Abstract
Murine macrophages effect potent antimycobacterial function via the production of nitric oxide by the inducible isoform of the enzyme nitric oxide synthase (NOS2). The protective role of reactive nitrogen intermediates (RNI) against Mycobacterium tuberculosis infection has been well established in various murine experimental tuberculosis models using laboratory strains of the tubercle bacillus to establish infection by the intravenous route. However, important questions remain about the in vivo importance of RNI in host defense against M. tuberculosis. There is some evidence that RNI play a lesser role following aerogenic, rather than intravenous, M. tuberculosis infection of mice. Furthermore, in vitro studies have demonstrated that different strains of M. tuberculosis, including clinical isolates, vary widely in their susceptibility to the antimycobacterial effects of RNI. Thus, we sought to test rigorously the protective role of RNI against infection with recent clinical isolates of M. tuberculosis following both aerogenic and intravenous challenges. Three recently isolated and unique M. tuberculosis strains were used to infect both wild-type (wt) C57BL/6 and NOS2 gene-disrupted mice. Regardless of the route of infection, NOS2−/− mice were much more susceptible than wt mice to any of the clinical isolates or to either the Erdman or H37Rv laboratory strain of M. tuberculosis. Mycobacteria replicated to much higher levels in the organs of NOS2−/− mice than in those of wt mice. Although the clinical isolates all exhibited enhanced virulence in NOS2−/− mice, they displayed distinct growth rates in vivo. The present study has provided results indicating that RNI are required for the control of murine tuberculous infection caused by both laboratory and clinical strains of M. tuberculosis. This protective role of RNI is essential for the control of infection established by either intravenous or aerogenic challenge.
Tuberculosis was responsible for over 1.5 million deaths worldwide in 1999 (26). Mycobacterium tuberculosis infects and replicates within macrophages. To date, the only mechanism by which macrophages have been shown to kill M. tuberculosis is the generation of reactive nitrogen intermediates (RNI) via the enzyme inducible nitric oxide synthase (NOS2; reviewed in reference 3). The importance of NOS2 in mediating control over M. tuberculosis infection has been demonstrated in mice in several studies. Administration of NOS2 inhibitors to C57BL/6 mice greatly increased their susceptibility to an intravenous challenge of M. tuberculosis, reducing the mean survival time (MST) from over 300 days to just 28 days (4). NOS2 gene-disrupted mice exhibited profound susceptibility to acute M. tuberculosis infection established intravenously (13). In a murine model of latent tuberculosis in which mice were infected for 6 months, the NOS2 inhibitor aminoguanidine resulted in recrudescence of the chronic infection and led to fatal tuberculosis (6). Recent studies also suggest that NOS2-derived RNI play a protective role in human tuberculosis (10, 14, 16, 18, 22, 24, 25).
A factor that may affect the ability of RNI to limit the replication of M. tuberculosis is variability in the sensitivity of different M. tuberculosis strains to RNI-mediated cytotoxicity. Several studies have demonstrated that different isolates of M. tuberculosis can vary in sensitivity to RNI (7, 19, 21, 27). For instance, the M. tuberculosis C strain, which was responsible for over 20% of the tuberculosis cases in New York City between 1992 and 1993 (8), was the most RNI-resistant M. tuberculosis strain among 38 tested with an acellular in vitro system (acidified nitrite) (7). O'Brien et al. also measured the in vitro RNI sensitivities of several clinical and laboratory strains of M. tuberculosis and showed a positive correlation between RNI resistance in vitro and virulence in guinea pigs in vivo (19). In another study, 13 M. tuberculosis strains were tested for both sensitivity to cell-free acidified nitrite and the ability to replicate in gamma interferon (IFN-γ)-primed murine macrophages producing RNI; substantial variation in RNI sensitivity was observed among the strains in both systems (21). Several of these strains were tested for the ability to replicate in C57BL/6 mice following aerosol infection and exhibited a wide range (5 × 101 to 2 × 105) in the number of bacteria in the lungs 20 and 40 days postinfection (20). However, there was no correlation between RNI tolerance and in vivo virulence, as assessed by the rate of replication of M. tuberculosis in the lungs of infected animals (20, 21). The inconsistency in the correlation between RNI susceptibility and virulence in vivo (19, 20, 21) suggested that RNI sensitivity might not contribute to the outcome of an infection with M. tuberculosis clinical isolates. Finally, the requirement for NOS2 in the control of infection caused by recently isolated clinical strains of M. tuberculosis has not been tested in mice, nor has the significance of RNI in controlling aerogenic murine tuberculous infection caused by clinical isolates been examined. Indeed, a recent report (5) suggests that NOS2−/− mice are much more resistant to tuberculosis following aerosol rather than intravenous infection. Therefore, the general significance of RNI in host defense against M. tuberculosis remains to be determined.
In the present study, we tested the importance of the NOS2-mediated effector function against both laboratory and clinical strains of M. tuberculosis. We compared the intravenous and aerosol routes of infection of NOS2−/− and C57BL/6 mice. Our data strongly support the requirement for RNI production in vivo for control of M. tuberculosis infection caused by both clinical and laboratory strains, regardless of the route of inoculum delivery. The results also show that the clinical and laboratory strains varied in the ability to replicate and cause disease in vivo in both NOS2−/− and wild-type mice.
MATERIALS AND METHODS
Mice.
Eight- to 10-week-old C57BL/6 female mice were obtained from The Jackson Laboratory, Bar Harbor, Maine. NOS2−/− breeding pairs (12) were kindly provided by Timothy Billiar at the University of Pittsburgh School of Medicine and bred as homozygotes. NOS2−/− mice (11) were also obtained from The Jackson Laboratory. These two different strains of NOS2−/− mice gave similar results in the experiments. All mice were maintained in biosafety level 3, specific-pathogen-free animal facilities. All mice were observed daily, and those judged to be moribund were humanely sacrificed and counted as dead.
Bacteria and infections.
The M. tuberculosis Erdman strain (Trudeau Institute, Saranac Lake, N.Y.) was passed through mice, grown in culture once, and frozen in aliquots. H37Rv was grown in culture and frozen in aliquots. Three anonymous clinical isolates of M. tuberculosis (CI3, CI4, and CI7) were obtained directly from Lowenstein-Jensen slant cultures of specimens obtained from patients with active tuberculosis at the Montefiore Medical Center of the Albert Einstein College of Medicine, Bronx, N.Y. All three isolates are drug susceptible and have distinct IS6110 restriction fragment length polymorphism patterns (1; data not shown). These isolates were expanded by, at most, three passages in 7H9 liquid medium and frozen in aliquots. Prior to infection, an aliquot was thawed, diluted in phosphate-buffered saline (PBS) containing 0.05% Tween 80, and briefly sonicated in a cup-horn sonicator. Intravenous infection was achieved via a tail vein at a dose of 105 to 106 viable CFU per mouse. For aerosol infection, mice were placed in a closed-air aerosolization system (In-Tox Products, Albuquerque, N.Mex.) and exposed for 20 min to nebulized M. tuberculosis at a concentration calibrated to deliver approximately 10 to 50 CFU to the lungs (23).
Determination of CFU.
At regular intervals, mice were humanely sacrificed and one-quarter or one-half of the lungs, liver, and spleen were homogenized in PBS containing 0.05% Tween 80. Serial dilutions of the homogenates were plated onto 7H10 agar, the plates were incubated at 37°C in a 5% CO2 atmosphere, and the colonies were enumerated after 21 days.
Histopathology.
Formalin-fixed, paraffin-embedded tissues sections were stained with hematoxylin and eosin for histological analysis and for acid-fast bacilli by Kinyoun's method (Difco, Detroit, Mich.) in accordance with the manufacturer's directions.
M. tuberculosis growth in broth.
From frozen stocks of each strain, 7H9 broth was inoculated at 106 CFU/ml, grown to an optical density at 600 nm of 1.0 (∼3 × 108 CFU/ml), and used to inoculate a fresh culture at 106 CFU/ml. The cultures were incubated at 37°C with gentle shaking. Daily aliquots were diluted and plated on 7H10 plates for viable CFU counting. The optical density at 600 nm of each culture was determined daily. In vitro growth was monitored for 8 days. Doubling time of the strains was determined by the formula 1/k, where k = (log10xt − log10x0)/0.301(t) and where x0 is the initial concentration of bacteria and xt is the concentration after time t.
M. tuberculosis growth in macrophages.
Thioglycolate-elicited peritoneal macrophages or bone marrow-derived macrophages were obtained from C57BL/6 and NOS2−/− mice. To obtain bone marrow-derived macrophages, cells were extracted from the femurs of mice in cold Dulbecco modified Eagle medium (Life Technologies, Grand Island, N.Y.) medium and washed twice in the same medium. Cells (2.5 × 106) were added to LabTek PS petri dishes (Fisher Scientific, Pittsburgh, Pa.) in Dulbecco modified Eagle medium containing 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, and 33% L-cell fibroblast supernatant. The medium was changed on day 3. On day 5, adherent cells were washed with ice-cold PBS, detached for 20 min on ice, harvested with cell scrapers, and plated in a 96-well plate at 1.5 × 105 cells/well in 200 μl. For activation of macrophages, recombinant murine IFN-γ at 100 U/ml was added and the mixture was incubated for 12 to 18 h (Genentech, South San Francisco, Calif.) and then lipopolysaccharide (LPS; Sigma, St. Louis, Mo.) at 1 μg/ml was added. M. tuberculosis cultures were washed and resuspended in macrophage medium, sonicated, and then added to the macrophages at a multiplicity of infection of 2 to 4. The inoculum was removed 4 h after infection, the monolayers were washed once, and the cells were refed with 200 μl of medium/well. To enumerate viable intracellular mycobacteria, infected macrophages were lysed in 1% saponin and 10-fold serial dilutions were plated onto 7H10 agar plates. Colonies were counted after 21 days. Nitrites in the supernatants were measured by the Griess assay as previously described (9).
RESULTS
Clinical M. tuberculosis strains in NOS2−/− mice infected intravenously.
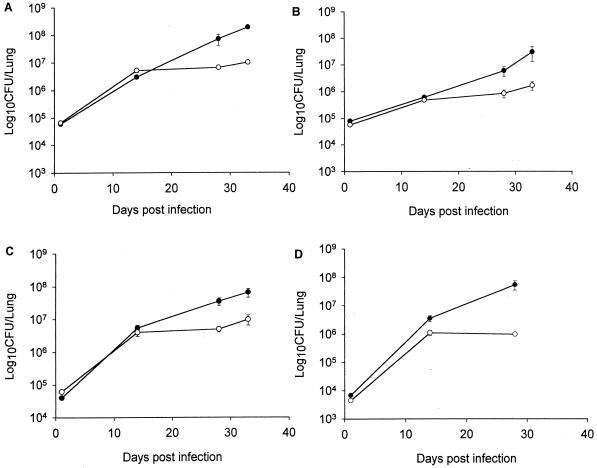
The abilities of NOS2−/− mice to control intravenous infections (inoculum, 105 to 106 CFU per mouse) with three clinical strains (CI3, CI4, and CI7) and with laboratory strain Erdman were compared. All three clinical strains, as well as Erdman, grew to significantly higher numbers in the lungs of NOS2−/− mice (Fig. 1). By 28 days postinfection, the lung bacterial burdens in NOS2−/− mice infected with the various strains of M. tuberculosis were 7- to 56-fold higher than that in wild-type C57BL/6 animals. The difference between tissue bacterial burdens in NOS2−/− and C57BL/6 mice infected with all of the strains tested was also observed in the spleen and liver (data not shown). These data indicated that NOS2 is required for containment of the laboratory Erdman strain of M. tuberculosis and the three clinical isolates with distinct restriction fragment length polymorphism patterns.
FIG. 1.
Bacterial burdens in lungs of NOS2−/− (closed circles) and wild-type C57BL/6 (open circles) mice following intravenous infection with M. tuberculosis Erdman (A) or clinical isolate CI3 (B), CI4 (C), or CI7 (D). Mice were infected with 105 to 106 CFU of M. tuberculosis, and lung homogenates were plated at the times indicated to determine the number of viable bacilli. Each point is the mean of three mice, and the bar is the standard error and is representative of two experiments. By 4 weeks postinfection and beyond, the bacterial burden in the lungs of NOS2−/− mice is significantly greater than that of wild-type C57BL/6 animals for all of the M. tuberculosis strains examined (P < 0.05).
Individual clinical isolates exhibited unique replication kinetics in vivo. Regardless of NOS2 status, strain CI3 replicated at the slowest rate in the lungs of infected mice while CI7 grew most rapidly (Fig. 1B and D). During the initial 28 days postinfection, the pulmonic bacterial loads of NOS2−/− mice infected with strains CI3 and CI7 increased by 75-and 8,100-fold, respectively (Fig. 1B and D). In the same period postinfection, the bacterial numbers in the lungs of wild-type C57BL/6 mice infected with strains CI3 and CI7 increased by 15- and 230-fold, respectively. These results indicated that the virulence of the clinical isolates, as measured by in vivo growth in the lungs, varies in both NOS2−/− and wild-type mice. Of the four isolates of M. tuberculosis tested in these studies, including the Erdman laboratory strain, it appears that CI7 is the most virulent and CI3 is the least virulent, as assessed by in vivo growth in the lungs of infected mice.
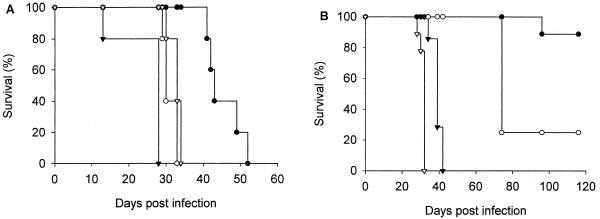
To determine the importance of NOS2 in preventing progressive fatal tuberculosis, mice infected intravenously with either one of the clinical isolates or with the Erdman strain were monitored for death (Fig. 3A). If an animal was judged to be moribund, it was humanely sacrificed and counted as dead. No wild-type C57BL/6 mouse succumbed to infection with any of the isolates during the experimental period. In contrast, infection with each clinical isolate, as well as the Erdman strain, proved fatal to NOS2−/− mice (Fig. 3A). However, the MSTs varied among the clinical isolates (Table 1), with CI7 being the most virulent and CI3 being the least virulent. The MSTs of CI3 (P = 0.005) and CI7 (P = 0.03) were significantly different from that of the Erdman strain. There was a direct correlation between the MST of NOS2−/− mice (Table 1) and the ability of the isolates to replicate in NOS2−/− mouse lungs (Fig. 1).
FIG. 3.
Survival of NOS2-deficient mice following infection with 105 to 106 CFU intravenously (A) or with approximately 50 CFU aerogenically (B). The M. tuberculosis strains used for infection included Erdman (open triangle) and clinical isolates CI3 (closed circle), CI4 (open circle), and CI7 (closed triangle). No wild-type C57BL/6 mouse succumbed to infection. There were five mice per group. Similar results were obtained in a second experiment.
TABLE 1.
Mean survival times of NOS2−/− mice infected with M. tuberculosis strains and doubling time of each strain in 7H9 brotha
| Strain | MST (days) ± SD
|
In vitro doubling time (h) | |
|---|---|---|---|
| Intravenous injection | Aerosol | ||
| CI3 | 45 ± 5 | NDb | 30 |
| CI4 | 31 ± 1 | ND | 43 |
| CI7 | 25 ± 6 | 39 ± 1 | 25.5 |
| Erdman | 33 ± 1 | 39 ± 1 | 19 |
Mice were humanely sacrificed when moribund.
ND, not determined; MST cannot be calculated since not all of the mice succumbed to the infection by 116 days, at which time the experiment was concluded.
Clinical M. tuberculosis strains in NOS2−/− mice infected aerogenically.
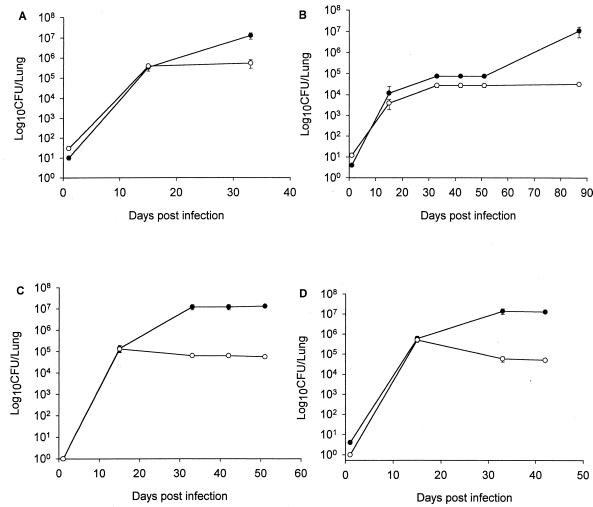
To rigorously test the significance of RNI in defense against the tubercle bacillus, the requirement for NOS2 for controlling murine tuberculosis established by the natural aerogenic route of infection was evaluated. C57BL/6 (wild type) and NOS2−/− mice from two different sources were aerogenically infected with M. tuberculosis Erdman and three clinical isolates, CI3, CI4, and CI7. Approximately 10 to 50 CFU per mouse were delivered aerogenically, as determined by viable CFU counts in lungs at 1 day postinfection. As with the intravenous infections, each strain replicated to much higher numbers in the lungs of NOS2−/− mice than in those of C57BL/6 mice (Fig. 2). This requirement of NOS2 for control of an aerogenic tuberculous infection was also demonstrated when H37Rv—another commonly used laboratory strain of M. tuberculosis—was used as the infecting organism (data not shown). These data indicated that RNI are required for the control of aerogenic infection caused by two laboratory strains (Erdman and H37Rv), as well as by three distinct clinical isolates of M. tuberculosis.
FIG. 2.
Bacterial burdens in lungs of NOS2−/− (closed circles) and wild-type C57BL/6 (open circles) mice following aerosol infection with M. tuberculosis Erdman (A) or clinical isolate CI3 (B), CI4 (C), or CI7 (D). Mice were infected by aerosol with approximately 50 viable bacilli, and lung homogenates were plated at the times indicated to determine the number of viable bacilli. Each point is the mean of three mice (except on day 1 postinfection, when two mice were examined), and the bar is the standard error. M. tuberculosis Erdman and clinical isolate CI3 were evaluated three times with similar results. Isolates CI4 and CI7 were studied once. By 33 days postinfection and beyond, the bacillary load in the lungs of NOS2−/− mice was significantly greater than that of wild-type C57BL/6 animals for all of the strains of M. tuberculosis tested (P < 0.05)
The growth advantage exhibited by CI7 over the other strains tested in the intravenous model (Fig. 1D) was not apparent in mice infected aerogenically (Fig. 2D). In contrast, the growth of strain CI3 remained remarkably attenuated in both NOS2−/− and C57BL/6 mice infected by aerosols (Fig. 2B), as had been observed in the intravenous infection model (Fig. 1B). Concordant with the slow-growing phenotype of CI3 in the lungs of infected mice, the development of pulmonic pathology caused by this isolate was significantly slower than that observed with other strains of M. tuberculosis examined (data not shown). However, histopathologic examination of tissues from mice infected with the various M. tuberculosis strains that harbored comparable number of bacilli revealed similar degrees of granulomatous inflammation (data not shown).
C57BL/6 and NOS2−/− mice aerogenically infected with either the Erdman strain or one of the clinical isolates of M. tuberculosis were monitored for death. No wild-type mouse succumbed to the infections during the 116-day study period. In NOS2−/− mice, CI7 again was the most virulent of the clinical isolates (MST, 39 ± 1 days); in contrast, aerogenic infection with CI3 resulted in the loss of only one of five infected NOS2−/− mice (Table 1 and Fig. 3B). These results demonstrated that NOS2−/− mice were very susceptible to aerogenic infection with the laboratory Erdman strain of M. tuberculosis and with certain clinical isolates (Table 1; Fig. 3B).
Growth of clinical strains in vitro.
The distinct virulence patterns associated with the individual isolates may be related to the inherent growth rate of the isolates, either in vitro or in vivo. The in vitro doubling time of each strain was determined by monitoring growth in cultures, seeded at ∼106 CFU/ml of 7H9 broth, for 8 days (Table 1). Although there was a range of doubling times, the growth rate in vitro did not correlate with growth in C57BL/6 or NOS2−/− mice or with deaths of NOS2−/− mice (Table 1).
Growth of clinical strains in macrophages.
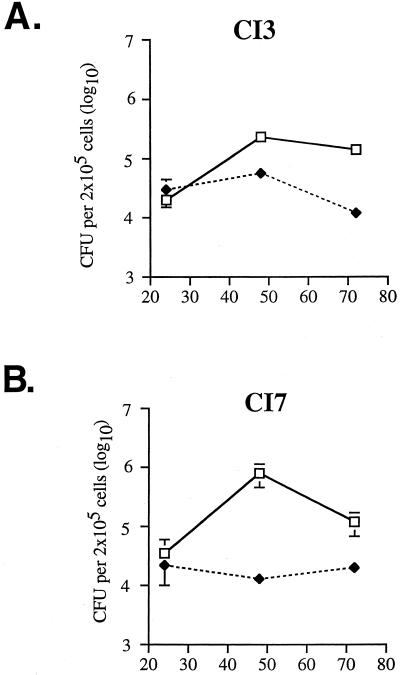
The abilities of the most and least virulent clinical isolates, CI3 and CI7, to grow in peritoneal macrophages obtained from both wild-type C57BL/6 and NOS2−/− mice were compared. In unactivated wild-type macrophages, both CI3 and CI7 replicated over 48 h (Fig. 4). Wild type C57BL/6 macrophages activated with IFN-γ and LPS controlled the replication of both CI3 and CI7, and there was no significant difference (P > 0.05) between the numbers of viable intracellular bacilli in cells infected with CI3 and in cells infected with CI7 (Fig. 4). Similarly, there was no significant difference (P > 0.05) in the growth of CI3 compared to that of CI7 in unactivated, NOS2-deficient macrophages (data not shown). Activation of NOS2-deficient macrophages with IFN-γ and LPS did not induce RNI production or potentiate antimycobacterial effects (data not shown). Thus, the replication kinetics of the two clinical isolates tested were similar in NOS2−/− and wild-type murine macrophages in vitro, suggesting that this in vitro culture system does not predict virulence.
FIG. 4.
Growth of clinical isolates CI3 (A) and CI7 (B) in C57BL/6 macrophages. Thioglycolate-elicited peritoneal macrophages were left unactivated (solid line) or were activated with IFN-γ and LPS (dashed line) and then infected with one of the clinical isolates of M. tuberculosis at a multiplicity of infection of 2 to 4. The number of intracellular bacteria was determined over 3 days by plating cell lysates onto 7H10 agar plates and enumerating bacterial colonies 21 days later. Each point is the mean of three wells; bars are standard errors. The x axis denotes hours postinfection.
To determine the capacity of clinical isolates CI3 and CI7 to induce RNI production in macrophages, nitrite accumulation was measured in the supernatant of infected, bone marrow-derived macrophages. IFN-γ- and LPS-activated C57BL/6 wild-type macrophages infected with both clinical isolates produced similar amounts of RNI (Table 2). Interestingly, the more virulent isolate, CI7, compared to the less virulent isolate, CI3, induced significantly less RNI in unactivated macrophages 5 days postinfection (Table 2).
TABLE 2.
RNI production induced by M. tuberculosis isolates in bone marrow-derived macrophages 5 days postinfection
| M. tuberculosis strain | Macrophage nitrite/production (μg/ml)b
|
|
|---|---|---|
| Unactivated | IFN-γ/LPS activated | |
| Erdman | 0.22 ± 0.02 | 1.50 ± 0.10 |
| CI3 | 0.23 ± 0.07 | 1.29 ± 0.03 |
| CI7 | 0.04 ± 0.04a | 1.39 ± 0.16 |
P = 0.05 versus either Erdman or CI3.
Data are means ± standard deviations.
DISCUSSION
The data presented here demonstrate that NOS2 is required for the control of murine infections with recent clinical isolates, as well as laboratory strains, of M. tuberculosis, regardless of the route of infection. Without the ability to produce NOS2-derived RNI, mice rapidly succumbed to intravenous infection with all of the strains of M. tuberculosis tested. Following low-dose (<50 CFU) aerosol infection, the bacterial numbers of all of the strains examined (three clinical strains and two laboratory strains, Erdman and H37Rv) were higher in the lungs of NOS2−/− mice than in those of C57BL/6 mice. Although two clinical isolates and laboratory strain Erdman proved rapidly fatal following low-dose aerosol infection of NOS2−/− mice, one clinical isolate (CI3) did not cause death in the majority of infected NOS2−/− mice. This strain also grew more slowly in both NOS2−/− and C57BL/6 mouse lungs, suggesting that it was less virulent.
In a recently published study, NOS2−/− mice infected with a small inoculum of M. tuberculosis laboratory strain Erdman via the aerosol route were not markedly more susceptible than wild-type mice, particularly in the early phase of infection (5). Bacterial numbers in the lungs of aerosol-infected NOS2−/− mice were only moderately increased compared to those of wild-type mice, and the NOS2−/− mice survived for over 180 days (5). The reasons for the discrepancy between our results and those of Cooper et al. (5) with respect to aerosol infection of NOS2−/− mice are not clear, but the discrepancy may stem from differences in the virulence of the Erdman strain used for these studies. We confirmed our results with another laboratory strain, H37Rv (data not shown), whose enhanced virulence in NOS2−/− mice infected by aerosols has been recently reported (15). Our studies used two independent and genetically different NOS2−/− mouse strains (11, 12) with very similar results; one of these mouse strains was also used by Cooper et al., making it unlikely that the mouse strain is responsible for the differences seen.
All of the clinical isolates used are from patients with active tuberculosis, are not drug resistant, and exhibit unique IS6110 banding patterns, indicating that they are distinct from each other. There were differences in the isolates with respect to virulence in vivo and growth rates in vitro. Of the three clinical isolates tested, CI7 was the most virulent, as evident by its robust rate of replication in the lungs of infected mice and the rapidly fatal tuberculosis it caused. The least virulent strain, CI3, showed attenuated growth in vivo, and infection with this isolate did not result in a high mortality rate. CI4 was intermediate compared to the other two strains with respect to virulence. Based on histology, the disparate virulence appears not to be due to a difference in the ability to induce inflammation and tissue destruction in the lungs. Doubling times in 7H9 broth ranged from 19 to 43 h and did not correlate with bacterial growth in wild-type or NOS2−/− mice or with the ability of a strain to progress to fatal tuberculosis in NOS2−/− mice.
The least and most virulent clinical isolates were compared for growth in macrophage cultures. Both strains grew in unactivated wild-type macrophages, and this replication was arrested when the macrophages were activated with IFN-γ and LPS. The inhibition of growth and killing of the bacteria were at least partially NOS2 mediated, as NOS2−/− macrophages treated with IFN-γ and LPS were unable to inhibit the replication of the two clinical strains. We reported obtaining similar results with C57BL/6 and NOS2−/− macrophages and strain Erdman (2). Thus, the clinical strains tested here were sensitive to RNI production, both in vivo and in vitro. RNI production can be induced by M. tuberculosis infection, albeit at a much lower level than in IFN-γ-primed macrophages. Comparison of CI3 and CI7 indicated that the more virulent CI7 isolate induced lower levels of RNI in unactivated macrophages. Although limited, these data raise the possibility that the enhanced virulence of CI7 is due, in part, to its capacity to avoid inducing mycobacteristatic and/or mycobactericidal levels of RNI soon after infection. With respect to the attenuated virulence of CI3, it is possible that this strain, while susceptible to RNI, is more sensitive to RNI-independent antimycobacterial mechanisms than are the other isolates tested. These antimycobacterial mechanisms may assume an important role in defense against M. tuberculosis in an RNI-deficient environment, such as that in NOS2−/− mice. Alternatively, it is possible that the relative avirulence of CI3 is due to an innate growth deficiency in the lungs of infected mice. Several studies have investigated the relationship between the mycobacterial replication rate within lungs or within cultured macrophages and virulence. The growth rate of tubercle bacilli within the lung was originally claimed to be a virulence factor by North and Izzo (17). This was supported by a comparison of the ability of individual M. tuberculosis strains to spread within a community and their replication rate in human macrophages in vitro (28). M. tuberculosis strain 210, which is responsible for 27% of the tuberculosis cases in central Los Angeles, grew faster than did strains that caused small outbreaks or isolated cases (28). Our finding that the growth rate in the lungs of infected mice correlated well with mortality further confirms the relationship between in vivo replication and virulence.
In summary, this study has established the important role of NOS2-generated RNI in protecting mice against clinical isolates, as well as laboratory strains, of M. tuberculosis. In addition, the protective role of RNI is operative in tuberculous infections established via the intravenous and aerogenic routes. These results therefore demonstrate the general in vivo significance of NOS2 in defense against M. tuberculosis in the mouse. Interestingly, the growth kinetics in mice and the lethality of the clinical strains varied greatly and appear to be independent of their in vitro replication rate. Additional studies, including bacterial genotypic analysis, are under way to identify the determinant(s) of the disparate virulence of these strains.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant ROI 36990 (J.L.F. and J.C.).
We are grateful to Amy Caruso and Heather Joseph for technical assistance and to the members of the Chan and Flynn laboratories for helpful discussions.
REFERENCES
- 1.Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City, an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar K A, Serbina N V, Flynn J L. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Flynn J L. Nitric oxide in Mycobacterium tuberculosis infection. In: Fang F, editor. Nitric oxide and infection. New York. N.Y: Plenum Publishers; 1999. pp. 281–310. [Google Scholar]
- 4.Chan J, Tanaka K, Carroll D, Flynn J L, Bloom B R. Effect of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Pearl J E, Brooks J V, Ehlers S, Orme I M. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68:6879–6882. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn J L, Scanga C A, Tanaka K E, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 7.Friedman C R, Quinn G C, Kreiswirth B N, Perlman D C, Salomon N, Schluger N, Lutfey M, Berger J, Poltoratskaia N, Riley L W. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis I. J Infect Dis. 1997;176:478–484. doi: 10.1086/514067. [DOI] [PubMed] [Google Scholar]
- 8.Friedman C R, Stoeckle M Y, Kreiswirth B N, Johnson W D J, Manoach S M, Berger J, Sathianathan K, Hafner A, Riley L W. Transmission of multi-drug resistant tuberculosis in a large urban setting. Am J Respir Crit Care Med. 1995;152:355–359. doi: 10.1164/ajrccm.152.1.7599845. [DOI] [PubMed] [Google Scholar]
- 9.Green L C, Wagner D A, Glogowske J A, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrite, nitrate, and [15N]-nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 10.Jagannath C, Actor J K, Hunter R L., Jr Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide. 1998;2:174–186. doi: 10.1006/niox.1998.9999. [DOI] [PubMed] [Google Scholar]
- 11.Laubach V E, Shesely E G, Smithies O, Sherman P A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacMicking J D, Nathan C, Hom G, Chartrain N, Trumbauer M, Stevens K, Xie Q-W, Sokol K, Fletcher D S, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Means T K, Jones B W, Schromm A B, Shurtleff B A, Smith J A, Keane J, Golenbock D T, Vogel S N, Fenton M J. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 15.Mogues T, Goodrich M E, Ryan L, LaCourse R J, North R J. The relative importance of T cell subsets in immunity and immunopathogenesis of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson S, Bonecini-Almeida M, Silva J R L, Nathan C, Xie Q-W, Mumford R, Weidner J R, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J L. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;184:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism for nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–3647. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien L, Carmichael J, Lowrie D B, Andrew P W. Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect Immun. 1994;62:5187–5190. doi: 10.1128/iai.62.11.5187-5190.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ordway D J, Sonnenberg M G, Donahue S A, Belisle J T, Orme I M. Drug-resistant strains of Mycobacterium tuberculosis exhibit a wide range of virulence for mice. Infect Immun. 1995;63:741–743. doi: 10.1128/iai.63.2.741-743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich E A, Torres M, Sada E, Finegan C K, Hamilton B D, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tubercle Lung Dis. 1997;78:247–255. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 23.Serbina N V, Liu C-C, Scanga C A, Flynn J L. CD8+ cytotoxic T lymphocytes from lungs of M. tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 24.Wang C H, Lin H C, Liu C Y, Huang K H, Huang T T, Yu C T, Kuo H P. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int J Tubercle Lung Dis. 2001;5:283–291. [PubMed] [Google Scholar]
- 25.Wang C H, Liu C Y, Lin H C, Yu C T, Chung K F, Kuo H P. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11:809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The world health report 1999. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 27.Yu K, Mitchell C, Xing Y, Magliozzo R S, Bloom B R, Chan J. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tubercle Lung Dis. 1999;79:191–198. doi: 10.1054/tuld.1998.0203. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Gong J, Yang Z, Samten B, Cave M D, Barnes P F. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J Infect Dis. 1999;179:1213–1217. doi: 10.1086/314738. [DOI] [PubMed] [Google Scholar]