Abstract
Vitamin D deficiency is associated with a variety of age-related diseases and is becoming increasingly more prevalent in the population over time. Some diseases associated with deficiency are cardiovascular disease, cancer, and neurodegeneration. This association, as well as the fact that vitamin D has been demonstrated to play an important role in a variety of extraskeletal processes, has led some to claim that vitamin D is an essential longevity vitamin. However, the role of vitamin D in healthy aging has been difficult to determine. In order to study vitamin D in the context of aging, the model organism, Caenorhabditis elegans (C. elegans), was employed. To study vitamin D’s impact on aging and age-related disease, lifespan and health span were measured across three different genetic strains of C. elegans. Strains investigated were wildtype (N2), worms with a mutant vitamin D receptor ortholog (nhr-8), and worms engineered to represent Alzheimer disease (gnals2). Bioinformatic analysis of available public data was also performed in order to identify the transcriptional response produced in N2 worms treated with vitamin D3. Treatment with vitamin D3 significantly extended the lifespan of N2 worms and rescued nhr-8 worms, which typically have decreased lifespans compared to N2. Treatment with vitamin D3 minimally extended the lifespan of gnals2 worms. Similar results were obtained for measures of health span, quantified as motility through time. Differentially expressed genes upon treatment with vitamin D3 were largely associated with biological processes such as the innate immune response and metabolism of xenobiotic compounds in the worms, which may explain the observed increase in lifespan and health span.
Keywords: Vitamin D, Lifespan, Extension, Longevity, NHR-8, Alzheimer disease, C. elegans
Introduction
Vitamin D deficiency is associated with a variety of age-related diseases and is becoming increasingly more prevalent in the population through time [1–3]. Some diseases associated with deficiency are cardiovascular disease, cancer, neurodegeneration, and more [3]. This association, as well as the fact that vitamin D has been demonstrated to play an important role in a variety of extraskeletal processes [4], has led some to claim that vitamin D is an essential longevity vitamin [5]. However, despite these associations, whether or not vitamin D deficiency is causative of such diseases has been hard to determine. While some clinical trials have shown promising results [6, 7], the majority of trials have failed to identify a beneficial effect of supplementation [8]. This discrepancy has led vitamin D to become a highly contentious topic across research and medicine, resulting in two opposing opinions across the field.
The first opinion posits that the lack of significant results in clinical trials is erroneous, as studies designed using guidelines devised for drugs, rather than nutrients, can dilute results [9]. For example, if the initial study population set to receive vitamin D treatment is largely replete with the vitamin, the effects of supplementation would likely be masked. Only those deficient in the nutrient would likely gain the benefit of supplementation, while a drug is typically a novel compound to all individuals in a given study. Therefore, adjustments to the clinical trial process would need to be implemented, including an initial screening for vitamin blood serum levels at the start of the study with potential exclusion criteria limiting those replete with the vitamin. It could also be true that single nucleotide polymorphisms could impact the absorption/conversion efficiency in particular individuals – thus rendering the given dosage strategy ineffective [10]. For this reason, the monitoring of blood serum levels throughout a clinical trial would also be necessary.
On the other hand, however, others have asserted that the association of age-related disease with vitamin D deficiency is at high risk of confounding and reverse causation [8]. Considering that factors such as body mass index (BMI), hospitalizations, and age increase susceptibility to vitamin D deficiency [4, 11], it is easy to understand how such associative studies could be subject to confounding. Thus, many claim that vitamin D deficiency may not truly be causative of age-related disease, but rather, an innocent bystander.
To resolve these discrepancies, more research into the matter is critical. While properly designed randomized controlled trials will remain the gold standard, laboratory research into vitamin D’s role as a potential longevity vitamin can help determine mechanisms and future areas of interest to inform further research. One powerful model that can be used to study aging and longevity is the nematode, Caenorhabditis elegans, which has a sequenced genome, has a relatively short lifespan, and can be easily manipulated [12]. For these reasons and more, many of the frontier discoveries into the genetic determinants of aging have been uncovered through work in C. elegans [13]. Beyond their convenience as a model organism, work done in the worms can be translatable to humans, as the C. elegans genome possesses homologs of around two-thirds of all genes known to cause human disease [13]. Work in C. elegans has resulted in numerous translatable discoveries, uncovering a key role for dietary and genetic factors in determining lifespan.
In humans, vitamin D3 is produced in the skin when 7-dehydrocholesterol is exposed to ultraviolet B (UVB) radiation. Vitamin D3 can also be sourced from the diet, found naturally in foods like fatty fish and mushrooms. Vitamin D3 must then be hydroxylated two times in the body before becoming the potent steroid hormone that binds the vitamin D receptor to enact transcriptional action. The first hydroxylation step occurs in the liver and is largely performed by the cytochrome P450 enzyme, CYP2R1 [14]. However, studies have shown that CYP2R1 is not the only enzyme capable of performing this function, rather, CYP27A1, CYP3A4, CYP2D25, and others have the ability to perform this first step [14]. Once this initial hydroxylation step is completed, vitamin D3 becomes 25-hydroxyvitamin D (25(OH)D) and is then hydroxylated again by CYP27B1 into 1,25 dihydroxyvitamin D (1,25(OH)2D) [14]. This is the major steroid hormone that binds the vitamin D receptor in order to modulate transcription of vitamin D-regulated genes. Interestingly, C. elegans have the core proteins and produce the same nutritional precursors necessary for vitamin D endocrinology. For example, C. elegans have orthologs to many of the CYP proteins involved in human vitamin D metabolism, with CYP3A4 being represented by cyp-13A4 and CYP27B1 being represented by cyp-44A1 in C. elegans. Further, there are three nuclear hormone receptors in C. elegans that are considered orthologs to the human vitamin D receptor. These are encoded by the genes, daf-12, nhr-48, and nhr-8. Previous studies have investigated DAF-12’s role in vitamin D induced lifespan extension and have found no clear role in C. elegans [15]. However, NHR-8 and NHR-48 have been unstudied in this context. Finally, C. elegans are known to produce 7-dehydrocholesterol, the precursor to vitamin D in humans, which would convert to vitamin D3 spontaneously in the presence of UVB radiation. This conversion likely happens in nature, as C. elegans are found in habitats such as rotting fruit which could be exposed to sunlight. The presence of core aspects involved with vitamin D endocrinology in C. elegans is not surprising, as the vitamin D system is highly conserved across the eukaryotic tree of life [16].
Previous studies have identified that vitamin D seems to increase the lifespan of C. elegans [15, 17]. Vitamin D has also been demonstrated to alleviate paralysis in genetic strains of C. elegans engineered to represent Alzheimer disease [15, 18]. However, although the genetic mechanisms have begun to be teased out, many questions about vitamin D’s longevity promoting effects in C. elegans remain. For example, although lifespan extension in the worms was found to be independent of the DAF-12 nuclear hormone receptor, and dependent on the longevity-associated transcription factor, SKN-1, the other orthologs to the human VDR receptor that exist in the worms have not been investigated. Additionally, whether or not vitamin D can relieve age related phenotypes, such as a decline in motility, has not been investigated.
This study aimed to address some of the questions about vitamin D’s effects on the longevity of C. elegans. The first fundamental question investigated was whether or not vitamin D could extend the lifespan of C. elegans, and this question was tested across three genetic strains of worms. First, the wildtype (N2) strain of C. elegans was investigated in order to determine if previous studies reporting lifespan extension could be replicated. Additionally, a strain (nhr-8) containing an approximate 1.3 kb deletion in the coding region of nhr-8 was tested in order to see if functional NHR-8 was implicated in vitamin D induced lifespan extension. The last strain investigated was a strain (gnals2) of worms engineered to represent Alzheimer disease, different from strains that have been previously studied. This strain expresses pan-neuronal amyloid beta and exhibits impaired neuromuscular and sensorimotor behavior. It was hypothesized that vitamin D3 would extend the lifespan of N2 and gnals2 worms, but would not induce lifespan extension in nhr-8 worms, rationalized by the hypothesis that NHR-8 may be the functional vitamin D receptor in C. elegans, equipped to accommodate vitamin D. Beyond lifespan, whether or not vitamin D3 could reduce the burden of age-related decline was also investigated. A proxy used to represent age-related decline was motility, as C. elegans generally decline in motility with increasing age. Once the effects of vitamin D3 on lifespan and health span were quantified, its impact on oxidative stress resistance was also measured. Finally, a bioinformatic approach was utilized in order to interrogate pathways enriched in worms treated with vitamin D3 compared to the controls.
Methods
Chemicals and reagents
Vitamin D3 was obtained from Fisher Scientific in the form of a crystalline powder. Vitamin D3 was stored in an amber bottle under refrigeration in order to minimize degradation of the compound. Stock concentrations of vitamin D3 (250 and 400 μM) were produced by dissolving appropriate amounts of D3 into 95% ethanol. All vitamin D3 concentrations were stored in plastic conical tubes, covered in aluminum foil to block light, under refrigeration. The control solution used in all experiments was comprised of the solvent, 95% ethanol, treated/stored in the same way as D3 solutions.
5-Fluoro-2′-deoxyuridine (FUDR) was obtained from Sigma-Aldrich in the form of a powder. FUDR was stored at room temperature until dissolved into distilled water to produce a stock concentration (2.5 mM) that was stored in the freezer until ready for use.
Paraquat dichloride tetrahydrate (paraquat) was obtained from SPEX CertiPrep dissolved in methanol at a concentration of 1000 μg/mL. Paraquat was pipetted directly onto FUDR plates under fume hood using proper safety precautions.
C. elegans and Escherichia coli OP50 culture conditions
The C. elegans strains used were N2 (Bristol), AE501 (nhr-8(ok186) IV), and GRU102 (gnaIs2 [myo-2p::YFP + unc-119p::Abeta1-42]), which were purchased from the Caenorhabditis Genetics Center (CGC). Escherichia coli OP50 (OP50) was also obtained from the CGC and was propagated into liquid cultures grown in Luria Broth. In order to grow worm populations, 120 μL of live OP50 was spotted onto Nematode Growth Media (NGM) plates and was allowed to grow at least one night before use. For experiments in which worms were treated with vitamin D3, plates were spotted with 80 μL of live OP50 and were allowed to grow at least one night to form a circular bacterial lawn in the center of the plates. All strains were maintained at 20 °C and on standard NGM plates. Populations were synchronized by bleach preparation if stock strains were contaminated; otherwise, populations were synchronized by picking young adult worms onto fresh plates.
Lifespan assay
All lifespan assays were performed on FUDR plates at a concentration of 50 μM in order to prevent reproduction. NGM plates were made following standard procedures [19], and FUDR was added to the NGM solution along with salts and cholesterol after autoclaving and cooled to 55 °C. Prior to the start of lifespan assays, FUDR plates that had been spotted with 80 μL of OP50 were treated with 100 μL of vitamin D3 or control (95% ethanol). 100 μL of treatment was used in order to cover the entire surface of the plates and was allowed to dry overnight before use. Lifespan analyses were performed for N2, AE501, and GRU102 strains at both a concentration of 250 μM and 400 μM of D3. Worms were scored every other day until day 19, after which time the worms were scored daily. Worms that died of nonnatural deaths, such as accidental deaths or internal hatching of progeny, were censored. The results represent the survival rate from a minimum of four biological replicates with 25 worms per replicate.
Motility assay
All motility assays were performed on FUDR plates at a concentration of 50 μM in order to prevent reproduction. NGM plates were made following standard procedures [19], and FUDR was added to the NGM solution along with salts and cholesterol after autoclaving and cooled to 55 °C. Prior to the start of motility assays, FUDR plates that had been spotted with 80 μL of OP50 were treated with 100 μL of vitamin D3 or control (95% ethanol). 100 μL of treatment was used in order to cover the entire surface of the plates and was allowed to dry overnight before use. Motility assays were performed for N2 and AE501 strains at concentration of 400 μM of D3, while gnals2 worms were tested under a concentration of 250 μM of D3. L4s were placed onto treated plates and were observed on days 1, 3, 5, 7, 11, and 13 of the adult worm lifespan. Worms were not observed past day 13 since the worms generally stopped moving beyond that time. In order to quantify motility, worms were moved one at a time to a clean FUDR plate (no bacteria or treatment) and were observed for a time of one minute. During the one-min time interval, the ability of the worm to complete one cycle from crest to crest of their sinusoid movement pattern was quantified as one beat. The number of times the worms completed this cycle over the time of 1 min constituted the motility of the particular worm being measured. During this assay, movements in reverse were also counted, so long as the worm completed one cycle from crest to crest. The results represent the mean motility from three biological replicates, with 10 worms per replicate.
Oxidative stress assay
N2 and AE501 worms were synchronized and L4s were transferred to FUDR plates (50 μM) treated with either 200 μL of D3 (400 μM) or control (95% ethanol). Worms were grown on treated plates until day 5 and were then subsequently transferred onto plates containing the treatment condition (vitamin D3 or control) plus 200 μL of paraquat (3 mM). Worms remained on paraquat plates for 5 days and then were transferred back to vitamin D3 or control treated plates. Worms were scored daily, censoring nonnatural deaths as appropriate. Worms were censored after day 30. The results represent the survival rate from three biological replicates of at least 30 worms per replicate.
Bioinformatic analysis
All data analysis was performed using the Galaxy web platform, utilizing the public server at usegalaxy.org [20]. RNA sequencing data from Mark et al. (2016) were obtained from the NCBI Gene Expression Omnibus (GEO) under the accession number, GSE86493 [15, 21]. Raw reads were first evaluated using FastQC to generate quality reports [22]. After analysis with FastQC, adapter sequences were cut using Cutadapt [23]. Reads were then aligned to WBcel235/ce11 using HISAT2 [24]. After alignment, the number of aligned reads were quantified using StringTie [25]. Differential expression analysis was then performed using DESeq2 [26]. DESeq2 produced a list of all differentially expressed genes which was further sorted in Microsoft Excel using filtering tools. Genes that were expressed with a log2 fold change (lg2FC) greater than 0, at adjusted p values less than or equal to 0.05 (padj < 0.05), were filtered as upregulated genes. p values were adjusted for multiple testing with the Benjamini–Hochberg procedure which controls for false discovery rate (FDR). Finally, pathway enrichment analysis was performed using gProfiler for both significantly upregulated and downregulated genes [27].
Statistical analysis
For lifespan assays, Kaplan–Meier curves were generated, and the nonparametric test, log-rank (Mantel-Cox), was utilized in order to identify differences in overall lifespans. To investigate differences in lifespan at specific times points, Fisher’s exact test was utilized.
For motility assays, a two-way ANOVA was utilized to test the omnibus hypothesis that there was an effect of treatment, time, and/or interaction between the two. A plate containing 10 worms was defined as a replicate, and the average motility scores from the 10 worms on the plate was used to represent motility in the statistical test. Assumptions of ANOVA were satisfied before use; Spearman’s test for heteroscedasticity was used to test for equal variance and Shapiro–Wilk to assess normality. Sidak’s multiple comparisons test was performed for post hoc analysis in order to control for accumulation of error associated with multiple comparisons.
Lifespan, motility, and oxidative stress assays were analyzed using GraphPad Prism 9.1.1. Alpha was defined as 0.05 for each statistical test. Figures for these assays were also generated in GraphPad Prism.
Results
Vitamin D3 induces lifespan extension and rescues nhr-8 mutants
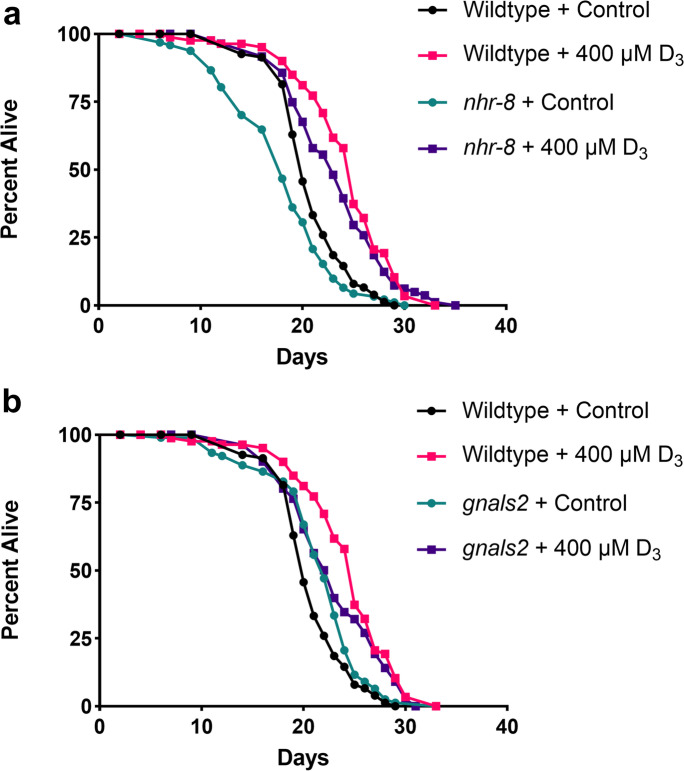
The first hypothesis investigated was whether vitamin D3 would induce a lifespan extending benefit in wildtype (N2) C. elegans. Previous studies have already identified a concentration of 250 µM, as well as 10 and 1000 μg/mL, to induce lifespan extension [15, 17]. There is likely a threshold of effective vitamin D3 concentrations that extend lifespan; we found that worms treated with 400 μM of vitamin D3 throughout adulthood experienced a substantial lifespan extension benefit (Fig. 1, Table 1, log-rank test, p < 0.001), although slightly less than the extension found in Mark et al. [15], supporting the existing hypothesis that vitamin D3 can extend lifespan in C. elegans.
Fig. 1.
Kaplan–Meier survival curves of hermaphrodite C. elegans treated with 400 μM of vitamin D3 from day 1 of adulthood. Treatment with vitamin D3 significantly extended the lifespan of wildtype (N2) worms and worms that lack the fully functional nuclear hormone receptor, NHR-8 (nhr-8) (a, log-rank test, p < 0.001). Treatment with D3 rescued deficits caused by nhr-8 mutation, resulting in a lifespan similar to N2 worms (a, log-rank test, p = 0.04). Vitamin D3 minimally extended the lifespan of worms engineered to represent a model for Alzheimer disease (gnals2); however, D3 seemed to increase late-life lifespan (b, Fisher’s exact test, p value at 75% = 0.013). The results represent the survival of 100 worms per strain/treatment
Table 1.
Lifespan of wildtype, nhr-8, and gnals2 ± vitamin D3
| Strain/condition | Mean ± SE (days) | p values | Median (days) | Deathsa |
|---|---|---|---|---|
| WT | 20.7 ± 0.4 | 20 | 80 | |
| WT + VD3 | 24.8 ± 0.5 | < 0.001 | 25 | 75 |
| nhr-8 | 17.8 ± 0.5 | 18 | 94 | |
| nhr-8 + VD3 | 23.1 ± 0.5 | < 0.001 | 23 | 82 |
| gnals2 | 21.4 ± 0.5 | 22 | 81 | |
| gnals2 + VD3 | 22.8 ± 0.5 | 0.013 | 23 | 79 |
a “Deaths” refers to scored age-related (i.e., non-censored) deaths
Considering that vitamin D must bind a functional vitamin D receptor in order to modulate transcription, the role of a homologous C. elegans receptor was investigated [28]. Utilizing a mutant strain, AE501 (nhr-8), that contains a 1.3 kb deletion in the coding region of the nhr-8 receptor, lifespan analyses were performed in order to determine if vitamin D3 would have an effect. The hypothesis was that vitamin D3 would have no lifespan inducing effect on nhr-8 worms, implicating this receptor in the mechanism behind vitamin D3 lifespan extension. The results did not support this hypothesis; nhr-8 worms treated with D3 demonstrated considerable lifespan extension (Fig. 1a, Table 1, log-rank test, p < 0.001). Interestingly, compared to wildtype worms, nhr-8 worms had a shorter lifespan, exhibiting an increased mortality rate likely due to the deleterious effects induced upon mutation of this receptor (Fig. 1a, log-rank test, p = 0.003). However, when treated with 400 μM of vitamin D3, the lifespan of nhr-8 worms was rescued, leading to a comparable lifespan to N2 worms treated with D3 (Fig. 1a, Table 1, log-rank test, p = 0.04). This finding was dose-dependent, with 250 μM D3 leading to a rescuing back to N2 control lifespan (data not shown) and 400 μM matching the lifespan extension observed in N2 worms treated with D3 (Fig. 1a).
After confirmation that vitamin D3 could indeed extend the lifespan of C. elegans, whether vitamin D3 could be beneficial in a model for Alzheimer’s disease was then evaluated. Previous studies have identified vitamin D3 to improve pathologies, such as paralysis, associated with strains of C. elegans engineered to represent models for Alzheimer’s disease [15, 18]. With that in mind, lifespan analyses were performed in a strain, GRU102 (gnals2), which is engineered to express pan-neuronal amyloid beta expression. When treated with vitamin D3, gnals2 worms received a slight increase in lifespan, however, gains in lifespan were more apparent in late life (Fig. 1b, Table 1, Fisher’s exact test, p value at 75% = 0.013). These results suggest a delay in the onset of disease associated with accumulated amyloid beta; however, they do not support that vitamin D3 appreciably slows the rate of aging like observed in other strains (Fig. 1a). Surprisingly, we found gnals2 worms actually had slightly longer lifespans than wildtype controls (Fig. 1b, Table 1, log-rank test, p = 0.029).
Vitamin D3 leads to gains in health span
While vitamin D3 treatment is known to increase the lifespan of C. elegans, whether or not it would increase metrics of health span was also of interest. Motility, a measure of movement in the worms, generally declines with age [29]. It was hypothesized that vitamin D3 would not only extend the lifespan of the worms but simultaneously increase the motility of the worms as they aged.
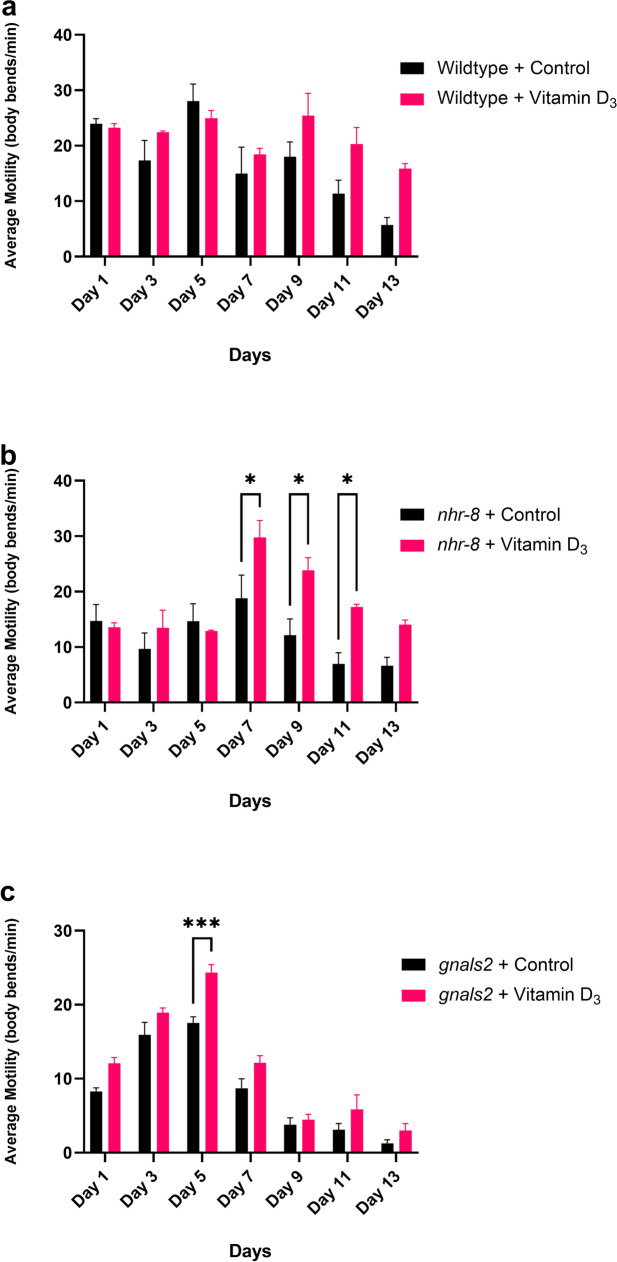
In wildtype worms, there was a significant effect of time on the motility of the worms, with worms declining in motility as they aged (Fig. 2a, two-way ANOVA, F6,28 = 8.563, p < 0.001). While worms treated with vitamin D3 generally seemed to have increased motility into late life compared to control worms, post hoc analysis did not identify any significant differences in motility at specific time points (Fig. 2a). At day 13, there was a trend for the motility of worms treated with D3 to be higher than control worms; however, these differences were not quite significant (Fig. 2a, Sidak’s multiple comparisons test, p = 0.06).
Fig. 2.
Average motility (body bends/minute) of hermaphrodite worms treated with 400 μM of vitamin D3 from day 1 of adulthood. Motilities did not differ between the two treatment groups from days 1 to 7 in N2 and nhr-8 worms (a and b). At day 9, a divergence in motility was observed, with vitamin D3-treated worms trending higher motility scores compared to controls in late life (a and b). Despite trends, post hoc analysis did not identify significant differences in wildtype motility between treatment groups at specific time points (a). However, in nhr-8 worms, vitamin D3 treated worms maintained significantly higher motilities at days 7, 9, and 11 compared to controls (b, Sidak’s multiple comparisons, p = 0.03, p = 0.02, p = 0.05). Gnals2 worms treated with D3 had an increased motility compared to controls at day 5 (c, Sidak’s multiple comparisons, p < 0.001). Motility in both treatment groups declined rapidly as worms aged (c). The results represent means ± SE of 3 biological replicates containing 10 worms per replicate [0.12 (ns), 0.033 (*), 0.002 (**), < 0.001 (***)]
Considering the results of the lifespan analysis in nhr-8 worms, it was hypothesized that vitamin D3 would increase the motility of these mutants compared to controls. Compared to wildtype worms, nhr-8 mutants generally seemed to exhibit lower motility scores, possibly due to deficits caused by mutation of this receptor. Again, as expected there was a significant effect of time on motility, with worms declining in motility as they aged (Fig. 2b, two-way ANOVA, F6,28 = 7.536, p < 0.001). There was also a significant effect of treatment on the motility of the worms (Fig. 2b, two-way ANOVA, F1,28 = 19.75, p < 0.001). Treatment with vitamin D generally seemed to improve the deficits in motility exhibited with nhr-8 mutation, with D3-treated worms scoring higher motility scores than controls. Post hoc analysis identified late life differences on days 7 (p = 0.028), 9 (p = 0.017), and 11 (p = 0.045), to be significant (Fig. 2b). Qualitatively, nhr-8 worms treated with vitamin D3 seemed more active on their plates, even on the last day of observation (day 13), which is typically when worms begin to become immobile.
While considerable lifespan extension was not observed in gnals2 worms, a significant late-life extension was detected. This finding was suggestive that vitamin D3 may delay the deleterious onset of paralysis that is induced by the accumulation of amyloid beta proteins in these worms. Therefore, it was hypothesized that gnals2 mutants treated with vitamin D3 would have increased late-life motility compared to control worms. Generally, gnals2 worms exhibited lower motility scores than wildtype worms, probably attributed to amyloid beta induced paralysis. Similar to the other two strains, a significant effect of time on the motility of gnals2 worms was identified; worms declined substantially in motility as they aged (Fig. 2c, two-way ANOVA, F6,28 = 90.93, p < 0.001). There was also a significant effect of treatment identified (Fig. 2c, two-way ANOVA, F1,28 = 32.14, p < 0.001). Post hoc analysis found worms treated with vitamin D3 to have higher motility scores at day 5 (p < 0.001), but no other significant differences were identified (Fig. 2c). However, these experiments were performed at a concentration of 250 μM, and more notable differences may have been identified under higher concentrations.
Vitamin D3 promotes oxidative stress resistance in nhr-8 mutants
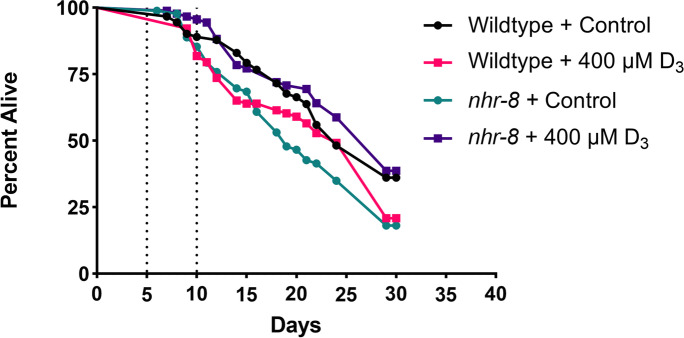
Considering the lifespan and motility response mounted under treatment with vitamin D3, it was hypothesized that vitamin D3 may help C. elegans, particularly nhr-8 mutants, withstand oxidative stress. Worms were treated with a transient exposure to the oxidative stressor, paraquat, for a time of 5 days, while simultaneously remaining exposed to either D3 or control solutions. The results from this assay mirror results from previous studies [15], indicating that vitamin D3 may not help wildtype C. elegans handle oxidative stress induced by paraquat (Fig. 3, log-rank test, p = 0.56). The lifespans of N2 worms exposed to 400 μM D3 compared to those exposed to control were not significantly different upon exposure to a transient oxidative stressor. However, in nhr-8 worms, there was a significant difference identified between D3 treated and control treated worms exposed to paraquat (Fig. 3, log-rank test, p < 0.001). Unlike the wildtype worms, D3 treatment resulted in lifespan extension in nhr-8 worms, potentially indicating vitamin D3 may promote resistance to oxidative stress in this strain (Fig. 3).
Fig. 3.
Oxidative stress assays of hermaphrodite C. elegans treated with 400 μM of vitamin D3 from day 1 of adulthood. Worms were exposed to 3-mM paraquat from days 5 to 10 of adulthood (indicated by dotted lines), representing transient exposure to an oxidative stressor. In wildtype worms, treatment with vitamin D3 did not induce resistance to oxidative stress (a, log-rank test, p = 0.56). In nhr-8 mutants, vitamin D3 did induce resistance to oxidative stress, resulting in a similar lifespan to wildtype worms even when exposed to paraquat (a, log-rank test, p < 0.01). Worms were censored after day 30. The results represent at least 60 worms per strain/treatment
Bioinformatic approach reveals upregulation of xenobiotic response pathways
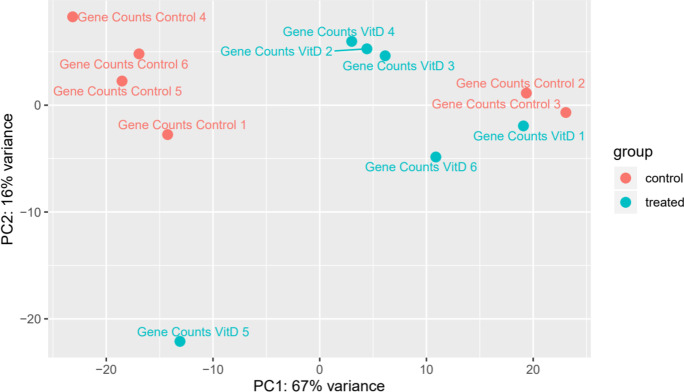
Since vitamin D3 induces dramatic gains to lifespan and marginal gains to health span, the genetic mechanisms that may be governing these responses were investigated. Interestingly, a previous study conducted an RNA sequencing (RNAseq) experiment in order to identify the transcriptional response mounted in C. elegans upon treatment with vitamin D3 [15]. While this study comprehensively studied genetic mechanisms that govern lifespan extension, the full list of differentially expressed genes (DEGs) was not published with the associated manuscript. Additionally, although gene ontology was performed on the DEGs, only a summary of these results was discussed in the manuscript. Using a bioinformatics approach, the sequencing data was analyzed in order to obtain a more comprehensive look at pathways that are enriched during vitamin D3 treatment. Principle component analysis of the data identified distinct clustering of the treatment groups, with vitamin D3 treated worms mounting an aggregate response more similar to each other than to control treated worms (Fig. 4).
Fig. 4.
Principal component analysis (PCA) of gene counts between control and vitamin D3 treated worms. Blue circles indicate worms treated with vitamin D3, and red circles indicate control worms. Clustering of treatment groups indicates a degree of similarity between expression patterns
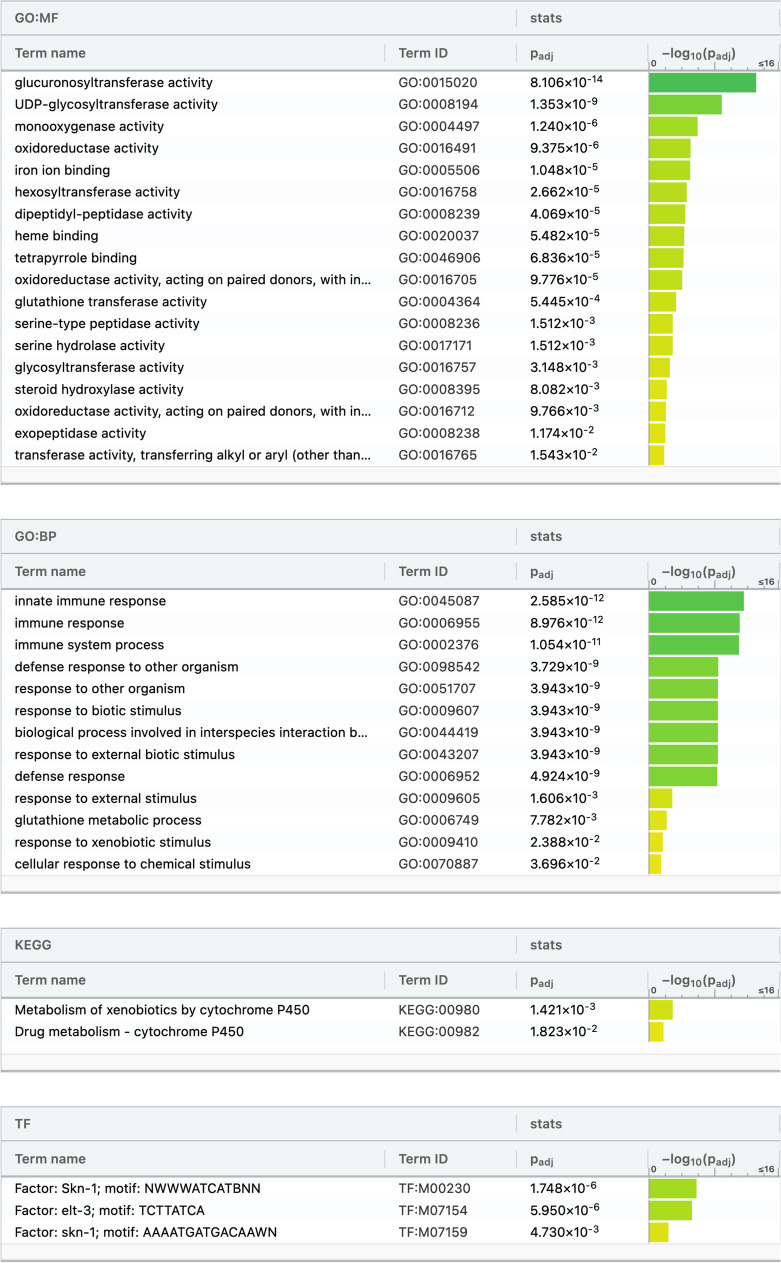
Further analysis revealed that vitamin D3 treatment resulted in 399 significantly upregulated genes. This is different from the number of upregulated genes identified by Mark et al. (2016), which may be due to differences in bioinformatic workflows and/or differences in lg2FC cutoffs. In order to identify the molecular processes, biological processes, and KEGG pathways associated with the differentially expressed genes, gProfiler functional analysis was utilized to evaluate the DEGs. gProfiler analysis revealed that vitamin D3 treatment resulted in an upregulation of genes involved in molecular functions such as glucuronosyltransferase activity, UDP-glycosyltransferase activity, glutathione transferase activity, and steroid hydroxylase activity (Fig. 5). Biological processes that were enriched included processes involved in the C. elegans immune response, defense response, and xenobiotic metabolism (Fig. 5). Notably, this analysis also identified a significant enrichment for the stress response transcription factor (TF), SKN-1, which was previously published in the original analysis by Mark et al. (15), as well as the TF, ELT-3. Enriched KEGG pathways associated with upregulated genes included metabolism of xenobiotics by cytochrome P450 proteins (Fig. 5).
Fig. 5.
gProfiler gene ontology representing the molecular functions (MF), biological processes (BP), KEGG pathways, and transcription factors (TF) enriched after treatment with vitamin D3 compared to controls. Molecular functions that were significantly enriched included glucuronosyltransferase activity, UDP-glycosyltransferase activity, and steroid hydroxylase activity. Biological processes that were significantly enriched included the innate immune response, defense responses, and xenobiotic metabolism. Enriched KEGG pathways involved metabolism of xenobiotics by cytochrome P450 proteins
Discussion
Vitamin D deficiency has been associated with a variety of age-related diseases, sparking debate about whether or not this vitamin is an essential longevity vitamin. While further human studies are necessary, the results of this study support existing evidence that vitamin D3 promotes longevity in C. elegans [15, 17]. In vertebrate vitamin D endocrinology, 7-dehydrocholesterol is converted to vitamin D3 upon exposure to UVB radiation. Vitamin D3 is subsequently converted to the bioactive steroid hormone, 1,25(OH)2D, through two hydroxylation reactions mediated by CYP proteins. Interestingly, C. elegans have the proteins and nutritional precursors required to undergo this process and have been demonstrated to produce the bioactive steroid hormone, 1,25(OH)2D, when supplemented with the precursor, vitamin D3 [15]. This leads to the rationale that vitamin D metabolism may be a part of normal C. elegans endocrinology. Due to the fact that C. elegans experiments are conducted in a lab environment, it could be true that the organisms used for common lifespan studies are deficient in this vitamin, as they are not experiencing active exposure to UVB radiation.
This study also sought to identify whether vitamin D3 supplementation in C. elegans could alleviate some of the burden associated with Alzheimer disease, which is one of the specific age-related diseases that vitamin D deficiency has been associated with [30]. Previous work in C. elegans has found vitamin D3 supplementation to alleviate paralysis in strains of worms engineered to represent Alzheimer disease through proposed mechanisms such as an increase in protein homeostasis and decreased nutrient signaling [15, 18]. In this study, vitamin D3 supplementation was found to extend late-life survival rates of gnals2 worms, which exhibit impaired neuromuscular and sensorimotor behavior (Fig. 1d). These results are suggestive that vitamin D3 treatment may alleviate some of the late life disease present in this particular strain of worms. In terms of motility, vitamin D3 treatment did not seem to significantly improve the decline in movement observed in these worms with advancing age (Fig. 2c). While there was a significant effect at day 5, other observed differences with increasing age were not significantly different. These results could mean that improvements in paralysis reported in previous studies could be strain specific or perhaps improvements in life- and health span would be observed in altered conditions such as different concentrations of D3 or a larger sample size.
The next goal of this study was to investigate a possible vitamin D receptor in the worms. In vertebrates, vitamin D endocrinology relies upon a functional vitamin D receptor, which interacts with various target genes to regulate transcription. In C. elegans, there are three receptors considered homologous to the human vitamin D receptor, DAF-12, NHR-8, and NHR-48. DAF-12 has the highest homology to the human VDR but has been previously determined to not be involved in the mechanism by which vitamin D induces lifespan extension [15]. In this study, NHR-8 was hypothesized to be involved in this process due to its high homology to DAF-12, with significant homology in DNA- and ligand-binding domains and identical residues in the P box of the first zinc finger that functions in DNA recognition [28]. Previous work has identified that nhr-8 mutants exhibit decreased lifespans, which this study also supported; nhr-8 mutants had significantly shorter lifespans compared to wildtype worms (Fig. 1c). However, the interesting finding in this study was that vitamin D3 supplementation completely rescued nhr-8 mutants, with lifespans similar to wildtype worms treated with vitamin D3 (Fig. 1c). Previous work in nhr-8 worms has implicated NHR-8 in the Δ7 dafachronic acid (Δ7-DA) pathway, with NHR-8 regulating cholesterol and bile acid homeostasis [28]. One key step in sterol and bile acid homeostasis is the conversion of cholesterol to 7-dehydrocholesterol, and it has been supported that NHR-8 promotes this conversion [28]. The conversion of cholesterol into 7-dehydrocholesterol is a first key step in Δ7-DA synthesis, but it is also the first step in vitamin D3 synthesis. This posits that nhr-8 worms lack the critical precursor necessary to produce not only key dafachronic acids but also vitamin D. With this in mind, D3 supplementation may be acting as a form of cholesterol supplementation in these worms or could be providing the worms with derivatives such as vitamin D that they may be deficient in. Further studies investigating the amount of vitamin D produced in nhr-8 worms compared to wildtype controls when exposed to UVB would be interesting and may help further support the notion that nhr-8 worms are deficient in vitamin D.
The finding that vitamin D3 could induce lifespan and health span benefits in nhr-8 mutants led to investigation of its ability to correct other known deficits, such as sensitivity to oxidative stress. C. elegans treated with control or D3 were exposed to paraquat for a time of five days, which is known to induce mitochondrial oxidative stress [31]. In wildtype worms, the hypothesis that vitamin D3 would increase resistance to paraquat was not supported; there was no observed difference in lifespans between the two treatment groups (Fig. 3). However, in nhr-8 worms, vitamin D3 treatment did seem to increase resistance to oxidative stress, as treated worms had an increased lifespan compared to control (Fig. 3). As mentioned, nhr-8 worms are known to be more susceptible to oxidative stress [28]; however, the finding that D3 treatment imparts resistance comparable to wildtype worms suggests, again, that D3 may be alleviating the negative consequences associated with nhr-8 mutation (Fig. 3). One important note to consider when evaluating these results is that there are variable types of reactive oxygen species (ROS) that contribute to oxidative stress in various subcellular compartments [31]. Due to this, these results do not support that D3 is unable to impart oxidative stress resistance in C. elegans generally, rather, that it may not provide protection from mitochondrial oxidative stress specific to paraquat exposure. This distinction is important, as other studies have found variable amounts/types of oxidative damage to be caused by different oxidative stressors. Further studies testing vitamin D3’s ability to protect against oxidative stressors, such as juglone, hydrogen peroxide, hyperoxia, and more, will need to be done in order to determine whether or not it can truly protect against oxidative stress in wildtype C. elegans.
Whether there is a true vitamin D receptor in C. elegans that accommodates vitamin D is still undetermined, however, it is clear that vitamin D treatment modulates the transcription of C. elegans, which may be causal of the observed gains in longevity. Pathway enrichment analysis found upregulated genes to be related to biological processes such as the C. elegans immune response and xenobiotic metabolism, similar to what was identified in Mark et al. (2016) (Fig. 4). It is notable that this observed transcriptional response is similar to the response mounted across the tree of life with respect to vitamin D3 endocrinology. For example, evidence suggests that the vitamin D receptor in the sea lamprey (Petromyzon marinus) may function to induce cytochrome P450 enzymes for xenobiotic detoxification [32]. Due to its evolutionary relationship to modern vertebrates, this insight allows us to speculate that the early function of the VDR receptor was detoxification [33]. With that in mind, it is interesting to note that C. elegans mounted a transcriptional response highly associated with the metabolism of xenobiotics, with vitamin D3 treatment inducing the expression of many CYP and glutathione S-transferase (GST) proteins. It is also interesting to note that the transcriptional response exhibited in C. elegans was similar to responses identified in higher organisms such as the laboratory rat when treated with vitamin D [34]. Specifically, these responses included the upregulation of CYP proteins, steroid hormone metabolism genes, UDP-glucuronosyltransferases, and glutathione S-transferases in the rat intestine [34]. This response is strikingly similar to what was observed in C. elegans treated with vitamin D3, suggesting that the functions of vitamin D may be highly conserved across organisms.
Ultimately, this study supports the established notion that vitamin D3 promotes longevity in C. elegans and rescues deficits in nhr-8 mutants. While the relevance of lifespan extension in nematodes for human longevity may seem unclear, the use of C. elegans to screen for longevity modulating compounds has thus far been surprisingly promising [35]. Compounds that extend the lifespan of C. elegans have later been demonstrated to modulate longevity in higher organisms, leading to the hypothesis that various compounds may act on highly conserved pathways to modulate the aging process [35]. Considering the transcriptional response identified in C. elegans upon treatment with D3 was similar to responses observed across other organisms, it is suggestive that the pathways that promote longevity in the worms may be similar across eukaryotes. Moving forward, C. elegans may be a good model to further study the longevity promoting effects of vitamin D. Future studies could investigate the range of D3 concentrations C. elegans can tolerate as well as the role NHR-48 may play in vitamin D3-induced lifespan extension. Additionally, considering the well-established immunomodulatory effects of vitamin D in mammals [36], as well as the expression of immune related genes in C. elegans treated with D3, vitamin D3’s effect on worms challenged with pathogens would be interesting to investigate. While much debate will remain about whether or not vitamin D is a longevity vitamin in humans, C. elegans are a good model moving forward to help identify the role of vitamin D in longevity promotion.
Acknowledgements
Thank you to Arijit Mukherjee, Ben Cash, Erin Wiley, Mick Yoder, Steve Karafit, Autumn Kennedy, and Julian Stobaugh for equipment use and project assistance.
Author contribution
BH performed all experiments. MF supervised the project. BH and MF wrote the manuscript.
Funding
Funding provided by the University of Central Arkansas, University Research Council and the College of Natural Sciences and Mathematics Student Research Fund.
Data availability
Survival and motility data is available via GraphPad Prism data file upon request.
Code availability
All bioinformation analysis was performed in the Galaxy platform at usegalaxy.org. Code used to run analysis was pulled directly from this platform, and associated references are described in the methods.
Declarations
Consent for publication
The authors consent for publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Billy Huggins, Email: bhuggins@uchc.edu.
Mindy Farris, Email: mfarris@uca.edu.
References
- 1.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US Population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omeed Sizar, Swapnil Khare, Amandeep Goyal, Pankaj Bansal AG. Vitamin D deficiency. Treasure Island, FL: StatPearls Publishing; 2021.Available from: https://www.ncbi.nlm.nih.gov/books/NBK532266/ [PubMed]
- 3.Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009;114(1–2):78–84. doi: 10.1016/j.jsbmb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, TmavaBerisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74(11):1498–513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames BN. Prolonging healthy aging: longevity vitamins and proteins. 2018;115(43). 10.1073/pnas.1809045115 [DOI] [PMC free article] [PubMed]
- 6.Raed A, Bhagatwala J, Zhu H, Pollock NK, Parikh SJ, Huang Y, et al. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: a placebo controlled randomized trial. PLoS ONE. 2017;12(12):1–13. doi: 10.1371/journal.pone.0188424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97(8):2670–2681. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guessous I. Role of vitamin D deficiency in extraskeletal complications: predictor of health outcome or marker of health status? Biomed Res Int. 2015;2015. 10.1155/2015/563403 [DOI] [PMC free article] [PubMed]
- 9.Pilz S, Pilz S, Zittermann A, Trummer C, Theiler-schwetz V, Lerchbaum E, et al. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8:27–43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18–29. doi: 10.1016/j.jsbmb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28(6):2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 12.Olsen A, Vantipalli MC, Lithgow GJ. Using Caenorhabditis elegans as a model for aging and age-related diseases. Ann N Y Acad Sci. 2006;1067(1):120–128. doi: 10.1196/annals.1354.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Li F, Zhou T, Wang G, Li Z. Caenorhabditis elegans as a useful model for studying aging Mutations. Front Endocrinol (Lausanne) 2020;11(October):1–9. doi: 10.3389/fendo.2020.554994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones G, Kottler ML, Schlingmann KP. Genetic diseases of vitamin D metabolizing enzymes. Endocrinol Metab Clin North Am. 2017;46(4):1095–1117. doi: 10.1016/j.ecl.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Mark KA, Dumas KJ, Bhaumik D, Schilling B, Davis S, Oron TR, et al. Vitamin D promotes protein homeostasis and longevity via the stress response pathway genes skn-1, ire-1, and xbp-1. Cell Rep. 2016;17(5):1227–1237. doi: 10.1016/j.celrep.2016.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouillon R, Suda T. Vitamin D: calcium and bone homeostasis during evolution. Bonekey Rep. 2013;2014(3):1–10. doi: 10.1038/bonekey.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messing J, Heuberger R, Schisa J. Effect of Vitamin D3 on lifespan in Caenorhabditis elegans. Curr Aging Sci. 2014;6(3):220–224. doi: 10.2174/18746098113066660038. [DOI] [PubMed] [Google Scholar]
- 18.Leiteritz A, Schmiedl T, Baumanns S, Wenzel U. Amyloid-beta induced paralysis is reduced by cholecalciferol through inhibition of the steroid-signaling pathway in an Alzheimer model of Caenorhabditis elegans. Nutr Neurosci. 2019;0(0):1–8. doi: 10.1080/1028415X.2019.1596371. [DOI] [PubMed] [Google Scholar]
- 19.Stiernagle T. Maintenance of C. elegans. WormBook. 1999;2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Čech M, et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44(W1):W3–10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: Archive for functional genomics data sets - Update. Nucleic acids Res. 2013;41(D1):991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingett SW, Andrews S. Fastq screen: A tool for multi-genome mapping and quality control [version 1; referees: 3 approved, 1 approved with reservations] F1000Research. 2018;7:1–14. doi: 10.12688/f1000research.15931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.j. 2011;17(1):10–2. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 24.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):1–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. G:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47(W1):W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magner DB, Wollam J, Shen Y, Hoppe C, Li D, Latza C, et al. The NHR-8 nuclear receptor regulates cholesterol and bile acid homeostasis in C. elegans. Cell Metab. 2013;18(2):212–24. doi: 10.1016/j.cmet.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son HG, Altintas O, Kim EJE, Kwon S, Lee SJV. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell. 2019;18(2):1–11. doi: 10.1111/acel.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: Evidence from meta-analysis. Nutr J. 2015;14(1):1–5. doi: 10.1186/s12937-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senchuk M, Dues D, Van Raamsdonk J. Measuring oxidative stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio-Protoc. 2017;7(1):1–16. doi: 10.21769/BioProtoc.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitfield GK, Dang HTL, Schluter SF, Bernstein RM, Bunag T, Manzon LA, et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinol. 2003;144(6):2704–2716. doi: 10.1210/en.2002-221101. [DOI] [PubMed] [Google Scholar]
- 33.Hanel A, Carlberg C. Vitamin D and evolution: Pharmacologic implications. Biochem Pharmacol. 2020;173. 10.1016/j.bcp.2019.07.024 [DOI] [PubMed]
- 34.Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol. 2007;218(1):37–44. doi: 10.1016/j.taap.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Gómez-Linton DR, Alavez S, Alarcón-Aguilar A, López-Diazguerrero NE, Konigsberg M, Pérez-Flores LJ. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology. 2019;20(5):583–603. doi: 10.1007/s10522-019-09817-2. [DOI] [PubMed] [Google Scholar]
- 36.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Survival and motility data is available via GraphPad Prism data file upon request.
All bioinformation analysis was performed in the Galaxy platform at usegalaxy.org. Code used to run analysis was pulled directly from this platform, and associated references are described in the methods.