Abstract
A robust, accurate, and standardized approach to measurement of the aorta is critical to improve the predictive accuracy of these aortic measurements, and to investigate other aortic imaging biomarkers. Developing a comprehensive and generic schema for characterization of the aorta to enable investigators to standardize data that are collected across all aorta research. A systematic review of the literature was conducted to identify and assess schemata of aortic measurement and description. The schemata were reported and discussed to guide the synthesis of a comprehensive schema. We propose the International College of Angiology Aortic Research Schema as a comprehensive design that fills the gaps left behind by previously reported schemata. It is intended to be applicable for all clinically relevant purposes, including endograft development for aneurysm repair and for the accurate characterization of the aortic anatomy. This schema divides the aorta into 14 segments and 2 sections (thoracic and abdominal aortas). The segmentation proposed can be used in addition to specific measurements taken for any aneurysm including the neck, and maximal and minimal diameters of the aneurysm.
Keywords: abdominal aortic aneurysm, endovascular repair, hyopgastric aneurysm, aortic dissection, vessel repair, thoracoabdominal aortic aneursym, repair
An aneurysm is defined as an increase in the diameter of a vessel despite gross preservation of the integrity of all three layers of the vessel wall. As the diameter of a vessel increases, the tensile strength of the vessel wall decreases, and the vessel becomes more prone to rupture. Consequently, aneurysms of the aorta are of particular concern due to the large diameter and high physiologic pressure within the vessel. Aortic aneurysm (AA) rupture remains a significant cause of morbidity and mortality in the United States. 1 2 The mortality of a ruptured abdominal aortic aneurysm approaches 90%. 3 Aortic aneurysms and dissections are ranked as the 15th leading cause of death in people above the age of 65 years. 4
Prior research has shown that aortic diameter and its rate of change correlates with the risk of aortic rupture. 5 More recent data are emphasizing the volume of the aneurysm rather than the absolute diameter as better predictive parameter for the risk of aortic rupture. 6 To improve the predictive accuracy of these aortic measurements and investigate other aortic imaging biomarkers, a robust, accurate, and standardized approach to measurement of the aorta is critical.
Three-dimensional (3D) postprocessing of imaging data can provide nuanced analysis of aortic morphology. Volume rendering, double oblique multiplanar reformatting, and curved planar reformatting along central lumen lines (CLL) are just a few of the advanced methods available to quantify aortic morphology. 7 Several descriptive schema have been proposed to characterize the aorta in an advanced fashion. By and large, these schemata aim to improve clinical decision-making and provide pre and perioperative guidance. The inability to generalize these findings to a broader patient population or research context limits their utility in developing universal standards for surgical planning. 8 No single existing schema is suited for comprehensive research including multivariate analyses to identify useful imaging biomarkers.
Comparison of data from aortic studies is limited by the fact that each existing schema describes and measures the aorta using different parameters. We believe that there is value in establishing a comprehensive and generic schema for characterization of the aorta to enable investigators to standardize data that are collected across all aorta research. For this communication, we identified and assessed schemata of aortic measurement and description and synthesize the best schema into a comprehensive schema, which we have named the International College of Angiology Aortic Research Schema (ICAARS).
Methods
The PRISMA record review workflow guided our investigation and assessment of the medical and scientific literature. 9 the PubMed database was were queried with multiple permutations of the following terms: “aorta” OR “aortic” AND “measurement” OR “morphology.” For the sake of practicality, studies prior to 1960 and non-English language records were excluded. The abstracts were screened for relevance to aortic measurement or description schemata and irrelevant records were discarded. The full-text articles corresponding to the abstracts deemed relevant to aortic measurement and description were retrieved. Those records not attainable online or via interlibrary loan were excluded. During the review, the following criteria were considered: number of citations, inclusiveness, logic, practicality of use, and societal endorsement. Based on these criteria, six clinical schemata, which are discussed and summarized below, were selected, and used as the foundational material for the drafting of a unified and comprehensive schema for measurement and description of aorta (ICAARS schema hereafter) by the investigative group.
Results
CE-CMRA Protocol
The schema proposed by Kaiser et al in children and then updated by Kawel-Boehm et al in adults subdivides the aorta into nine different zones of measurements and is commonly known as the contrast-enhanced cardiovascular magnetic resonance-angiography (CE-CMRA) protocol. As indicated in Fig. 1 , the diameter is measured at the aortic sinus (AS), sinotubular junction (STJ), ascending aorta at the level of the right pulmonary artery (AA), proximal to the brachiocephalic artery (BCA), first transverse segment (T1), second transverse segment (T2), isthmic region (IR), descending aorta at the level of the left pulmonary artery (DA), and the thoracoabdominal aorta at the level of diaphragm (D). 10 11
Fig. 1.
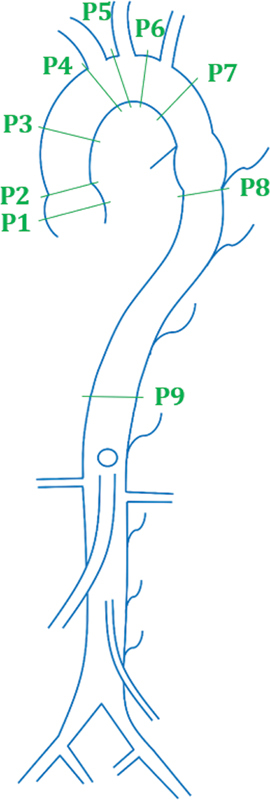
Segmentation proposed by Kiaser et al. ( 1 ) Aortic sinus (AS). ( 2 ) Sinotubular junction (STJ). ( 3 ) Ascending aorta (AA). ( 4 ) Proximal to the origin of brachiocephalic artery (BCA). ( 5 ) First transverse segment (T1). ( 6 ) Second transverse segment (T2). ( 7 ) Isthmic region (IR). ( 8 ) Descending aorta (DA). ( 9 ) Thoracoabdominal aorta at the level of the diaphragm (D).
Compared with its echocardiography predecessor, 12 the CE-CMRA protocol is not affected by the geometry of the patient's thoracic cavity, and thus provide an unlimited choice of 3D image planes or measurement of diameters in both cross-sectional and longitudinal planes. Because the protocol emphasizes the aortic sinus and arch, this procedure can be a great tool in detecting early stages of aneurysm in Marfan's syndrome, connective tissue diseases of the aorta, or even post aortic surgery using the Ross procedure or arterial switch operation. 13 In addition, CE-CMRA has been shown to be ideal for obtaining data on aneurysms or residual stenosis in pre- and post-surgical procedures as well as catheter-guided treatment. 14
Unified Protocol
The schema by Asch et al focuses on the aortic valve annulus and is known as the Unified protocol. 15 The segmentation schema was designed as a unified baseline comparison of three different aortic diameter measurement methods: (1) transthoracic echocardiography (TTE), (2) computed tomography (CT), and (3) magnetic resonance imaging (MRI). As indicated by Fig. 2 , the Unified protocol calls for diameter measurements at the aortic valve annulus, sinuses of Valsalva, sinotubular junction, ascending aorta at pulmonary artery bifurcation, transverse arch between the first and second vessels, isthmus ∼1 cm distal to the last neck vessel, descending thoracic aorta at pulmonary artery, and supra-renal aorta below the celiac artery.
Fig. 2.
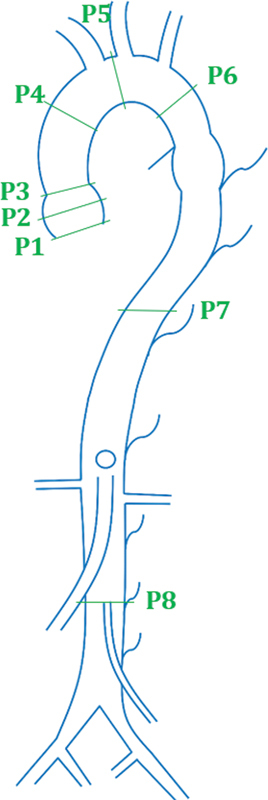
Segmentation proposed by Asch et al. ( 1 ) Aortic valve annulus. ( 2 ) Sinus of Valsalva. ( 3 ) Sinotubular junction. ( 4 ) Ascending aorta. ( 5 ) Transverse arch. ( 6 ) Isthmus. ( 7 ) Descending thoracic. ( 8 ) Infrarenal.
The goal of the Unified protocol is to compare the reproducibility of CT imaging results against TTE and MRI. While the overall reproducibility of CT was confirmed in the study, CT imaging at the arch was not reproducible when compared with TTE. As a result, the study proposed a combination, or a mixed model, to be used by the Unified protocol, with different techniques standardized to different segments of the vessel (i.e., TTE measurements preferred at the proximal aortic segment). 15
Expanded Unified Protocol
Erbel et al proposes a schema that serves as an expansion to the Unified protocol. As indicated in Fig. 3 , measurements are recorded at the sinus of Valsalva, the sinotubular junction, mid-ascending aorta, proximal aortic arch (at the origin of innominate artery), mid-aortic arch (between left common carotid and subclavian arteries), proximal descending aorta (∼2 cm distal to left subclavian artery), mid-descending aorta (at pulmonary arteries), diaphragm, celiac axis to origin, and proximal to bifurcation. 16 Because this method has identical measurement locations in the aortic proximal area (the sinus and arch) as the Unified protocol, it shares the same drawback as its predecessor. In contrast, additional measurements at the descending aorta provided by Erbel et al can be used in the diagnosis of aneurysms in the celiac, distal descending, and bifurcation zones on the aorta. Note that due to a lack of segmentation between the celiac axis origin and the bifurcation zone, an aneurysm in this location may not be diagnosed correctly, and as a result may subsequently lead to a suboptimal aneurysm repair.
Fig. 3.
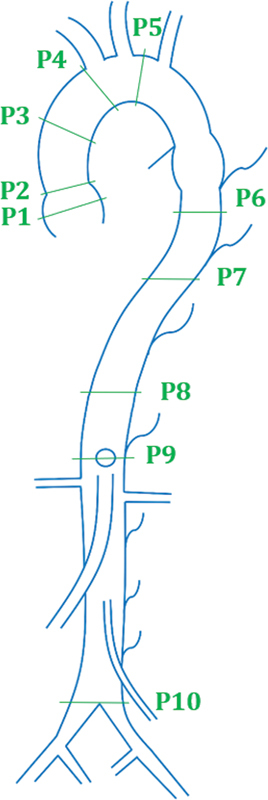
Segmentation proposed by Erbel et al. ( 1 ) Sinus of Valsalva. ( 2 ) Sinotubular junction. ( 3 ) Mid ascending aorta. ( 4 ) Proximal aortic arch (at the origin of innominate). ( 5 ) Mid-aortic arch. ( 6 ) Proximal descending thoracic aorta. ( 7 ) Mid-descending aorta (at the level of pulmonary arteries). ( 8 ) Diaphragm. ( 9 ) Celiac axis origin. ( 10 ) Proximal to aortic bifurcation.
Protocol by Agarwal et al
An alternative method proposed by Agarwal et al focuses on obtaining measurements at the sinus section of the aorta and the section beneath the mid-descending aorta, as shown in Fig. 4 . The study provides more specific measurements for more inferior aortic aneurysms, calling for measurements in regions such as the aorta at the diaphragm, abdominal aorta at the most superior and inferior renal arteries, and the infrarenal abdominal aorta. 17 Unlike the previously mentioned studies, this schema lacks specificity when measuring the diameters at the aortic arch and the aortic isthmus. However, similar to the other schemata, this protocol once again does not provide a complete profile of the aorta or an accurate diagnosis of the aneurysm in those locations.
Fig. 4.
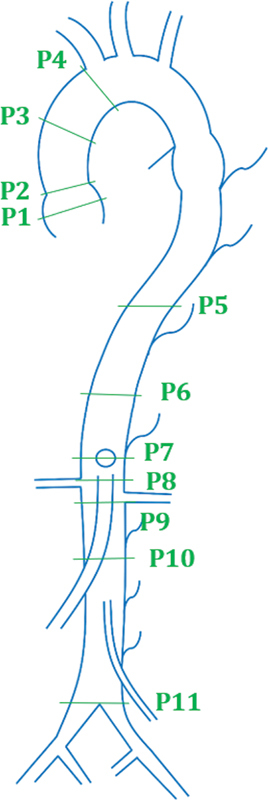
Segmentation proposed by Agarwal et al. ( 1 ) Sinus. ( 2 ) Sinoaortic junction. ( 3 ) Mid-ascending aorta. ( 4 ) Proximal aortic arch. ( 5 ) Mid descending aorta. ( 6 ) Aorta at diaphragm. ( 7 ) Abdominal aorta at origin of celiac axis. ( 8 ) Abdominal aorta at the most superior renal artery. ( 9 ) Abdominal aorta at most Inferior renal artery. ( 10 ) Infrarenal abdominal aorta. ( 11 ) Aorta proximal to bifurcation.
TEVAR Protocol
Fillinger et al proposes a schema for the diagnosis and treatment of thoracic endovascular aneurysm utilizing the TEVAR protocol. 7 The protocol incorporates a prior protocol to describe proximal zones of attachments and adds a description for distal zones of attachments. 18 19 As shown in Fig. 5 , TEVAR protocol obtains measurements that divides the aorta in 12 zones: the proximal edge of covered endograft (zone 0), proximal transverse aorta (zone 1), distal transverse aorta (zone 2), < 2 cm within the origin of the left subclavian artery without covering it (zone 3), proximal end of endograft >2 cm distal to the left subclavian artery within proximal descending thoracic aorta (zone 4), distal descending thoracic aorta but proximal to celiac artery (zone 5), celiac segment and proximal juxtavisceral segment (zone 6), SMA segment and distal juxtavisceral segment (zone 7), covers at least 1 renal artery (zone 8), infrarenal arteries (zone 9), and the iliac arteries–common and external (zones 10 and 11).
Fig. 5.
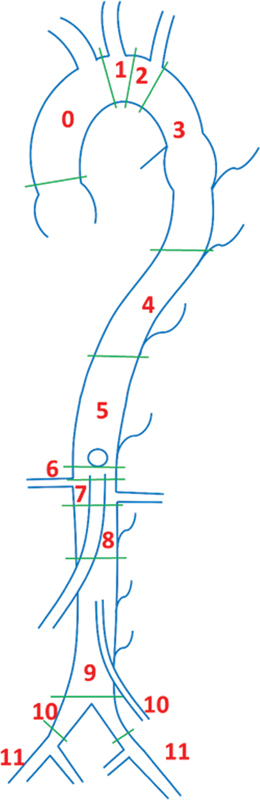
Tevars protocol by Fillinger et al. ( 0 ) Proximal edge of covered endograft. ( 1 ) Proximal transverse aorta. ( 2 ) Distal transverse aorta. ( 3 ) < 2 cm within origin of the left subclavian artery. ( 4 ) Proximal extent of endograft >2 cm distal to the left subclavian artery, within proximal descending thoracic aorta. ( 5 ) Distal descending thoracic aorta but proximal to celiac artery. ( 6 ) Celiac segment and proximal juxtavisceral segment. ( 7 ) SMA segment and distal juxtavisceral segment. ( 8 ) Covers at least 1 renal artery. ( 9 ) Infrarenal arteries. ( 10 ) Common iliac arteries. ( 11 ) External iliac arteries.
The TEVAR protocol has been the most complete schema proposed to date. It provides a consistent and reproducible interpretation of thoracic aortic aneurysms and hence can be utilized to differentiate time-dependent diseases, such as penetrating aortic ulcer, intramural aortic hematoma, and dissection. 20 21 In addition to measuring the diameter of the aorta at the centerline, the zonal schema allows for the estimation of irregularities and tortuosity based on the landmarks close to the aneurysm. The proximal and distal attachment zones have been linked with neurological deficits and renal failure due to their direct linkage with perfusion and CSF fluid drainage. 22 23
The TEVAR protocol can be utilized to determine the suitable candidates for revascularization through the measurement of aneurysms pre- and postoperatively. Hence, it can be extended to partially evaluate other pathologies; measurements which are usually laborious to obtain—aortic attachment site diameter change and apposition length, device migration, inter-component relationships, and aortic/device-related tortuosity—can be obtained with comparatively less difficulty. 7 24 These measurements also have a direct bearing on associated patient risk, reliability of the selected device, procedure type, and complexity. 25
Reporting Standards for Complex AAA Repair
The schema proposed by the TEVARS protocol and the 2002 EVAR and the 2010 TEVAR reporting standards where used as a foundation for the 2020 study by Oderich et al. 7 26 The updated measurement guidelines include specific considerations for complex abdominal aortic aneurysm (AAA), which are defined as aneurysms involving the renal or mesenteric arteries at a level below the level of the thoracic aorta. 26 Because endovascular repair of complex AAA's requires the use of fenestrated, branched, or parallel stent grafts to account for branch arteries of the aorta, unique considerations must be made prior to surgery. The proposed guidelines include multiple factors such as 1) branch incorporation, 2) seal-zone specifications, 3) renal artery angulation and tortuosity measurements, 4) renal volume and perfusion, 5) aortic wall thrombus index, and 6) iliac access measurements. 27
Discussion and ICAARS Schema
We propose the ICAARS schema as a comprehensive design that fills the gaps left behind by the above schemata and has been illustrated in Fig. 6 . It is intended to be applicable for all clinically relevant purposes, including endograft development for aneurysm repair and for the accurate characterization of anatomic anomalies that commonly occur along the aorta. ICAARS divides the aorta into 14 segments and 2 sections (thoracic and abdominal aortas). Measurements are taken at the root, ascending aorta, proximal and distal transverse arteries, isthmus, spindle, descending aorta (both proximal and distal), juxtavisceral, juxtarenal, infrarenal (proximal, mid, and distal), and supra-iliac segment. Various vessels and landmarks have been used to delineate these segments. The segmentation proposed by ICAARS can be used in addition to specific measurements taken for any aneurysm including the neck, and maximal and minimal diameters of the aneurysm. This schema also allows for adequate measurements of aneurysms that overlap multiple sections (i.e., thoracoabdominal or those located as a continuation of the abdominal aorta into its bifurcation into the common iliac arteries).
Fig. 6.
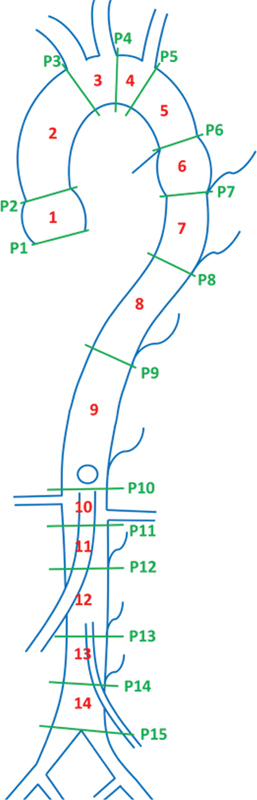
Proposed schemata by ICAARS. Segments (1–14) ( 1 ) Root. ( 2 ) Ascending. ( 3 ) Proximal transverse. ( 4 ) Distal transverse. ( 5 ) Isthmus. ( 6 ) Spindle. ( 7 ) Proximal descending. ( 8 ) Distal descending. ( 9 ) Juxtavisceral. ( 10 ) Juxtarenal. ( 11 ) Proximal infrarenal. ( 12 ) Mid infrarenal. ( 13 ) Distal infrarenal. ( 14 ) Suprailiac. Planes (P1-P15). ( 1 ) Annulus. ( 2 ) Sinotubular. ( 3 ) Pre-innominate. ( 4 ) Post-carotid. ( 5 ) Post-subclavian. ( 6 ) Ligamentum arteriosum. ( 7 ) T3 intersegmental artery. ( 8 ) T7 intersegmental artery. ( 9 ) T12 intersegmental artery. ( 10 ) Pre-renal. ( 11 ) Post-renal. ( 12 ) L2 intersegmental artery. ( 13 ) L3 intersegmental artery. ( 14 ) L4 intersegmental artery. ( 15 ) Aortic bifurcation.
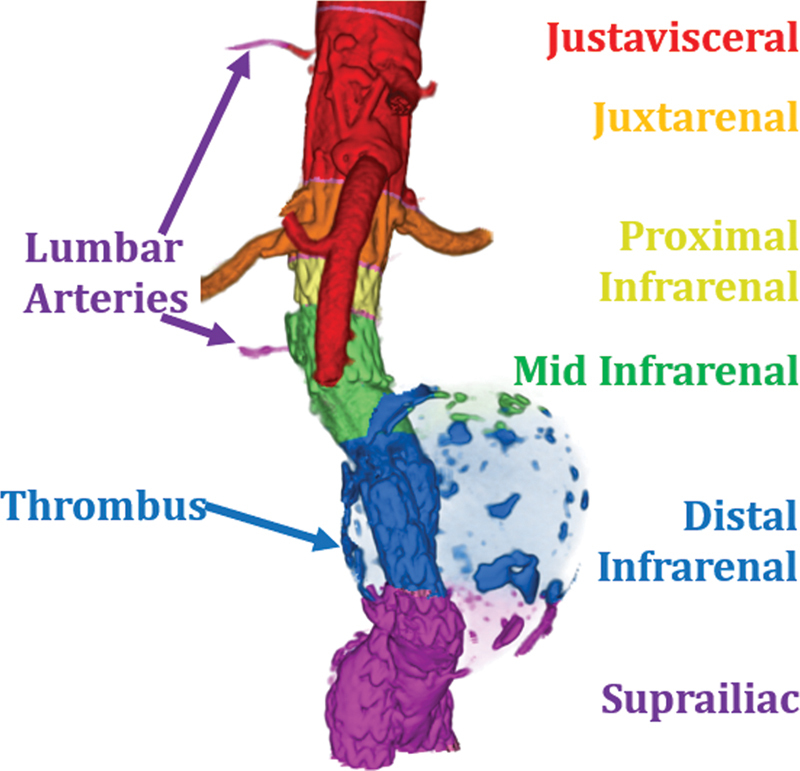
Fig. 7 depicts the segmentation of thoracic and abdominal aorta by describing the landmarks associated with each segment. These landmarks correlate with the embryological development of the aorta and its branches. When characterizing an aneurysm, each segment can be treated as unique, dimensions specific to its anatomic location and its overlap with aortic branches. ICAARS also takes an additional step in describing the morphology of the aneurysm extending beyond the typical “saccular” or “fusiform” subtypes, depicting the various morphologies that are proposed in describing aneurysms. These additional morphologies include juxtavisceral, apple-on-a-stick, and aortoiliac in addition to saccular and fusiform subtypes ( Figs. 8 9 10 11 ).
Fig. 7.
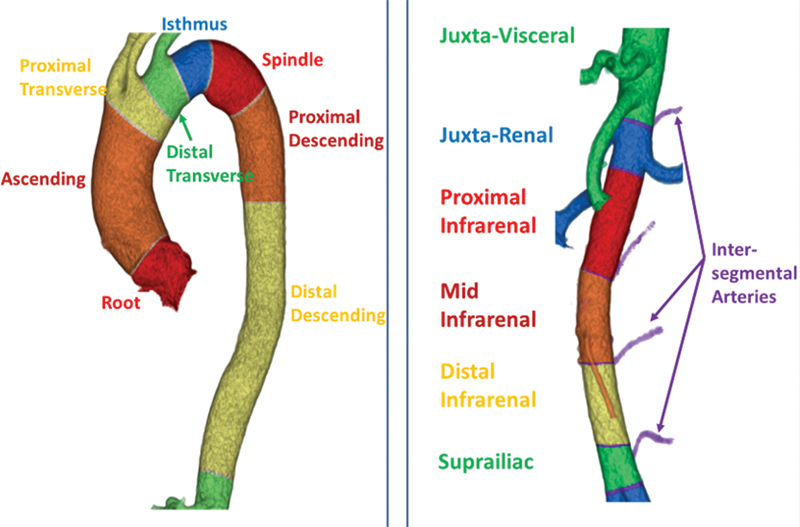
Normal view of thoracic and abdominal aortas.
Fig. 8.
Apple-on-a-stick AAA. The above figure displays the morphology of the aneurysm. In addition, 3D reconstruction was used to display the aorta and its segmentation as it related to the anatomical location of the aneurysm. This patient is status post endovascular graft placement.
Fig. 9.
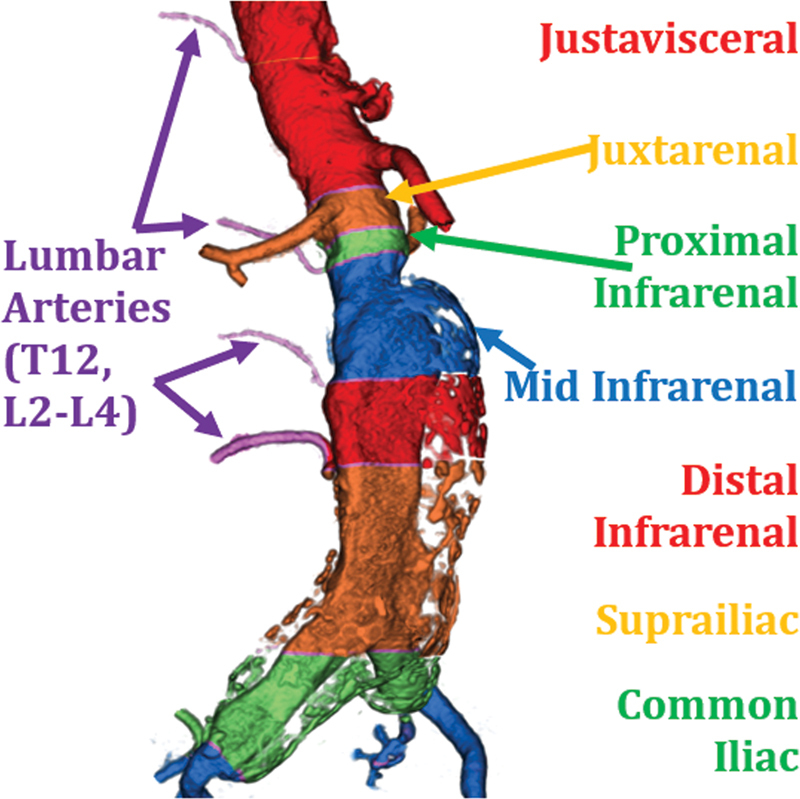
Aortoiliac AAA. The above figure displays the morphology of the aneurysm extending into bilateral common iliac arteries. This patient had diffuse peripheral calcifications.
Fig. 10.
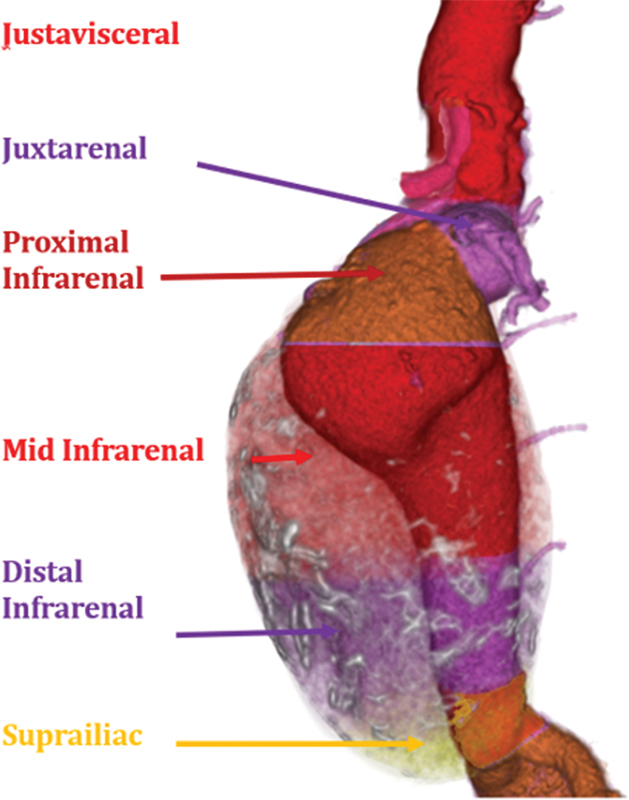
Fusiform AAA. The above figure displays the morphology of this aneurysm with the thrombus rendered in transparency.
Fig. 11.
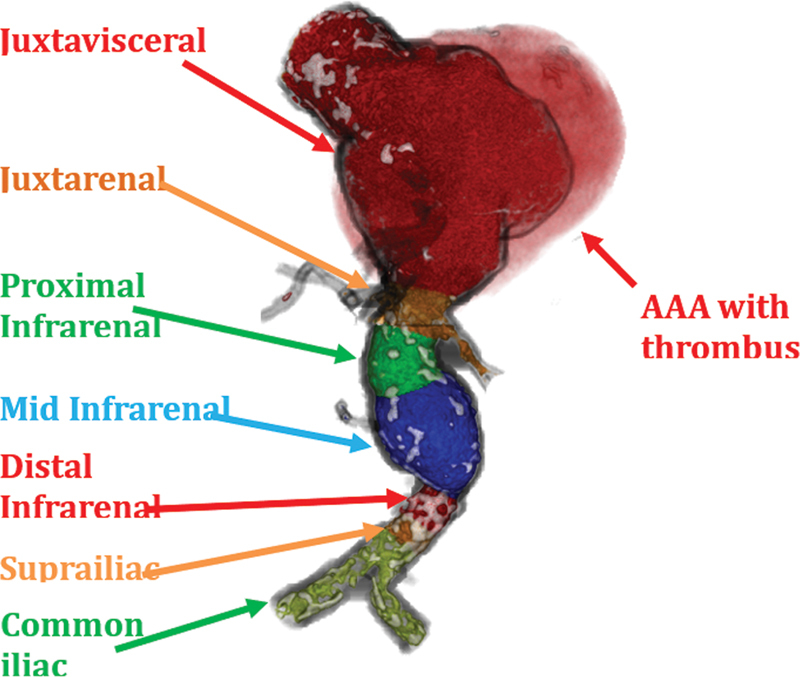
Juxtavisceral AAA. The above figure displays the morphology of this aneurysm with the thrombus rendered in transparency.
Unlike the CE-CMRA protocol, which does not account for lower aortic or branch aneurysms, the ICAARS schema accounts for measurements at the juxtavisceral, juxtarenal, infrarenal, and the iliac zones. These additional measurements allow for the identification of aneurysms that predominate the abdominal aorta, which often occur secondary to atherosclerosis and smoking. Similarly, ICAARS addresses the drawbacks of the Unified protocol by accounting for measurements of the aortic isthmus, spindle, distal descending aorta, infrarenal, and the supra-iliac zones. Another weakness of the Unified protocol lies in its lack of specificity in measurements at the aortic arch. The variability in measurement location, lack of additional arch segmentation, and profile of the arch all contribute to common arch measurement errors. As a result, aneurysms or diseases at the arch seen with Marfan's syndrome, Ehlers–Danlos syndrome, and other connective tissue diseases cannot be diagnosed with accuracy. Lastly, while the TEVAR protocol focuses on the aortic arch and the lower sections, such as infrarenal, celiac, and aortic bifurcation, it fails to focus on the sinus region, the isthmus, and the descending sections of the aorta, all of which are included in the ICAARS schema.
By integrated the strengths of the established schemata as discussed in the results section, utilization of the ICAARS schema would obtain a comprehensive range of measurements, including measurements at the aortic isthmus, spindle, distal and proximal descending aorta, infrarenal, and the supra-iliac zones. Overall, the schema provides measurements at multiple strategic locations, some of which can be used as imaging biomarkers. This well-structured and comprehensive schema is more comprehensive and inclusive of the various types of aneurysms.
The schemata discussed above were intended for specific types of aneurysm, but ICAARS can be used for any type of aneurysm. One major drawback is the increased amount of time and resources necessary to make additional measurements. Additionally, the direct correlation between aortic diameter and the root mean squared of body surface area weighs heavily on the interpretation of the data. Therefore, patients with very low or very high body surface areas will require extrapolation, resulting in increased errors associated with the measurements. As such, caution needs to be exercised using data in patients with very low or high body surface area. 28
Conclusion
Aortic morphology guides surgical management. There are complex hemodynamic, genetic, and hormonal factors that influence aortic morphology. Analysis of aortic morphology, both within a population and for specific individuals, may reveal underlying disease mechanisms and guide medical interventions, such as drug therapies, surgical repair, and genetic counseling. ICAARS is an aortic morphology classification schema that is comprehensive, standardized, and anatomically based. Clear and consistent descriptions of aortic morphology are necessary to ensure rigor and reproducibility in aortic research and to optimize grafts for aneurysm repair.
Footnotes
Conflict of Interest None declared.
References
- 1.Centers for Disease Control and Prevention Underlying Cause of Death 1999–2019 on CDC WONDER Online Database, released 2020 Data are from the Multiple Cause of Death Files, 1999–2019, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative ProgramAccessed Feb 15, 2022 from:https://wonder.cdc.gov/ucd-icd10.html
- 2.Aortic AFS Division for Heart Disease and Stroke Prevention [Web Page] 2016. 6/16/2016 [cited 2017; Available from:https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_aortic_aneurysm.htm
- 3.Kent K C. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371(22):2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 4.Karthikesalingam A, Vidal-Diez A, Holt P J. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med. 2016;375(21):2051–2059. doi: 10.1056/NEJMoa1600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RESCAN collaborators . Sweeting M J, Thompson S G, Brown L C, Powell J T. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99(05):655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 6.Lindquist Liljeqvist M, Hultgren R, Gasser T C, Roy J. Volume growth of abdominal aortic aneurysms correlates with baseline volume and increasing finite element analysis-derived rupture risk. J Vasc Surg. 2016;63(06):1434–1.442E6. doi: 10.1016/j.jvs.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards Fillinger M F, Greenberg R K, McKinsey J F, Chaikof E L.Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg 201052041022–1033., 1033.e15 [DOI] [PubMed] [Google Scholar]
- 8.Rylski B, Schofer F, Beyersdorf F.Aortic arch anatomy in candidates for aortic arch repairSemin Thorac Cardiovasc Surg 2021 [DOI] [PubMed]
- 9.Page M J, McKenzie J E, Bossuyt P M. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel E R. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser T, Kellenberger C J, Albisetti M, Bergsträsser E, Valsangiacomo Buechel E R. Normal values for aortic diameters in children and adolescents–assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson. 2008;10:56. doi: 10.1186/1532-429X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Association of Echocardiography ; Document Reviewers . Evangelista A, Flachskampf F A, Erbel R. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11(08):645–658. doi: 10.1093/ejechocard/jeq056. [DOI] [PubMed] [Google Scholar]
- 13.Nollen G J, Groenink M, Tijssen J G, Van Der Wall E E, Mulder B J. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J. 2004;25(13):1146–1152. doi: 10.1016/j.ehj.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Godart F, Labrot G, Devos P, McFadden E, Rey C, Beregi J P. Coarctation of the aorta: comparison of aortic dimensions between conventional MR imaging, 3D MR angiography, and conventional angiography. Eur Radiol. 2002;12(08):2034–2039. doi: 10.1007/s00330-001-1260-7. [DOI] [PubMed] [Google Scholar]
- 15.GenTAC Investigators . Asch F M, Yuriditsky E, Prakash S K. The need for standardized methods for measuring the aorta: multimodality core lab experience from the GenTAC registry. JACC Cardiovasc Imaging. 2016;9(03):219–226. doi: 10.1016/j.jcmg.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ESC Committee for Practice Guidelines ; The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) . Erbel R, Aboyans V, Boileau C. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal P P, Chughtai A, Matzinger F R, Kazerooni E A. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29(02):537–552. doi: 10.1148/rg.292075080. [DOI] [PubMed] [Google Scholar]
- 18.Criado F J, Clark N S, Barnatan M F. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg. 2002;36(06):1121–1128. doi: 10.1067/mva.2002.129649. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell R S, Ishimaru S, Ehrlich M P. First International Summit on Thoracic Aortic Endografting: roundtable on thoracic aortic dissection as an indication for endografting. J Endovasc Ther. 2002;9 02:II98–II105. [PubMed] [Google Scholar]
- 20.Nauta F K. Arnoud; Trimarchi, Santi . Penetrating aortic ulcer and intramural hematoma. Endovascular Today 2014 [Google Scholar]
- 21.Eggebrecht H, Plicht B, Kahlert P, Erbel R. Intramural hematoma and penetrating ulcers: indications to endovascular treatment. Eur J Vasc Endovasc Surg. 2009;38(06):659–665. doi: 10.1016/j.ejvs.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Safi H J, Miller C C, III, Huynh T T.Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection Ann Surg 200323803372–380., discussion 380–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Criado F J, Clark N S, Barnatan M F. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg. 2002;36(06):1121–1128. doi: 10.1067/mva.2002.129649. [DOI] [PubMed] [Google Scholar]
- 24.Fillinger M F. New imaging techniques in endovascular surgery. Surg Clin North Am. 1999;79(03):451–475. doi: 10.1016/s0039-6109(05)70018-9. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg R K, Lu Q, Roselli E E. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118(08):808–817. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- 26.Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery . Chaikof E L, Blankensteijn J D, Harris P L. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35(05):1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 27.Writing Committee Group Oderich G S, Forbes T L, Chaer R.Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries J Vasc Surg 202173(1S):4S–52S. 10.1016/j.jvs.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 28.Sluysmans T, Colan S D. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985) 2005;99(02):445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]


