Abstract
Abstract
Hypertrophic carotid geometric phenotypes (h-CGP) are predictors of incident cardiovascular disease (CVD). While arterial aging is hypothesized as a contributor to this associated risk, the association of CGPs with chronological age is not clear. In this manuscript we examine whether hypertrophic CGPs represent accelerated biological, rather than chronological, aging by examining their association with carotid-femoral pulse wave velocity (PWV), the hallmark of arterial aging. We analyzed data from 5516 participants of the SardiNIA study with a wide range of age at baseline (20–101 years), and a median follow-up time of 13 years (mean 11.5 years; maximum 17.9 years). Baseline CGPs were defined based on the common carotid lumen diameter, wall thickness, and their ratio. Subject-specific rates of change of PWV, blood pressure parameters, body mass index, glucose, and lipids were estimated using linear mixed effects models. Compared to those with typical(t-) CGP, those with dilated hypertrophy (dh-) CGP had a greater longitudinal increase in PWV; this increase was significantly greater among older individuals and men. The greater PWV longitudinal increase in dh-CGP remained significant after adjusting for baseline values and rates of change of covariates. Dilated hypertrophic CGP is independently associated with accelerated increase in age-associated arterial stiffening over time, with a strong association in men than in women. Future studies are needed to examine if this association mediates the increased risk for CVD observed in individuals with hypertrophic cardiac remodelling and the role of retarding it to reduce this risk.
Highlights
• Individuals with dilated hypertrophic geometric phenotypes of the common carotid artery (increased age- and sex-specific wall thickness and lumen diameter) have greater future central arterial stiffening, independently of other determinants of arterial stiffening.
• The dilated hypertrophic phenotype group has a greater age-specific arterial dilation, wall thickening, and stiffness (the arterial aging triad). This suggests that this phenotype is a form of accelerated aging that might explain the worse clinic outcomes observed in this group.
• Understanding the natural history of the carotid geometric phenotype across the lifespan and the determinants of the deleterious progression towards the dilated hypertrophic phenotype are needed to develop interventions that reduce the adverse clinical outcomes associated with it.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00699-w.
Keywords: Arterial stiffness, Longitudinal study, Pulse wave velocity, Rate of change, Vascular remodelling
Introduction
The arterial wall, especially that of the elastic arteries, is an active organ that continuously undergoes structural and functional alterations with aging [1, 2]. As arterial structural remodelling manifests in wall thickening [3] and lumen dilatation [4], variation in the two processes result in different geometric patterns with different wall-to-lumen ratios [5]. We have previously characterized different carotid geometric phenotypes (CGPs) using the common carotid artery (CCA), an artery easily assessed by ultrasonography and frequently used a proxy of central arterial stiffness [5].
CGP is not only based on having greater absolute values of carotid geometric parameters; instead, it categorizes individuals based on whether they were in the upper sex-age specific 10th percentile of these parameters. Previous studies have shown that, compared to those with typical CGP (t-CGP), individuals with concentric hypertrophic CGP (ch-CGP), i.e., greater arterial wall mass due to wall thickening, or dilated hypertrophic CGPs (dh-CGPs), i.e., greater arterial wall mass due to dilatation, have higher risk for incident cardiovascular disease (CVD) independently of other CVD risk factors. These associations highlight the biological relevance of these phenotypes beyond the raw parameters from which they are derived. [6]
While hypertrophic CGPs demonstrate signature characteristics of arterial aging [2], their prevalence, at first glance, is not increased with chronological age [6]. These patterns, however, are developed based on exceeding age- and sex-specific diameter and wall-to-lumen ratio values. Hence, hypertrophic CGPs are likely to inform on accelerated arterial aging processes for one’s chronological age, and subsequently are associated with other makers of arterial aging. In fact, previous cross-sectional studies support this hypothesis as concentric and dilated hypertrophic CGPs were shown to have greater pulse wave velocity (PWV), the best available measure of arterial stiffness, and the hallmark of arterial aging [7]. Whether having hypertrophic CGPs at baseline is associated with prospective accelerated arterial aging, i.e., greater longitudinal increase in PWV over time, has not been evaluated.
In this analysis, we examine the hypothesis that compared to other geometric patterns, hypertrophic CGP, with or without relative dilatation, is associated with accelerated arterial aging expressed as greater longitudinal increase in PWV. We also examine whether the established sex-difference of arterial aging [3, 7] affects the association between hypertrophic CGP and the longitudinal association with PWV. Such information would help explain the biological implications of CGPs and guide its potential utility in risk stratification for CVD beyond traditional cardiovascular risk factors.
Methods
Study population
The SardiNIA study investigates the genetics and epidemiology of complex traits/phenotypes, including CV risk factors and arterial properties, in a community-dwelling Sardinian founder population [8]. The subset of the SardiNIA population for the present analysis consists of 5516 volunteers (2326 men and 3190 women) who entered the study over of a wide age range (20 to 101 years) with a median follow-up of 13 years (mean 11.5 years and maximum 17.9 years).
Measurements of baseline carotid geometric pattern (CGP)
High-resolution B-mode carotid ultrasonography was performed by use of a linear-array 5- to 7.5-MHz transducer (HDI 3500-ATL Ultramark Inc) as previously described [8]. Briefly, the right CCA is examined with a transducer positioned so that the near and far walls of the CCA are parallel to the transducer footprint and the lumen diameter was maximized in the longitudinal plane. While wall-thickness includes the adventitia, however, demarcation of the adventitial layer is extremely limited on ultrasound (US); hence, intima-medial thickness was used as the best available proxy for full wall thickness. A region 1.5 cm proximal to the carotid bifurcation was identified, and the IMT of the far wall was evaluated as the distance between the luminal-intimal interface and the medial-adventitial interface. IMT was measured on the frozen frame of a suitable longitudinal image with the image magnified to achieve a higher resolution of detail in areas without plaques or calcification (at least 1 mm distance from the plaque shoulder if plaque was present); no individuals were excluded due to the presence of plaques. The IMT measurement was obtained from 5 contiguous sites at 1-mm intervals, and the average of the 5 measurements was used for analyses. All the measurements were performed by a single reader (AS). CCA systolic and diastolic radius (half diameter) (r and R, respectively) were identified via ECG gating.
CCA wall-to-lumen ratio (%) was calculated as:
| 1 |
CCA cross-sectional area (CSA) was calculated, as:
| 2 |
where ρ is the arterial wall density (ρ = 1.06), Re = IMT + 2 × R.
90th percentile values for CCA W/L and CCA CSA were defined as those within the age- (10-year intervals) and sex-specific 90th percentile of subjects who were normotensive (BP < 140/90 mm Hg and no antihypertensive medication), without diabetes mellitus (fasting glucose < 126 mg/dl and without antidiabetic therapy), and who were not obese (BMI < 30 kg/m2) at baseline.
Four carotid geometric patterns (or phenotypes) (CGP) were defined as follows:
Longitudinal analysis: trajectories of outcome variables measured over time
The following variables were measured serially over time following a baseline measurement.
Blood pressure
Blood pressure was measured in both arms with a mercury sphygmomanometer using an appropriately sized cuff after 15-min rest in a dark, quiet room. Values for systolic blood pressure (SBP) and diastolic blood pressure (DBP) were defined by Korotkoff phase I and V, respectively. The average of second and third measurements on both the right and left arm were used in the analysis. Pulse pressure was computed as PP = (SBP-DBP); mean BP was computed as MBP = DBP + (PP / 3).
Anthropometry
Height, weight, and waist circumference were determined for all participants. Body mass index (BMI) was calculated as body weight (kg)/height (m2).
Fasting plasma lipids
Blood samples were drawn from the antecubital vein between 7 and 8 AM after an overnight fast. Subjects were not allowed to smoke, engage in significant physical activity, or take medications prior to the collection of the samples. Plasma triglycerides and total cholesterol were determined by an enzymatic method (Abott Laboratories ABA-200 ATC BiochromaticAnalyzer, Irving, TX 75,015). HDL-cholesterol was determined by a dextran sulfate-magnesium precipitation. LDL-cholesterol concentrations were estimated by the Friedewald formula. Fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments Inc., Fullerton, CA 92,634).
Aortic stiffness
Carotid-femoral PWV was measured as previously described [9] using nondirectional transcutaneous Doppler probes (Model 810A, 9 to 10-MHz probes, Parks Medical Electronics, Inc, Aloha, OR).
Statistical analyses
All analyses were performed using R version 4.0.2. Data are presented as mean ± SD unless otherwise specified. One-way ANOVAs were used to compare the means of each variable across the baseline CGP groups. For significant variables, the false discovery rate (FDR) approach was used to identify which means differ from each other. Average values of intima-medial thickness and carotid diameters for CGP groups by age groups (< 40, 40–65, and > 65), and by sex are provided in Table 1s and illustrated in Fig. 1. A roadmap of the analyses performed is depicted in Fig. S1.
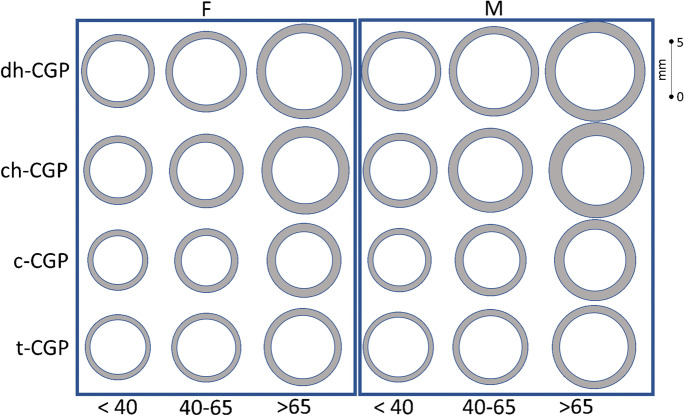
Fig. 1.
Geometric configuration of carotid geometric phenotype by age group in females (F) and males (M) using group-specific average intima-medial thickness and carotid diameter. t-CGP: typical CGP c-CGP: concentric ch-CGP: concentric hypertrophic CGP dh-CGP: dilated hypertrophic CGP
Linear mixed-effects models (LME) are used to estimate subject-specific rates of change (ROC) for PWV and potential covariates including BMI, DBP, SBP, and fasting glucose levels. This method has been explained previously [3, 10]. In summary, the LME approach fits a linear mixed-effects model to the variable as a function of follow-up time with both fixed and random effects. The estimated person-specific random-effect and fixed-effect are used to compute the subject-specific rates of change (ROC).
Given the available data on CGP at baseline only along with longitudinal PWV measruements, a series of multiple linear regression models were fitted to examine the association between baseline CGP groups (as independent variables) and PWVROC (as the dependent variable); Model 1 included baseline age and sex, with baseline CGP as dummy categorical variables with t-CGP (i.e., the most common pattern) as the reference group. Baseline age and sex interaction terms with CGP dummy variables were included to assess the effect of baseline age and sex on the association between CGP and PWVROC. In Model 2, baseline common carotid artery wall-to-lumen ratio and wall cross-sectional area, and their interaction, were included to examine whether the CGPs are associated with PWVROC independently of the individual carotid parameters used to construct them. In subsequent models, baseline PWV and covariates, and rates of changes of covariates were subsequently added to the model to examine whether the associations between CGP groups and PWVROC were independent of baseline PWV and baseline covariate values and their rates of changes. Backward elimination (keeping CGP group in the model even if not significant) was used to reach final models. To explore whether the determinants of PWV change varied across the CGPs, additional regression analyses were stratified by CGPs.
Results
Baseline descriptive characteristics and follow-up information of the sample by CGP phenotypes
We studied 5516 subjects over a median follow-up time of 13 years (mean 11.5 years) for a total of 21,577 observations. There was no difference in the distribution of number of visits among CGP groups (chi-square P-value = 0.11). Average baseline characteristics of subjects within each of the four CGP groups are listed in Table 1. At baseline and compared to t-CGP (normal CCA), subjects with ch-CGP (i.e., CCA hypertrophy due to thickening) were on average 5.5 years older, while subjects with c-CGP (greater relative wall thickness without hypertrophy) were on average 3.5 years younger. No difference in sex distribution or in mean follow-up time was observed among CGPs. Subjects with ch-CGP and dh-CGP (i.e., CCA hypertrophy mainly due to dilatation) had a greater BMI and greater SBP, DBP, and use of antihypertensive medications than t-CGP (normal CCAW/L and normal CSA), whereas the opposite was observed for subjects with c-CGP. Average baseline PWV values increased from c-CGP → t-CGP → ch- /dh-CGP (Table 1).
Table 1.
Baseline characteristics and length of follow-up of subjects according to their specific baseline CGP group (mean ± SD or %)
| t-CGP (1) | c-CGP (2) | ch-CGP (3) | dh-CGP-y (4) | ANOVA P Value | Post hoc comparisons among baseline CGPs [1, 2] | |
|---|---|---|---|---|---|---|
| n | 4419 | 352 | 214 | 531 | ||
| Age (years) | 42.90 ± 16.9 | 40.1 ± 17.3 | 47.0 ± 15.0 | 44.6 ± 15.7 | < .0001 | 2 < 1 < 4, 3 |
| Women (%) | 58.2 | 57.7 | 50.9 | 57.3 | 0.2064 | |
| Follow-up time (years) | 11.7 ± 3.8 | 11.2 ± 4.0 | 11.5 ± 3.9 | 11.4 ± 3.9 | 0.0428 | No pairwise differences |
| Number of visit (%) | 0.1115 | |||||
| 2 | 14.6 | 19.9 | 17.8 | 15.3 | ||
|
3 4 5 |
17.8 27.6 40.1 |
17.9 29.3 33.0 |
18.2 25.7 38.3 |
18.1 30.1 36.5 |
||
| BMI (Kg/m2) | 25.1 ± 4.5 | 24.0 ± 4.6 | 27.1 ± 4.7 | 27.0 ± 5.3 | < .0001 | 2 < 1 < 4, 3 |
| Waist circumference (cm) | 83.9 ± 12.6 | 80.8 ± 12.6 | 90.3 ± 13.1 | 88.6 ± 14.2 | < .0001 | 2 < 1 < 4, 3 |
| Fasting glucose (mg/dl) | 89.0 ± 22.0 | 88.9 ± 24.6 | 93.7 ± 25.5 | 91.7 ± 26.1 | 0.0029 | 2, 1 < 4, 3; |
| Total cholesterol (mg/dl) | 208.3 ± 41.9 | 201.5 ± 41.9 | 216.9 ± 36.8 | 213.1 ± 43.6 | < .0001 | 2 < 1 < 4, 3 |
| LDL cholesterol (mg/dl) | 126.3 ± 35.2 | 121.6 ± 34.7 | 136.3 ± 32.2 | 130.5 ± 36.0 | < .0001 | 2 < 1 < 4 < 3 |
| HDL cholesterol (mg/dl) | 64.7 ± 14.9 | 63.2 ± 14.9 | 61.1 ± 14.0 | 63.7 ± 15.4 | 0.0016 | 3 < 1; 3 < 4 |
| Triglycerides (mg/dl) | 86.8 ± 66.9 | 83.4 ± 54.8 | 97.5 ± 60.6 | 94.7 ± 90.5 | 0.2038 | 2, 1 < 4, 3 |
| SBP (mmHg) | 124.4 ± 17.6 | 122.0 ± 16.0 | 129.2 ± 18.6 | 131.1 ± 21.5 | < .0001 | 2 < 1 < 3, 4 |
| DBP (mmHg) | 76.5 ± 10.6 | 74.6 ± 10.0 | 79.6 ± 10.5 | 80.5 ± 11.9 | < .0001 | 1 < 2 < 3, 4 |
| PP (mmHg) | 47.9 ± 12.2 | 47.5 ± 11.4 | 49.6 ± 12.8 | 50.6 ± 14.8 | < .0001 | 1 2, < 4 |
| MBP (mmHg) | 92.4 ± 12.1 | 90.4 ± 11.1 | 96.2 ± 12.4 | 97.4 ± 14.2 | < .0001 | 1 < 2 < 3, 4 |
| Serum creatinine (mg/ml) | 0.80 ± 0.18 | 0.81 ± 0.16 | 0.81 ± 0.16 | 0.81 ± 0.28 | 0.7782 | |
| PWV (m/sec) | 6.6 ± 2.1 | 6.3 ± 1.9 | 7.1 ± 2.2 | 7.0 ± 2.3 | < .0001 | 2 < 1 < 4, 3 |
| Antihypertensive medication (%) | 8.0 | 6.5 | 12.6 | 14.1 | < .0001 | 2 < 1 < 4, 3 |
Notes: Age: baseline age; t-CGP: typical CGP c-CGP: concentric ch-CGP: concentric hypertrophic CGP dh-CGP: dilated hypertrophic CGP 1post hoc comparisons use FDR adjustments. 2For post hoc comparisons, for example,( 2 < 1 < 4, 3) means that group 2 differs from 1, 1 differs from 4 and 3, but 4 does not differ from 3
Geometric configuration of CGPs by age groups
Figure 1 and Table 1s illustrate carotid geometry of the different CGPs by age group and sex using the average carotid measurements (i.e., IMT and diameter) for each subgroup. In both men and women there are significant trends of increasing IMT with advancing age, and from t-CGP, to c-CGP, to ch-CGP, to dh-CGP across all age groups; Carotid diameter showed the same pattern of increasing diameter with advancing age; considering CGPS, however, carotid diameter was smallest in the c-CGP, followed by c-CGP, to ch-CGP, and dh-CGP.
Baseline CCA geometry and baseline PWV, PWVROC, and aging.
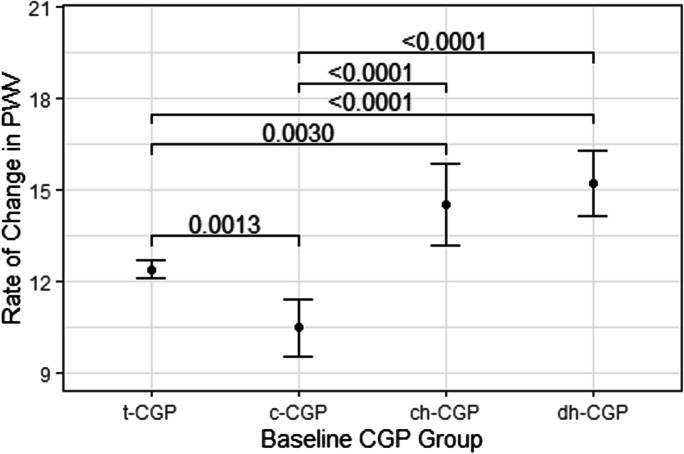
Figure 2 demonstrates that dh-CGP and ch-CGP had higher PWVROC than c-CGP and t-CGP; in addition, c-CGP had lower PWVROC than t-CGP (Fig. 2).Simple multiple linear regression models examining the association between baseline CGP and PWVROC, adjusting for age showed that among men and women aged < 40, c-CGP was associated with lower PWVROC, while ch-GGP, and dh-CGP were marginally associated with greater PWVROC, compared to those with t-CGP. In the older groups, however, dh-CGP was the only group to have a significant association with greater PWVROC (Table 2).
Fig. 2.
Differences in longitudinal PWV rate of change by baseline CGP. Rate of change of PWV (cm/s/year)
Table 2.
Associations between baseline CGP with future longitudinal rate of change in PWV (PWVROC) by age groups (cm/s/year)
| < 40 Years | 40-65 Years | > = 65 Years | ||||
|---|---|---|---|---|---|---|
| Baseline Var | Estimate | P-value | Estimate | P-value | Estimate | P-value |
| (Intercept) | 1.08 | 0.058 | − 6.54 | 0.000 | − 12.46 | 0.063 |
| FAge | 0.21 | 0.000 | 0.41 | 0.000 | 0.50 | 0.000 |
| Sex | 0.94 | 0.001 | 0.61 | 0.084 | 1.40 | 0.139 |
| c-CGP (Ref = t-CGP) | − 1.11 | 0.037 | 0.09 | 0.901 | − 0.48 | 0.851 |
| ch-CGP (Ref = t-CGP) | 1.45 | 0.086 | 0.66 | 0.396 | 0.29 | 0.898 |
| dh-CGP (Ref = t-CGP) | 0.92 | 0.066 | 3.40 | 0.000 | 4.38 | 0.012 |
Var, variables; Age, baseline age; CGP, carotid geometric phenotype
Baseline CCA geometry and future longitudinal changes in PWV, BP, and other CV risk factors
To further explore whether this age association was independent of other covariate baseline and rates of change values, we first performed pairwise comparisons of means and standard deviations of rates of changes of traditional CV risk factors between CGPs (Table S2). Rates of change of SBP, DBP, BMI, waist circumference, glucose, and LDL were significantly different between the CGPs. These variables were included in the subsequent multiple linear regression models to examine whether the differences observed in PWV rates of change across the CGPs groups were independent of the differences observed in the rates of change of the aforementioned covariates.
The independent associations between baseline CGP groups and PWV rate of change
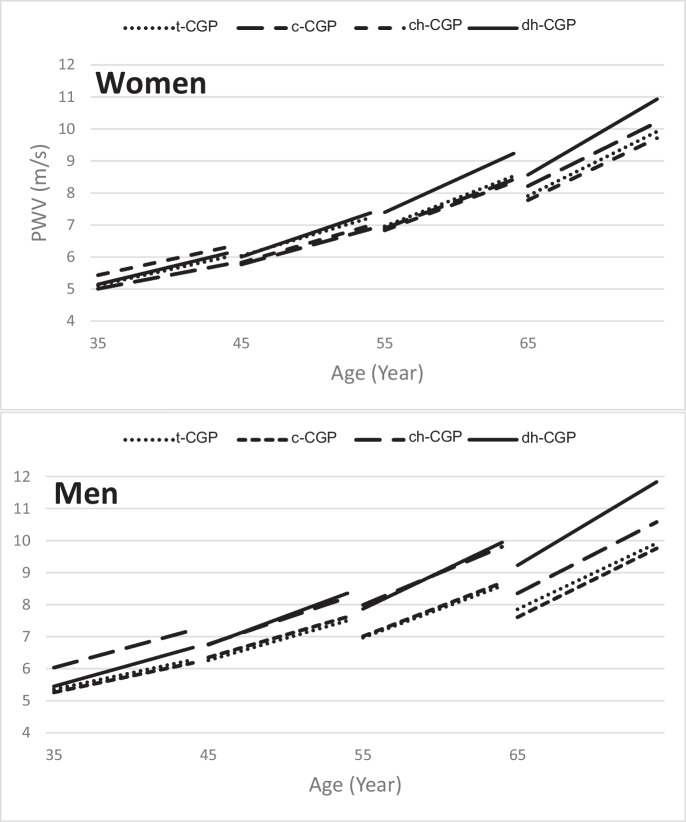
A series of multiple linear regression models were fitted to examine the independent association between baseline CGP groups and PWVROC (Table 3). In Model 1, including baseline age, baseline age [2], sex, and their interaction with CGP baseline groups, dh-CGP (hypertrophy with dilation) was associated with greater PWVROC, compared to t-CGP, with significant baseline age-dh-CGP and sex-dh-CGP interactions indicating that older individuals and men with dh-CGP group had the greatest PWVROC compared to their younger and female counterparts, respectively. In addition, the sex-h-CGP interaction was significant, indicating that among those with ch-CGP, men had greater PWV rate of change compared to women. Figure 3 illustrates the results of this model.
Table 3.
Independent associations between baseline CGP with future longitudinal rate of change in PWV (cm/s/year)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline Var | Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value |
| Intercept | 3.3 | 0.0000 | 2.0 | 0.0000 | − 19.9 | 0.0000 | − 18.0 | 0.0000 |
| Age | 0.040 | 0.2504 | 0.064 | 0.0814 | − 0.032 | 0.4033 | − 0.034 | 0.3512 |
| Age × age | 0.003 | 0.0000 | 0.003 | 0.0000 | 0.002 | 0.0000 | 0.002 | 0.0000 |
| Sex (male) | 0.505 | 0.0469 | 0.035 | 0.8997 | − 1.039 | 0.0003 | − 0.880 | 0.0009 |
| c-CGP (Ref = t-CGP) | − 2.369 | 0.0729 | − 1.576 | 0.2548 | − 1.527 | 0.2245 | − 0.416 | 0.4002 |
| ch-CGP (Ref = t-CGP) | 0.727 | 0.7087 | 2.440 | 0.2527 | 1.841 | 0.3438 | − 1.121 | 0.0857 |
| dh-CGP (Ref = t-CGP) | − 2.220 | 0.0604 | − 1.773 | 0.1378 | − 0.460 | 0.6739 | 1.022 | 0.0168 |
| FAge × c-CGP | 0.034 | 0.2449 | 0.022 | 0.4695 | 0.023 | 0.3954 | ||
| FAge × ch-CGP | − 0.023 | 0.5307 | − 0.084 | 0.0541 | − 0.070 | 0.0788 | ||
| FAge × dh-CGP | 0.089 | 0.0002 | 0.057 | 0.0224 | 0.022 | 0.3425 | ||
| Sex × c-CGP | 1.061 | 0.2579 | 1.024 | 0.2785 | 0.575 | 0.5028 | ||
| Sex × ch-CGP | 2.500 | 0.0276 | 1.960 | 0.0874 | 0.495 | 0.6367 | ||
| Sex × dh-CGP | 1.908 | 0.0150 | 1.693 | 0.0320 | 1.502 | 0.0374 | ||
| W/L, % | − 18.112 | 0.0963 | − 17.518 | 0.0796 | − 10.942 | 0.2244 | ||
| CSA, mm2 | 0.079 | 0.7110 | − 0.426 | 0.0310 | − 0.282 | 0.1133 | ||
| W/L × CSA | 1.046 | 0.2273 | 2.045 | 0.0103 | 1.447 | 0.0344 | ||
| WC, cm | 0.118 | 0.0000 | 0.120 | 0.0000 | ||||
| DBP, mmHg | − 0.075 | 0.0020 | − 0.077 | 0.0014 | ||||
| SBP, mmHg | 0.116 | 0.0000 | 0.117 | 0.0000 | ||||
| Glucose, mg/dL | 0.014 | 0.0050 | 0.014 | 0.0080 | ||||
| LDL, mg/dL | 0.002 | 0.6958 | ||||||
| PWV, m/s | 0.015 | 0.0000 | 0.015 | 0.0000 | ||||
| Heart rate, bpm | 0.041 | 0.0000 | 0.040 | 0.0000 | ||||
| Ever smoking | 0.556 | 0.0371 | 0.573 | 0.0316 | ||||
| Hypertension RX | 0.381 | 0.3521 | ||||||
| Longitudinal Var | ||||||||
| WC-ROC | 3.666 | 0.0003 | 3.833 | 0.0001 | ||||
| DBP-ROC | 6.409 | 0.0150 | 6.561 | 0.0128 | ||||
| SBP-ROC | 2.111 | 0.0000 | 2.122 | 0.0000 | ||||
| Glucose-ROC | 0.195 | 0.0904 | 0.229 | 0.0449 | ||||
| LDL-ROC | 0.148 | 0.1518 | ||||||
Var, variables; Age, baseline age; CGP, carotid geometric phenotype; W/L, wall-to-lumen ratio of the common carotid artery; CSA, cross-sectional area of the wall of the common carotid artery; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; PWV, pulse wave velocity; ROC, rate of change per year
Fig. 3.
Model predicted trajectories of PWV of the different baseline CGP groups in women and men using age-specific mean baseline PWV values and model predicted slopes at the given baseline age. Refer to basic model in Table 2 for statistics on the significance of differences in slopes
In Model 2, the association between CGP and PWVROC was not significantly changed after adjusting for W/L, CSA, and their interaction. In Model 3, we included baseline and rates of changes of relevant covariate and history of HTN treatment and ever smoking assessed at baseline; after backward elimination of nonsignificant terms, the final Model (Model 4) showed that dh-CGP was significantly associated with greater PWVROC, after adjusting for CV risk factors associated with PWVROC, namely baseline and rates of change of SBP, DBP, glucose, and waist circumference.
Determinants of PWV rate of change within each CGP group at baseline
Table 4 shows differences in the determinants of PWV longitudinal change in each of the CGP baseline groups. PWVROC was positively associated with baseline PWV and with advancing age in all CGP groups, although the association with age was quadratic in the t-CGP and c-CGP (association with Age [2].) t-CGP demonstrated an association in the total sample; in both c-CGP and dh-CGP, greater baseline SBP was associated with greater PWVROC. SBPROC was positively associated with PWVROC only in dh-CGP. However, in ch-CGP, PWVROC was positively associated with DBPROC and not with SBPROC.
Table 4.
Determinants of future longitudinal rate of change in PWV within each groups at baseline (reduced models after backward eliminaton in each group)
| t-CGP | c-CGP | ch-CGP | dh-CGP | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | P | Estimate | p | Estimate | P | Estimate | P | |
| Intercept | − 21.503 | < 0.0001 | − 18.949 | < 0.0001 | 6.059 | 0.3242 | − 21.629 | < 0.0001 |
| Age | − 0.068 | 0.0812 | − 0.041 | 0.6787 | 0.185 | 0.0000 | 0.128 | 0.0003 |
| Age × age | 0.002 | < 0.0001 | 0.002 | 0.0330 | ||||
| Sex | − 1.310 | < 0.0001 | ||||||
| Initial WC | 0.127 | < 0.0001 | 0.116 | 0.0006 | ||||
| Initial DBP | − 0.086 | 0.0011 | − 0.222 | 0.0298 | ||||
| Initial SBP | 0.121 | < 0.0001 | 0.071 | 0.0072 | 0.118 | 0.0028 | ||
| Initial glucose | 0.017 | 0.0022 | ||||||
| Initial PWV | 0.015 | < 0.0001 | 0.013 | < 0.0001 | 0.014 | 0.0000 | 0.023 | < 0.0001 |
| WCROC | 3.478 | 0.0015 | ||||||
| DBPROC | 7.234 | 0.0104 | 24.823 | 0.0174 | ||||
| SBPROC | 2.125 | < 0.0001 | 2.731 | 0.0124 | ||||
| GlucoseROC | 0.867 | 0.0431 | 1.725 | 0.0049 | ||||
Age, baseline age; ROC, rate of change cm/s/year; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; PWV, pulse wave velocity
Discussion
Principal findings
In this cohort, hypertrophic CGPs (i.e., ch-CGP and dh-CGP) were associated with greater PWV at baseline, but only hypertrophic CGP with dilation, (i.e.) dh-CGP, was independently associated with prospective increases in PWV over time, with a more pronounced increase in older than in younger individuals. Among those with hypertrophic CGPs with and without dilatation, men had a greater prospective PWV increase than women. The greater longitudinal increase in PWV in dh-CGP was independent of the carotid geometric parameters used to construct CGP, of baseline PWV as well as baseline and rates of changes of covariates. Interestingly, in younger men and women, concentric CGP (greater thickness that dilatation for age) was associated with less baseline and longitudinal arterial stiffness compared to the typical CGP.
CGPs and arterial aging
Our findings shed light on a fundamental issue regarding CGPs with aging. CGPs did not vary by age [5, 6]; however, within a given age, hypertrophic deviants (i.e. dh-CGP and ch-CGP) demonstrated accelerated aging patterns [3, 4] with increased wall and mass (through thickening of dilatation), compared to the typical CGP. Unlike increased wall mass due to thickening, however, increased wall mass through dilatation was associated accelerated with greater wall stiffening with aging. On the other hand, concentric CGP, while demonstrated increased wall to lumen ratio, but without increased mass, was associated a smaller diameter and favorable stiffness profile indicating attenuated age-associated processes.
That CGPs have not been traditionally associated with chronological age omits that CGPs are weakly associated with chronological age by design (CGP are based on deviating from normal, age- and sex- specific wall thickness and diameter ranges). Instead, their construction represents accelerated forms of arterial aging for a given chronological age. This is consistent with our findings of accelerated arterial stiffening in in subjects with dilated hypertrophic CGP, demonstrating the triad of arterial aging of wall hypertrophy [3], relative luminal dilation [2], and functional stiffening [7, 9].
Interestingly, the greater PWVROC in individuals in the dh-CGP group was independent of W/L, CSA, and their interaction, indicating that the biological condition represented by dh-CGP cannot be inferred only from the carotid geometric parameters used to construct dh-CGP. One possible explanation is that the CGP categorizes individuals based on whether they were in the upper sex-age specific 10th percentile of carotid geometric parameters, and not merely based on having greater absolute values of these parameters. As such, an individual in the dh-CGP does not only have greater relative wall thickness and an increase in wall mass, but they have greater values of these elements compared to their counterparts of the same sex and age group, representing accelerated aging phenotype. On the end of the spectrum, it seems the concentric CGP with increased relative wall thickness but without a real increase in wall mass, which comes as a result of smaller diameter, was associated with attenuated arterial aging, which suggests that this phonotype might represent the other pole of attenuated arterial aging. This in fact supported by the lower risk associated with this profile in prior studies. [6]
Differences in the determinants of PWV rate of change between CGP groups at baseline
These analyses showed similarities and differences in the determinants of PWV future change between the different groups. On one hand, this analysis redemonstrated the increasing of PWV at increasing rates with advancing age and among those with greater baseline PWV, across all CGP groups. Changes in SBP was a significant determinant of future increase in PWV in t-CGP and dh-CGP, while changes in DBP was a significant determinant of future increase in PWV in t-CGP and ch-CGP. The different associations between the 4 groups can be attributed to the smaller number of participants in c-CGP, 3, and 4 compared to t-CGP. However, that PWVROC was associated with GlucoseROC only in ch-CGP, the group that the highest increase in GlucoseROC suggests some biological role of glucose metabolism in this ch-CGP group (hypertrophy without dilatation).
Waist circumference, an established determinant of PWV increase with aging in the general population [7], was associated with PWVROC only in the t-CGP and c-CGP groups, but not in the ch-CGP and dh-CGP groups. Exploring the link between obesity and PWV is beyond the scope of this analysis; however, the lack of the association between WC and PWV future change in the groups with hypertrophy is interesting. One explanation is that the impact of differences in WC on PWV future change is weaker after the development of structural remodelling, which becomes the major determinant of arterial stiffening. Alternative explanations include that the impact of WC in the referenced groups, which have the highest average WC, has already occurred.
Potential mechanisms linking geometric phenotypes and future functional changes
The link between CCA geometric phenotypes and future increases in arterial stiffening may reflect changes in wall composition that modify the arterial stress–strain relationship and/or the response to hemodynamic and metabolic changes associated with obesity. Indeed, a few decades ago, Folkow proposed that small arterial concentric hypertrophic structural changes amplify the arterial response to the distending blood pressure [11]. However, at the central arterial level, the structural–functional relationship appears more complex with dilatory remodelling that might alter this relationship. For example, we found in the concentric hypertrophy CGP group that PWVROC was associated with DBPROC, which is in line with the changes Folkow proposed in smaller arteries. In dilated hypertrophic CGP, however, PWVROC was not associated with distending DBP, but with SBPROC which is often a result of central arterial stiffness.
Novel experimental evidence suggests that, beyond the classical elastin/collagen components of the arterial wall, regulation of vascular smooth muscle cells (VSMC) tone, extracellular matrix composition, and cell–matrix interactions are key factors in age-associated arterial remodelling [12–14]. Age associated changes in these factors are linked to a proinflammatory profile in the aged arterial wall [15]. Arterial geometry may also impact on the optimal energy transfer along the arterial system [16]. Modulation of pulsatile load impacts on arterial stiffness [9] and the coupling between left ventricle and arterial system. [17, 18]
Although we were not able to accurately establish the temporal sequence in the natural history of CCA remodelling, because CGP could only be measured at baseline, we can speculate on possible mechanisms underlying our observations on the role of vascular geometry.
The CCA geometric phenotype, in part, may be the result of permissive genome [19, 20], ethnicity [21], and concomitant clinical conditions, such as hypertension, diabetes, and dyslipidaemia [22]. Arterial geometry is associated with specific arterial functional profiles [5] in order to maintain parietal stress within certain limits [23] and/or at damping the pulsatility downstream and, thus, to protect microcirculation and tissue perfusion [24] while modulating mean blood pressure and arterial stiffness. In normotensive volunteers from the Baltimore Longitudinal Study on Aging (BLSA), aortic root diameter impacted arterial stiffening and the onset of systolic hypertension [25]. It is not clear under which circumstances hypertrophy or concentric remodelling without dilation the remodelling is adaptive or maladaptive. In addition, its role in slowing or accelerating arterial aging [26, 27] needs to be clarified.
A previous study had reported that in subjects with the metabolic syndrome, changes in blood pressure and stiffness preceded and drove changes in arterial geometry [28]. However, several of the arterial parameters adopted to describe arterial geometry and function in that study were calculated from carotid diameter and intima-media thickness only, and thus may be impacted by some certain degree of collinearity. Of note, that study also reported that changes in BP were correlated with carotid CSA but not with carotid IMT, highlighting the relevance of vascular geometry in modulating arterial function(s) [28].
The present study has some limitations. The study population consisted of a large cohort of “not health seeking” participants. Although this makes the findings more representative of a general population, it does not help to identify higher risk subjects (specifically “affected” individuals, or patients). The homogenous ethnic background (Founder population of SardiNIA) might limit its generalizability to other ethnicities. However, our previous analysis of two cohorts with different ethnic background, SardiNIA and BLSA [7, 9], yielded similar results indicating the low impact of the ethnic background on the general findings observed. Characterizing carotid geometry is limited by various factors including noisy images, delineation of anatomical boundaries (including the inability to demarcate the adventitia, which plays a major role in arterial wall stiffness), and assumptions of geometric regularities to apply area formulae. Also, EKG timed measurements of carotid diameter might introduce an effect of PWV, higher image quality; we believe the short heart-carotid transit distance makes any effect of PWV minute and within the error margin of manual measurements. While we believe these factors might have introduced noise, however, such noise would have resulted in underestimation in the associations observed, which further strengthens the validly of these findings.
An additional limitation is that, despite the longitudinal population design of the present study, longitudinal CGP data are not currently available for analysis. This limits the ability to explore the natural history of CGP starting with individuals with normal and concentric remodelling patterns; such a study will help determine the temporal relationships between structural and functional alterations, prospectively, and identify early interventions to prevent the development of accelerated aging phenotypes. Another limitation is that due to the smaller number of participants in the different classes of medications, we did not have the statistical power to assess the effect of the various classes of medications on the associations observed. However, this is beyond the scope of this study and future studies powered to detect such associations would help elucidate this issue.
In conclusion, the results of the present study highlight the importance of studying arterial geometric phenotypes to reveal accelerated aging phenotypes and improve clinical risk stratification to guide interventions. Detailed investigation of the mechanisms that underlie arterial geometry is required in order to inform the prognostic value of arterial geometric phenotype in CVD risk stratification and guide the process to develop novel therapeutic interventions for intertwined reality of arterial aging and disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AGEs
Advanced glycation end-products
- BMI
Body mass index
- BP
Blood pressure
- CCA
Common carotid artery
- CGP
Carotid geometric phenotype
- t-CGP
Typical CGP
- c-CGP
Concentric
- ch-CGP
Concentric hypertrophic CGP
- dh-CGP
Dilated hypertrophic CGP
- CV
Cardiovascular
- DBP
Diastolic blood pressure
- HDL
High density lipoprotein
- HT
Hypertension/hypertensive
- IMT
Intima-media thickness
- LDL
Low density lipoprotein
- MBP
Mean blood pressure
- MMP
Matrix metalloproteinase
- PP
Pulse pressure
- PWV
Pulse wave velocity
- SBP
Systolic blood pressure
- VSMC
Vascular smooth muscle cells
Funding
This research was supported in part by the Intramural Research Program of the US National Institutes of Health, National Institute on Aging (No. HHSN271201600005C).
Data Availability
Data associated with this study are available on written request through the corresponding author.
Declarations
Ethics approval
The present study complies with the Declaration of Helsinki that the locally appointed ethics committee has approved the research protocol and that informed consent has been obtained from the subjects (or their legally authorized representative).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Majd AlGhatrif, Email: Majd.alghatrif@nih.gov.
Angelo Scuteri, Email: angeloelefante@interfree.it.
References
- 1.Lacolley P, Regnault V, Avolio PA. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 2018;114:513–528. doi: 10.1093/cvr/cvy009. [DOI] [PubMed] [Google Scholar]
- 2.AlGhatrif M, Lakatta EG. The reality of aging viewed from the arterial wall. In: Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. London: Springer London. 2014; 137–153.
- 3.Karikkineth AC, Alghatrif M, Oberdier MT, Morrell C, Palchamy E, Strait JB, Ferrucci L, Lakatta EG. Sex differences in longitudinal determinants of carotid intima medial thickening with aging in a community-dwelling population: the Baltimore longitudinal study on aging. J Am Heart Assoc. 2020;9. 10.1161/JAHA.119.015396. [DOI] [PMC free article] [PubMed]
- 4.Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–278. doi: 10.1161/01.CIR.56.2.273. [DOI] [PubMed] [Google Scholar]
- 5.Scuteri A, Chen C-H, Yin FCP, Chih TT, Spurgeon HA, Yin FCPP, Chih-Tai T, Spurgeon HA, Lakatta EG. Functional correlates of central arterial geometric phenotypes. Hypertension. 2001;38:1471–1475. doi: 10.1161/hy1201.099291. [DOI] [PubMed] [Google Scholar]
- 6.Scuteri A, Manolio TA, Marino EK, Arnold AM, Lakatta EG. Prevalence of specific variant carotid geometric patterns and incidence of cardiovascular events in older persons the Cardiovascular Health Study (CHS E-131) J Am Coll Cardiol. 2004;43:187–193. doi: 10.1016/j.jacc.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 7.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal Trajectories of Arterial Stiffness and the Role of Blood Pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–951. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scuteri A, Najjar SS, Orru’ M, Albai G, Strait J, Tarasov KV, Piras MG, Cao A, Schlessinger D, Uda M, et al. Age- and gender-specific awareness, treatment, and control of cardiovascular risk factors and subclinical vascular lesions in a founder population: the SardiNIA Study. Nutr Metab Cardiovasc Dis. 2009;19:532–41. doi: 10.1016/j.numecd.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberdier MT, Morrell CH, Lakatta EG, Ferrucci L, AlGhatrif M. Subclinical longitudinal change in ankle‐brachial index with aging in a community‐dwelling population is associated with central arterial stiffening. J Am Heart Assoc 2019;8. 10.1161/jaha.118.011650. [DOI] [PMC free article] [PubMed]
- 10.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LAP, Loi F, Pilia MG, Delitala A, Spurgeon H, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkow B. Structural factor in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.HYP.16.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Lacolley P, Regnault P, Laurent S. Mechanisms of arterial stiffening. From Mechanotransduction to Epigenetics. Arter Thromb Vasc Biol. 2020;40:1055–1062. doi: 10.1161/ATVBAHA.119.313129. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz HH, LópezDíez R, Arivazahagan L, Ramasamy R, Metabolism SAM. Obesity, and diabetes mellitus. Recent studies in cellular and animal models and human subjects highlight mechanisms and consequences of metabolic dysfunction. Arter Thromb Vasc Biol. 2019;39:e166–e174. doi: 10.1161/ATVBAHA.119.312005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Zhao X, Stiffness WHA. A focus on vascular calcification and its link to bone mineralization. Arter Thromb Vasc Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Jiang L, Monticone RE, Proinflammation LEG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, Nakayama M, Nevo E, Fetics BJ, Lowell Maughan W, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/S0735-1097(98)00374-X. [DOI] [PubMed] [Google Scholar]
- 17.AlGhatrif M, Wang M, Fedorova OV, Bagrov AY, Lakatta EG. The pressure of aging. Med Clin North Am. 2017;101:81–101. doi: 10.1016/j.mcna.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlGhatrif M, Morrell CH, Becker LC, Chantler PD, Najjar SS, Ferrucci L, Gerstenblith G, Lakatta EG. Longitudinal uncoupling of the heart and arteries with aging in a community dwelling population. Geroscience. 2021;43:551–561. doi: 10.1007/s11357-020-00321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesh SK, Chasman DI, Larson MG, Guo X, Verwoert G, Bis JC, Xiangjun GU, Smith AV, Yang ML, Zhang Y, et al. Effects of long-term averaging of quantitative blood pressure traits on the detection of genetic associations. Am J Hum Genet. 2014;95:49–65. doi: 10.1016/j.ajhg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calimport S, Bentley BL, Stewart CE, Pawelec G, Scuteri A, Vinciguerra M. To help aging populations, classify organismal senescence. Science(80- ). 2019;366:576–578. [DOI] [PMC free article] [PubMed]
- 21.Schutte AE, Kruger R, Gafane-Matemane LF, Breet Y, Strauss-Kruger M, Ethnicity CJK, Stiffness A. Ethnicity and Arterial Stiffness. Arter Thromb Vasc Biol. 2020;40:1044–1054. doi: 10.1161/ATVBAHA.120.313133. [DOI] [PubMed] [Google Scholar]
- 22.Watase H, Sun J, Hippe DS, Balu N, Li F, Zhao X, Mani V, Fayad ZA, Fuster V, Hatsukami TS, et al. Carotid artery remodeling is segment specific: an in vivo study by vessel wall magnetic resonance imaging. Arter Thromb Vasc Biol. 2018;38:927–934. doi: 10.1161/ATVBAHA.117.310296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 24.Scuteri A, Wang H. Pulse wave velocity as a marker of cognitive impairment in the elderly. J Alzheimers Dis. 2014;42(Suppl 4):S401–S410. doi: 10.3233/JAD-141416. [DOI] [PubMed] [Google Scholar]
- 25.Farasat SM, Morrell CH, Scuteri A, Ting CT, Yin FC, Spurgeon HA, Chen CH, Lakatta EG, Najjar SS. Pulse pressure is inversely related to aortic root diameter implications for the pathogenesis of systolic hypertension. Hypertension. 2008;51:196–202. doi: 10.1161/HYPERTENSIONAHA.107.099515. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson PN, Laurent S, Cunha PG, Olsen MH, Rietzschel E, Franco OH, Ryliskyte L, Strazhesko I, Vlachopoulos C, Chen CHM, Boutouyrie P, Cucca F, Lakatta EG, Scuteri A. Characteristics of Healthy Vascular Ageing (HVA) in pooled population-based cohort studies: the global MARE consortium. J Hypertens. 2018;36:2340–2349. doi: 10.1097/HJH.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha P, Cotter J, Oliveira P, Vila I. Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hyperten. 2015;33:1438–1445. doi: 10.1097/HJH.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 28.Brown I, Diederich L, Good ME, De Lalio LJ, Murphy SA, Cortese-Krott MM, Hall JL, Le TH, Isakson BE. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arter Thromb Vasc Biol. 2018;38:1969–1985. doi: 10.1161/ATVBAHA.118.311229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study are available on written request through the corresponding author.