Abstract
Lewy body diseases, such as Parkinson’s disease and dementia with Lewy bodies, vary in their clinical phenotype but exhibit the same defining pathological feature, α-synuclein aggregation. Microbiome–gut–brain dysfunction may play a role in the initiation or progression of disease processes, though there are multiple potential mechanisms. We discuss the need to evaluate gastrointestinal mechanisms of pathogenesis across Lewy body diseases, as disease mechanisms likely span across diagnostic categories and a ‘body first’ clinical syndrome may better account for the heterogeneity of clinical presentations across the disorders. We discuss two primary hypotheses that suggest that either α-synuclein aggregation occurs in the gut and spreads in a prion-like fashion to the brain or systemic inflammatory processes driven by gastrointestinal dysfunction contribute to the pathophysiology of Lewy body diseases. Both of these hypotheses posit that dysbiosis and intestinal permeability are key mechanisms and potential treatment targets. Ultimately, this work can identify early interventions targeting initial disease pathogenic processes before the development of overt motor and cognitive symptoms.
Keywords: Idiopathic REM sleep behavior disorder, Parkinson’s disease, Dementia with Lewy bodies, Gut–brain axis, Dysbiosis, Intestinal permeability
Introduction
The spectrum of Lewy body diseases ranges from incidental Lewy body disease, Parkinson’s disease (PD) with varying degrees of cognitive impairment, to dementia with Lewy bodies (DLB) at the most severe end [1]. PD is characterized by motor symptoms (bradykinesia, rigidity, and tremor [2]), with recent, increased appreciation that non-motor symptoms are common and significantly impact quality of life [3]. Estimated prevalence of PD ranges between 100 and 200 per 100,000 people and an annual incidence of 15 per 100,000 [4]. DLB is characterized by fluctuating cognition, recurrent visual hallucinations, REM sleep behavior disorder, and parkinsonism (including bradykinesia, rigidity, and tremor) [5]. Point and period prevalence estimates of DLB range from 0.02 to 63.5 per 1000 persons [6], with considerable variability likely related to underdiagnosis of the disorder [7, 8].
As PD and DLB are diagnosed after there has been significant degeneration (e.g. PD results in 50–70% loss of neurons in the substantia nigra before clinical diagnosis occurs [9]), it is crucial to develop diagnostic tools and interventions that can be used early in the disease course. Idiopathic REM sleep behavior disorder (iRBD) is one of the earliest and most specific prodromal indicators of Lewy body diseases [10–12] with one in three idiopathic iRBD patients converting to a Lewy body disease within 5 years, 82.4% at 10.5 years, and 96.6% at 14 years. Of the converters, 44% will convert to PD and 25% to dementia with Lewy bodies [13]. Therefore, iRBD cohorts provide a unique opportunity to identify early disease mechanisms and develop neuroprotective interventions for Lewy body diseases.
The phenotypic presentation of Lewy body diseases largely depends on the location of the pathological initiation and progression [14–16]. While the clinical phenotypes of Lewy body diseases differ, the aggregation of alpha-synuclein (α-syn) is a defining feature [17, 18]. Through careful assessment of the propagation pattern of PD pathology, Braak and colleagues proposed that PD may be caused by an intestinal pathogen [19]. Several reviews have discussed the evidence to suggest that the gut plays an important role in the initiation or progression of pathological processes [20–24], though many questions remain regarding the mechanisms by which gut dysfunction leads to the development of Lewy body diseases. In the current review, we discuss two main hypotheses by which gastrointestinal dysfunction contributes to pathogenesis or disease progression. The first builds on Braak’s initial theory and posits that Lewy body diseases are caused by α-syn aggregation in the gut which travels in a prion-like fashion to the central nervous system (CNS). The second, more recent hypothesis emphasizes chronic intestinal proinflammatory processes as key mechanisms [24]. These theories are not mutually exclusive as there are likely interactions between immune responses and α-syn at all stages of the disease process. How early in the disease course a proinflammatory response versus α-syn aggregation occurs is an area of controversy and active investigation. Notably, both of these hypotheses emphasize dysbiosis (an imbalance in the microbiota) and intestinal permeability (translocation of lumen products across the gut wall, also referred to as “leaky gut”) as key mechanisms in the initiation and progression of pathophysiology in Lewy body diseases.
Gastrointestinal dysfunction
Historically, PD research had focused on the motor symptoms of the disorder, though it is increasingly appreciated that gastrointestinal dysfunction, most commonly constipation, is one of the earliest prodromal symptoms of PD [25]. PD patients are three times more likely to experience constipation [26]. Constipation can precede motor deficits of PD by decades [27, 28] and is associated with worse outcomes, including earlier onset of dementia [29, 30]. In addition, patients may also experience sialorrhea and dysphagia, gastroparesis, and small intestinal bacterial overgrowth (SIBO), demonstrating pan-gut involvement [31, 32]. LBD patients experience similar, if not more severe gastrointestinal symptoms, with evidence that gastric emptying is slower in DLB patients relative to PD [33], though there is currently limited examination in DLB. Of note, objective colonic dysfunction is far more prevalent than subjective constipation in PD, highlighting the need to incorporate objective assessments to detect gastrointestinal dysfunction in Lewy body diseases [34].
SIBO was historically viewed as a cause of malabsorption and required invasive aspiration to obtain cultures of jejunal aspirate to diagnose [35]. Over time, it was recognized that intestinal bacteria were the sole source of certain gases, such as hydrogen and methane, that could be detected in exhaled breath. This was leveraged to develop glucose and lactulose breath tests for SIBO [36]. Using this approach, ~ ½ of PD patients test positive for SIBO [37, 38] and the occurrence of SIBO was associated with more severe motor fluctuations [38, 39]. SIBO eradication led to a significant improvement in patients OFF time and delayed ON episodes each day, though there is a high rate of SIBO relapse at 6 months (43%) [38]. SIBO was not directly related to small bowel transit delays [40] nor associated with worse gastrointestinal symptoms, but independently predicted worse motor function [41]. Gastrointestinal disorders that exhibit increased rates of SIBO, such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome are associated with an increased risk of PD [42, 43], suggesting SIBO may increase risk of PD or may play a role in the pathogenesis of Lewy body diseases. Evaluation of SIBO has been primarily conducted in more advanced patients. Given potential improvement in motor functioning, it is important to evaluate SIBO within prodromal, early stage PD, and DLB cohorts to understand the impact across phenotypes. For a detailed review of gastrointestinal symptoms in PD, refer to [26, 27, 32].
Pathological processes
Lewy bodies and Lewy neurites are the defining neuropathological characteristics of PD and DLB [44–46]. Point mutations in the gene encoding α-syn (SNCA) were found to be pathogenic for familial forms of PD [47], which led to the subsequent discovery that α-syn is the principal component of Lewy bodies [17]. α-syn in its normal form is found within the presynaptic regions of neurons, either unfolded or contained in alpha-helical membrane-bound forms. Aggregation refers to the process by which α-syn becomes partially folded and aggregates to form oligomers, protofibrils, fibrils, and mature Lewy bodies [17, 48, 49]. It is unclear whether these variants of protein structure reflect distinct pathologies or a continuum of conformations reflecting the different stages of Lewy body diseases. Further, it is likely that in addition to a “triggering” event that initiates α-syn aggregation, it is likely that additional mechanisms are necessary to facilitate (allowing the disease to spread to the CNS) and aggravate the disease process (promote neurodegeneration beyond the basal ganglia) [50].
As noted, Braak and colleagues proposed that PD may be caused by an intestinal pathogen which travels through enteric neurons before entering the CNS via the vagus nerve [19]. Increasing evidence supports this hypothesis, including evidence of α-syn pathology in the intestinal wall examined both antemortem [51, 52] and postmortem [53, 54]. The α-syn deposits have been observed up to 20 years prior to a PD diagnosis [55]. Examination of colonic biopsies in iRBD cohorts has also demonstrated the presence of α-syn in these prodromal cohorts [56]. Recent work has demonstrated that gut microbes are able to promote α-syn-mediated motor deficits, brain pathology, and neuroinflammation in a mouse model of PD [57]. This evidence has led to an interest in identifying how intestinal dysbiosis and intestinal permeability, may play a mechanistic role in the initiation of α-syn aggregation at the level of the gut.
However, neuropathological studies using large cohorts have failed to find evidence of cases in which α-syn pathology is present in the peripheral nervous system in the absence of CNS pathology [58, 59]. Alternatively, intestinal inflammation may be the driver of disease pathogenesis in the periphery [24, 60]. It is well established that neuroinflammation is present in PD, though it was initially considered a response to α-syn aggregation rather than a primary mechanism of disease initiation [61]. More recently, it is hypothesized that intestinal dysbiosis and inflammation are the earliest disease processes that initiate both innate and adaptive immune system activation [60]. Specifically, a toxic trigger or changes in microbiota may contribute to dysbiosis and facilitate a proinflammatory environment. These processes increase intestinal permeability, resulting in increased levels of circulating proinflammatory cytokines, innate and adaptive immune cell activation, increased blood–brain barrier permeability, peripheral cell infiltration of the central nervous system, and neuroinflammation. While this process upregulates native α-syn expression which could potentially trigger its aggregation in the peripheral nervous system, the mechanistic emphasis is on the inflammatory processes.
Clinical phenotypes
Clinically, the distinction between PD and DLB is made based on the temporal onset of motor versus cognitive symptoms (e.g., motor symptoms occur first = PD; cognitive symptoms occur first = DLB). However, both PD (including PDD) and DLB patients can exhibit similar clinical symptoms as the disease progresses. For instance, while hallucinations and RBD are diagnostic criteria for DLB, these same symptoms are present in 40–50% [62, 63] and 39–50% [64, 65] of PD patients, respectively. Additionally, cognitive impairment is common in PD, with up to 83% of patients exhibiting dementia after 20 years [66]. It is well established that the motor and cognitive symptoms of the disorders are closely linked to α-syn aggregation suggesting that disease mechanisms are the same across the disorders [1, 67], with differences in the clinical presentation related to the anatomical location of disease initiation and progression [16, 68].
Revisions of Braak’s original pathological staging have addressed some of the heterogeneity in pathological progression within the brain that leads to PDD versus DLB [69]. Given the evidence for a possible gut origin in some patients, a recent revision to Braak’s pathological staging has proposed two distinct paths of pathological initiation and progression. Either α-syn aggregation originates in the CNS (brain first) or the peripheral nervous system (body first) [15, 70], with the spread of pathology in a bidirectional manner. The neural connectome thus plays a crucial role in determining how α-syn propagates through the nervous system [16]. This theory is particularly useful for understanding the heterogeneity in phenotypes across Lewy body diseases. For example, in the body-first subtype, the α-syn pathology presumably originates in the enteric or autonomic nervous system and spreads to the CNS via the vagus and sympathetic connectome. These patients develop iRBD in the prodomal phase, have more autonomic and gastrointestinal symptoms, significant hyposmia, and faster motor and non-motor symptoms progression. This clinical presentation also largely overlaps with many symptoms observed in DLB, such as iRBD, autonomic dysfunction, and more severe cognitive dysfunction. Given the common neuropathology (α-syn aggregation) across these disorders and overlap in symptoms, this highlights the need to evaluate gastrointestinal mechanisms of pathogenesis across Lewy body diseases, as disease mechanisms likely span across diagnostic categories and may better account for the heterogeneity of clinical phenotypes.
Dysbiosis
Microorganisms that live inside and on humans, referred to as microbiota, have symbiotic relationships with the human host. However, dysbiosis can lead to many disease processes, such as SIBO, Crohn’s disease, and inflammatory bowel disease [71, 72], with more recent evidence supporting the role of microbiota in neurodegenerative conditions [73]. Initial estimates suggested a ratio of 10:1 between bacteria and human cells, with more recent evidence suggesting a 1:1 ratio with approximately 3.9 × 1013 bacteria in/on the human body [74]. The sheer number of microbiota leads to complex dynamics that raises considerable challenges in evaluating the patterns of microbiota variation and the impact of dysbiosis.
The most common methodological approach includes the use of 16S rRNA sequencing of either fecal or intestinal samples. The 16S rRNA gene is conserved in all bacteria allowing for taxonomic identification. Despite over 25 studies using this approach in fecal samples in PD, there is considerable variability among findings and over 100 differently abundant taxa between PD patients and controls [75, 76]. A meta-analysis and pooled re-analysis of ten available studies that used 16S rRNA-gene amplicon sequencing indicated that the gut microbiome significantly differs between PD patients and controls, though the interstudy variability was the main factor driving bacterial community structures and only 1% of the total variance was accounted for by group status [76]. When inconsistencies across studies (country of origin, sampling protocols, sample storage, DNA extractions, and sequencing strategies) were accounted for, PD patients exhibited a reduction of the genera Roseburia, Fusicategnibacter, Blautia, Anaerostipes (Lachnospiraceae family), and Faecalibacterium (Ruminococcaceae family) in addition to enrichment of the Lactobacillus, Akkermansia, and Bifidobacterium genera. No significant differences in enterotypes were observed. Similar reports were observed in a recent review [75]. Increased Akkermansia (14 out of 30 studies), followed by Lactobacillus (7 out of 30 studies), and Bifidobacterium (10 out of 30 studies) whereas decreased abundance of Roseburia (8 out of 30), Faecalibacterium (8 out of 30 studies), and Blautia (7 out of ten studies) were most consistently observed.
There are numerous reasons for variability across studies, including collection and assaying methods, with poor control of confounding factors, such as antibiotic use in early life or previous gastrointestinal infections [77]. There is an enormous amount of individual and geographic variability that raises challenges when attempting to quantify group differences in the microbiome [78, 79]. Finally, the majority of prior evaluations of the microbiome quantify microbial taxa and metabolic pathways as fractions of the sample sequence generated by each analysis, rather than disease-associated imbalances that may occur [77, 80].
Increases in rare species of microbiota
While the prior studies often detect changes in the more prevalent microbiota species, it can be challenging to detect rare species that, when they increase in number, can exert adverse biological effects. As part of the pooled re-analysis by Romano et al. [76] discussed above, microbial alpha-diversity and abundances of rare taxa were significantly increased in PD relative to control samples. This suggests a reduction in dominant species and an increase in rare/low abundant ones. When commensal bacteria increase in number to exert adverse biologic effects, they are known as pathobionts. For example, sulfate-reducing bacteria are rare members of the gut microbiome under normal conditions (a fraction of a percent) and help to support microbial fermentation by converting its metabolite, hydrogen, to hydrogen sulfide (H2S). However, when dysbiosis occurs, sulfate-reducing bacteria can increase in number (bloom in sulfate-reducing bacteria), becoming pathogenic as an increase can impair intestinal barrier and increase levels of potentially toxic H2S. The Desulfovibrionaceae family, the most prominent family of sulfate-reducing bacteria [81], is elevated in PD patients [82, 83] and the concentration of Desulfovibrio species correlates with the severity of PD [83]. As a consequence, PD patients may exhibit excess H2S. H2S can be beneficial as it acts as a gaseous neurotransmitter produced in small quantities by the host regulating a number of body functions including gastrointestinal, neuronal, cardiovascular, endocrine, respiratory, renal, and hepatic systems [84]. However, elevated levels of H2S produced by a bloom in sulfate-reducing bacteria can become harmful to the host and is associated with gastrointestinal disorders such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome [84–86]. As noted, these disorders are linked with an increased risk of PD [42, 43].
Consequences of dysbiosis
Reduction in short-chain fatty acids
Roseburia, Fusicategnibacter, Blautia, and Anaerostipes are butyrate producers, a short-chain fatty acid (SCFA). SCFAs are produced by the fermentation of dietary fiber by microbiota and are exclusively produced in the intestine. They exhibit anti-oxidant and anti-inflammatory processes and regulate the expression of tight junction proteins, which can impact intestinal barrier integrity [87]. Absolute concentrations of SCFAs are significantly reduced in human PD fecal samples, including butyrate, acetate, and propionate [88]. A decrease in fecal levels of butyrate has been associated with intestinal inflammation in PD patients [89]. Examination of plasma SCFAs suggested opposite effects, with increased SCFAs in PD relative to a matched cohort [90]. Taken together, the observed reductions in Roseburia, Fusicategnibacter, Blautia, and Anaerostipes may contribute to proinflammatory shifts in microbiota composition in PD.
Increase in self-peptides
Lactobacillus, Akkermansia, and Bifidobacterium genera are typically considered to be beneficial bacteria, suggesting either a role in PD or simply that these bacteria are well adapted to thrive in the context of dysbiosis. However, an enrichment of Akkermansia has been observed in multiple sclerosis patients and is associated with a proinflammatory response [91–93]. Recent evidence suggests that peptides produced by Akkermansia may interact with autoreactive T cells in multiple sclerosis [94]. Specifically, the human leukocyte antigen (HLA)-DR15 haplotype has been associated with the pathogenesis of multiple sclerosis [95] via the abundant production of HLA-DR-derived self-peptides. Akkermansia may mimic these peptides ultimately sensitizing activated CD4 T cells in the periphery which leads to pathogenic autoreactive T cells in the brain [94]. Given the increases in Akkermansia, it is possible that similar mechanisms may occur in Lewy body diseases.
Increase in lipopolysaccharide
The observed increase in the density of Gram-negative bacterial strains (including Akkermansia and Desulfovibrio) in PD fecal samples [96, 97] corresponds to an increase in endotoxin lipopolysaccharide (LPS) content, as LPS is a cell wall component of Gram-negative bacteria [98]. LPS is a known endotoxin that can lead to increased intestinal permeability [99–101]. Plasma and serum lipopolysaccharide-binding protein is reduced in PD [102–104]. Lipopolysaccharide-binding protein increases when LPS is elevated acutely [105, 106], but decreased when there has been chronic exposure, suggesting PD patients experience prolonged elevated LPS. LPS in this context may directly contribute to the initiation of α-synuclein at the level of the gut [107] as it has demonstrated the ability to promote α-synuclein aggregation via the formation of intermediate nucleating species [108–110]. Alternately, LPS may lead to systemic inflammatory processes in the context of increased intestinal permeability that contribute to disease pathogenesis, as it is a potent stimulator of microglial activation and has been associated with degeneration of the neurons in the SNc and motor deficits [111]. Specifically, LPS activates toll-like receptors initiating an innate immune response stimulating the production of inflammatory cytokines (such as IL-1, TNF-α, and IL-6) and reactive oxygen species [112, 113].
Increase in H2S
Several pathological processes may occur when Desulfovibrio colonizes the intestine, including increased generation of H2S in amounts that exceed detoxification capacity, increased inflammatory responses, and increased intestinal permeability (leaky gut) [114, 115]. Desulfovibrio also has the ability to produce magnetite (Fe3O4) [116], which may accelerate α-syn aggregation [117]. A recent model has proposed that the increase in H2S concentrations causes leaky membrane resulting in the release of cytochrome c from the mitochondria and an increase in cytosolic iron levels. This, in combination with magnetite nanoparticles originating from Desulfovibrio species may result in α-syn aggregation via production of reactive oxygen species [118].
Increase in curli protein
Preclinical studies have suggested that the amyloid protein, curli, produced by Escherichia coli (E. coli), may play an important role in the initiation of α-syn initiation. Specifically, in the context of increased proteobacteria (Gram-negative bacteria), there will be more E. coli, a bacterium that secretes curli. Rats exposed to curli-producing bacteria (E. coli) displayed increased neuronal α-syn deposition in both the gut and brain as well as enhanced microgliosis and astrogliosis. Together, this suggests that curli, a gut bacterial amyloid protein, may trigger the initiation of α-syn aggregation [119]. This role of curli was supported by the finding that curli expression was required for E. coli to exacerbate α-syn-induced behavioral deficits. In addition, oral treatment of mice with a gut-restricted inhibitor of amyloid prevented curli-mediated acceleration of PD-like pathology and behavioral abnormalities [120]. A separate line of work showed that LPS may contribute to the pathophysiology by accelerating the synthesis of curli fibrils [121].
Increased intestinal permeability
The intestinal barrier, which consists of physical (mucus, tight junction proteins), and chemical (anti-microbial peptides) components, shields the intestine from the contents of the lumen. Barrier integrity is reliant on the tight junctions, which include claudins, occludin, zonula occludens, adheren junctions, desmosomes, and gap junctions [122]. Damage to the barrier can allow α-syn, microbes, environmental toxins, or other luminal contents to gain access to the submucosal neuronal tissue or systemic circulation. Several studies have demonstrated that PD patients exhibit intestinal barrier dysfunction [89, 123–125], referred to as ‘leaky gut’ that is associated with microbial translocation across the intestinal mucosa. Factors resulting from dysbiosis, such as increases in LPS [99–101] and Desulfovibrio spp. [126] also lead to leaky gut. Recent work has demonstrated that Desulfovibrio spp. induced intestinal permeability via the snail pathway [126]. Snail is a transcription factor associated with increased intestinal permeability [127–129] via negatively regulating tight junctions [130, 131].
Leaky gut may contribute to the entry of known modulators of α-syn aggregation, such as curli, H2S, and LPS, into the systemic circulation and then beyond, to end organs such as the brain. For example, increased intestinal permeability and E. coli staining correlated with α-syn staining, supporting the contribution of leaky gut and this bacteria to α-syn aggregation [123]. Additional evidence has also demonstrated associations between intestinal permeability and pathological α-syn aggregation [132].
Inflammation
As we discussed above, many of the observations thus far suggest the observed patterns of altered microbiota composition facilitate a proinflammatory shift in PD, including a reduction in SCFAs and an increase in immune system activating peptides. LPS activates toll-like receptors that initiate an innate immune response and the production of inflammatory cytokines. The production of H2S functions as an endogenous regulator of the immune system [133] and thus an increase in H2S secondary to a bloom in sulfate-reducing bacteria could contribute to a proinflammatory response. Aspects of these processes can occur with intact intestinal barrier, though an increase in intestinal permeability facilitates the release of lumen products contributing to a systemic inflammatory response driven by the innate and adaptive immune systems [134, 135], which may play a key role in sustaining and exacerbating α-syn aggregation [24].
Pathways to the brain
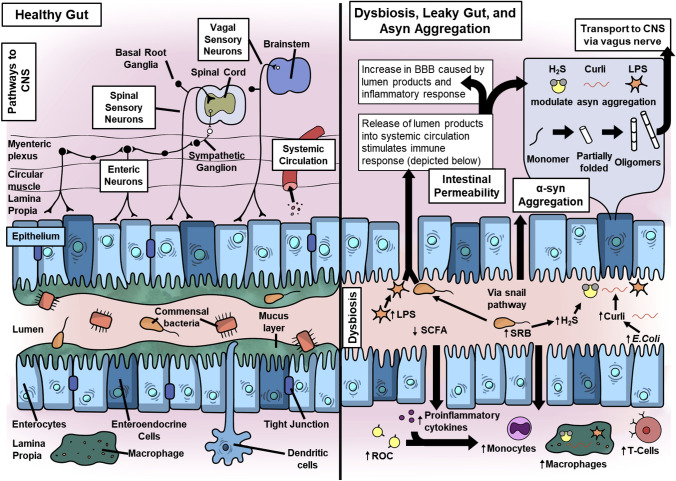
There are multiple paths for bidirectional gut–brain communications (Fig. 1), involving neural pathways as well as immune and endocrine mechanisms [136]. The vagal and spinal sensory neurons receive signals within the lamina propria and are directly connected to the brainstem and spinal cord, respectively. Additional pathways connect the enteric nervous system with CNS. For example, signaling from intrinsic primary afferent neurons are conveyed by intestinofugal nerves to the spinal cord via sympathetic ganglia.
Fig. 1.
Proposed mechanisms of early disease processes in Lewy body diseases. Left panel: healthy gut. In a healthy gut, the commensal microbes, epithelium and immune cells maintain an equilibrium. Right panel: dysbiosis includes decrease in Roseburia, Fusicategnibacter, Blautia, and Anaerostipes, leading to a reduction in the production of short-chain fatty acids (SCFAs). An increase in Lactobacillus, Akkermansia, and Bifidobacterium is considered beneficial bacteria, though Akkermansia may produce peptides that mimic self-peptides that sensitize T cells. An increase in Gram-negative bacteria strains increase lipopolysaccharide (LPS), an endotoxin that can damage the intestinal barrier and initiate inflammatory processes. A bloom in sulfate-reducing bacteria (SRB) increases hydrogen sulfide (H2S) production, which may facilitate α-syn aggregation, increase intestinal permeability, and initiate an inflammatory response. E. coli increases levels of curli protein, which has been implicated as a modulator of α-syn aggregation. α-Syn may aggregate locally via these mechanisms within the enteroendocrine cells and travel via the vagus nerve to the brainstem. Additionally, the activation of both innate and adaptive inflammatory responses increases the circulating proinflammatory cytokines, reactive oxygen species (ROC), monocytes, macrophages, and T cells. The release of lumen products and triggering of inflammatory processes can damage the blood–brain barrier (BBB), facilitating infiltration of the pathogenic processes into the central nervous system (CNS)
Braak’s initial theory posited that α-syn pathology has the ability to spread from the gastrointestinal tract to the brain via the vagus nerve [19]. Epidemiological evidence has demonstrated that a full truncal vagotomy decreases the risk of PD [137, 138]. Animal models have also shown that recombinant α-syn injected into the intestinal wall could be transported via the vagal nerve to reach the dorsal motor nucleus in the brainstem [139]. Additionally, the injection of preformed α-syn fibrils into the muscle layers of the pylorus and duodenum, which is densely innervated by the vagus nerve, leads to their propagation to the CNS following a path similar to that characterized by Braak [140]. The potential cellular mechanisms of α-syn propagation have been reviewed elsewhere [141], with increasing evidence supporting the notion that it propagates in a prion-like fashion [142], which would provide a mechanism by which α-syn aggregation in the gut could propagate to the CNS.
There are multiple potential sites within these pathways in which α-syn aggregation could initiate, including the enteric nervous system [143]. However, recent efforts have proposed that gut enteroendocrine cells may serve as sites for the initial emergence of pathogenic α-syn [144]. Enteroendocrine cells are chemosensory cells dispersed throughout the mucosal lining of the intestine and their apical surface is open to the lumen of the intestine. Historically, they were viewed as hormone-producing cells, but subsequent observations have demonstrated that they are electrically excitable, possess many neuronal features, and can communicate directly with the nervous system [145, 146]. Additionally, α-syn is expressed by the enteroendocrine cells, both in the small and large intestine, highlighting that they may serve as loci for the initial pathological α-syn aggregation [144].
This work largely supports the notion that it is physiologically feasible for α-syn aggregation to begin in the gut and travel to the CNS. However, as noted, there has been limited evidence using immunohistochemical staining of cases in which α-syn pathology was observed in the gut in the absence of CNS pathology [58, 59]. Alternatively, dysbiosis and intestinal permeability may lead to an increase in the entry of lumen products (including LPS, H2S, and curli proteins) as well as cytokines and immune cells into systemic circulation [147, 148]. Rather than initiating α-syn aggregation in the periphery, these processes may contribute to disease mechanisms by impacting the blood–brain barrier (BBB) and facilitating a prolonged immune response. This would facilitate peripheral cell infiltration across the BBB which contributes to neuroinflammation.
Increased blood–brain barrier permeability
The BBB is a physiological barrier that protects the brain from unwanted molecules in the blood. Similar to the intestinal barrier, the BBB leakage is driven by damage to endothelial tight junctions, which include occludin, claudins, zonula occludens, and adheren junctions, though the BBB has added complexity given the sensitivity of the brain to toxins and pathogens [149]. In addition to changes in tight junctions, BBB permeability can also be altered by damage to endothelial cells or astrocytes as well as degradation of extracellular matrix components. Disruption of the BBB likely plays an important role across neurodegenerative conditions [150], with emerging evidence that permeability of the BBB is increased in PD [151, 152]. Specifically, an increased ratio of cerebrospinal fluid albumin to serum albumin was observed in PD [153]. Thinning and fragmentation of tight junction proteins was observed in postmortem immunofluorescence staining evaluations of PD cases [154]. PET imaging has also indicated reduced P-glycopreotein 1 activity, suggestive of BBB dysfunction, in the midbrain in PD patients [155].
The gut microbiota can regulate the BBB via several potential mechanisms [156], including the direct impact of intestinal microbial metabolites such as LPS [157, 158], immune and endocrine responses [159], or upregulation of α-syn [160]. In terms of the consequences of dysbiosis discussed above, there are several potential mechanisms by which dysbiosis and intestinal permeability may lead to increased BBB in PD. Specifically, LPS has been used to study the impact of systemic inflammation on BBB function, indicating potential BBB dysfunction in 60% of studies [161], with BBB change observed more consistently in mice versus rats. These studies, however, typically use septic doses of LPS, which limits the generalizability to Lewy body diseases. While α-syn in its non-pathologic form can travel bi-directionally across the BBB, transportation is enhanced in the presence of LPS [162, 163], suggesting potential upregulation of α-syn in the brain. Additionally, increased desulfovibrio spp. can induce leaky gut via the activation of the snail pathway. This same pathway has also been found to disrupt BBB by impacting integrity of tight junctions [164].
Systemic inflammation induced by dysbiosis and intestinal permeability may also increase BBB permeability. For example, systemic inflammation induces migration of microglia to the cerebral vasculature to maintain BBB integrity by expressing tight-junction proteins and connecting with the endothelial cells. However, during sustained inflammation, microglia phagocytose the astrocytic end-feet of the BBB, impairing the BBB function [165]. Given the consistent finding of activated microglia in the postmortem brains of PD patients [166, 167], this would suggest that systemic inflammation may be driving these processes. Herein we focus our review on the potential mechanisms driven by gastrointestinal factors we have identified, however, for detailed review of immune dysfunction and neuroinflammation, please refer to [24, 60].
REM sleep behavior disorder
As noted above, iRBD is a prodrome of Lewy body diseases, with up to 96% of iRBD patients converting to a synucleinopathy, the majority would be diagnosed with either PD or dementia with Lewy bodies. In the “body first” Lewy body disease phenotype mentioned above, the pathology would theoretically progress from the dorsal motor nerve of the vagus to first impact the locus coeruleus [16], which is inferior to the substantia nigra and associated with the development of iRBD [168, 169]. As the disease progresses to the substantia nigra or broader regions, individuals may begin to exhibit symptoms consistent with either PD or LBD.
Self-reported gastrointestinal symptoms are elevated in iRBD cohorts relative to healthy controls, with significantly greater endorsement of constipation and straining for defecation [170, 171]. Total gastrointestinal transit time, colonic volume, and 3D-Transit colonic transit time were significantly increased in an iRBD cohort relative to controls, though not to the extent observed in medicated PD patients [172]. iRBD patients exhibit a microbiome similar to that seen in patients with PD [173] and colonic biopsies in iRBD cohorts showed the presence of α-syn [56], though SCFA were not reduced in iRBD as in PD [174].
Additionally, interleukin-10 levels are upregulated in iRBD relative to controls [175] in addition to tumor necrosis factor-α levels, which were found to predict phenoconversion to an α-synucleinopathy [176]. Increased microglial activation was detected by PET in the substantia nigra in addition to reduced dopaminergic function in the putamen [177]. iRBD patients’ blood monocytic cells showed increased expression of CD11b and decreased expression of HLA-DR. iRBD patients had increased classical monocytes and mature natural killer cells. The levels of expression of toll-like receptor 4 on blood monocytes was correlated with the nigral immune activation measured with PET [178].
Taken together, early gastrointestinal symptoms, such as constipation and dysbiosis, as well as systemic inflammation, are present in the prodromal stages of LBDs, however, there is a great need to understand the earliest changes, identify potential mechanisms, and whether these predict phenoconversion.
Early detection of pathological changes and targets for intervention
There are currently no disease-modifying treatments for Lewy body diseases, largely due to the lack of mechanistic understanding of disease pathogenesis and the challenges associated with targeting α-syn aggregates [179]. While there are numerous research questions to answer regarding the mechanisms of gastrointestinal dysfunction and the pathogenesis of Lewy body disease, there are several potential treatments that are readily available spanning antibiotics, probiotics, and fecal microbiota transplantation [180]. For example, Rifaximin is a broad-range, gastrointestinal-specific antibiotic used to treat SIBO, which improves motor symptoms [38]. Relatedly, numerous studies have evaluated the impact of probiotics on constipation symptoms in PD [181], with evidence of a reduced MDS-UPDRS total score [182]. Based on the emerging research presented herein, targeting specific bacteria offer intriguing possibilities.
Additionally, a major limitation of current PD medications is that they lose efficacy over time, with recent evidence that gut microbiota has been found to moderate the metabolism of Parkinson’s medication [183–185]. While not disease modifying, targeting these bacteria may significantly improve efficacy of PD medications [186]. Evolving evidence support PD and Lewy body diseases more generally as both central and peripheral diseases. Targeting the pathophysiology taking place in the gut offers exciting opportunities for early intervention. Could targeting dysbiosis or intestinal permeability prior to the development of α-syn aggregation be an effective way of forestalling Lewy body diseases before the disease is clinically diagnosable?
Acknowledgements
Dr. Ryman’s work is supported by the National Institutes of Health (P30 GM122734, R03 AG075408, UF1NS100598, P20 AG068077, R61 MH125126). Dr. Vakhtin’s work is supported by the National Institutes of Health (P30 GM122734). Dr. Pirio Richardson’s work is supported by the National Institutes of Health (R03 AG075408), Department of Defense, and industry (Pharma 2B, AEON, ADDEX, SCION). Dr. Lin’s work is supported by the Winkler Bacterial Overgrowth Research Fund.
Declarations
Conflicts of interest
Drs. Ryman, Vakhtin, and Lin declare they have no competing financial interests. Dr. Pirio Richardson has received honoraria for lectures from the International Parkinson’s Disease and Movement Disorders Society and the American Academy of Neurology. Dr. Pirio Richardson serves on the Scientific Advisory Boards for private foundations including the Benign Essential Blepharospasm Research Foundation and the Dystonia Medical Research Foundation. She has received royalties from Springer.
References
- 1.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018;16:34. doi: 10.1186/s12916-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2016;22:S119–S122. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Tysnes O-B, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 5.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan DB, Fiest KM, Roberts JI, et al. The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci. 2016;43:S83–S95. doi: 10.1017/cjn.2016.2. [DOI] [PubMed] [Google Scholar]
- 7.Mok W, Chow TW, Zheng L, et al. Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimer’s Dis Other Dementias. 2004;19:161–165. doi: 10.1177/153331750401900309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SAV, O’brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673–683. doi: 10.1017/S0033291713000494. [DOI] [PubMed] [Google Scholar]
- 9.Brooks DJ. The early diagnosis of Parkinson’s disease. Ann Neurol. 1998;44:S10–S18. doi: 10.1002/ana.410440704. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 11.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142:744–759. doi: 10.1093/brain/awz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postuma RB, Berg D. Prodromal Parkinson’s disease: the decade past, the decade to come. Mov Disord. 2019;34:665–675. doi: 10.1002/mds.27670. [DOI] [PubMed] [Google Scholar]
- 13.Galbiati A, Verga L, Giora E, et al. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev. 2019;43:37–46. doi: 10.1016/j.smrv.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Just MK, Gram H, Theologidis V, et al. Alpha-synuclein strain variability in body-first and brain-first synucleinopathies. Front Aging Neurosci. 2022;14:907293. doi: 10.3389/fnagi.2022.907293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsager J, Andersen KB, Knudsen K, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain. 2020;143:3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 16.Borghammer P. The α-synuclein origin and connectome model (SOC Model) of Parkinson’s disease: explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J Parkinsons Dis. 2021;11:455–474. doi: 10.3233/JPD-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spillantini MG, Schmidt ML, Lee VM-Y, et al. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 18.Baba M, Nakajo S, Tu P-H, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879. [PMC free article] [PubMed] [Google Scholar]
- 19.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 20.Lionnet A, Leclair-Visonneau L, Neunlist M, et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135:1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 21.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat Rev Neurol. 2015;11:625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 22.Breen DP, Halliday GM, Lang AE. Gut–brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov Disord. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 23.Baizabal-Carvallo JF, Alonso-Juarez M. The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience. 2020;432:160–173. doi: 10.1016/j.neuroscience.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Park Dis. 2017;3:1–9. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260:1332–1338. doi: 10.1007/s00415-012-6801-2. [DOI] [PubMed] [Google Scholar]
- 26.Lubomski M, Davis RL, Sue CM. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol. 2020;267:1377–1388. doi: 10.1007/s00415-020-09723-5. [DOI] [PubMed] [Google Scholar]
- 27.Fasano A, Visanji NP, Liu LWC, et al. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 28.Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811–826. doi: 10.1016/bs.irn.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Camacho M, Macleod AD, Maple-Grødem J, et al. Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Park Dis. 2021;7:1–7. doi: 10.1038/s41531-021-00191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leta V, Urso D, Batzu L, et al. Constipation is associated with development of cognitive Impairment in de novo Parkinson’s disease: a longitudinal analysis of two international cohorts. J Parkinsons Dis. 2021;11:1–11. doi: 10.3233/JPD-212570. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson’s disease. World J Gastroenterol. 2016;22:5742. doi: 10.3748/wjg.v22.i25.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warnecke T, Schäfer KH, Claus I, et al. Gastrointestinal involvement in Parkinson’s disease: pathophysiology, diagnosis, and management. NPJ Park Dis. 2022;8:1–13. doi: 10.1038/s41531-022-00295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakakibara R, Masuda M, Tateno F, et al. Gastrointestinal function in dementia with Lewy bodies: a comparison with Parkinson disease. Clin Auton Res. 2019;29:633–638. doi: 10.1007/s10286-019-00597-w. [DOI] [PubMed] [Google Scholar]
- 34.Knudsen K, Fedorova TD, Bekker AC, et al. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: a colon transit and volume study. J Parkinsons Dis. 2017;7:359–367. doi: 10.3233/JPD-161050. [DOI] [PubMed] [Google Scholar]
- 35.Quigley EMM. The spectrum of small intestinal bacterial overgrowth (SIBO) Curr Gastroenterol Rep. 2019;21:1–7. doi: 10.1007/s11894-019-0671-z. [DOI] [PubMed] [Google Scholar]
- 36.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112:775. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Feng X, Jiang Z, Jiang Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: a systematic review and meta-analysis. Gut Pathog. 2021;13:1–10. doi: 10.1186/s13099-021-00420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fasano A, Bove F, Gabrielli M, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;28:1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 39.Niu X-L, Liu L, Song Z-X, et al. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson’s disease. J Neural Transm. 2016;123:1381–1386. doi: 10.1007/s00702-016-1612-8. [DOI] [PubMed] [Google Scholar]
- 40.Su A, Gandhy R, Barlow C, Triadafilopoulos G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastroenterol. 2017;4:e000132. doi: 10.1136/bmjgast-2017-000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AH, Mahadeva S, Thalha AM, et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Svn Z, Liv M, et al. Association between irritable bowel syndrome and risk of Parkinson’s disease: a systematic review and meta-analysis. Front Neurol. 2021;12:2. doi: 10.3389/fneur.2021.720958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu F, Li C, Gong J, et al. The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2019;51:38–42. doi: 10.1016/j.dld.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki H, Lipkin LE, Aronson SM. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol. 1961;20:237–244. doi: 10.1097/00005072-196104000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka K, Oyanagi S, Matsushita M, Hori A. Presenile dementia with Alzheimer-, pick-and Lewy-body changes. Acta Neuropathol. 1976;36:221–233. doi: 10.1007/BF00685366. [DOI] [PubMed] [Google Scholar]
- 46.Ince PG, Perry EK, Morris CM. Dementia with lewy bodies. a distinct non-alzheimer dementia syndrome? Brain Pathol. 1998;8:299–324. doi: 10.1111/j.1750-3639.1998.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 48.Iwatsubo T, Yamaguchi H, Fujimuro M, et al. Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol. 1996;148:1517. [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138:1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson ME, Stecher B, Labrie V, et al. Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci. 2019;42:4–13. doi: 10.1016/j.tins.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon KM, Keshavarzian A, Dodiya HB, et al. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 52.Hilton D, Stephens M, Kirk L, et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 53.Wakabayashi K, Takahashi H, Takeda S, et al. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 54.Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal P arkinson disease patients. Ann Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 56.Sprenger FS, Stefanova N, Gelpi E, et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85:1761–1768. doi: 10.1212/WNL.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beach TG, Adler CH, Sue LI, et al. Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental Lewy body disease, and normal elderly subjects: evidence against the “body-first” hypothesis. J Parkinsons Dis. 2021;11:1–11. doi: 10.3233/JPD-212733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tansey MG, Wallings RL, Houser MC, et al. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022;2:1–17. doi: 10.1038/s41577-022-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harms AS, Ferreira SA, Romero-Ramos M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021;141:527–545. doi: 10.1007/s00401-021-02268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605–610. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- 63.Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Sun X, Wang J, et al. Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis. Neurol Sci. 2017;38:163–170. doi: 10.1007/s10072-016-2744-1. [DOI] [PubMed] [Google Scholar]
- 65.Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. doi: 10.1016/j.smrv.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hely MA, Reid WGJ, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 67.Walker L, Stefanis L, Attems J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies–current issues and future directions. J Neurochem. 2019;150:467–474. doi: 10.1111/jnc.14698. [DOI] [PubMed] [Google Scholar]
- 68.Aarsland D, Ballard CG, Halliday G. Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol. 2004;17:137–145. doi: 10.1177/0891988704267470. [DOI] [PubMed] [Google Scholar]
- 69.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33:48–57. doi: 10.1002/mds.27138. [DOI] [PubMed] [Google Scholar]
- 71.Baker PI, Love DR, Ferguson LR. Role of gut microbiota in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2009;3:535–546. doi: 10.1586/egh.09.47. [DOI] [PubMed] [Google Scholar]
- 72.Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 73.Marizzoni M, Provasi S, Cattaneo A, Frisoni GB. Microbiota and neurodegenerative diseases. Curr Opin Neurol. 2017;30:630–638. doi: 10.1097/WCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 74.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Tan AH, Lim SY, Lang AE. The microbiome–gut–brain axis in Parkinson disease—from basic research to the clinic. Nat Rev Neurol. 2022;18:1–20. doi: 10.1038/s41582-022-00681-2. [DOI] [PubMed] [Google Scholar]
- 76.Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Park Dis. 2021;7:1–13. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haikal C, Chen Q-Q, Li J-Y. Microbiome changes: an indicator of Parkinson’s disease? Transl Neurodegener. 2019;8:1–9. doi: 10.1186/s40035-019-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiwaki H, Ito M, Ishida T, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord. 2020;35:1626–1635. doi: 10.1002/mds.28119. [DOI] [PubMed] [Google Scholar]
- 79.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vandeputte D, Kathagen G, D’hoe K, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 81.Loubinoux J, Bronowicki J-P, Pereira IAC, et al. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 82.Lin A, Zheng W, He Y, et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Murros KE, Huynh VA, Takala TM, Saris PEJ. Desulfovibrio bacteria are associated with Parkinson’s disease. Front Cell Infect Microbiol. 2021;11:652617. doi: 10.3389/fcimb.2021.652617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh SB, Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms. 2015;3:866–889. doi: 10.3390/microorganisms3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barton LL, Ritz NL, Fauque GD, Lin HC. Sulfur cycling and the intestinal microbiome. Dig Dis Sci. 2017;62:2241–2257. doi: 10.1007/s10620-017-4689-5. [DOI] [PubMed] [Google Scholar]
- 86.Villumsen M, Aznar S, Pakkenberg B, et al. Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut. 2019;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 87.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 88.Unger MM, Spiegel J, Dillmann K-U, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Schwiertz A, Spiegel J, Dillmann U, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 90.Shin C, Lim Y, Lim H, Ahn T. Plasma short-chain fatty acids in patients with Parkinson’s disease. Mov Disord. 2020;35:1021–1027. doi: 10.1002/mds.28016. [DOI] [PubMed] [Google Scholar]
- 91.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:1–11. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Jelcic I, Mühlenbruch L, et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell. 2020;183:1264–1281. doi: 10.1016/j.cell.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin R, Sospedra M, Eiermann T, Olsson T. Multiple sclerosis: doubling down on MHC. Trends Genet. 2021;37:784–797. doi: 10.1016/j.tig.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 97.Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 98.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nighot M, Al-Sadi R, Guo S, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol. 2017;187:2698–2710. doi: 10.1016/j.ajpath.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo S, Nighot M, Al-Sadi R, et al. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J Immunol. 2015;195:4999–5010. doi: 10.4049/jimmunol.1402598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasegawa S, Goto S, Tsuji H, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pal GD, Shaikh M, Forsyth CB, et al. Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front Neurosci. 2015;9:306. doi: 10.3389/fnins.2015.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen S-J, Chi Y-C, Ho C-H, et al. Plasma lipopolysaccharide-binding protein reflects risk and progression of Parkinson’s disease. J Parkinsons Dis. 2021;11:1129–1139. doi: 10.3233/JPD-212574. [DOI] [PubMed] [Google Scholar]
- 105.Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39:989–993. doi: 10.1042/BST0390989. [DOI] [PubMed] [Google Scholar]
- 106.Ramadori G, Zum Buschenfelde K-HM, Tobias PS, et al. Biosynthesis of lipopolysaccharide-binding protein in rabbit hepatocytes. Pathobiology. 1990;58:89–94. doi: 10.1159/000163569. [DOI] [PubMed] [Google Scholar]
- 107.Bhattacharyya D, Bhunia A. Gut–brain axis in Parkinson’s disease etiology: the role of lipopolysaccharide. Chem Phys Lipids. 2021;235:105029. doi: 10.1016/j.chemphyslip.2020.105029. [DOI] [PubMed] [Google Scholar]
- 108.Bhattacharyya D, Mohite GM, Krishnamoorthy J, et al. Lipopolysaccharide from gut microbiota modulates α-synuclein aggregation and alters its biological function. ACS Chem Neurosci. 2019;10:2229–2236. doi: 10.1021/acschemneuro.8b00733. [DOI] [PubMed] [Google Scholar]
- 109.Bhattacharyya D, Bhunia A. Gut–Brain axis in Parkinson’s disease etiology: the role of lipopolysaccharide. Chem Phys Lipids. 2020;2:105029. doi: 10.1016/j.chemphyslip.2020.105029. [DOI] [PubMed] [Google Scholar]
- 110.Kim C, Lv G, Lee JS, et al. Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci Rep. 2016;6:1–12. doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deng I, Corrigan F, Zhai G, et al. Lipopolysaccharide animal models of Parkinson’s disease: recent progress and relevance to clinical disease. Brain Behav Immun Health. 2020;4:100060. doi: 10.1016/j.bbih.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 113.Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017;44:14–19. doi: 10.1016/j.coi.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh SB, Coffman CN, Carroll-Portillo A, et al. Notch signaling pathway is activated by sulfate reducing bacteria. Front Cell Infect Microbiol. 2021;643:2. doi: 10.3389/fcimb.2021.695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weglarz L, Dzierzewicz Z, Skop B, et al. Desulfovibrio desulfuricans lipopolysaccharides induce endothelial cell IL-6 and IL-8 secretion and E-selectin and VCAM-1 expression. Cell Mol Biol Lett. 2003;8:991–1004. [PubMed] [Google Scholar]
- 116.Pereira IAC, Ramos AR, Grein F, et al. A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea. Front Microbiol. 2011;2:69. doi: 10.3389/fmicb.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Joshi N, Basak S, Kundu S, et al. Attenuation of the early events of α-synuclein aggregation: a fluorescence correlation spectroscopy and laser scanning microscopy study in the presence of surface-coated Fe3O4 nanoparticles. Langmuir. 2015;31:1469–1478. doi: 10.1021/la503749e. [DOI] [PubMed] [Google Scholar]
- 118.Murros KE. Hydrogen sulfide produced by gut bacteria may induce Parkinson’s disease. Cells. 2022;11:978. doi: 10.3390/cells11060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen SG, Stribinskis V, Rane MJ, et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep. 2016;6:1–10. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sampson TR, Challis C, Jain N, et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife. 2020;9:e53111. doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swasthi HM, Mukhopadhyay S. Electrostatic lipid–protein interactions sequester the curli amyloid fold on the lipopolysaccharide membrane surface. J Biol Chem. 2017;292:19861–19872. doi: 10.1074/jbc.M117.815522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forsyth CB, Shannon KM, Kordower JH, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clairembault T, Leclair-Visonneau L, Coron E, et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun. 2015;3:1–9. doi: 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aho VTE, Houser MC, Pereira PAB, et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegener. 2021;16:1–14. doi: 10.1186/s13024-021-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singh SB, Coffman CN, Varga MG, et al. Intestinal alkaline phosphatase prevents sulfate reducing bacteria-induced increased tight junction permeability by inhibiting snail pathway. Front Cell Infect Microbiol. 2022;627:2. doi: 10.3389/fcimb.2022.882498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Forsyth CB, Tang Y, Shaikh M, et al. Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol Clin Exp Res. 2011;35:1635–1643. doi: 10.1111/j.1530-0277.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elamin E, Masclee A, Troost F, et al. Activation of the epithelial-to-mesenchymal transition factor snail mediates acetaldehyde-induced intestinal epithelial barrier disruption. Alcohol Clin Exp Res. 2014;38:344–353. doi: 10.1111/acer.12234. [DOI] [PubMed] [Google Scholar]
- 129.Liu W, Ruan T, Ji X, et al. The Gli1-snail axis contributes to salmonella typhimurium-induced disruption of intercellular junctions of intestinal epithelial cells. Cell Microbiol. 2020;22:e13211. doi: 10.1111/cmi.13211. [DOI] [PubMed] [Google Scholar]
- 130.Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 131.Martínez-Estrada OM, Cullerés A, Soriano FX, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kelly LP, Carvey PM, Keshavarzian A, et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dilek N, Papapetropoulos A, Toliver-Kinsky T, Szabo C. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res. 2020;161:105119. doi: 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- 134.Shannon K. Gut-derived sterile inflammation and Parkinson’s disease. Front Neurol. 2022;521:2. doi: 10.3389/fneur.2022.831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Q, Luo Y, Ray Chaudhuri K, et al. The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 136.Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol life Sci. 2013;70:55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of P arkinson’s disease. Ann Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 138.Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease: a Swedish register–based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 140.Kim S, Kwon S-H, Kam T-I, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fares MB, Jagannath S, Lashuel HA. Reverse engineering Lewy bodies: how far have we come and how far can we go? Nat Rev Neurosci. 2021;22:111–131. doi: 10.1038/s41583-020-00416-6. [DOI] [PubMed] [Google Scholar]
- 142.Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73. doi: 10.1007/s00401-015-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lebouvier T, Chaumette T, Paillusson S, et al. The second brain and Parkinson’s disease. Eur J Neurosci. 2009;30:735–741. doi: 10.1111/j.1460-9568.2009.06873.x. [DOI] [PubMed] [Google Scholar]
- 144.Chandra R, Hiniker A, Kuo Y-M, et al. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI insight. 2017;2:2. doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohórquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bohórquez DV, Samsa LA, Roholt A, et al. An enteroendocrine cell–enteric glia connection revealed by 3D electron microscopy. PLoS ONE. 2014;9:e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reale M, Iarlori C, Thomas A, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 148.Brodacki B, Staszewski J, Toczyłowska B, et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett. 2008;441:158–162. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 149.Sweeney MD, Zhao Z, Montagne A, et al. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gray MT, Woulfe JM. Striatal blood–brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Al-Bachari S, Naish JH, Parker GJ, et al. Blood-brain barrier leakage is increased in Parkinson’s disease. Front Physiol. 2020;2:2. doi: 10.3389/fphys.2020.593026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pisani V, Stefani A, Pierantozzi M, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflamm. 2012;9:1–4. doi: 10.1186/1742-2094-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pienaar IS, Lee CH, Elson JL, et al. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis. 2015;74:392–405. doi: 10.1016/j.nbd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 155.Kortekaas R, Leenders KL, Van Oostrom JCH, et al. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 156.Tang W, Zhu H, Feng Y, et al. The impact of gut microbiota disorders on the blood–brain barrier. Infect Drug Resist. 2020;13:3351. doi: 10.2147/IDR.S254403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Banks WA, Gray AM, Erickson MA, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12:1–15. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barton SM, Janve VA, McClure R, et al. Lipopolysaccharide induced opening of the blood brain barrier on aging 5XFAD mouse model. J Alzheimer’s Dis. 2019;67:503–513. doi: 10.3233/JAD-180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 160.Elabi O, Gaceb A, Carlsson R, et al. Human α-synuclein overexpression in a mouse model of Parkinson’s disease leads to vascular pathology, blood brain barrier leakage and pericyte activation. Sci Rep. 2021;11:1–14. doi: 10.1038/s41598-020-80889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Varatharaj A, Galea I. The blood–brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 162.Sui Y-T, Bullock KM, Erickson MA, et al. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jangula A, Murphy EJ. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett. 2013;551:23–27. doi: 10.1016/j.neulet.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim BJ, Hancock BM, Bermudez A, et al. Bacterial induction of Snail1 contributes to blood–brain barrier disruption. J Clin Invest. 2015;125:2473–2483. doi: 10.1172/JCI74159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:1–17. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 167.Imamura K, Hishikawa N, Sawada M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 168.Ehrminger M, Latimier A, Pyatigorskaya N, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139:1180–1188. doi: 10.1093/brain/aww006. [DOI] [PubMed] [Google Scholar]
- 169.Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. doi: 10.1016/S1474-4422(18)30162-5. [DOI] [PubMed] [Google Scholar]
- 170.Ferini-Strambi L, Oertel W, Dauvilliers Y, et al. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case–control study. J Neurol. 2014;261:1112–1118. doi: 10.1007/s00415-014-7317-8. [DOI] [PubMed] [Google Scholar]
- 171.Aguirre-Mardones C, Iranzo A, Vilas D, et al. Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol. 2015;262:1568–1578. doi: 10.1007/s00415-015-7742-3. [DOI] [PubMed] [Google Scholar]
- 172.Knudsen K, Fedorova TD, Hansen AK, et al. Objective intestinal function in patients with idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2019;58:28–34. doi: 10.1016/j.parkreldis.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 173.Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nishiwaki H, Hamaguchi T, Ito M, et al. Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. Msystems. 2020;5:e00797–e820. doi: 10.1128/mSystems.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim R, Jun J, Kim H, et al. Peripheral blood inflammatory cytokines in idiopathic REM sleep behavior disorder. Mov Disord. 2019;34:1739–1744. doi: 10.1002/mds.27841. [DOI] [PubMed] [Google Scholar]
- 176.Zhang H, Wang T, Li Y, et al. Plasma immune markers in an idiopathic REM sleep behavior disorder cohort. Parkinsonism Relat Disord. 2020;78:145–150. doi: 10.1016/j.parkreldis.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 177.Stokholm MG, Iranzo A, Østergaard K, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case–control study. Lancet Neurol. 2017;16:789–796. doi: 10.1016/S1474-4422(17)30173-4. [DOI] [PubMed] [Google Scholar]
- 178.Farmen K, Nissen SK, Stokholm MG, et al. Monocyte markers correlate with immune and neuronal brain changes in REM sleep behavior disorder. Proc Natl Acad Sci. 2021;118:e2020858118. doi: 10.1073/pnas.2020858118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vijiaratnam N, Simuni T, Bandmann O, et al. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 2021;20:559–572. doi: 10.1016/S1474-4422(21)00061-2. [DOI] [PubMed] [Google Scholar]
- 180.Dutta SK, Verma S, Jain V, et al. Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J Neurogastroenterol Motil. 2019;25:363. doi: 10.5056/jnm19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87:1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 182.Tamtaji OR, Taghizadeh M, Kakhaki RD, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 183.van Kessel SP, Frye AK, El-Gendy AO, et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun. 2019;10:1–11. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maini Rekdal V, Bess EN, Bisanz JE, et al. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364:6323. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Kessel SP, de Jong HR, Winkel SL, et al. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol. 2020;18:1–14. doi: 10.1186/s12915-020-00876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lubomski M, Davis RL, Sue CM. The gut microbiota: a novel therapeutic target in Parkinson’s disease? Parkinsonism Relat Disord. 2019;66:265–266. doi: 10.1016/j.parkreldis.2019.08.010. [DOI] [PubMed] [Google Scholar]