impairments (Mobility Loss) | Disability-related | 0.987 (0.985, 0.989) | 1.188 (1.181, 1.195) |
| Multiple Sclerosis and Transverse Myelitis (MS) | Disability-related | 1.002 (0.994, 1.010) | 1.211 (1.183, 1.239) |
| Muscular Dystrophy (MD) | Disability-related | 0.994 (0.976, 1.012) | 1.201 (1.139, 1.267) |
| Obesity | Other | 0.974 (0.972, 0.975) | 1.220 (1.215, 1.225) |
| Opioid Use Disorder (OUD) | Mental/behavioral | 1.007 (1.003, 1.010) | 1.135 (1.125, 1.145) |
| Osteoporosis | Age-related | 0.981 (0.980, 0.983) | 1.159 (1.154, 1.164) |
| Peripheral Vascular Disease (PVD) | Age-related | 0.994 (0.993, 0.995) | 1.200 (1.196, 1.204) |
| Personality Disorders (Personality Dis.) | Mental/behavioral | 1.022 (1.017, 1.026) | 1.263 (1.248, 1.277) |
| Post-Traumatic Stress Disorder (PTSD) | Mental/behavioral | 1.013 (1.007, 1.019) | 1.197 (1.178, 1.217) |
| Pressure Ulcers and Chronic Ulcers (Skin Ulcer) | Age-related | 1.004 (1.003, 1.006) | 1.198 (1.193, 1.203) |
| Prostate Cancer (Prostate Ca) | Age-related | 0.981 (0.979, 0.984) | 1.139 (1.130, 1.149) |
| Psychotic Disorders (Non-Schizophrenic) (Psychotic/Non-schizophrenic) | Mental/behavioral | 0.970 (0.968, 0.973) | 1.260 (1.250, 1.270) |
| Rheumatoid Arthritis and Osteoarthritis (Arthritis) | Age-related | 0.987 (0.986, 0.988) | 1.176 (1.172, 1.179) |
| Schizophrenia | Mental/behavioral | 1.035 (1.031, 1.040) | 1.384 (1.367, 1.402) |
| Sickle Cell Disease (Sickle Cell) | Other | 1.027 (0.998, 1.058) | 1.153 (1.059, 1.255) |
| Spina Bifida and Related (Spina Bifida) | Disability-related | 0.950 (0.941, 0.960) | 1.160 (1.125, 1.196) |
| Spinal Cord Injury (Spinal Injury) | Disability-related | 1.002 (0.998, 1.007) | 1.177 (1.163, 1.190) |
| Stroke | Age-related | 0.996 (0.995, 0.998) | 1.189 (1.184, 1.194) |
| Tobacco Use Disorders (Tobacco Dis) | Other | 0.994 (0.992, 0.996) | 1.141 (1.135, 1.146) |
| Traumatic Brain Injury and Related (TBI) | Disability-related | 0.987 (0.983, 0.992) | 1.227 (1.210, 1.244) |
| Viral Hepatitis (General) (Hepatitis) | Other | 1.001 (0.996, 1.005) | 1.177 (1.163, 1.192) |
The shorthand terms that were used for improved data visualization in Fig. 2 are denoted, where applicable, by the italicized terms that are shown in parentheses.
Table 1.
Study Population Characteristics of Beneficiaries Enrolled in Medicare Fee-For-Service (FFS) Parts A and B, by Year: 2016, 2019, 2020 – United States.⁎
| 2016 |
2019 |
2020 |
|
|---|---|---|---|
| N (%) |
N (%) |
N (%) |
|
| Total FFS Parts A and B Enrolled Study Population | 33,981,644 (100%) | 33,172,265 (100%) | 32,407,844 (100%) |
| Race & Ethnicity | |||
| American Indian/Alaskan Native | 190,392 (0.56%) | 185,243 (0.56%) | 175,876 (0.54%) |
| Asian/Pacific Islander | 838,204 (2.47%) | 896,370 (2.7%) | 903,018 (2.79%) |
| Non-Hispanic White | 27,037,631 (79.57%) | 26,368,784 (79.49%) | 25,784,260 (79.56%) |
| Black/African American | 3,218,260 (9.47%) | 2,900,963 (8.75%) | 2,730,698 (8.43%) |
| Hispanic | 1,994,862 (5.87%) | 1,942,729 (5.86%) | 1,895,342 (5.85%) |
| Other | 259,131 (0.76%) | 261,433 (0.79%) | 260,963 (0.81%) |
| Unknown/Missing | 443,164 (1.30%) | 616,743 (1.86%) | 657,687 (2.03%) |
| Age Group | |||
| ≤ 54 | 2,838,620 (8.35%) | 2,308,289 (6.96%) | 2,113,073 (6.52%) |
| 55–64 | 2,760,319 (8.12%) | 2,391,802 (7.21%) | 2,169,361 (6.69%) |
| 65–74 | 15,216,392 (44.78%) | 15,471,562 (46.64%) | 15,329,619 (47.3%) |
| 75–84 | 8,688,628 (25.57%) | 8,861,728 (26.71%) | 8,763,014 (27.04%) |
| 85+ | 4,477,685 (13.18%) | 4,138,884 (12.48%) | 4,032,777 (12.44%) |
| Unknown/Missing | 0 (0%) | 0 (0%) | 0 (0%) |
| Sex | |||
| Female | 18,650,709 (54.88%) | 18,102,031 (54.57%) | 17,666,581 (54.51%) |
| Male | 15,330,923 (45.12%) | 15,070,234 (45.43%) | 14,741,263 (45.49%) |
| Unknown/Missing | 12 (0%) | – | – |
| Dual Eligibility | |||
| Full-benefit | 5,224,875 (15.38%) | 4,724,570 (14.24%) | 4,509,095 (13.91%) |
| Partial-benefit | 1,801,665 (5.30%) | 1,534,516 (4.63%) | 1,318,733 (4.07%) |
| Non-dual | 26,955,104 (79.32%) | 26,913,179 (81.13%) | 26,580,016 (82.02%) |
| Region | |||
| Rural | 8,924,595 (26.26%) | 8,663,073 (26.12%) | 8,382,247 (25.86%) |
| Urban | 25,057,049 (73.74%) | 24,509,192 (73.88%) | 24,025,597 (74.14%) |
| Health Condition Groups# | |||
| Age-related Condition Categories | |||
| Acquired Hypothyroidism | 8,509,425 (25.04%) | 8,486,083 (25.58%) | 8,312,691 (25.65%) |
| Anemia | 16,137,721 (47.49%) | 15,531,680 (46.82%) | 15,068,153 (46.50%) |
| Asthma | 4,592,860 (13.52%) | 4,468,200 (13.47%) | 4,320,480 (13.33%) |
| Atrial Fibrillation | 4,468,535 (13.15%) | 4,444,036 (13.40%) | 4,341,244 (13.4%) |
| Benign Prostatic Hyperplasia^ | 5,605,572 (16.5%) | 5,743,506 (17.31%) | 5,689,937 (17.56%) |
| Breast Cancer^⁎ | 1,698,011 (9.10%) | 1,721,820 (9.51%) | 1,703,824 (9.64%) |
| Chronic Kidney Disease | 9,790,513 (28.81%) | 10,943,855 (32.99%) | 10,964,909 (33.83%) |
| Colorectal Cancer | 842,517 (2.48%) | 782,572 (2.36%) | 748,772 (2.31%) |
| COPD | 8,068,163 (23.74%) | 7,598,151 (22.91%) | 7,158,133 (22.09%) |
| Diabetes | 11,683,383 (34.38%) | 11,244,429 (33.9%) | 10,874,458 (33.56%) |
| Endometrial Cancer^ | 292,108 (0.86%) | 305,662 (0.92%) | 302,898 (0.93%) |
| Eye Cataract | 18,751,744 (55.18%) | 18,419,440 (55.53%) | 17,920,002 (55.30%) |
| Glaucoma | 6,750,091 (19.86%) | 6,652,286 (20.05%) | 6,465,916 (19.95%) |
| Heart Failure | 7,595,494 (22.35%) | 7,227,956 (21.79%) | 6,962,128 (21.48%) |
| Hip/Pelvic Fracture | 1,128,864 (3.32%) | 1,083,261 (3.27%) | 1,053,915 (3.25%) |
| Hyperlipidemia | 23,924,750 (70.4%) | 23,747,352 (71.59%) | 23,288,434 (71.86%) |
| Hypertension | 24,728,815 (72.77%) | 24,004,977 (72.36%) | 23,314,612 (71.94%) |
| Ischemic Heart Disease | 13,757,397 (40.48%) | 13,151,559 (39.65%) | 12,760,694 (39.38%) |
| Leukemias and Lymphomas | 681,900 (2.01%) | 730,093 (2.20%) | 732,297 (2.26%) |
| Liver Disease, Cirrhosis & Related (Non-Hep.) | 3,057,660 (9.00%) | 3,528,934 (10.64%) | 3,588,910 (11.07%) |
| Lung Cancer | 514,076 (1.51%) | 515,386 (1.55%) | 500,226 (1.54%) |
| Osteoporosis | 6,105,348 (17.97%) | 5,928,401 (17.87%) | 5,797,718 (17.89%) |
| Peripheral Vascular Disease | 6,622,026 (19.49%) | 6,582,587 (19.84%) | 6,429,870 (19.84%) |
| Pressure Ulcers and Chronic Ulcers | 2,782,231 (8.19%) | 2,663,917 (8.03%) | 2,607,234 (8.05%) |
| Prostate Cancer^ | 1,668,595 (4.91%) | 1,671,711 (5.04%) | 1,651,400 (5.1%) |
| Rheumatoid Arthritis, Osteoarthritis | 17,427,419 (51.28%) | 17,661,940 (53.24%) | 17,277,074 (53.31%) |
| Stroke | 4,369,601 (12.86%) | 4,131,235 (12.45%) | 3,987,067 (12.30%) |
| Disability-related Condition Categories | |||
| Alzheimer's | 1,753,243 (5.16%) | 1,561,211 (4.71%) | 1,459,760 (4.50%) |
| Alzheimer's Disease & Other Dementias | 4,400,422 (12.95%) | 4,333,510 (13.06%) | 4,217,382 (13.01%) |
| Autism Spectrum Disorders | 109,252 (0.32%) | 139,875 (0.42%) | 147,053 (0.45%) |
| Blindness and Visual Impairment | 501,976 (1.48%) | 390,816 (1.18%) | 361,112 (1.11%) |
| Cerebral Palsy | 174,033 (0.51%) | 172,820 (0.52%) | 168,620 (0.52%) |
| Cystic Fibrosis and Related | 591,249 (1.74%) | 722,972 (2.18%) | 736,909 (2.27%) |
| Deafness & Hearing Impairment | 4,074,926 (11.99%) | 4,536,294 (13.67%) | 4,568,282 (14.1%) |
| Developmental Delays | 114,799 (0.34%) | 130,025 (0.39%) | 131,989 (0.41%) |
| Epilepsy | 1,316,634 (3.87%) | 1,329,718 (4.01%) | 1,290,538 (3.98%) |
| Intellectual Disabilities and Related | 561,781 (1.65%) | 554,727 (1.67%) | 540,786 (1.67%) |
| Learning Disabilities | 106,941 (0.31%) | 161,065 (0.49%) | 171,314 (0.53%) |
| Mobility Impairments | 1,629,088 (4.79%) | 1,652,691 (4.98%) | 1,614,693 (4.98%) |
| Multiple Sclerosis and Transverse Myelitis | 270,947 (0.80%) | 270,786 (0.82%) | 263,835 (0.81%) |
| Muscular Dystrophy | 36,777 (0.11%) | 35,805 (0.11%) | 34,279 (0.11%) |
| Spina Bifida and Related | 118,898 (0.35%) | 121,339 (0.37%) | 120,887 (0.37%) |
| Spinal Cord Injury | 338,996 (1.00%) | 493,226 (1.49%) | 510,026 (1.57%) |
| Traumatic Brain Injury and Related | 416,176 (1.22%) | 415,889 (1.25%) | 399,972 (1.23%) |
| Mental & Behavioral Health Condition Categories | |||
| ADHD and Conduct Disorders | 641,561 (1.89%) | 699,483 (2.11%) | 694,156 (2.14%) |
| Alcohol Use Disorders | 1,574,802 (4.63%) | 1,575,874 (4.75%) | 1,522,581 (4.70%) |
| Anxiety Disorders | 8,396,286 (24.71%) | 9,241,457 (27.86%) | 9,230,929 (28.48%) |
| Bipolar Disorder | 1,913,515 (5.63%) | 1,948,395 (5.87%) | 1,894,114 (5.84%) |
| Major Depressive Disorder | 11,406,342 (33.57%) | 11,517,759 (34.72%) | 11,218,727 (34.62%) |
| Opioid use disorder (OUD) | 1,072,087 (3.15%) | 1,385,319 (4.18%) | 1,374,591 (4.24%) |
| Personality Disorders | 787,064 (2.32%) | 989,706 (2.98%) | 983,502 (3.03%) |
| Post-Traumatic Stress Disorder | 665,112 (1.96%) | 792,477 (2.39%) | 796,938 (2.46%) |
| Psychotic Disorders (Non-Schizophrenic) | 1,159,917 (3.41%) | 871,596 (2.63%) | 792,943 (2.45%) |
| Schizophrenia | 890,503 (2.62%) | 832,516 (2.51%) | 790,317 (2.44%) |
| Other Condition Categories | |||
| Chronic Pain/Fatigue, Fibromyalgia | 9,549,362(28.1%) | 11,678,963 (35.21%) | 11,750,977 (36.26%) |
| HIV/AIDS | 151,067(0.44%) | 138,190 (0.42%) | 130,541 (0.40%) |
| Migraine and Chronic Headache | 1,917,095(5.64%) | 2,331,958 (7.03%) | 2,370,649 (7.32%) |
| Obesity | 7,623,228(22.43%) | 9,802,605 (29.55%) | 9,838,276 (30.36%) |
| Sickle Cell Disease | 17,656(0.05%) | 16,470 (0.05%) | 15,667 (0.05%) |
| Tobacco Use Disorders | 4,856,757(14.29%) | 5,090,674 (15.35%) | 4,866,829 (15.02%) |
| Viral Hepatitis (General) | 686,672(2.02%) | 686,403 (2.07%) | 654,631 (2.02%) |
∼ Due to cell size suppression requirements, those Medicare beneficiaries with missing sex values in 2019 and 2020 were combined with the female sex group, summing to 12 total observations across the two years.
# Condition categories are not mutually exclusive at the beneficiary level, therefore summing is not appropriate.
^ Percentages for sex-specific conditions were calculated using appropriate sex-specific denominators.
Because breast cancer prevalence in males is less than 1% (47), percentages were calculated using the denominator for females. The percentages with breast cancer among combined female and male Medicare beneficiaries in this sample for 2016, 2019 and 2020 are respectively: 5.00%, 5.19%, 5.26%.
For illustration purposes, we sorted condition categories into four groups according to each condition category's development phase in the CCW [44]: “age-related” [i.e., heart, lung and cancer]; “mental and behavioral health” [i.e., psychiatric and substance use disorders; “disability-related” [i.e., cognitive, intellectual, developmental, physical]; and “other health conditions” [e.g., pain-related conditions, HIV/AIDS, hepatitis]). Exceptions to this logic were made in cases where a condition category fell more rationally into a different category than that of its CCW development phase (e.g., leukemia/lymphoma [cancer-related] and peripheral vascular disease were moved from “other” to “age-related”; alcohol use disorders and opioid use disorders were moved from “other” to “mental and behavioral health”). Note that the quantitative results were not affected by grouping decisions because statistics were not generated at the group-level.
2.3. Statistical analyses
For the first analytic component, we used the combined managed-care and fee-for-service populations to present annual all-cause mortality trends from 2009 through 2020. We also estimated the number of above-expected (i.e., “excess”) deaths from any cause in the first year of the COVID-19 pandemic (2020) based on the proportion of deaths due to any cause in 2019.
For the second component we used bivariate logistic regression models to generate odds ratios (ORs) and 95% confidence intervals (CIs) that quantify and compare relative changes in all-cause mortality across different subpopulations, between 2016 and 2019, and likewise between 2019 and 2020. Similar to percentage change, this method of measuring all-cause mortality in one year, relative to a prior year (e.g., 2020 versus 2019), separately for each subpopulation, allowed to assess and compare the subpopulation-specific impacts of time on all-cause mortality. The regression model is:
where P(Yi) is the probability of death for case i; β0 is the y intercept; β1 is the slope of the regression line; and Xi is the observation year (e.g., {2019, relative to 2016} and {2020, relative to 2019}) for case, i.
Separate models were run for each of the sociodemographic and condition-related variables included in the study. Note that, because the fee-for-service Parts A- and B-enrolled study sample encompasses the entire fee-for-service Parts A- and B-enrolled population, there should be no uncertainty in our estimates and therefore the confidence intervals should theoretically equal the odds ratios themselves. However, because Medicare's different demographic and health-condition subpopulations are not always equally engaged in, or able to participate fully in, their health care [45], in actuality, our confidence intervals reflect a small degree of uncertainty in our subpopulation estimates.
We conducted analyses using SAS Enterprise Guide 7.1 with data extracted in June 2022. This observational, secondary research study made use of routinely collected, deidentified, publicly available Medicare claims data, with findings reported in accordance with the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) [46,47]. Results are portrayed tabularly and graphically.
3. Results
3.1. All-cause mortality trends and excess deaths
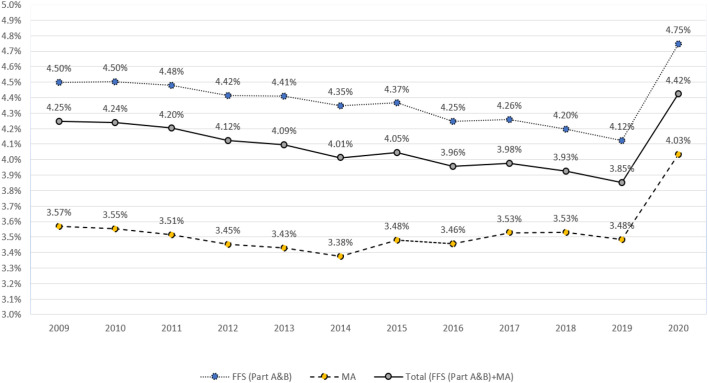
In 2020, the first year of the COVID-19 pandemic, 4.4% of Medicare's total 59,173,438 fee-for-service and managed-care beneficiaries died from any cause (Fig. 1 ), representing a one-year absolute increase of 0.6 percentage points, and a 15.5% relative increase (OR, 1.155 [95% CI 1.153 to 1.157]). These deaths include an estimated 338,494 excess deaths in 2020, above what we would have expected based on the proportion of deaths due to any cause in 2019. Over time, all-cause mortality has been consistently higher for those enrolled in fee-for-service versus Medicare Advantage, and the 2019-to-2020 increase was slightly greater, at 0.63 versus 0.55 percentage points, respectively.
Fig. 1.
Annual All-Cause Mortality (%) among United States Population of Beneficiaries Enrolled in Medicare Advantage (MA) and Medicare Fee-for-Service (FFS) Parts A and B (2009–2020)*^.
* The odds-ratio changes in all-cause mortality were statistically significant for all three groups, between 2009 and 2019 for FFS (OR, 0.991 [95% CI 0.991 to 0.991]), MA (OR, 0.998 [95% CI 0.997 to 0.998]), and FFS + MA (OR, 0.990 [95% CI 0.990 to 0.990]); and between 2019 and 2020 for FFS (OR, 1.159 [95% CI 1.156 to 1.162]), MA (OR, 1.164 [95% CI 1.161 to 1.167]), and FFS + MA (OR, 1.155 [95% CI 1.153 to 1.157]).
3.2. Relative change in all-cause mortality
In 2020, 54.8% (32,407,844) of Medicare beneficiaries were enrolled exclusively in fee-for-service Parts A and B. In this predominantly Non-Hispanic White, Medicare fee-for-service population, there was an overall 1% decline in all-cause mortality between 2009 and 2019 (OR, 0.991 [95% CI 0.991 to 0.991]). As shown in Fig. 1, this downward trend then reversed course in 2020, spiking by 15.9% in just one year (OR, 1.159 [95% CI, 1.156 to 1.162]). This 2019-to-2020 relative change was much greater than all of the other relative year-to-year increases over the prior decade, the largest of which was 2014-to-2015 (OR, 1.005 [95% CI, 1.002 to 1.007]). (Data not shown.)
3.3. Relative changes in all-cause mortality by beneficiary subpopulations
3.3.1. Health conditions
3.3.1.1. Pandemic-related changes (i.e., 2020 versus 2019)
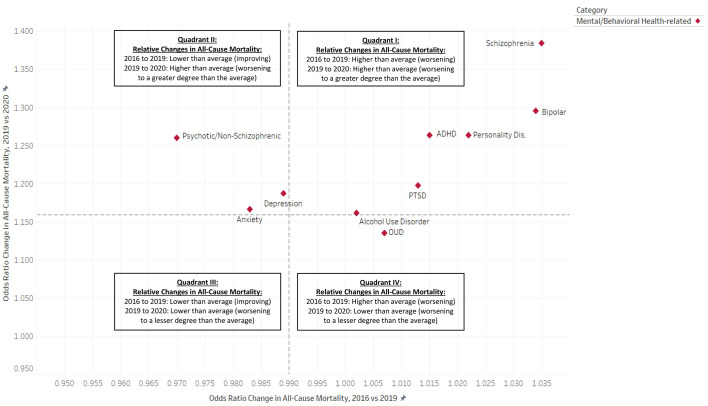
Fig. 2 displays the relative change results for psychiatric conditions only (Panel A), and for all 61 condition categories (Panel B). The conditions found above the horizontal line were most impacted by year-one of the pandemic in terms of changing annual all-cause mortality. Of all conditions studied, the most-impacted conditions were mental health and disability-related conditions, specifically: schizophrenia (OR, 1.384 [95% CI, 1.367 to 1.402]), developmental delays (OR, 1.337 [95% CI, 1.291 to 1.385]), Alzheimer's disease (OR, 1.320 [95% CI, 1.313 to 1.327]), intellectual disabilities (OR, 1.312 [95% CI, 1.289 to 1.336]), autism spectrum disorders (OR, 1.307 [95% CI, 1.245 to 1.373]), cerebral palsy (OR, 1.301 [95% CI, 1.262 to 1.341]), bipolar disorder (OR, 1.295 [95% CI, 1.285 to 1.305]), learning disabilities (OR, 1.285 [95% CI, 1.256 to 1.314]), personality disorders (OR, 1.263 [95% CI, 1.248 to 1.277]), ADHD and conduct disorder (OR, 1.263 [95% CI, 1.246 to 1.280]), and non-schizophrenic psychotic disorders (OR, 1.260 [95% CI, 1.250 to 1.270]).
Fig. 2.
(Panel A). Relative Pre-Pandemic (2016–2019) and Post-Pandemic (2009–2020) Changes in Annual All-Cause Mortality (Odds Ratio), among United States Population of Beneficiaries Enrolled in Medicare Fee-for-Service (FFS) Parts A and B, by Mental and Behavioral Health Condition Categories*#.
* Dashed lines signify population averages.
# Odds ratios greater than 1.000 signify increasing (i.e., worsening) all-cause mortality. Odds ratios less than 1.000 signify declining (i.e., improving) all-cause mortality.
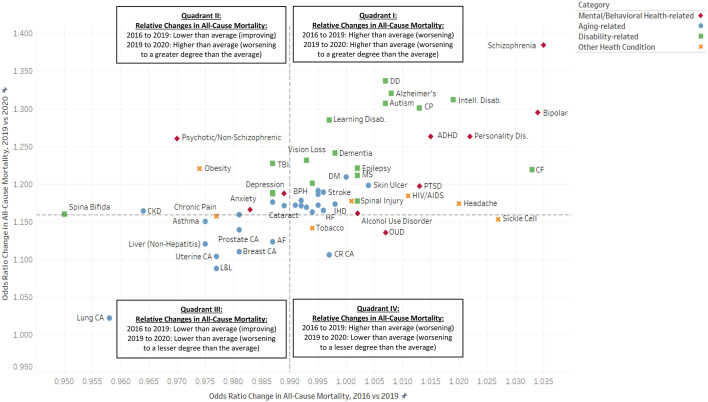
(Panel B). Relative Pre-Pandemic (2016–2019) and Post-Pandemic (2009–2020) Changes in Annual All-Cause Mortality (Odds Ratio), among United States Population of Beneficiaries Enrolled in Medicare Fee-for-Service (FFS) Parts A and B, by Mental and Behavioral Health, Aging-Related, Disability-Related and Other Health-Condition Categories*#^.
* Dashed lines signify population averages.
# Odds ratios greater than 1.000 signify increasing (i.e., worsening) all-cause mortality. Odds ratios less than 1.000 signify declining (i.e., improving) all-cause mortality.
^ Due to space limitations, this chart does not include all condition categories or all condition category full labels. The data for all condition categories, as well as the definitions for all abbreviated condition categories, are provided in Table 2.
3.3.1.2. Pre-pandemic changes (i.e., 2019 versus 2016)
Even though all-cause mortality had been declining for the population as a whole prior to the pandemic (OR, 0.990 [95% CI, 0.989 to 0.991]), we found that this was not uniform for all groups. Fig. 2, Panels A and B shows – to the right of the vertical axis – those conditions with worsening pre-pandemic all-cause mortality. This study uncovered a trend in which many of the health-condition categories that exhibited worsening, rather than improving, all-cause mortality prior to the pandemic also had above-average all-cause mortality increases between 2019 and 2020. This phenomenon is visible in upper right quadrant (i.e., Quadrant I) of both panels of Fig. 2, with the most pronounced manifestations being psychiatric and disability-related conditions, specifically: schizophrenia, developmental delays, Alzheimer's disease, intellectual disabilities, autism spectrum disorders, cerebral palsy, bipolar disorder, personality disorders, ADHD and conduct disorder, and cystic fibrosis. Of all conditions studied, the impact was the greatest for subpopulation of individuals with schizophrenia, for whom all-cause mortality was a relative 38.4% greater in 2020 than in 2019 (OR = 1.384, 95% [CI 1.367, 1.402]), after having also increased 3.5% (OR 1.035, 95% CI [1.031, 1.040]) in the four years prior to the pandemic. Quadrant I also includes the skin-ulcer condition, which, in spite of having been pre-categorized as age-related, can also be disabling; moreover, mobility-related disabilities (e.g., spinal cord injury) can increase the risk of developing pressure-ulcers, especially in the context of poor nursing care [48,49].
In contrast, some condition categories demonstrated both improving pre-pandemic all-cause mortality, and lower-than-average changes in the pandemic's first year. These conditions are found in the lower left quadrant (i.e., Quadrant III) of both panels of Fig. 2 and include all types of cancers studied, as well as liver disease and cirrhosis of the liver. Lung cancer was the most extreme example of this finding, and had the lowest change in all-cause mortality in the pandemic's first year (OR, 1.022 [95% CI, 1.013 to 1.032]) of all conditions studied.
3.3.2. Sociodemographic characteristics
3.3.2.1. Pandemic-related changes (i.e., 2020 versus 2019)
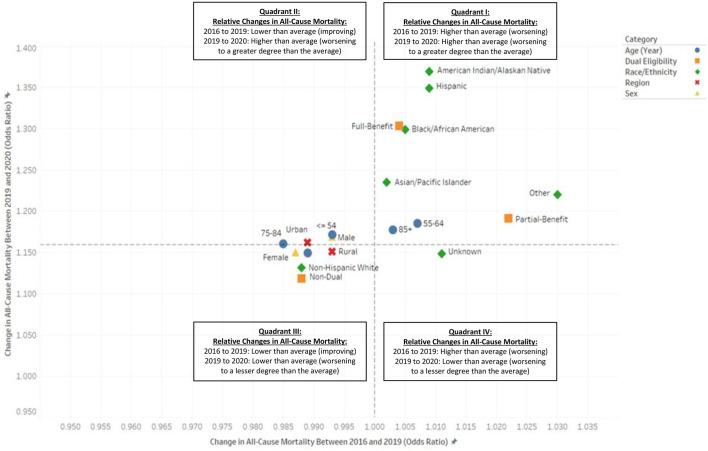
With the COVID-19 pandemic, one-year all-cause mortality grew by a relative 36.9% among American Indian and Alaska Native (AIAN) (OR, 1.369 [95% CI, 1.330 to 1.410]); 34.9% among Hispanic (OR, 1.349 [95% CI, 1.330 to 1.362]); 29.9% among Black/African American (OR, 1.299 [95% CI, 1.289 to 1.309]); 23.5% among Asian and Pacific Islander (API) (OR, 1.235 [95% CI, 1.215 to 1.255]); and 22.0% among “Other” beneficiary populations based on this social construct (OR, 1.220 [95% CI, 1.185 to 1.257]) (Table 3 ). Taken together, among all groups representing people of color, all-cause mortality was a relative 30.1% greater in 2020 than it had been in 2019 (OR, 1.301 [95% CI, 1.294 to 1.308]). (Data not shown.) This is over double the relative 13.1% increase experienced by the Non-Hispanic White beneficiary group (OR, 1.131 [95% CI, 1.128 to 1.134]).
Table 3.
Relative Differences in All-Cause Mortality, by Sociodemographic Characteristic, within the Study Population of Beneficiaries Enrolled in Medicare Fee-For-Service (FFS) Parts A and B: 2019 vs. 2016 and 2020 vs. 2019.
| Sociodemographic Characteristic | Sociodemographic Category | Change in all-cause mortality (2019 vs 2016) OR (95% CI) | Change in all-cause mortality (2020 vs 2019) OR (95% CI) |
|---|---|---|---|
| Total FFS Parts A and B Enrolled Study Population | 0.990 (0.989, 0.991) | 1.159 (1.156, 1.620) | |
| American Indian/Alaskan Native | Race & Ethnicity | 1.009 (0.999, 1.019) | 1.369 (1.330, 1.410) |
| Asian/Pacific Islander | Race & Ethnicity | 1.002 (0.997, 1.008) | 1.235 (1.215, 1.255) |
| Non-Hispanic White | Race & Ethnicity | 0.988 (0.987, 0.989) | 1.131 (1.128, 1.134) |
| Black/African American | Race & Ethnicity | 1.005 (1.002, 1.007) | 1.299 (1.289, 1.309) |
| Hispanic | Race & Ethnicity | 1.009 (1.005, 1.012) | 1.349 (1.330, 1.362) |
| Other | Race & Ethnicity | 1.030 (1.019, 1.041) | 1.220 (1.185, 1.257) |
| Unknown/Missing | Race & Ethnicity | 1.011 (1.000, 1.023) | 1.148 (1.116, 1.181) |
| ≤ 54 | Age group | 0.993 (0.989, 0.998) | 1.171 (1.155, 1.188) |
| 55–64 | Age group | 1.007 (1.004, 1.010) | 1.185 (1.173, 1.197) |
| 65–74 | Age group | 0.989 (0.987, 0.990) | 1.149 (1.143, 1.155) |
| 75–84 | Age group | 0.985 (0.983, 0.986) | 1.160 (1.155, 1.165) |
| 85+ | Age group | 1.003 (1.002, 1.005) | 1.177 (1.173, 1.182) |
| Unknown/Missing | Age group | ||
| Female | Sex | 0.987 (0.986, 0.988) | 1.150 (1.146, 1.154) |
| Male | Sex | 0.993 (0.992, 0.994) | 1.169 (1.165, 1.173) |
| Unknown/Missing | Sex | ||
| Full-benefit | Dual status | 1.004 (1.002, 1.006) | 1.303 (1.297, 1.309) |
| Partial-benefit | Dual status | 1.022 (1.018, 1.026) | 1.191 (1.178, 1.205) |
| Non-dual | Dual status | 0.988 (0.987, 0.989) | 1.118 (1.115, 1.121) |
| Rural | Region | 0.993 (0.992, 0.995) | 1.151 (1.146, 1.157) |
| Urban | Region | 0.989 (0.988, 0.990) | 1.162 (1.159, 1.165) |
Between 2019 and 2020, all-cause mortality was up by 30.3% (OR, 1.303 [95% CI, 1.297 to 1.309]) and 19.1% (OR, 1.191 [95% CI, 1.178 to 1.205]) among full-benefit and partial-benefit lower-income, dually eligible beneficiaries, respectively. This is in contrast to the lower-than-average 11.8% increase for Medicare beneficiaries who did not have concomitant Medicaid eligibility (OR, 1.118 [95% CI, 1.115 to 1.121]).
Regarding age, we found the highest 2019-to-2020 increases in all-cause mortality among fee-for-service Medicare's most senior beneficiaries, ages 85 and older (OR, 1.177 [95% CI, 1.173 to 1.182]), and among those younger than 65 and thus eligible for Medicare predominately on the basis of disability (ages 55 and younger: OR, 1.171 [95% CI, 1.155 to 1.188]; ages 55 to 64: OR, 1.185 [95% CI, 1.173 to 1.197]). Relative changes in all-cause mortality were slightly below average for females (OR, 1.150 [95% CI, 1.146 to 1.154]) and rural-dwelling individuals (OR, 1.151 [95% CI, 1.146 to 1.157]) and slightly above average for males (OR, 1.169 [95% CI, 1.165 to 1.173]) and urban-dwelling individuals (OR, 1.162 [95% CI, 1.159 to 1.165]).
3.3.2.2. Pre-pandemic changes (i.e., 2019 versus 2016)
Similar to the health-conditions findings, the overall pre-pandemic declines in all-cause mortality were not uniform across Medicare's different sociodemographic subpopulations. In fact, 2016-to-2019 all-cause mortality had actually been worsening for all race and ethnicity groups except the Non-Hispanic White group, as well as for low-income individuals with Medicare-Medicaid dual eligibility (Table 3). Moreover, in plotting the relative changes in all-cause mortality from before the pandemic (i.e., 2019 versus 2016) against those occurring in its first year (i.e., 2020 versus 2019), we observed a pattern similar to that found for health-condition categories (Fig. 3 ). The upper-right quadrant (i.e., Quadrant I) of Fig. 3, shows that many of the sociodemographic characteristics with worsening all-cause mortality prior to the pandemic also demonstrated higher-than-average worsening in 2020 (i.e., AIAN, Hispanic, Black/African American, API, and dual-eligible). In contrast, the bottom left corner (i.e., Quadrant III) of the same figure reveals that those subpopulations with the lowest pandemic-related increases in 2020, most notably Non-Hispanic White beneficiaries and those not dually eligible for Medicaid, had improving all-cause mortality prior to the pandemic.
Fig. 3.
Relative Pre-Pandemic (2016–2019) and Post-Pandemic (2009–2020) Changes in Annual All-Cause Mortality (Odds-Ratio) among United States Population of Beneficiaries Enrolled in Medicare Fee-for-Service (FFS) Parts A and B, by Sociodemographic Characteristics*#.
* Dashed lines signify population averages.
# Odds ratios greater than 1.000 signify increasing (i.e., worsening) all-cause mortality. Odds ratios less than 1.000 signify declining (i.e., improving) all-cause mortality.
4. Discussion
In this observational study of individuals enrolled in Medicare Parts A and B, we found large differences in the direction and/or magnitude of changes in all-cause mortality across a number of subpopulations, both prior-to and in year-one of the COVID-19 pandemic. The greatest differences were by race and ethnicity, dual eligibility and certain health-condition categories. For example, despite overall, population-wide improvements in all-cause mortality between 2016 and 2019, all-cause mortality had been worsening for individuals of color during this same timeframe. Then, in 2020, all-cause mortality for beneficiaries of color jumped by a relative 30.1%, versus 13.1% among Non-Hispanic White beneficiaries. Likewise, lower-income Medicare-Medicaid dually eligible beneficiaries experienced pre-pandemic increases in all-cause mortality, and pandemic-related increases that were significantly larger than the population average, while the majority group of non-dually eligible Medicare beneficiaries had declining pre-pandemic all-cause mortality, followed by an uptick in 2020 that was lower than the population average.
The health-conditions analysis found that beneficiaries with certain psychiatric and disability-related conditions experienced the very greatest increases in all-cause mortality out of all 61 health conditions studied. In addition, this analysis displayed a similar pattern as the sociodemographic characteristics in that virtually all of the chronic health conditions with worsening pre-pandemic mortality also had above-average spikes in the pandemic's first year (Fig. 2, Quadrant I). These conditions were generally those related to mental and behavioral health and disability, with the most pronounced being among Medicare beneficiaries with schizophrenia, developmental delays, Alzheimer's disease, intellectual disabilities, autism spectrum disorders, cerebral palsy, bipolar disorder, personality disorders, ADHD and conduct disorder, and cystic fibrosis. Conversely, many of the conditions with improving pre-pandemic all-cause mortality had below-average increases in 2020. Given these findings, it is evident that viewing the pandemic-associated changes in all-cause mortality in the context of preexisting trends contributes an important and unique depth of analysis to the existing literature.
In addition to depth of analysis, this study contributes a unique breadth of analysis to the literature. Specifically, the comprehensive, population-level fee-for-service Medicare claims data afforded us the power to include a wide array of beneficiary characteristics, including the less populous ones. This broad view allowed us to identify those sociodemographic and clinical characteristics that convey the greatest vulnerability, including those lower in frequency whose true effects would have remained undetected in reports of overall population-level statistics. As such, our study was able to overcome the common, but perilous, limitation of studies that present findings only for the full study sample or study population, as well as those that include common health conditions but omit conditions of lower prevalence, often psychiatric and disability-related [[2], [3], [4], [5], [6]]. Thus, with a wide array of beneficiary characteristics, this study was equipped to identify the most vulnerable subpopulations in fee-for-service Medicare.
Given the novel and evolving nature of the virus, the all-cause mortality measure was ideal in elucidating the overall toll of the pandemic on human life, as it was able to capture both direct and indirect effects of the COVID-19 pandemic. Equally important, our focus on measuring the relative changes in all-cause mortality across two points in time, for different subpopulations, rather than simply studying cross-sectional mortality-related risk factors in 2020, allowed us to identify subpopulation-specific trajectories prior to the pandemic, and additionally, the impact that the pandemic may have had on these trajectories. Measures of relative change between two points in time hold constant the factors that typically affect all-cause mortality within each specific subpopulation, year over year, thereby isolating the impact of time itself on the outcome and facilitating comparisons between subpopulations.
Despite these advantages, our study does have some limitations. While we believe that the majority of our 2019-to-2020 odds-ratio change results are attributable to the COVID-19 pandemic [6], the possibility exists that non-pandemic-related factors played a change-exacerbating or change-attenuating role for any of our subpopulations. An example might be the rapid introduction of a non-COVID-19-related diagnostic tool, treatment or technology for a chronic condition in 2020. To our knowledge, however, no such sweeping medical advancements were introduced in 2020 for any of the condition categories included in our study. That said, our finding that lung cancer demonstrated the smallest increase in 2019-to-2020 all-cause mortality may be an artifact of its well-documented declining mortality and increasing survival in the general U.S. population over the last two decades resulting from advancements in targeted immunotherapies [50,51]. We similarly found all-cause mortality among beneficiaries with lung cancer to have declined by 4.2% between 2016 and 2019 (OR, 0.958 [95% CI, 0.956 to 0.961]). If this downward trend had continued naturally into 2020, as expected, this may have served to counterbalance the forces of the COVID pandemic on our lung-cancer findings. As a result, while it may appear as though the pandemic only mildly affected the lung-cancer population, its true impact on this population is unknown. Moreover, it was beyond our scope to ascertain why the pandemic impacted the different conditions differently.
One constraining factor in this study is that the only available race or ethnicity variable for the Medicare fee-for-service population was an SSA-sourced, already imperfect variable that combines both of these social constructs into one variable [52,53], an amalgamation that fails to account for individuals who are bi- or multi-racial and/or bi- or multi-ethnic. In spite of this unavoidable limitation, our findings corroborate other reports of racial and ethnic disparities in all-cause mortality in the COVID-19 pandemic era [[31], [32], [33], [34], [35]].
Another limitation is that the observed effects associated with any given subpopulation may have been driven by characteristics of subgroups within that subpopulation. Three examples of this could include: 1) the subgroup of individuals residing in a residential care setting [unmeasurable with our data] among all individuals with an intellectual disability; 2) the subgroup of individuals with two or more comorbid conditions [e.g., intellectual disability and schizophrenia] versus just one of these conditions; 3) and the various race and ethnicity subgroups among people with mental and behavioral health and disability-related conditions. In addition, because condition categories do not include details on prognosis, remission-status, treatment-engagement, etc., there may be some unobserved clinical variability within and between the condition categories. Finally, while the use of a relative measure has many advantages, large relative changes may be associated with small, absolute population-level differences, and small relative changes may be associated with large absolute population-level differences, depending on subpopulation size.
This analysis quantified changes in all-cause mortality by different subpopulations and identified those with evidence of concerning trajectories (i.e., individuals of color, dually eligible beneficiaries, and patients with psychiatric and disability-related conditions). Some of these subpopulation characteristics may have a disproportionate institutional component (e.g., intermediate-care facility for individuals with intellectual disabilities, residential substance abuse treatment facility, psychiatric residential treatment center, etc.). For example, one study found that 27.7% of persons with intellectual and other related disabilities live in a residential facility or nursing home [54]. We unfortunately did not have the ability to study residential setting in this population-based study because Medicare administrative data lacks this information for all beneficiaries. However, future investigations using other data sources should explore the potential role of institutional residence on all-cause mortality and other outcomes and identify the underlying, modifiable factors (e.g., staffing and care delivery in different residential care settings) within these groups, and their intersections, that could ultimately be the targets of future public health improvement policies and programs.
In conclusion, our study highlights the vastly different experiences of Medicare's different sociodemographic and health-condition subpopulations. Our findings shed light on Medicare's more vulnerable beneficiary subgroups, both large and small, whose worsening pre-pandemic, and elevated pandemic-related all-cause mortality outcomes would have otherwise gone undetected in a study focusing solely on the population as a whole. In addition, our findings contribute a unique depth of analysis by presenting pandemic-associated changes in all-cause mortality in the context of preexisting trends. The unique subpopulation vulnerabilities revealed by our study signify a need for additional research to better understand the factors contributing to these disparities, as well as the timely development of appropriate and effective policies and programs to address them in the context of the ever-evolving COVID-19 pandemic as well as future epidemics.
CRediT authorship contribution statement
Karyn Kai Anderson: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Project administration. Sha Maresh: Methodology, Data curation, Formal analysis. Andrew Ward: Supervision, Writing – review & editing. Elizabeth A. Koller: Writing – review & editing. Philip Connor: Software, Validation. Melissa Evans: Writing – review & editing. Zippora Kiptanui: Project administration, Writing – review & editing, Visualization. Meghana M. Raja: Writing – review & editing, Visualization, Validation. Serena Thomas: Writing – review & editing, Visualization, Validation. Thomas Wolfe: Project administration. Christine S. Gill: Methodology, Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors of this manuscript have no conflicts of interest, activities or relationships – financial or otherwise – to declare.
Acknowledgments
Lee A. Fleisher, M.D., Director of the Centers for Clinical Standards and Quality, Centers for Medicare and Medicaid Services.
Shari M. Ling, M.D., Deputy Chief Medical Officer, Centers for Medicare and Medicaid Services. Tamara Syrek Jensen, J.D., Director of the Coverage and Analysis Group, Centers for Clinical Standards and Quality, Centers for Medicare and Medicaid Services.
Data availability
Data will be made available on request.
References
- 1.Centers for Disease Control and Prevention Basics of COVID-19. Updated November 4, 2021. https://www.cdc.gov/coronavirus/2019-ncov/your-health/about-covid-19/basics-covid-19.html Accessed September 18, 2022.
- 2.Tromans S., Kinney M., Chester V., et al. Priority concerns for people with intellectual and developmental disabilities during the COVID-19 pandemic. BJPsych Open. 2020 Oct 29;6(6) doi: 10.1192/bjo.2020.122. PMID: 33118913; PMCID: PMC7609203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Immunization and Respiratory Diseases (NCIRD) DoVD Science brief: evidence used to update the list of underlying medical conditions associated with higher risk for severe covid-19. 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html Accessed September 18, 2022. [PubMed]
- 4.Tarazi W.W., Finegold K., Sheingold S.H., Wong Samson L., Zuckerman R., Bosworth A. COVID-19-related deaths and excess deaths among Medicare fee-for-service beneficiaries. Health Aff (Millwood) 2021 Jun;40(6):879–885. doi: 10.1377/hlthaff.2020.02521. [PMID: 34097514] [DOI] [PubMed] [Google Scholar]
- 5.Clark A., Jit M., Warren-Gash C., et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020 Aug;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. Epub 2020 Jun 15. PMID: 32553130; PMCID: PMC7295519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf S.H., Chapman D.A., Sabo R.T., Weinberger D.M., Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020 Aug 4;324(5):510–513. doi: 10.1001/jama.2020.11787. PMCID: PMC7330820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langmuir A.D., Schoenbaum S.C. The epidemiology of influenza. Hosp Pract. 1976 Oct;11(10):49–56. doi: 10.1080/21548331.1976.11707011. PMID: 67988. [DOI] [PubMed] [Google Scholar]
- 8.Sabin A.B. Mortality from pneumonia and risk conditions during influenza epidemics. High influenza morbidity during nonepidemic years. JAMA. 1977 Jun 27;237(26):2823–2828. [PMID: 577246] [PubMed] [Google Scholar]
- 9.Kruse-Thomas K. Pandemic deaths are almost impossible to count. He invented a way to estimate them. The Johns Hopkins Bloomberg school of public health expert insights. May 15, 2020. https://www.jhsph.edu/covid-19/articles/pandemic-deaths-are-almost-impossible-to-count-he-invented-a-way-to-estimate-them.html Accessed September 18, 2022.
- 10.National Center for Health Statistics . Centers for Disease Control and Prevention; 2023. Excess deaths associated with COVID-19. Provisional death counts for coronavirus disease (COVID-19)https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm Accessed September 18, 2022. [Google Scholar]
- 11.Magnani C., Azzolina D., Gallo E., Ferrante D., Gregori D. How large was the mortality increase directly and indirectly caused by the COVID-19 epidemic? An analysis on all-causes mortality data in Italy. Int J Environ Res Public Health. 2020 May 15;17(10):3452. doi: 10.3390/ijerph17103452. PMID: 32429172; PMCID: PMC7277828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossen L.M., Branum A.M., Ahmad F.B., Sutton P., Anderson R.N. Excess deaths associated with COVID-19, by age and race and ethnicity - United States, January 26-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020 Oct 23;69(42):1522–1527. doi: 10.15585/mmwr.mm6942e2. PMID: 33090978; PMCID: PMC7583499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown T.S., Walensky R.P. Serosurveillance and the COVID-19 epidemic in the US: undetected, uncertain, and out of control. JAMA. 2020 Aug 25;324(8):749–751. doi: 10.1001/jama.2020.14017. [PMID: 32692350] [DOI] [PubMed] [Google Scholar]
- 14.Zheng K.I., Feng G., Liu W.Y., Targher G., Byrne C.D., Zheng M.H. Extrapulmonary complications of COVID-19: a multisystem disease? J Med Virol. 2021 Jan;93(1):323–335. doi: 10.1002/jmv.26294. Epub 2020 Jul 22. PMID: 32648973; PMCID: PMC7405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanova E.S., Vasilyev V.V., Startseva G., Karev V., Rybakova M.G., Platonov P.G. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J Intern Med. 2021 Sep;290(3):655–665. doi: 10.1111/joim.13300. Epub 2021 May 20. PMID: 33872433; PMCID: PMC8250818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen van Vuren E., Steyn S.F., Brink C.B., Möller M., Viljoen F.P., Harvey B.H. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed Pharmacother. 2021 Mar;135:111200. doi: 10.1016/j.biopha.2020.111200. Epub 2021 Jan 1. PMID: 33421734; PMCID: PMC7834135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V., Clarke J., Beaney T. Association between democratic governance and excess mortality during the COVID-19 pandemic: an observational study. J Epidemiol Community Health. 2022 Jun 29 doi: 10.1136/jech-2022-218920. jech-2022-218920. Epub ahead of print. PMID: 35768188; PMCID: PMC9271843. [DOI] [PubMed] [Google Scholar]
- 18.Findling M.G., Blendon R.J., Benson J.M. Delayed care with harmful health consequences—reported experiences from national surveys during coronavirus disease 2019. JAMA Health Forum. 2020;1(12) doi: 10.1001/jamahealthforum.2020.1463. [DOI] [PubMed] [Google Scholar]
- 19.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020 Aug;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. Epub 2020 Jul 20. Erratum in: Lancet Oncol. 2021 Jan;22(1):e5. PMID: 32702310; PMCID: PMC7417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masroor S. Collateral damage of COVID-19 pandemic: delayed medical care. J Card Surg. 2020 Jun;35(6):1345–1347. doi: 10.1111/jocs.14638. Epub 2020 May 17. PMID: 32419177; PMCID: PMC7276840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y., Li H., Luo G., et al. Antiretroviral treatment interruption among people living with HIV during COVID-19 outbreak in China: a nationwide cross-sectional study. J Int AIDS Soc. 2020 Nov;23(11) doi: 10.1002/jia2.25637. PMID: 33247541; PMCID: PMC7645858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Şen Doğan R., Deveci Şirin H. Death anxiety and satisfaction with life among the adults in the social isolation process of Covid-19 pandemic: the mediating role of perceived stress. J Ment Health. 2022 Jun 30:1–10. doi: 10.1080/09638237.2022.2069689. Epub ahead of print. PMID: 35770825. [DOI] [PubMed] [Google Scholar]
- 23.Matthay E.C., Duchowny K.A., Riley A.R., Galea S. Projected all-cause deaths attributable to COVID-19-related unemployment in the United States. Am J Public Health. 2021 Apr;111(4):696–699. doi: 10.2105/AJPH.2020.306095. Epub 2021 Feb 18. PMID: 33600244; PMCID: PMC7958047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Keefe E.L., Torres-Acosta N., O’Keefe J.H., Sturgess J.E., Lavie C.J., Bybee K.A. Takotsubo syndrome: cardiotoxic stress in the COVID era. Mayo Clin Proc Innov Qual Outcome. 2020 Dec;4(6):775–785. doi: 10.1016/j.mayocpiqo.2020.08.008. Epub 2020 Nov 30. PMID: 33283161; PMCID: PMC7704068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petterson S., Westall J.M., Miller B.F. Projected deaths of despair from COVID-19. Providence St. Joseph health digital commons. May 8, 2020. https://digitalcommons.psjhealth.org/cgi/viewcontent.cgi?article=3991&context=publications
- 26.Czeisler M.É., Lane R.I., Petrosky E., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020 Aug 14;69(32):1049–1057. doi: 10.15585/mmwr.mm6932a1. PMID: 32790653; PMCID: PMC7440121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettman C.K., Abdalla S.M., Cohen G.H., Sampson L., Vivier P.M., Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020 Sep 1;3(9) doi: 10.1001/jamanetworkopen.2020.19686. PMID: 32876685; PMCID: PMC7489837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo Y.H., Zou B., Cheung R., Nguyen M.H. Increased mortality of patients with alcohol-related liver diseases during the COVID-19 pandemic in the United States. J Intern Med. 2022 Jul 22 doi: 10.1111/joim.13545. Epub ahead of print. PMID: 35869603; PMCID: PMC9350353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavova S., Rock P., Bush H.M., Quesinberry D., Walsh S.L. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend. 2020 Sep 1;214:108176. doi: 10.1016/j.drugalcdep.2020.108176. Epub 2020 Jul 10. PMID: 32717504; PMCID: PMC7351024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadhera R.K., Wang Y., Figueroa J.F., Dominici F., Yeh R.W., Joynt Maddox K.E. Mortality and hospitalizations for dually enrolled and nondually enrolled medicare beneficiaries aged 65 years or older, 2004 to 2017. JAMA. 2020 Mar 10;323(10):961–969. doi: 10.1001/jama.2020.1021. PMID: 32154858; PMCID: PMC7064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochieng N., Cubanski J., Neuman T., Artiga S., Damico A. Kaiser Family Foundation; Feb 16, 2021. Racial and ethnic health inequities and medicare.https://www.kff.org/medicare/report/racial-and-ethnic-health-inequities-and-medicare/ Accessed September 18, 2022. [Google Scholar]
- 32.Polyakova M., Udalova V., Kocks G., Genadek K., Finlay K., Finkelstein A.N. Racial disparities in excess all-cause mortality during the early COVID-19 pandemic varied substantially across states. Health Aff (Millwood) 2021 Feb;40(2):307–316. doi: 10.1377/hlthaff.2020.02142. PMID: 33523748; PMCID: PMC7996479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seligman B., Ferranna M., Bloom D.E. Social determinants of mortality from COVID-19: a simulation study using NHANES. PLoS Med. 2021 Jan 11;18(1) doi: 10.1371/journal.pmed.1003490. e1003490. Erratum in: PLoS Med. 2021 Dec 29;18(12):e1003888. PMID: 33428624; PMCID: PMC7799807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millett G.A., Jones A.T., Benkeser D., et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020 Jul;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. Epub 2020 May 14. PMID: 32419766; PMCID: PMC7224670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selden T.M., Berdahl T.A. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood) 2020 Sep;39(9):1624–1632. doi: 10.1377/hlthaff.2020.00897. Epub 2020 Jul 14. PMID: 32663045. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services Chronic conditions overview. Page last modified December 1, 2021. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions Accessed September 18, 2022.
- 37.Chronic Conditions Warehouse CCW white paper: impact of transition from 27 to 30 CCW chronic conditions. March 2022. https://gcc02.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww2.ccwdata.org%2Fdocuments%2F10280%2F19002256%2Fccw-condition-categories-impact-of-transition-from-27-to-30.pdf&data=05%7C01%7CKaryn.Anderson%40cms.hhs.gov%7C14e00a9ecf994c1344b708da5ace7e70%7Cd58addea50534a808499ba4d944910df%7C0%7C0%7C637922140346992516%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=tL%2FzKeeZWT3FrI1tksANVK%2FF3HV7vsZBu3Bh0C6VK3o%3D&reserved=0 Accessed September 18, 2022.
- 38.Research Data Assistance Center Death information in the research identifiable medicare data. University of Minnesota. Page last modified July 11, 2018. https://resdac.org/articles/death-information-research-identifiable-medicare-data Accessed September 18, 2022.
- 39.Research Data Assistance Center . University of Minnesota; 2023. Research triangle institute (RTI) race code.https://resdac.org/cms-data/variables/research-triangle-institute-rti-race-code#:~:text=Research%20Triangle%20Institute%20%28RTI%29%20Race%20Code%20%20,%20%20OTHER%20%203%20more%20rows%20 Accessed September 18, 2022. [Google Scholar]
- 40.Eicheldinger C., Bonito A. More accurate racial and ethnic codes for medicare administrative data. Health Care Financ Rev. 2008 Spring;29(3):27–42. PMID: 18567241; PMCID: PMC4195038. [PMC free article] [PubMed] [Google Scholar]
- 41.Healthcare Cost and Utilization Project Central Distributor SID: Description of Data Elements Agency for healthcare research and quality. https://www.hcup-us.ahrq.gov/db/vars/siddistnote.jsp?var=pl_ruca4_2005 Updated August 12, 2008. Accessed September 18, 2022.
- 42.Chronic Conditions Warehouse. CCW condition algorithms. June 2019. Page last modified June 2019. https://www2.ccwdata.org/documents/10280/19139421/other-condition-algorithms-reference-list.pdf Accessed September 18, 2022.
- 43.Chronic Conditions Data Warehouse Condition categories. Centers for Medicare and Medicaid Services. https://www2.ccwdata.org/web/guest/condition-categories Accessed September 18, 2022.
- 44.Chronic Conditions Warehouse Medicare-medicaid linked enrollees analytic data source (MMLEADS V2.0) user guide. June 2019. https://www2.ccwdata.org/documents/10280/19002246/mmleads-user-guide-v2-0.pdf Accessed September 18, 2022.
- 45.2021 national healthcare quality and disparities report. Agency for Healthcare Research and Quality; Rockville, MD: December 2021. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2021qdr.pdf AHRQ Pub. No. 21(22)-0054-EF. Accessed September 18, 2022. [PubMed] [Google Scholar]
- 46.Code of Federal Regulations Title V, Subtitle A, Subchapter A, Part 45, Subpart A §46.104(d)(4)(iv) https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46/subpart-A/section-46.104 Accessed September 18, 2022.
- 47.Benchimol E.I., Smeeth L., Guttmann A., et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015 Oct 6;12(10) doi: 10.1371/journal.pmed.1001885. PMID: 26440803; PMCID: PMC4595218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprigle S., McNair D., Sonenblum S. Pressure ulcer risk factors in persons with mobility-related disabilities. Adv Skin Wound Care. 2020 Mar;33(3):146–154. doi: 10.1097/01.ASW.0000653152.36482.7d. PMID: 32058440. [DOI] [PubMed] [Google Scholar]
- 49.Mäki-Turja-Rostedt S., Leino-Kilpi H., Korhonen T., Vahlberg T., Haavisto E. Consistent practice for pressure ulcer prevention in long-term older people care: a quasi-experimental intervention study. Scand J Caring Sci. 2021 Sep;35(3):962–978. doi: 10.1111/scs.12917. Epub 2020 Nov 8. PMID: 33164226. [DOI] [PubMed] [Google Scholar]
- 50.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020 Aug 13;383(7):640–649. doi: 10.1056/NEJMoa1916623. PMID: 32786189; PMCID: PMC8577315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Cancer Statistics Working Group . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2023. U.S. Cancer Statistics Data Visualizations Tool, based on 2021 submission data (1999–2019)www.cdc.gov/cancer/dataviz Released June 2022. Accessed September 18, 2022. [Google Scholar]
- 52.Martin P.A. Why researchers now rely on surveys for race data on OASDI and SSI programs: a comparison of four major surveys. Research and statistics note no. 2016–01. https://www.ssa.gov/policy/docs/rsnotes/rsn2016-01.html released January 2016.
- 53.Jarrín OF, Nyandege A.N., Grafova I.B., Dong X., Lin H. Validity of race and ethnicity codes in medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care. 2020 Jan;58(1):e1–e8. doi: 10.1097/MLR.0000000000001216. PMID: 31688554; PMCID: PMC6904433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esteban L., Navas P., Verdugo M.Á., Arias V.B. Community living, intellectual disability and extensive support needs: a rights-based approach to assessment and intervention. Int J Environ Res Public Health. 2021 Mar 19;18(6):3175. doi: 10.3390/ijerph18063175. PMID: 33808617; PMCID: PMC8003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.