Abstract
Purpose of Review
To review recent literature on Malassezia folliculitis and explore its association with COVID-19.
Recent Findings
Reports of Malassezia folliculitis in the setting of COVID-19 are scarce. Shared characteristics between affected individuals include male sex, obesity, intensive care, and administration of systemic antibiotics and systemic steroids. Dexamethasone can potentially stimulate sebum production and therefore lead to Malassezia proliferation. The clinical picture of Malassezia folliculitis accompanying COVID-19 is similar to classic descriptions but tends to spare the face and predominates in occlusion sites.
Summary
Malassezia folliculitis is under-recognized. Fever, sweating, occlusion, immobility, antibiotics, and dexamethasone contribute to COVID-19 patients developing Malassezia folliculitis. Antifungal therapy, together with correcting predisposing factors, is the mainstay of management. Future research should explore the relationship between systemic steroids and other acneiform reactions.
Keywords: Malassezia folliculitis, Pityrosporum folliculitis, Fungal folliculitis, COVID-19, Dexamethasone, Acneiform
Introduction
The genus Malassezia is composed of lipophilic yeasts found in mammals’ skin and mucosae. The current taxonomy recognizes 18 species [1]. In humans, these yeasts play both the role of commensals and pathogens. Malassezia represents the most common eukaryote in the human cutaneous microbiome and prevails in seborrheic regions, but also plays a clear role in skin diseases such as seborrheic dermatitis, pityriasis versicolor, head and neck dermatitis, and Malassezia folliculitis [2•]. Malassezia folliculitis (MF) is an inflammatory process centered on the pilosebaceous unit due to an overproliferation of the yeasts. It is still occasionally termed Pityrosporum folliculitis in recent publications, despite the incorporation since the 1980s of Pityrosporum into the Malassezia genus [3]. Several recent reports have described its development accompanying COVID-19 infection [4•, 5•, 6•, 7•]. This report seeks to review the literature regarding MF with a focus on recent papers and analyze its association with COVID-19.
A Review on Malassezia Folliculitis
MF usually presents as a monomorphic acneiform eruption of seborrheic regions. The distribution of sex and age varies across reports, but three recent studies coincide with a predilection for young adult males. A large multi-centric cohort from Indonesia that included 353 individuals with MF showed a 2:1 male-to-female ratio, with 44.5% of those affected composing the group of 17 to 25 years old [8••]. A study from Singapore produced comparable results: 214 individuals with confirmed MF through Gram staining showed a 3:1 male-to-female ratio and a predominance of 35% in the 21 to 30 years group [9]. A retrospective cohort from Taiwan that included pediatric and adult patients with confirmed MF reported a median age of 28 years and a male predominance of 76% when averaging both age groups [10•]. However, a fourth cohort focused on pediatric patients with MF showed a female predominance [11].
Risk factors attributed to the development of MF are varied. Regarding climate, MF tends to develop in tropical regions [12••] or during the summer months in more temperate ones, suggesting that sweating and humidity can be predisposing factors. Summer was the season with the highest MF diagnoses in a Taiwan study [10•]. A classic study from the Philippines reported that it represented 16% of outpatient consults in a single private center [13]. The investigators explicitly reference the region’s warm and humid climate as a contributing factor, as well as exercise and using clothes that increase body heat as additional triggers. A study focused on individuals 21 years or younger highlights the pathogenesis MF shares with acne by citing sebum production and follicular occlusion as predisposing factors to both [11]. In the same paper, antibiotic use was reported by 75% of those affected by MF, proposing it as an additional factor that can lead to Malassezia proliferation. Immunosuppression is a risk factor for developing skin diseases related to Malassezia [14]. In this setting, conditions associated with MF include diabetes, living with HIV, systemic steroid use, malignancies, and solid organ and bone marrow transplants [12••]. The previously mentioned study from Taiwan retrospectively addressed some of these predisposing factors: patients reported frequencies of excessive sweating (4%), use of antibiotics (24%), and malignancy (1.6%) with no statistically significant difference between age groups [10•].
Vlachos et al. succinctly summarize the pathogenesis of MF as a series of predisposing factors that lead to Malassezia proliferation in the pilosebaceous unit [12••]. These include follicular occlusion, increased sebum production, alterations in the cutaneous microbiome, and a disrupted immune response, integrating the previously commented risk factors. The yeast’s proliferation results in an inflammatory response and subsequent clinical manifestations. Multiple papers coincide that M. globosa is the most commonly identified species from samples obtained from lesions of MF (69 to 84%) [15–17]. Interestingly, M. globosa responds favorably in vitro over other species to high temperatures and the components of sweat [18], which suggests a link between this observed predominance and risk factors associated with MF. A recent study observed M. globosa’s heightened ability to induce inflammasome-mediated inflammation [19].
Recent cohorts describe similar clinical presentations [8••, 9, 10•, 20]. The most affected body region is the trunk, followed by the upper limb [8••, 9, 20] or the neck [10•]. In contrast, a pediatric cohort reported a predominance of facial lesions [11]. Reports consistently describe lesions as monomorphic, 1 to 2-mm follicular papules and pustules. Pruritus was variably present (36 to 83%). Where reported, other conditions associated with Malassezia, such as seborrheic dermatitis or pityriasis versicolor, accompanied MF in approximately 20% [11, 20]. Tsai et al. described atypical presentations of biopsy-proven MF reminiscent of eosinophilic folliculitis, pityriasis lichenoides, prurigo, rosacea, and mucinosis [21]. The primary differential diagnosis of MF is acne, and comedones, nodules, and cysts are notably absent in MF. Other differentials include bacterial folliculitis, acneiform eruptions related to drugs, rosacea, demodicosis, and perioral dermatitis [22].
Some studies have explored the relationship between MF and other acneiform eruptions and their distinguishing features. Yu et al. studied whether steroid acne could be related to Malassezia: the authors reported that direct microscopic examination of expressed follicular contents and skin biopsy showed findings consistent with MF in 76% and 37% of steroid acne cases, respectively [23]. Pürnak et al. reported that in 217 patients with a clinical diagnosis of acne vulgaris, MF was diagnosed in 25.3% through cytological examination of follicular contents [24]. An et al. compared clinical and histopathological findings between MF and other acneiform [22]. They found that MF was more common in the chest, back, and neck and other acneiform eruptions predominated on the face. Lesional morphology showed no differences. In histopathology, investigators observed Malassezia spores only in skin biopsies from MF. The biopsies from other acneiform eruptions showed significantly more frequent nuclear dust, intrafollicular inflammatory infiltrate, and necrotic keratinocytes in the follicular wall.
The gold standard for the diagnosis of MF is histopathology. Skin biopsies from MF lesions show a dilated pilosebaceous follicle with plugged ostium and a perifollicular mononuclear inflammatory infiltrate [12••]. Occasionally, a brisker infiltrate is present when the follicular contents are discharged into the surrounding dermis. The key to diagnosis is the identification of spherical yeasts within the pilosebaceous unit, highlighted with fungal stains. Investigators have proposed a threshold of 10 or more yeasts per high-power field (400 × magnification) to establish the diagnosis [25]. Others have suggested that performing transverse sections of the pilosebaceous unit may increase the diagnostic yield [23].
Other less invasive tests are helpful to support a diagnosis of MF. Direct microscopic examination of samples aids in evidencing the fungi. Malassezia presents as tiny, ovoid, thick-walled yeasts that can feature unipolar budding [12••]. In MF, observing hyphae is infrequent [16]. Jacinto-Jamora et al. described using a comedone extractor to obtain the full content of the inflamed follicle instead of acquiring more superficial samples by only scraping lesions [13]: subsequent studies replicate this technique. In order to highlight the yeasts, multiple helpful solutions and stains exist. These include KOH 10 to 20% with or without Parker ink, Albert’s stain, May-Grunwald-Giemsa stain, Gram stain, lactophenol blue, methylene blue, and if fluorescent microscopy is available, calcofluor white [16, 26•]. Tu et al. evaluated the utility of Gram staining to identify cases of MF compared to a set of diagnostic criteria and identified a sensitivity of 84.6% and a specificity of 100% with this technique [26•]. The established cutoff point was 30 or more yeasts or short hyphae identified in three high-power fields. Notably, the investigators sampled up to five lesions per patient. By demonstrating the presence of bacteria, Gram staining was additionally helpful in identifying cases of bacterial or mixed folliculitis. Other auxiliary non-invasive techniques to diagnose MF include dermoscopy [20, 27], reflectance confocal microscopy, and optical coherence tomography [28]. The most common dermoscopic finding is folliculocentric lesions surrounded by erythema.
Correcting predisposing factors, when possible, is fundamental in managing MF. These include avoiding heat, sweat-predisposing activities, and habits that lead to the occlusion of hair follicles, such as using tight-fitting clothing or heavy emollients or oils. The medical management of MF includes keratolytics and topical and systemic antifungals, but solid evidence for guidance is sparse. Vlachos et al. recently reviewed the treatment of MF [12••]. They recommend topical antifungals as first-line therapy, except in refractory cases or immunocompromised individuals, where systemic therapy is warranted. A recent meta-analysis and systematic review did not observe a statistically significant difference between cure rates obtained from systemic or topical medication [29]. However, significant heterogeneity between studies was present. Investigators concluded that topical treatment is as effective as oral for managing MF but insisted on the need for more robust evidence. Topical options include ketoconazole, clotrimazole, ciclopirox olamine, selenium sulfide, and zinc pyrithione. Systemic alternatives encompass itraconazole and fluconazole.
Malassezia Folliculitis in the Setting of COVID-19
Table 1 summarizes the reports describing folliculitis caused by Malassezia in individuals with concomitant infection by SARS-CoV-2 [4•, 5•, 6•]. Histopathology confirmed the diagnosis in every case, and fungal stains were employed to highlight the yeasts. All subjects were male adult patients with severe COVID-19 that required inpatient management. Where specified, shared features among some individuals included obesity, type 2 diabetes, the need for intensive care and mechanical ventilation, and the use of systemic steroids and systemic antibiotics. The clinical presentation was an acneiform eruption composed of monomorphic papules and pustules. The affected regions included the chest, abdomen, and upper limbs, with one paper highlighting the development in sites under occlusion (central venous catheter (CVC) insertion site). The reports do not include information regarding pruritus as an accompanying symptom or results from fungal culture. The only mentioned treatment was fluconazole 100 mg daily in one of the communications, but the response was not specified. Of the three reported outcomes, two patients expired. An additional study includes two cases of Malassezia folliculitis but does not describe any clinical data [7•]. Figure 1 portrays the clinical and histopathological findings from a skin biopsy demonstrating MF in a patient admitted for severe COVID-19.
Table 1.
A summary of reports describing Malassezia folliculitis in the setting of COVID-19
| Authors | Number of cases | Sex (age in years) | Associated conditions | Sites of lesions | Treatment |
|---|---|---|---|---|---|
| Barrera-Godínez et al. [4•] | 4 | Male (51) | T2D, Ob, SS, AB | Chest, abdomen, upper limbs | N/A |
| Male (58) | Ob, SS | ||||
| Male (33) | SS, AB | ||||
| Male (48) | T2D, Ob, SS, AB | ||||
| Sandoval-Tres et al. [5•] | 3 | Male (65) | Ob, SS | CVC insertion site, trunk, upper limbs | Fluconazole 100 mg qd |
| Male (50) | Ob, HTN, RF, SS | ||||
| Male (35) | SS, hypercholesterolemia | ||||
| Peres et al. [6•] | 3 | Male (52) | Ob, T2D, SS, AB, MV, RF | Chest, abdomen, upper limbs | N/A |
| Male (46) | Ob, SS, AB, MV | ||||
| Male (39) | Ob, SS, AB |
*AB, antibiotics; HTN, hypertension; MV, mechanical ventilation; RF, renal failure; Ob, obesity; SS, systemic steroids; T2D, type 2 diabetes mellitus
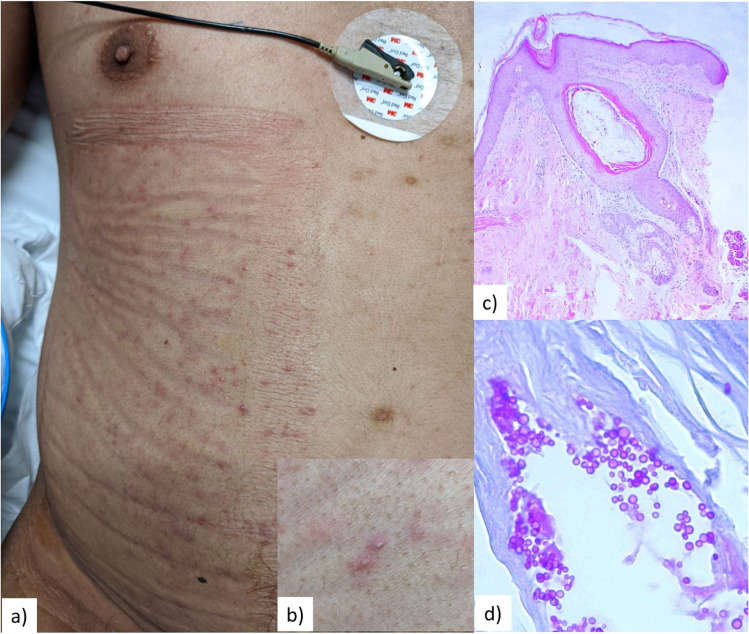
Fig. 1.
Clinical and histopathological findings in an individual with COVID-19 who developed Malassezia folliculitis in the ICU. Panel (a) shows the stark predominance of lesions under a site of occlusion, below a dressing used to protect the skin during for prone positioning. Panel (b) highlights the monomorphic, follicular pustules. Panel (c) exhibits the skin biopsy findings, including a dilated follicle with sparse inflammatory infiltrate (H&E, 10x). Panel (d) displays multiple yeasts and occasional budding highlighted with fungal stains (PAS, 60x)
Discussion
Few reports describing MF in the setting of COVID-19 exist, likely due to under-recognition. Multiple entities can present with acneiform eruptions in which skin biopsies are rarely performed because a diagnosis is usually established clinically. Without the proper investigations, clinicians can miss MF. Therefore, adequate suspicion is imperative to prompt its search. Acneiform eruptions accompanied by pruritus, with extension to arms or abdomen, or with no evidence of comedones warrant further study.
Unsurprisingly, MF can develop in patients admitted because of COVID-19 since these individuals present many of the previously reviewed risk factors, especially in the ICU. First, COVID-19 is a febrile illness that leads to sweating and a rise in body temperature. Second, cutaneous occlusion is not unusual in inpatients, partly due to dressings and medical devices but also immobility. Individuals requiring respiratory support with mechanical ventilation have limited mobility, particularly those in prone ventilation. Additionally, dressings that protect the skin from pressure-induced injury further aggravate occlusion and sweating. Third, as seen in Table 1, the concomitant use of antibiotics was frequent in these cases, mainly for managing healthcare-associated infections, which can allow species of Malassezia to proliferate due to dysbiosis. However, fever, immobility, occlusion, and antibiotic exposure are typical in the intensive care setting, whereas MF is not.
Managing severe COVID-19 requires systemic steroids, one of the most recurrent characteristics described in Table 1, and their use is probably the tipping point in the development of MF. Their role is twofold. On the one hand, they can lead to the altered immune response associated with diseases in which Malassezia has a role, as seen in immunosuppressed individuals. On the other hand, dexamethasone could increase sebum production and therefore stimulate yeast proliferation. Lee et al. demonstrated increased lipid synthesis of cultured sebocytes after in vitro exposure to dexamethasone [30]. These results establish a plausible explanation that links dexamethasone with Malassezia proliferation and, therefore, MF. These findings also highlight the need to discern between steroid acne, steroid folliculitis, and MF, which could represent a continuum. Nonetheless, ascertaining dexamethasone’s role in the development of MF requires further investigations.
A striking finding in Table 1 is the predominance of males across reports. As discussed, recent MF cohorts consistently show a higher proportion of men in their samples. However, the cases in Table 1 probably reflect the epidemiology of severe COVID-19, which is more common in males. The same could be said about accompanying comorbidities, such as obesity and type 2 diabetes. Proper cohorts are needed to calculate the risk of developing MF attributed to these conditions.
The clinical presentation of MF accompanying COVID-19 is consistent with previous descriptions. Notably, the cases summarized in Table 1 did not present facial involvement but highlighted occlusion’s role in MF. Pruritus was not reported, although evaluating it is difficult in an intensive care setting. The principal differential diagnosis was acneiform eruptions secondary to the use of steroids.
When MF is suspected, the authors of this paper prefer obtaining lesional samples from expressed follicular contents instead of superficial scrapings. However, comedone extractors are uncommon in the inpatient setting, and introducing them into COVID-19 areas may be undesirable. An alternative is to use a disposable sterile syringe with a removable needle. First, the needle is used to deroof a follicular pustule. Then, with the needle removed, the tip of the syringe is centered on the follicle, and perpendicular pressure is applied. The expressed follicular contents will be collected inside the syringe’s tip, where they can be retrieved with the needle. Then, they can be mounted on a slide for microscopic examination. Finally, the contaminated syringe can be discarded. Sampling more than one lesion is desirable.
The management of MF accompanying COVID-19 is not different from typical cases. Predisposing factors should be addressed and corrected. Interventions include control of fever, relief of occlusion, encouragement of mobility, antibiotic cessation if unneeded, and withdrawal of dexamethasone after ten days of treatment. Topical treatment avoids pharmacological interactions and systemic effects and may be preferred in mild cases. Systemic therapy can be used as first-line therapy in extensive lesions or additional immunosuppression.
Conclusions
Malassezia folliculitis is an under-recognized entity. Shared predisposing factors explain its development in the setting of COVID-19, which include fever, sweating, immobility, occlusion, systemic antibiotics, and systemic steroids. The use of dexamethasone could represent the critical step by stimulating sebum production and Malassezia proliferation and clarify what makes the relationship between MF and COVID-19 unique. Further research is needed to explore this association.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Vijaya Chandra SH, Srinivas R, Dawson TL, Jr, Common JE. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2021;10:614446. doi: 10.3389/fcimb.2020.614446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;20(10):112. doi: 10.3389/fcimb.2020.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25(1):106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.• Barrera-Godínez A, Méndez-Flores S, Gatica-Torres M, Rosales-Sotomayor A, Campos-Jiménez KI, Carrillo-Córdova DM, Durand-Muñoz MC, Mena-Hernández GL, Melchor-Mendoza YK, Ruelas-Villavicencio AL, García-Irigoyen A, Acatitla-Acevedo GA, Toussaint-Caire S, Domínguez-Cherit J. Not all that glitters is COVID-19: a case series demonstrating the need for histopathology when skin findings accompany SARS-CoV-2 infection. J Eur Acad Dermatol Venereol. 2021;35(9):1865–1873. 10.1111/jdv.17381. A case series that includes 4 cases of MF confirmed histologically. [DOI] [PMC free article] [PubMed]
- 5.Sandoval C, Rodríguez F, Bonifaz A. Foliculitis por Malassezia en pacientes con COVID-19 portadores de catéter venoso central. Dermatología Revista Mexicana. 2021;65(6):1021–1023. [Google Scholar]
- 6.• Peres FLX, Bonamigo RR, Bottega GB, Staub FL, Cartell AS, Bakos RM. Pityrosporum folliculitis in critically ill COVID-19 patients. J Eur Acad Dermatol Venereol. 2022;36(3):e186–e188. 10.1111/jdv.17842. A short communication that includes 3 cases of MF and explores associated clinical conditions. [DOI] [PMC free article] [PubMed]
- 7.• Giavedoni P, Podlipnik S, Pericàs JM, Fuertes de Vega I, García-Herrera A, Alós L, Carrera C, Andreu-Febrer C, Sanz-Beltran J, Riquelme-Mc Loughlin C, Riera-Monroig J, Combalia A, Bosch-Amate X, Morgado-Carrasco D, Pigem R, Toll-Abelló A, Martí-Martí I, Rizo-Potau D, Serra-García L, Alamon-Reig F, Iranzo P, Almuedo-Riera A, Muñoz J, Puig S, Mascaró JM Jr. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med. 2020;9(10):3261. 10.3390/jcm9103261. A large series that includes 2 cases of MF with no accompanying clinical information. [DOI] [PMC free article] [PubMed]
- 8.•• Niode NJ, Suling PL, Adji A, Miranda E, Bramono K, Astari L, Ervianti E, Sondakh ORL, Rusmawardiana, Yenny SW, Widasmara D, Lubis FM, Widaty S. Clinico-laboratory findings of Malassezia folliculitis in Indonesia: a multicentre study. Mycoses. 2022;65(10):953–9. 10.1111/myc.13511. The largest published cohort of MF. [DOI] [PubMed]
- 9.Yong AM, Tan SY, Tan CL. An update on pityrosporum folliculitis in Singapore from a single tertiary care dermatological centre. Singapore Med J. 2021;62(10):526–528. doi: 10.11622/smedj.2020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai YC, Wang JY, Wu YH, Wang YJ. Clinical differences in pediatric and adult Malassezia folliculitis: retrospective analysis of 321 cases over 9 years. J Am Acad Dermatol. 2019;81(1):278–280. doi: 10.1016/j.jaad.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Prindaville B, Belazarian L, Levin NA, Wiss K. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78(3):511–514. doi: 10.1016/j.jaad.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Vlachos C, Henning MAS, Gaitanis G, Faergemann J, Saunte DM. Critical synthesis of available data in Malassezia folliculitis and a systematic review of treatments. J Eur Acad Dermatol Venereol. 2020;34(8):1672–1683. doi: 10.1111/jdv.16253. [DOI] [PubMed] [Google Scholar]
- 13.Jacinto-Jamora S, Tamesis J, Katigbak ML. Pityrosporum folliculitis in the Philippines: diagnosis, prevalence, and management. J Am Acad Dermatol. 1991;24(5 Pt 1):693–696. doi: 10.1016/0190-9622(91)70104-a. [DOI] [PubMed] [Google Scholar]
- 14.Tragiannidis A, Bisping G, Koehler G, Groll AH. Minireview: Malassezia infections in immunocompromised patients. Mycoses. 2010;53(3):187–195. doi: 10.1111/j.1439-0507.2009.01814.. [DOI] [PubMed] [Google Scholar]
- 15.Cheikhrouhou F, Guidara R, Masmoudi A, Trabelsi H, Neji S, Sellami H, Makni F, Ayadi A. Molecular identification of Malassezia species in patients with Malassezia folliculitis in Sfax. Tunisia Mycopathologia. 2017;182(5–6):583–589. doi: 10.1007/s11046-017-0113-0. [DOI] [PubMed] [Google Scholar]
- 16.Durdu M, Güran M, Ilkit M. Epidemiological characteristics of Malassezia folliculitis and use of the May-Grünwald-Giemsa stain to diagnose the infection. Diagn Microbiol Infect Dis. 2013;76(4):450–457. doi: 10.1016/j.diagmicrobio.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Akaza N, Akamatsu H, Sasaki Y, Kishi M, Mizutani H, Sano A, Hirokawa K, Nakata S, Nishijima S, Matsunaga K. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47(6):618–624. doi: 10.1080/13693780802398026. [DOI] [PubMed] [Google Scholar]
- 18.Akaza N, Akamatsu H, Takeoka S, Sasaki Y, Mizutani H, Nakata S, Matsunaga K. Malassezia globosa tends to grow actively in summer conditions more than other cutaneous Malassezia species. J Dermatol. 2012;39(7):613–616. doi: 10.1111/j.1346-8138.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 19.Park HR, Oh JH, Lee YJ, Park SH, Lee YW, Lee S, Kang H, Kim JE. Inflammasome-mediated Inflammation by Malassezia in human keratinocytes: a comparative analysis with different strains. Mycoses. 2021;64(3):292–299. doi: 10.1111/myc.13214. [DOI] [PubMed] [Google Scholar]
- 20.Jakhar D, Bhatia V, Gupta RK, Kaur I. Dermoscopy as an auxiliary tool in the assessment of Malassezia folliculitis: an observational study. Actas Dermosifiliogr. 2022;113(1):T78–T81. doi: 10.1016/j.ad.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Tsai YC, Wang JY, Wu YH, Wang YJ. Atypical clinical presentations of Malassezia folliculitis: a retrospective analysis of 94 biopsy-proven cases. Int J Dermatol. 2018;57(3):e19–e20. doi: 10.1111/ijd.13880. [DOI] [PubMed] [Google Scholar]
- 22.An MK, Hong EH, Cho EB, Park EJ, Kim KH, Kim KJ. Clinicopathological differentiation between Pityrosporum folliculitis and acneiform eruption. J Dermatol. 2019;46(11):978–984. doi: 10.1111/1346-8138.15070. [DOI] [PubMed] [Google Scholar]
- 23.Yu HJ, Lee SK, Son SJ, Kim YS, Yang HY, Kim JH. Steroid acne vs. Pityrosporum folliculitis: the incidence of Pityrosporum ovale and the effect of antifungal drugs in steroid acne. Int J Dermatol. 1998;37(10):772–7. doi: 10.1046/j.1365-4362.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Pürnak S, Durdu M, Tekindal MA, Güleç AT, Seçkin D. The prevalence of Malassezia folliculitis in patients with papulopustular/comedonal acne, and their response to antifungal treatment. Skinmed. 2018;16(2):99–104. [PubMed] [Google Scholar]
- 25.Sei Y. Malassezia infections. Med Mycol J. 2012;53(1):7–11. doi: 10.3314/mmj.53.7. [DOI] [PubMed] [Google Scholar]
- 26.Tu WT, Chin SY, Chou CL, Hsu CY, Chen YT, Liu D, Lee WR, Shih YH. Utility of Gram staining for diagnosis of Malassezia folliculitis. J Dermatol. 2018;45(2):228–231. doi: 10.1111/1346-8138.14120. [DOI] [PubMed] [Google Scholar]
- 27.Jakhar D, Kaur I, Chaudhary R. Dermoscopy of pityrosporum folliculitis. J Am Acad Dermatol. 2019;80(2):e43–e44. doi: 10.1016/j.jaad.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 28.Andersen AJB, Fuchs C, Ardigo M, Haedersdal M, Mogensen M. In vivo characterization of pustules in Malassezia folliculitis by reflectance confocal microscopy and optical coherence tomography. A case series study. Skin Res Technol. 2018;24(4):535–541. doi: 10.1111/srt.12463. [DOI] [PubMed] [Google Scholar]
- 29.Chaitidis N, Festas C, Aritzi I, Kyriazopoulou M. Oral treatment with/without topical treatment vs topical treatment alone in Malassezia folliculitis patients: a systematic review and meta-analysis. Dermatol Ther. 2020;33(3):e13460. doi: 10.1111/dth.13460. [DOI] [PubMed] [Google Scholar]
- 30.Lee SE, Kim JM, Jeong MK, Zouboulis CC, Lee SH. 11β-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. Br J Dermatol. 2013;168(1):47–55. doi: 10.1111/bjd.12009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.