Abstract
Purpose
To demonstrate a need for improved health insurance coverage for anti-obesity medications (AOMs) by comparing clinical and economic benefits of obesity treatments to covered medications for selected therapeutic areas.
Methods
Using a grey literature search, we identified and prioritized therapeutic areas and treatment analogues for comparison to obesity. A targeted literature review identified clinical and economic outcomes research across the therapeutic area analogues. Associated comorbidities, clinical evidence, indirect costs (ie, absenteeism and productivity loss), and direct medical costs were evaluated to determine the relative value of treating obesity.
Results
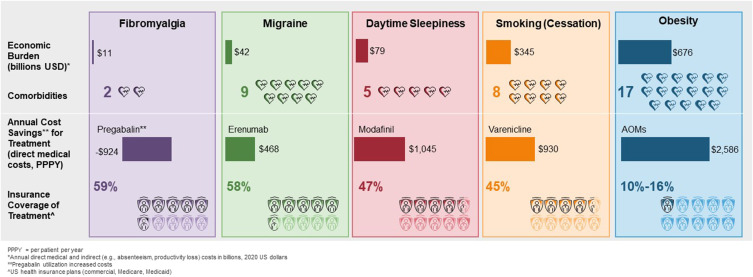
Four therapeutic areas/treatment analogues were selected for comparison to obesity: smoking cessation (varenicline), daytime sleepiness (modafinil), migraines (erenumab), and fibromyalgia (pregabalin). Obesity was associated with 17 comorbidities, more than migraine (9), smoking (8), daytime sleepiness (5), and fibromyalgia (2). Economic burden was greatest for obesity, followed by smoking, with yearly indirect and direct medical costs totaling $676 and $345 billion, respectively. AOMs resulted in cost savings of $2586 in direct medical costs per patient per year (PPPY), greater than that for varenicline at $930 PPPY, modafinil at $1045 PPPY, and erenumab at $468 PPPY; pregabalin utilization increased costs by $924 PPPY. AOMs were covered by 10–16% of United States health insurance plans, compared to 45–59% for the four comparators.
Conclusion
Compared to four therapeutic analogues, obesity represented the highest economic burden and was associated with more comorbidities. AOMs provide greater cost savings compared to selected analogues. However, AOMs have limited formulary coverage. Improved coverage of AOMs may increase access to these treatments and may help address the clinical and economic burden associated with obesity and its comorbidities.
Keywords: obesity, anti-obesity drugs, health care costs, cost savings, health insurance
Graphical Abstract
Plain Language Summary
Obesity is a chronic disease affecting over 40% of United States adults. It can lead to serious health risks and substantial medical costs. Although prescription anti-obesity medications (AOMs) can be effective for treating obesity and are recommended by medical guidelines, most health insurance plans do not cover them. We conducted a targeted literature review of published studies and reports to compare the relative value of AOMs to treatments for smoking, daytime sleepiness, migraines, and fibromyalgia (a condition that causes pain all over the body). We found that AOMs resulted in greater direct medical cost savings than the treatments for the other conditions. However, AOMs were covered by fewer health insurance plans than the other treatments.
Introduction
Obesity is highly prevalent in the United States (US), affecting over 40% of the adults,1 with prevalence rates expected to increase to nearly 50% by 2030.2 It is associated with significant disease burden by increasing the risk for many comorbidities including cancer, stroke, osteoarthritis, sleep apnea, type 2 diabetes, and other cardiometabolic conditions.3–7 Additionally, obesity poses considerable economic burden, both in direct medical costs8–12 and indirect costs including absenteeism and productivity loss.13–18 Obesity-related complications accounted for almost half of the medical and productivity costs of chronic diseases in the US as of 2016, totaling $1.72 trillion.19
Sustained weight loss of at least 5% body weight is recommended by obesity treatment guidelines20,21 and is associated with a significant reduction in the development and delayed onset of type 2 diabetes, hypertension, hyperlipidemia, and osteoarthritis.22,23 Additionally, weight loss has been shown to reduce healthcare costs.24,25 However, weight loss is difficult to maintain over time.26–28 Pharmacotherapy, as recommended by treatment guidelines,20,29 can be effective in managing obesity.30–33 Anti-obesity medications (AOMs), as an adjunct to a reduced calorie diet and increased physical activity, can provide greater weight loss than lifestyle changes alone.34–37
Although AOMs have been shown to be cost-effective38–41 and cost-saving,24,25,42 less than 2% of eligible patients have been prescribed AOMs.43–48 Low use of AOMs may be due to many reasons including lack of, or limited, health insurance coverage.48–52 Coverage of AOMs is currently lower than treatments for other chronic conditions such as migraine and fibromyalgia, for which over 50% of the commercial, Medicare, and Medicaid health plans provide formulary coverage.53 Increased coverage for AOMs may lead to substantial societal value, as demonstrated by a simulation model by Kabiri et al.54
The objective of this study is to demonstrate a need for improved health insurance coverage for AOMs by comparing clinical and economic benefits of obesity treatments to medications for other comparable chronic diseases. We conducted a literature review to identify the most appropriate therapeutic areas to compare to obesity and evaluated these across key economic factors.
Methods
Study Design
The study was conducted in three steps: 1) grey literature review to identify treatment areas similar to obesity and of concern for US payers and employers, 2) prioritization of therapeutic area and treatment analogues, and 3) targeted literature review to compare the relative value of AOMs to other therapies. Clinical outcomes, patient-reported endpoints, associated comorbidities, direct medical costs, and indirect costs (absenteeism and productivity loss) were evaluated for the selected analogues to assess the relative value compared to obesity.
Analogue Identification
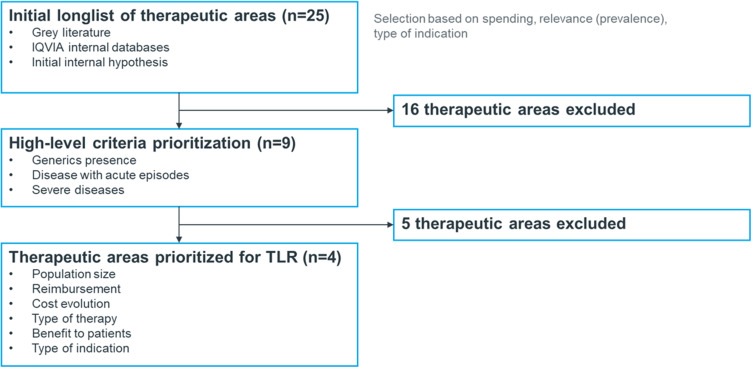
We conducted a grey literature search to create a list of candidate therapeutic areas. A preliminary list was developed using information on US medication spending and utilization trends from resources including IQVIA Institute databases and reports from the pharmaceutical industry, health plans, employer organizations, and the Centers for Disease Control and Prevention (CDC).55–63 Twenty-five therapeutic areas were selected and screened based on pre-specified criteria including healthcare cost spending, relevance to obesity, type of indication, and duration of treatment (Table S1).
Analogue Prioritization
The list of 25 therapeutic areas were further evaluated; 16 were eliminated due to having primarily generic options in the market, acute nature of the condition or episodes, falling outside medical/pharmacy budgets (eg, dental care and vision care), and overlapping with obesity (eg, diabetes, and hypercholesterolemia) (Table S2). The remaining nine therapeutic areas were selected for further analysis and prioritization: daytime sleepiness, asthma/chronic obstructive pulmonary disease (respiratory), dermatology, fibromyalgia, gastrointestinal, hypoactive sexual desire disorder, smoking cessation, vaccines (human papillomavirus), and migraine. These therapeutic areas were assessed using seven dimensions: population size, reimbursement type/history, cost evolution, type of therapies, benefit to patients, barriers, and lifestyle indication. See Table S3 for more information about each dimension.
We characterized each therapeutic area based on the prioritization criteria using secondary research including academic, peer-reviewed, business, and scientific reports and databases. The top four areas were selected based on their ranking and included the following therapeutic areas and one analogue/representative product each: smoking cessation (varenicline [Chantix]), daytime sleepiness (modafinil [Provigil]), migraine (erenumab [Aimovig]), and fibromyalgia (pregabalin [Lyrica]) (Table 1). These were included in the targeted literature review (Figure 1).
Table 1.
Detailed Prioritization Matrix and Prioritized Treatment Areas
| Therapeutic Area (Drug) | US Prevalence | Coveragea (% Plans) | Coverage | Cost Evolutionb | Types of Therapyc | Number of Products Available | Benefit to Patientsd | Payer Perception | Monthly WAC ($)e | Durationf | Opt-in Requiredg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity (Saxenda) | 35%1 | 16% | Not covered | 2.19% | Oral, injectable | 3–5 products | Large Improvement | Lifestyle indication | 1367.00 | Chronic | X |
| Daytime sleepiness (Provigil) | 23%65 | 47% | Generally covered with restrictions | 5.00% | Oral | 5–7 products | Large Improvement | Lifestyle indication | 1563.00 | Episodic | |
| Smoking cessation (Chantix) | 14%93 | 45% | Generally covered with restrictions | 10.02% | Oral, injectable | 3–5 products | Minor / No improvement | Lifestyle indication | 673.90 | Short course | X |
| Migraine (Aimovig) | 17%92 | 58% | Generally covered with restrictions | 22.48% | Oral, injectable | >10 products | Minor / No improvement | Lifestyle indication | 682.10 | Chronic | |
| Fibromyalgia (Lyrica) | 4%94 | 59% | Covered | 9.03% | Oral | 3–5 products | Mild improvement | Lifestyle indication | 694.50 | Chronic |
Notes: aPercentage of commercial, Medicare, and Medicaid plans that cover the product as preferred (IQVIA SMART). bCost variations in response to innovative treatments entry or other relevant events in the disease area (IQVIA SMART, 2015–2021). cRepresents the routes of administration that account for 60–70% of the share. dQualitative rating summarizing the improvement on key endpoints; Data from package insert by product. eIQVIA MIDAS, last reported price; Price RX; Monthly cost calculated based on prescribing information, each product label. fBased on product label. Short-term <1 year; Mid-term 1–3 years; Long-term >3 years. gBased on GoodRx coverage by insurance; X = Employer’s opt-in required at current state.
Abbreviation: WAC, wholesale acquisition cost.
Figure 1.
Therapeutic area analogue identification flow chart.
Abbreviation: TLR, targeted literature review.
Targeted Literature Review
We conducted a targeted literature review of published studies available in online databases or accessible via Google or PubMed. This was not meant to be an exhaustive review, but rather aimed to identify the most relevant, current, and high-quality evidence. The study considered MEDLINE and Embase using combinations of keywords, indexing terms, and Boolean operators based on predefined PICOS (Population, Intervention, Comparator, Outcomes, and Study Design) criteria for the therapeutic area analogues (Table S4).
The search strategy was developed in collaboration with an experienced information specialist/medical librarian. We searched for published materials in the English language without geographic limits. Publications were limited to the past 5 years. Supplemental Google searches were conducted to identify older, seminal studies (eg, used for health technology assessment and reimbursement decisions). A total of 2956 articles were identified. See Tables 5–8 for the search strategies for each analogue.
References were screened in an Excel workbook and marked for inclusion/exclusion for consideration for full-text review. Filters were applied to reduce the list of articles, removing duplicates and studies outside the relevant geography (ie, US). The 89 articles meeting the eligibility criteria were included in the full-text screening, data extraction, and synthesis. Data extraction included study population, data source, study setting, study design, outcomes, year/age of data, sample size, and stakeholder relevance (eg, employer, payer, clinician).
The full-text review yielded 39 articles considered most relevant in terms of time frame, stakeholder representation, granularity of data, and cost details. Nine of these articles were discarded due to lack of information or relevance regarding geography (eg, nationwide vs regional), type of data (eg, claims, real-world studies), and comparators considered in our analysis. Nine references for obesity were previously compiled and based on the same parameters regarding recency, geography, as well as the outcomes and study design PICOS criteria. Clinical and patient-reported endpoints were reported for subcutaneous semaglutide 2.4 mg (Wegovy). However, due to the recency of this medication’s approval by the Food and Drug Administration (FDA) for the treatment of obesity, analysis of economic burden of obesity and formulary coverage were based on research examining other FDA-approved prescription AOMs for long-term treatment of overweight and obesity. Cost savings of AOMs was based on assumptions of 10–20% weight reduction in a cohort of patients at 1 year and the direct medical cost impact and direct medical cost impact,25 which is consistent with 15% weight loss seen in the STEP 4 68-week clinical trial evaluating weekly subcutaneous semaglutide.64
Relative Value Analysis
The results of the 39 articles included in the analysis were summarized and tallied descriptively (eg, range of values) and qualitatively. The relative value analysis started with the collection of clinical and financial outcomes (clinical trials, claims databases, real-world studies). After identifying the most comparable endpoints and outcomes, results were compared against obesity to assess the relative value of treating obesity versus other treatment areas with covered products. To assess the broader impact of each condition, we identified comorbidities from the literature for obesity and for each of the four selected therapeutic analogues.
Results
Comparison of Analogues to Anti-Obesity Medications
Smoking cessation, daytime sleepiness, migraines, and fibromyalgia were selected as analogues based on similarities to AOMs including healthcare cost spending, chronicity, type of indication, and duration of treatment. Daytime sleepiness is defined as excessive sleepiness during the daytime stemming from causes such as obstructive sleep apnea.65 Migraines are characterized by recurrent headaches that can last from 4 to 72 hours and are often accompanied by other disabling symptoms such as nausea, vomiting, and/or photophobia.66 Fibromyalgia is a cause of widespread musculoskeletal pain and is often accompanied by irregular sleep patterns, fatigue, and substantial limitations in physical function and daily living.67 Treatment analogues were selected due to their clinical efficacy,68–77 coverage challenges at launch,78–85 and/or cost-effectiveness.86–91
Clinical Burden and Formulary Coverage
Prevalence of the four analogue conditions ranged from 4% to 23%,65,92–94 lower than that of obesity.1 Obesity was associated with 17 comorbidities,5 migraine was associated with 10,95 smoking with 8,96 daytime sleepiness with 5,97 and fibromyalgia with 298 (Table S9). The economic impact of obesity-related comorbidities ranged from $486 for dyslipidemia to $1665 for pulmonary embolism, for an estimated total of $139 million in a population of 100,000 individuals.5
The new generation of AOMs (subcutaneous semaglutide 2.4 mg) demonstrated significant improvement in four clinical outcomes, compared to improvement in one clinical endpoint for each of the four treatment analogues.64 Benefits provided by therapies for daytime sleepiness, migraine, and fibromyalgia were based more on patient-reported outcomes (Table S10).99–102 Insurance coverage among commercial, Medicare, and Medicaid plans ranges from 45% for varenicline, 47% for modafinil, 58% for erenumab, and 59% for pregabalin; coverage for liraglutide 3.0 mg and subcutaneous semaglutide 2.4 mg is 16% and less than 10%, respectively (Table 1).103
Economic Burden and Direct Medical Savings
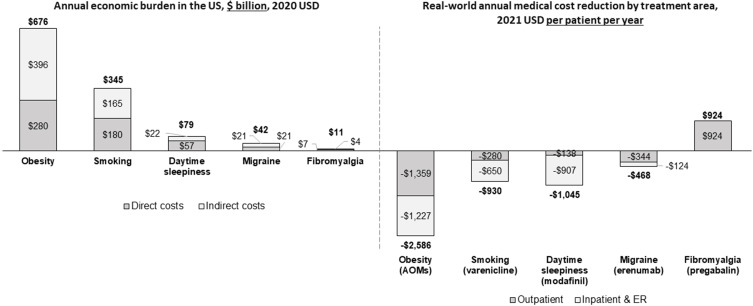
Across the areas evaluated, obesity accounted for the largest total economic burden (direct and indirect costs) at $676 billion8,104 annually, followed by smoking at $345 billion87 (Figure 2). Total economic burden was lower for the other conditions, ranging from $11 billion for fibromyalgia,105,106 $42 billion for migraine,107,108 and $79 billion for daytime sleepiness.109,110 Indirect costs primarily included absenteeism and productivity loss as these were the most common and consistent indirect cost measures for comparison across therapeutic areas. Direct and indirect costs for each therapeutic area are presented in Figure 2.
Figure 2.
Annual economic burden in the US and real-world annual medical cost reduction by treatment area.
Notes: Direct costs are medical and pharmacy costs attributable to the burden of the disease. Indirect costs are costs not directly associated with treatment but are produced by the disease (eg, productivity loss).
Abbreviations: AOMs, anti-obesity medications; ER, emergency room.
Weight loss medications accounted for almost $2600 savings in direct medical costs per patient per year (PPPY) assuming 15% weight loss,25,64 compared to approximately $1000 PPPY for smoking (varenicline)87 and daytime sleepiness (modafinil),111 and $468 PPPY for migraine (erenumab)112 (Figure 2). The main drivers of these cost savings were reductions in outpatient, inpatient, and emergency room (ER) costs. There was an increase of $924 PPPY in medical costs for fibromyalgia (pregabalin), primarily due to increased physician visits for dose titration and monitoring.113 Obesity and smoking treatments provided the greatest reduction in critical healthcare resource use utilization (eg, hospitalizations and ER admissions).25,87 AOMs showed the greatest cost reduction potential, as they can be attributed to a decrease in inpatient and outpatient visits for musculoskeletal, digestive, and circulatory disorders.25
Productivity Loss
To understand the financial implications in an average mid-size company defined as having 100 to 999 employees,114 we calculated indirect costs by multiplying the number of yearly average additional absenteeism days for each condition by the prevalence in an average mid-size company (assuming 500 employees), using the 2021 US national average hourly wage.115 The annual workday loss was highest for obesity and daytime sleepiness, resulting in >520 days for an average mid-sized employer, and approximately $130,000 in annual productivity loss for both conditions (Table 2).
Table 2.
Annual Workday Loss Associate with the Disease, for a Mid-Sized Company
| Therapeutic Area | Average Additional Absenteeism (Per Person Per Year) | Prevalence in an Average Mid-Size Company (Number of Employees) | Annual Workday Loss (Days) | Annual Productivity Loss Due to Absenteeism (USD) |
|---|---|---|---|---|
| Obesity | 3.0 days13 | 175 | 525 | 129,000 |
| Smoking | 2.3 days120 | 70 | 161 | 40,000 |
| Daytime sleepiness | 4.6 days121 | 115 | 529 | 130,000 |
| Migraine | 1.7 days122 | 85 | 145 | 36,000 |
| Fibromyalgia | 13 days123 | 20 | 260 | 93,000 |
Notes: Baseline of annual average absenteeism is 2.34 days in workers with normal weight.13 Annual workday loss was calculated using the assumption of 500 employees for an average mid-size employer and reported prevalence rates for each condition. Productivity loss to absenteeism assumes the US national average hourly wage, US Bureau of Labor and Statistics 2021.
Abbreviation: USD, United States Dollar.
Discussion
Obesity contributes a significant burden on the US healthcare system. Although diet, exercise, and bariatric surgery are available to address obesity, they are not viable and effective options for every person living with obesity. AOMs offer another treatment option in adjunct to diet and exercise to help patients achieve their obesity treatment goals. Despite demonstrated clinical efficacy of AOMs22,23,30–33 and reduced costs associated with weight loss,24,25 payers have been slow to include AOMs on their formularies. To better understand the potential value of increased health insurance coverage of AOMs, we compared AOMs to treatment analogues which have broad formulary coverage. Smoking cessation, daytime sleepiness, migraine, and fibromyalgia were deemed appropriate comparators due to factors such as their chronicity.
In our study, we found that obesity is associated with the greatest number of comorbidities as compared to the other health conditions evaluated. Each comorbidity related to obesity also contributes its own economic burden to the US healthcare system.5 Our analysis showed that among the therapeutic areas evaluated, the greatest economic burden was seen for obesity at $676 billion annually,8,104 nearly twice that of smoking,87 9 times that of daytime sleepiness,109,110 16 times that of migraine,108 and 61 times that of fibromyalgia.106 When considering the impact of all chronic diseases associated with obesity, the economic burden is even greater, estimated at $1.72 trillion in 2016, 72% of which was indirect costs due to lost productivity.19 Our research also demonstrated that AOMs have greater direct medical cost savings than the therapeutic analogues.
Treatments for obesity, smoking cessation, daytime sleepiness, migraine, and fibromyalgia all provide clinical and economic benefits.5,25,68–71,73,75,76,86–91,112 In addition to decreasing the risk for many obesity-related comorbidities64 and recommended by clinical guidelines,20,29 weight loss among patients with obesity is likely to decrease inpatient, outpatient, and ER visits, thus reducing the disease burden overall.24,25 Furthermore, this may contribute to fewer absenteeism days that may improve overall productivity.13 In a large study of patients treated to lose weight, Bilger et al found that weight loss of >5% could lead to an annual reduction of 3 days of absenteeism, translating to potential cost savings of $2.1 million.116 In 2019, the societal value of increased access to currently available and next-generation AOMs was estimated at $1.9 to $2.5 trillion, varying by the level of uptake ranging from 15% to 30% of the patients eligible for chronic weight management.54 AOMs offer benefits to payers and employers and may also have a positive impact on patients.117
Treatment of obesity demonstrates greater potential for a return on investment in decreasing comorbidity costs, all-cause medical expenditure, and absenteeism as compared to the four analogues studied. However, as of today, AOMs are only covered by 10–16% of health insurance plans, whereas the associated medications for the four analogues have at least 45% coverage.118 Acknowledging the benefits of AOMs, the National Institute for Health and Care Excellence (NICE) in the United Kingdom recently recommended that subcutaneous semaglutide 2.4 mg be available to eligible patients as a weight management option.119 Similar to the other treatments evaluated, coverage and adoption of AOMs may be a gradual process in the US, but our research demonstrates that broader coverage and increased utilization of AOMs can potentially reduce the economic burden associated with obesity.
This study consisted of a targeted literature review and was thus not a comprehensive review of the available literature for obesity and the four therapeutic area analogues. The analysis was limited to publicly available data. The data for daytime sleepiness is specific to sleep apnea based on available information in the literature; information on daytime sleepiness due to other reasons such as narcolepsy was not assessed. Data available in the literature and secondary sources were analyzed qualitatively; indirect comparisons were made from the extracted data, without any adjustments or meta-analysis. Data analyzed in this study to assess the economic burden of obesity were based on treatments approved by the FDA prior to 2021; thus, newer generation AOMs with greater weight loss effects were not considered, which could underestimate the economic benefits of treatment. Lastly, in-depth analysis of formulary positioning was not evaluated due to limited public information available to perform this comparison. This study estimated healthcare cost savings from weight loss of 15% which was recently reported in the STEP 4 clinical trial comparing weekly subcutaneous semaglutide against placebo. AOMs may not be suitable for all patients and may not produce weight loss of 15% in real-world populations. AOMs may not be suitable for patients with liver and kidney issues, and Ding et al excluded patients with cirrhosis and patients undergoing dialysis from their study on healthcare costs associated with weight loss.25 AOMs and the comparator treatments included in our analysis can have negative side-effects in some patients, and these negative side-effects could be associated with increased healthcare costs. This study did not capture costs that could be associated with negative side effects of AOMs or any of the comparator treatments. Further work is needed to evaluate how all-cause healthcare costs may be affected by weight loss specifically attributable to AOMs. Further work is needed to evaluate long-term effects of AOMs on health and healthcare costs.
Conclusions
The treatment of obesity showed a substantial potential for a return on investment by decreasing comorbidity costs, all-cause medical expenditure, and absenteeism. Ensuring increased patient access to obesity treatment options, including AOMs, may help address the obesity epidemic and decrease the overall disease burden of obesity, which will benefit patients, payers, and employers.
Acknowledgments
The authors thank Rebecca Hahn, MPH and Elizabeth Tanner, PhD, of KJT Group, Inc., Rochester, NY, for providing medical writing support, which was funded by Novo Nordisk, Inc., Plainsboro, NJ, in accordance with Good Publication Practice (GPP3) guidelines. This research has been presented as a poster presentation at ISPOR 2022 (May 15–18, 2022; Washington, DC) and the abstract was published in Value in Health.
Funding Statement
Novo Nordisk Inc. funded the study and had a role in the study design, data collection, analysis, and interpretation of data, as well as writing support of the manuscript.
Abbreviations
US, United States; AOMs, anti-obesity medications; PPPY, per patient per year; ER, emergency room, CDC, Centers for Disease Control and Prevention; PICOS, Population, Intervention, Comparator, Outcomes, and Study Design; FDA, Food and Drug Administration; NICE, National Institute for Health and Care Excellence.
Author Contributions
A Ruseva, A Ramasamy, C Blanchette, N Kim, and C Burudpakdee were responsible for study conception and design. J Estrada and I Chow were responsible for data analysis. All authors were responsible for analysis and interpretation of the data. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Aleksandrina Ruseva, Abhilasha Ramasamy, and Christopher M. Blanchette are employees of Novo Nordisk. Nina Kim and Chakkarin Burudpakdee were employees of Novo Nordisk, Inc. at the time the study was conducted. Isabella Chow is an employee of IQVIA, Inc., which received funding to conduct the research. Joaquin Estrada was an employee of IQVIA, Inc. at the time the study was conducted. The authors report no other conflicts of interest in this work.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS. 2020;360:1–8. [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 3.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase CL, Eriksen KT, Lopes S, Satylganova A, Schnecke V, McEwan P. Body mass index and risk of obesity-related conditions in a cohort of 2.9 million people: evidence from a UK primary care database. Obes Sci Pract. 2020;7(2):137–147. doi: 10.1002/osp4.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Blume SW, Huang JC, Hammer M, Ganz ML. Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ. 2015;18(12):1020–1028. doi: 10.3111/13696998.2015.1067623 [DOI] [PubMed] [Google Scholar]
- 6.Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med. 2019;25(6):602–608. doi: 10.1097/mcp.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 7.Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–1058. doi: 10.15585/mmwr.mm6639e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawley J, Biener A, Meyerhoefer C, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27(3):354–366. doi: 10.18553/jmcp.2021.20410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28(5):w822–W831. doi: 10.1377/hlthaff.28.5.w822 [DOI] [PubMed] [Google Scholar]
- 10.Kamble PS, Hayden J, Collins J, et al. Association of obesity with healthcare resource utilization and costs in a commercial population. Curr Med Res Opin. 2018;34(7):1335–1343. doi: 10.1080/03007995.2018.1464435 [DOI] [PubMed] [Google Scholar]
- 11.Kim DD, Basu A. Estimating the medical care costs of obesity in the United States: systematic review, meta-analysis, and empirical analysis. Value Health. 2016;19(5):602–613. doi: 10.1016/j.jval.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 12.Suehs BT, Kamble P, Huang J, et al. Association of obesity with healthcare utilization and costs in a Medicare population. Curr Med Res Opin. 2017;33(12):2173–2180. doi: 10.1080/03007995.2017.1361915 [DOI] [PubMed] [Google Scholar]
- 13.Cawley J, Biener A, Meyerhoefer C, et al. Job absenteeism costs of obesity in the United States: National and State-level estimates. J Occup Environ Med. 2021;63(7):565–573. doi: 10.1097/jom.0000000000002198 [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein E, Fiebelkorn C, Wang G. The costs of obesity among full-time employees. Am J Health Promot. 2005;20(1):45–51. doi: 10.4278/0890-1171-20.1.45 [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein EA, DiBonaventura M, Burgess SM, Hale BC. The costs of obesity in the workplace. J Occup Environ Med. 2010;52(10):971–976. doi: 10.1097/JOM.0b013e3181f274d2 [DOI] [PubMed] [Google Scholar]
- 16.Goetzel RZ, Gibson TB, Short ME, et al. A multi-worksite analysis of the relationships among body mass index, medical utilization, and worker productivity. J Occup Environ Med. 2010;52(Suppl1):S52–S58. doi: 10.1097/JOM.0b013e3181c95b84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard JT, Potter LB. An assessment of the relationships between overweight, obesity, related chronic health conditions and worker absenteeism. Obes Res Clin Pract. 2014;8(1):e1–e15. doi: 10.1016/j.orcp.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Kleinman N, Abouzaid S, Andersen L, Wang Z, Powers A. Cohort analysis assessing medical and nonmedical cost associated with obesity in the workplace. J Occup Environ Med. 2014;56(2):161–170. doi: 10.1097/jom.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 19.Waters H, Graf M. The costs of chronic disease in the U.S; 2018. Available from: https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed October 20, 2021.
- 20.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/ep161365.Gl [DOI] [PubMed] [Google Scholar]
- 21.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25):2985–3023. doi: 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Bailey-Davis L, Wood GC, Benotti P, et al. Impact of sustained weight loss on cardiometabolic outcomes. Am J Cardiol. 2022;162:66–72. doi: 10.1016/j.amjcard.2021.09.018 [DOI] [PubMed] [Google Scholar]
- 23.Wood GC, Bailey-Davis L, Benotti P, et al. Effects of sustained weight loss on outcomes associated with obesity comorbidities and healthcare resource utilization. PLoS One. 2021;16(11):e0258545. doi: 10.1371/journal.pone.0258545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US Adults with obesity, by diabetes status. Pharmacoeconomics. 2015;33(7):707–722. doi: 10.1007/s40273-014-0230-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Fan X, Blanchette CM, Smolarz BG, Weng W, Ramasamy A. Economic value of non surgical weight loss in adults with obesity. J Manag Care Spec Pharm. 2021;27(1):37–50. doi: 10.18553/jmcp.2020.20036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. 2005;29(10):1153–1167. doi: 10.1038/sj.ijo.0802982 [DOI] [PubMed] [Google Scholar]
- 27.Ghanemi A, Yoshioka M, St-Amand J. Broken Energy homeostasis and obesity pathogenesis: the surrounding concepts. J Clin Med. 2018;7(11). doi: 10.3390/jcm7110453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1Suppl):222s–225s. doi: 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 29.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray GA, Ryan DH. Evidence-based weight loss interventions: individualized treatment options to maximize patient outcomes. Diabetes Obes Metab. 2021;23(Suppl 1):50–62. doi: 10.1111/dom.14200 [DOI] [PubMed] [Google Scholar]
- 31.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754–762. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 35.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, Phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/s0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 36.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/s0140-6736(10)60888-4 [DOI] [PubMed] [Google Scholar]
- 37.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. NEJM. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 38.Ara R, Blake L, Gray L, et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess. 2012;16(5):iii–xiv, 1–195. doi: 10.3310/hta16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacey LA, Wolf A, O’Shea D, Erny S, Ruof J. Cost-effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes. 2005;29(8):975–982. doi: 10.1038/sj.ijo.0802947 [DOI] [PubMed] [Google Scholar]
- 40.Neovius M, Narbro K. Cost-effectiveness of pharmacological anti-obesity treatments: a systematic review. Int J Obes. 2008;32(12):1752–1763. doi: 10.1038/ijo.2008.189 [DOI] [PubMed] [Google Scholar]
- 41.Zohrabian A. Clinical and economic considerations of antiobesity treatment: a review of orlistat. Clinicoecon Outcomes Res. 2010;2:63–74. doi: 10.2147/ceor.s5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenway FL, Ryan DH, Bray GA, Rood JC, Tucker EW, Smith SR. Pharmaceutical cost savings of treating obesity with weight loss medications. Obes Res. 1999;7(6):523–531. doi: 10.1002/j.1550-8528.1999.tb00709.x [DOI] [PubMed] [Google Scholar]
- 43.Ciciurkaite G, Moloney ME, Brown RL. The incomplete medicalization of obesity: physician office visits, diagnoses, and treatments, 1996–2014. Public Health Rep. 2019;134(2):141–149. doi: 10.1177/0033354918813102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy. 2013;33(12):1299–1307. doi: 10.1002/phar.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacEwan J, Kan H, Chiu K, Poon JL, Shinde S, Ahmad NN. Antiobesity medication use among overweight and obese adults in the United States: 2015–2018. Endocr Pract. 2021;27(11):1139–1148. doi: 10.1016/j.eprac.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 46.Samaranayake NR, Ong KL, Leung RY, Cheung BM. Management of obesity in the National Health and Nutrition Examination Survey (NHANES), 2007–2008. Ann Epidemiol. 2012;22(5):349–353. doi: 10.1016/j.annepidem.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 47.Saxon DR, Iwamoto SJ, Mettenbrink CJ, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009–2015. Obesity. 2019;27(12):1975–1981. doi: 10.1002/oby.22581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, Kelton CML, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity. 2015;23(8):1721–1728. doi: 10.1002/oby.21136 [DOI] [PubMed] [Google Scholar]
- 49.Baum C, Andino K, Wittbrodt E, Stewart S, Szymanski K, Turpin R. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmacoeconomics. 2015;33(7):643–653. doi: 10.1007/s40273-015-0264-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237–248. doi: 10.1016/s2213-8587(17)30236-x [DOI] [PubMed] [Google Scholar]
- 51.Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes. 2018;42(3):495–500. doi: 10.1038/ijo.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jannah N, Hild J, Gallagher C, Dietz W. Coverage for obesity prevention and treatment services: analysis of Medicaid and state employee health insurance programs. Obesity. 2018;26(12):1834–1840. doi: 10.1002/oby.22307 [DOI] [PubMed] [Google Scholar]
- 53.IQVIA Insitute. IQVIA SMART internal database; 2022.
- 54.Kabiri M, Sexton Ward A, Ramasamy A, et al. The societal value of broader access to antiobesity medications. Obesity. 2020;28(2):429–436. doi: 10.1002/oby.22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Health Action Council (UnitedHealth Care U, OptumRx). Finding the uncommon: revealing disparities in care and prescribing for common conditions; 2021. Available from: https://healthactioncouncil.org/getmedia/cd194241-db0a-4db0-81c9-cfedc980b3f5/HAC-White-Paper.pdf. Accessed January 12, 2023.
- 56.Evernorth. Drug trend report; 2020. Available from: https://www.evernorth.com/drug-trend-report. Accessed January 12, 2023.
- 57.IQVIA Insitute. Medicine spending and affordability in the United States: understanding patients’ costs for medicines; 2020. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us. Accessed January 12, 2023.
- 58.Blue Cross Blue Shield Association and Blue Health Intelligence. Prescription drug costs trend update; 2018. Available from: https://www.bcbs.com/sites/default/files/file-attachments/health-of-america-report/HoA-Rx-Costs-Trend-Update.pdf. Accessed January 12, 2023.
- 59.IngenioRx. Taking a total view: linking pharmacy + medical drug trends. Commercial Report; 2017. Available from: https://uploads-ssl.webflow.com/59d2aa5c83887e00019716cc/5aec7315d0dbd43962508a77_2017%20Total%20View%20Drug%20Trend%20Report20180504.pdf. Accessed January 1, 2023.
- 60.Kaiser Family Foundation. How does prescription drug spending and use compare across large employer plans, medicare part D, and Medicaid?; 2019. Available from: https://www.kff.org/medicare/issue-brief/how-does-prescription-drug-spending-and-use-compare-across-large-employer-plans-medicare-part-d-and-medicaid/. Accessed January 12, 2023.
- 61.Kaiser Family Foundation. 2020 employer health benefits survey; 2020. Available from: https://files.kff.org/attachment/Report-Employer-Health-Benefits-2020-Annual-Survey.pdf. Accessed January 12, 2023.
- 62.Centers for Disease Control and Prevention (CDC). Health-Plan and employer-based wellness programs to reduce diabetes risk: the Kaiser Permanente Northern California NEXT-D study; 2013. Available from: https://www.cdc.gov/pcd/issues/2013/pdf/12_0146.pdf. Accessed January 12, 2023. [DOI] [PMC free article] [PubMed]
- 63.Abraham JM, Feldman R, Nyman JA, Barleen N. What factors influence participation in an exercise-focused, employer-based wellness program? INQUIRY. 2011;48(3):221–241. doi: 10.5034/inquiryjrnl_48.03.01 [DOI] [PubMed] [Google Scholar]
- 64.Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolla BP, He JP, Mansukhani MP, Frye MA, Merikangas K. Excessive sleepiness and associated symptoms in the U.S. adult population: prevalence, correlates, and comorbidity. Sleep Health. 2020;6(1):79–87. doi: 10.1016/j.sleh.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olesen J, Dodick DW, Ducros A, et al. Headache classification committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 67.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203 [DOI] [PubMed] [Google Scholar]
- 68.Burke MV, Hays JT, Ebbert JO. Varenicline for smoking cessation: a narrative review of efficacy, adverse effects, use in at-risk populations, and adherence. Patient Prefer Adherence. 2016;10:435–441. doi: 10.2147/PPA.S83469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tonstad S, Arons C, Rollema H, et al. Varenicline: mode of action, efficacy, safety and accumulated experience salient for clinical populations. Curr Med Res Opin. 2020;36(5):713–730. doi: 10.1080/03007995.2020.1729708 [DOI] [PubMed] [Google Scholar]
- 70.Chapman JL, Kempler L, Chang CL, et al. Modafinil improves daytime sleepiness in patients with mild to moderate obstructive sleep apnoea not using standard treatments: a randomised placebo-controlled crossover trial. Thorax. 2014;69(3):274. doi: 10.1136/thoraxjnl-2013-203796 [DOI] [PubMed] [Google Scholar]
- 71.Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Med Sci Monit. 2010;16(8):Ra177–Ra186. [PubMed] [Google Scholar]
- 72.Goadsby PJ, Reuter U, Lanteri-Minet M, et al. Long-term efficacy and safety of erenumab. Neurology. 2021;96(22):e2724. doi: 10.1212/WNL.0000000000012029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ornello R, Casalena A, Frattale I, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32. doi: 10.1186/s10194-020-01102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reuter U, Ehrlich M, Gendolla A, et al. Erenumab versus topiramate for the prevention of migraine - a randomised, double-blind, active-controlled Phase 4 trial. Cephalalgia. 2022;42(2):108–118. doi: 10.1177/03331024211053571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhusal S, Diomampo S, Magrey MN. Clinical utility, safety, and efficacy of pregabalin in the treatment of fibromyalgia. Drug Healthc Patient Saf. 2016;8:13–23. doi: 10.2147/DHPS.S95535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9(9):Cd011790. doi: 10.1002/14651858.CD011790.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology. 2010;49(4):706–715. doi: 10.1093/rheumatology/kep432 [DOI] [PubMed] [Google Scholar]
- 78.Suehs BT, Davis C, Galaznik A, Joshi AV, Zou KH, Patel NC. Association of out-of-pocket pharmacy costs with adherence to varenicline. J Manag Care Spec Pharm. 2014;20(6):592–600. doi: 10.18553/jmcp.2014.20.6.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yue X, Guo JJ, Wigle PR. Trends in utilization, spending, and prices of smoking-cessation medications in Medicaid programs: 25 Years Empirical Data Analysis, 1991–2015. Am Health Drug Benefits. 2018;11(6):275–285. [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng F, Chen CI, Mastey V, Zou KH, Harnett J, Patel BV. Effects of copayment on initiation of smoking cessation pharmacotherapy: an analysis of varenicline reversed claims. Clin Ther. 2011;33(2):225–234. doi: 10.1016/j.clinthera.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 81.Hypersomnia Foundation. Our struggle for prescription drug coverage – 2019 survey results. Available from: https://www.hypersomniafoundation.org/our-struggle-for-prescription-drug-coverage-2019-survey-results/. Accessed March 9, 2022.
- 82.Beasley D. Amgen’s new migraine drug hits insurance hurdles. Reuters. Available from: https://www.reuters.com/article/us-amgen-pricing-migraine/amgens-new-migraine-drug-hits-insurance-hurdles-idUSKBN1KG0CT. Accessed March 9, 2022. [Google Scholar]
- 83.Pearson SD, Beinfeld M, Fluetsch N, Grady M, Shah K, Emond SK. Assessment of barriers to fair access; 2021. Available from: https://icer.org/wp-content/uploads/2021/05/Barriers-to-Fair-Access-Assessment-Final-Report-120121.pdf. Accessed January 12, 2023.
- 84.Tepper DE. Calcitonin gene-related protein (CGRP)-targeted treatments for migraine prevention. Headache. 2019;59(3):477–480. doi: 10.1111/head.13492 [DOI] [PubMed] [Google Scholar]
- 85.Stacey BR, Liss J, Behar R, et al. A systematic review of the effectiveness of policies restricting access to pregabalin. BMC Health Serv Res. 2017;17(1):600. doi: 10.1186/s12913-017-2503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barnett PG, Ignacio RV, Kim HM, et al. Cost-effectiveness of real-world administration of tobacco pharmacotherapy in the United States Veterans Health Administration. Addiction. 2019;114(8):1436–1445. doi: 10.1111/add.14621 [DOI] [PubMed] [Google Scholar]
- 87.Lee LJ, Li Q, Bruno M, et al. Healthcare costs of smokers using varenicline versus nicotine-replacement therapy patch in the United States: evidence from real-world practice. Adv Ther. 2019;36(2):365–380. doi: 10.1007/s12325-018-0858-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lipton RB, Brennan A, Palmer S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–675. doi: 10.1080/13696998.2018.1457533 [DOI] [PubMed] [Google Scholar]
- 89.Sussman M, Benner J, Neumann P, Menzin J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia. 2018;38(10):1644–1657. doi: 10.1177/0333102418796842 [DOI] [PubMed] [Google Scholar]
- 90.Lloyd A, Boomershine CS, Choy EH, Chandran A, Zlateva G. The cost-effectiveness of pregabalin in the treatment of fibromyalgia: US perspective. J Med Econ. 2012;15(3):481–492. doi: 10.3111/13696998.2012.660254 [DOI] [PubMed] [Google Scholar]
- 91.Skaer TL. Fibromyalgia: disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics. 2014;32(5):457–466. doi: 10.1007/s40273-014-0137-y [DOI] [PubMed] [Google Scholar]
- 92.Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496–505. doi: 10.1111/head.13281 [DOI] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention (CDC). Smoking and tobacco use. Available from: https://www.cdc.gov/tobacco/data_statistics/index.htm. Accessed May 25, 2021.
- 94.National Fibromyalgia Association. Fibromyalgia Prevalence. Available from: https://www.fmaware.org/fibromyalgia-prevalence/. Accessed May 27, 2021.
- 95.Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700–714. doi: 10.1111/head.13275 [DOI] [PubMed] [Google Scholar]
- 96.Rojewski AM, Baldassarri S, Cooperman NA, et al. Exploring issues of comorbid conditions in people who smoke. Nicotine Tob Res. 2016;18(8):1684–1696. doi: 10.1093/ntr/ntw016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benca RM. Narcolepsy and excessive daytime sleepiness: diagnostic considerations, epidemiology, and comorbidities. J Clin Psychiatry. 2007;68(Suppl 13):5–8. [PubMed] [Google Scholar]
- 98.Kim SC, Landon JE, Solomon DH. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care Res. 2013;65(11):1813–1819. doi: 10.1002/acr.22071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pfizer Inc. Chantix: package insert; 2019.
- 100.Pfizer Inc. Lyrica: package insert; 2020.
- 101.Amgen, Inc. Aimovig: package insert; 2021.
- 102.Teva Pharmaceuticas USA, Inc. Provigil: package insert; 2015.
- 103.IQVIA. US claims–IQVIA PharMetrics Plus. Available from: https://www.iqvia.com/library/fact-sheets/iqvia-pharmetrics-plus. Accessed March 7, 2022.
- 104.Dee A, Kearns K, O’Neill C, et al. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes. 2014;7:242. doi: 10.1186/1756-0500-7-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frech FQC, Gore M, Zhang Q. Cost implications of early treatment initiation among patients with newly diagnosed fibromyalgia. Am J Pharm Benefits. 2017;9(6):200–207. [Google Scholar]
- 106.Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One. 2015;10(9):e0138024–e0138024. doi: 10.1371/journal.pone.0138024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tepper SJ, Fang J, Vo P, et al. Impact of erenumab on acute medication usage and health care resource utilization among migraine patients: a US claims database study. J Headache Pain. 2021;22(1):27. doi: 10.1186/s10194-021-01238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yucel A, Thach A, Kumar S, Loden C, Bensink M, Goldfarb N. Estimating the economic burden of migraine on US employers. Am J Manag Care. 2020;26(12):e403–e408. doi: 10.37765/ajmc.2020.88547 [DOI] [PubMed] [Google Scholar]
- 109.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–458. doi: 10.1093/sleep/27.3.453 [DOI] [PubMed] [Google Scholar]
- 111.Carlton RR, Lunascek T, Dammerman O. Healthcare resource utilization before and after initiation of armodafinil treatment for wakefulness. Sleep. 2011;34:5. [Google Scholar]
- 112.Faust E, Pivneva I, Yang K, et al. Real-world treatment profiles, clinical outcomes, and healthcare resource utilization of patients with migraine prescribed erenumab: a multicenter chart-review study of US headache centers. Neurol Ther. 2021;10(1):293–306. doi: 10.1007/s40120-021-00245-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harnett J, Margolis J, Cao Z, et al. Real-world evaluation of health-care resource utilization and costs in employees with fibromyalgia treated with pregabalin or duloxetine. Pain Pract. 2011;11(3):217–229. doi: 10.1111/j.1533-2500.2010.00440.x [DOI] [PubMed] [Google Scholar]
- 114.Gartner Glossary. Small and Midsize Business (SMB). Available from: https://www.gartner.com/en/information-technology/glossary/smbs-small-and-midsize-businesses. Accessed March 8, 2022.
- 115.U.S. Bureau of Labor Statistics. Occupational employment and wage statistics. Available from: https://www.bls.gov/oes/current/oes_nat.htm. Accessed May 25, 2021.
- 116.Bilger M, Finkelstein EA, Kruger E, Tate DF, Linnan LA. The effect of weight loss on health, productivity, and medical expenditures among overweight employees. Med Care. 2013;51(6):471–477. doi: 10.1097/MLR.0b013e318286e437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kolotkin RL, Gabriel Smolarz B, Meincke HH, Fujioka K. Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity. Clin Obes. 2018;8(1):1–10. doi: 10.1111/cob.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Managed Markets Insight & Technology LLC. Formulary Lookup; 2021. Available from: www.formularylookup.com. Accessed June 16, 2021.
- 119.National Institute for Health and Care Excellence (NICE). NICE recommends new drug for people living with obesity. Available from: https://www.nice.org.uk/news/article/nice-recommends-new-drug-for-people-living-with-obesity. Accessed March 10, 2022. [PubMed]
- 120.Halpern MT, Shikiar R, Rentz AM, Khan ZM. Impact of smoking status on workplace absenteeism and productivity. Tob Control. 2001;10(3):233–238. doi: 10.1136/tc.10.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reynolds AC, Appleton SL, Gill TK, et al. Sickness absenteeism is associated with sleep problems independent of sleep disorders: results of the 2016 Sleep Health Foundation national survey. Sleep Health. 2017;3(5):357–361. doi: 10.1016/j.sleh.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 122.Michel P, Dartigues JF, Duru G, Moreau J, Salamon R, Henry P. Incremental absenteeism due to headaches in migraine: results from the Mig-Access French national cohort. Cephalalgia. 1999;19(5):503–510. doi: 10.1046/j.1468-2982.1999.019005503.x [DOI] [PubMed] [Google Scholar]
- 123.Chandran A, Schaefer C, Ryan K, Baik R, McNett M, Zlateva G. The comparative economic burden of mild, moderate, and severe fibromyalgia: results from a retrospective chart review and cross-sectional survey of working-age U.S. adults. J Manag Care Pharm. 2012;18(6):415–426. doi: 10.18553/jmcp.2012.18.6.415 [DOI] [PMC free article] [PubMed] [Google Scholar]