Abstract
We investigated the importance of the host complement system in the pathogenesis of disease mediated by the intramacrophage pathogen Mycobacterium avium. Mycobacteria opsonized with complement are efficiently ingested by macrophages through various complement receptors. Furthermore, unlike other bacteria, mycobacteria can activate both the alternative and classical complement pathways in the absence of specific antibodies. Therefore, to examine the role of complement in the mycobacterial infection process in vivo, mice deficient in complement component C3 were infected with M. avium. Surprisingly, C3-deficient mice infected intravenously with M. avium displayed no difference in bacterial burden or granulomatous response compared to wild-type control mice. C3-sufficient mice and C3-deficient mice were equally susceptible to infection by M. avium regardless of the genotype at the bcg locus, a locus known to confer susceptibility to infection with intracellular pathogens. In vitro studies using mouse bone marrow-derived macrophages resulted in significant M. avium invasion of macrophages in the absence of C3; however, the kinetics of infection were delayed compared to complement-mediated invasion. The data indicate that complement does not play an essential role in mediating M. avium infections in the mouse and suggest either that other invasion mechanisms can compensate for the absence of complement-mediated entry or that complement is not a major mycobacterial opsonin in vivo.
Mycobacterium avium is an important human pathogen associated with morbidity and mortality in AIDS patients and chronic lung disease in non-AIDS patients (9). M. avium, like most infectious mycobacteria, is an intracellular pathogen whose major host cell is the monocyte or macrophage. In vitro studies have shown that mycobacteria can use a variety of macrophage receptors to mediate entry into the host cell, including the complement receptors, the mannose receptor, CD14, the scavenger receptor, and surfactant protein A receptors (for a review see reference 6). Phagocytic complement receptor 1 (CR1), CR3, and CR4 recognize and bind to cleavage products of complement component C3 that have been deposited on a surface following complement activation, and all three of these complement receptors have been shown to mediate ingestion of pathogenic mycobacteria (21, 22). Complement opsonization of mycobacteria and subsequent ingestion via complement receptors have been shown to be the components of a major mechanism by which mycobacteria invade macrophages in vitro (for a review see reference 19).
Recent studies using mice deficient in CR3 have shown that lack of this receptor, which recognizes the C3 cleavage fragment iC3b, does not affect the susceptibility of mice to intravenous infection with Mycobacterium tuberculosis (10, 15). Also, studies with mice deficient in beta 2 integrins, which include CR3 and CR4, have shown that these receptors are not required to initiate or control an M. avium infection (2). Although these studies showed that a lack of specific complement receptors did not alter susceptibility to mycobacterial infections, other complement receptors (e.g., CR1) may also mediate phagocytosis of complement-opsonized bacteria by mouse macrophages. Furthermore, activation of the complement system results in the production of multiple inflammatory mediators, including C3a and C5a, which may aid in recruiting leukocytes to sites of mycobacterial infection. Jagannath et al. have recently shown that mice deficient in complement component C5 are more susceptible to aerosolized M. tuberculosis infection than congenic control mice, which may be due in part to the inability of the C5-deficient macrophages to mount a sufficient protective cytokine response (11).
C3 is the central component of the complement system. In the absence of C3, some of the effector functions of complement that are lost or diminished include (i) production of anaphylatoxins C3a and C5a, (ii) opsonization by cleavage products of C3, and (iii) formation of the membrane attack complex. Therefore, we sought to investigate the role of complement in mediating infections with M. avium by using mice deficient in C3. We found that for C3-deficient mice from resistant (bcg rr) and susceptible (bcg ss) backgrounds there was no difference in the bacterial loads recovered from spleens and livers or in the granulomatous responses after 1 or 5 weeks of infection with M. avium. These results show that complement activation and the subsequent effector functions, which rely on the presence of C3, are not required to mediate either macrophage invasion or subsequent disease progression in M. avium-infected mice.
MATERIALS AND METHODS
Chemicals.
All chemicals were purchased from Sigma (St. Louis, Mo.) unless stated otherwise.
Mice.
C3-deficient C57BL/6 × 129/J mice were a gift from Harvey Colten, Northwestern University, Chicago, Ill. Six-week-old female C57BL/6 and 129/J mice used for backcrossing were purchased from Jackson Laboratories (Bar Harbor, Maine). C3−/− mice were bred one generation to C57BL/6 and 129/J mice to generate susceptible and resistant mice, respectively. C3 heterozygotes resulting from the initial cross were selected for susceptibility by PCR screening of the bcg locus by using previously published protocols (8, 14) and the following primers (University of Notre Dame Core Facility): 5′-TCG GGA CGG CTA TCT CCT TC and 5′-AAT GGT GAT CAG TAC ACC GC (resistant) or 5′-AAT GGT GAT CAG TAC ACC GT (susceptible). Bcg ss or Bcg rr C3+/− mice were crossed to generate C3−/− and C3+/+ littermate controls. Mice were PCR screened for C3 by using the following primers (GIBCO BRL, Rockville, Md.): 5′-CTT AAC TGT CCC ACT GCC AAG AAA CCG TCC CAG ATC and 5′-CTC TGG TCC CTC CCT GTT CCT GCA GGG ACT GCC CAA AAT TTC GCA AC. The mice were separately PCR screened for neomycin by using primers described previously (13). The mice were maintained at Frieman Life Science Center at the University of Notre Dame, and the infection experiments complied with the Institutional Animal Care and Use Committee guidelines.
Bacteria.
To generate M. avium stocks, bacteria were passaged through a mouse to ensure virulence, and a single colony was used to inoculate Middlebrook 7H10 media (Difco, Detroit, Mich.) supplemented with glucose, oleic acid, albumin, Tween 20, and NaCl (supplemented Middlebrook media). Bacteria were grown for 1 week at 37°C with vigorous shaking and resuspended in supplemented Middlebrook media containing 15% glycerol, and the preparations were divided into aliquots and stored at −70°C. Frozen stocks were quantitated by serial dilution on supplemented Middlebrook agar. Mycobacterium phlei was a generous gift from Tim Ratliff (University of Iowa). Mycobacterium smegmatis mc2155 was a generous gift from Eric Brown (University of California, San Francisco). Bacillus cereus and Escherichia coli were generous gifts from Charles Kulpa (University of Notre Dame). M. avium 724 and 2-151 were generous gifts from Andrea Cooper (Colorado State University, Fort Collins). M. avium 101 was a generous gift from David Russell (Cornell University, Ithaca, N.Y.).
Tissue culture.
Murine macrophage cell line J774 (American Type Culture Collection, Rockville, Md.) was cultured in Dulbecco modified Eagle medium (DMEM) (GIBCO BRL) supplemented with 5% fetal bovine serum (GIBCO BRL), 20 mM HEPES (Fisher Scientific, Pittsburgh, Pa.), 100 U of penicillin per ml, and 100 μg of streptomycin (BioWhittaker, Walkersville, Md.) per ml at 37°C in the presence of 5% CO2. Bone marrow macrophages were isolated and cultured as previously described (18). Briefly, bone marrow was isolated, and fibroblasts and mature macrophages were removed by selective adhesion. Bone marrow was cultured at 37°C in the presence of 5% CO2 in DMEM supplemented with 10% fetal bovine serum (GIBCO BRL), 20 mM HEPES (Fisher Scientific), 100 U of penicillin per ml, 100 μg of streptomycin (BioWhittaker) per ml, and 15% L-cell supernatant as a source of macrophage colony-stimulating factor. After 4 days in culture, macrophages were supplied with fresh media, and mature macrophages were used on day 7 of culture.
Determination of classical pathway activation.
A total of 107 bacteria per point were incubated with purified complement components (Advanced Research Technologies, San Diego, Calif.) at final concentrations of 0.4 μg/ml (C1), 19 μg/ml (C4), 0.14 μg/ml (C2), and 0.166 μg/ml (C3) in veronal-buffered saline (BioWhittaker) supplemented with 0.1% gelatin, 1 mM Mg2+, and 0.15 mM Ca2+ for 2 h at 37°C. Bacteria were pelleted, and the supernatants were assayed for C3a production by an enzyme-linked immunosorbent assay (ELISA) (Quidel, San Diego, Calif.) as described previously (24).
Mouse infection protocol.
Bacteria were resuspended by multiple passages through a 28-gauge needle and diluted into sterile phosphate-buffered saline (PBS) (GIBCO BRL). Mice were infected with bacteria in PBS retroorbitally. After a given time, mice were sacrificed, and organs were homogenized in 5 ml of sterile PBS with 1% Igepal. The homogenates were serially diluted in supplemented Middlebrook media and plated onto supplemented Middlebrook agar. The plates were incubated at 37°C for 10 to 12 days, and then colonies were counted.
Histology.
Liver sections from infected mice were fixed in 10% buffered formalin for 4 to 6 h and transferred to 70% ethanol. Samples were routinely processed and embedded in paraffin at the Keck Center for Transgene Research, Anatomic Pathology Lab, University of Notre Dame. Samples were stained with hematoxylin and eosin and with Ziehl-Neelsen by the Holburn Biomedical Corporation (Bowmanville, Ontario, Canada).
In vitro macrophage infection and TNF-α production.
Macrophages were plated on glass coverslips in tissue culture media and allowed to adhere overnight at 37°C in the presence of 5% CO2. Mycobacteria were preopsonized in DMEM–0.5% bovine serum albumin with 10% normal or complement-deficient serum (Advanced Research Technologies) for 2 h at 37°C. The media were removed from the macrophages, and the cells were cultured in DMEM–0.5% bovine serum albumin containing opsonized mycobacteria for the rest of the experiment. Preopsonized mycobacteria were added to the cells at a ratio of bacteria to cells of 10:1 for bone marrow macrophages and for J774 cells. After 2 h for J774 cells or at a specified time for bone marrow macrophages, monolayers were washed twice with PBS, fixed with methanol-acetone (1:1) for 2 min, and then washed two more times with PBS. The mycobacteria were stained with TB Auramine (Difco) and visualized by fluorescence microscopy. At least 100 macrophages per point were counted. The concentrations of tumor necrosis factor alpha (TNF-α) in macrophage supernatants were determined by an ELISA (BD Pharmingen, Franklin Lakes, N.J.) performed according to the manufacturer's instructions.
RESULTS
Pathogenic and nonpathogenic mycobacteria activate the classical complement pathway in the absence of specific antibody.
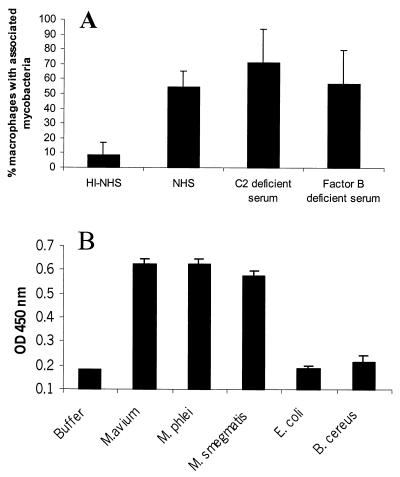
Complement-mediated ingestion of pathogenic mycobacteria by macrophages has been shown to be a major mechanism by which mycobacteria invade macrophages in vitro (for a review see reference 19). To illustrate this phenomenon, cells of J774 , a murine macrophage-like cell line, were infected with M. avium 724 in the presence of normal human serum or human serum depleted of complement activity by heat inactivation (Fig. 1A). Approximately 60% of J774 cells were associated with mycobacteria after a 2-h infection in the presence of normal human serum. The corresponding value was less than ∼10% in the presence of heat-inactivated serum. Furthermore, ingestion of mycobacteria was mediated by serum deficient in either the classical or lectin pathway (C2-deficient serum) or the alternative complement pathway (factor B-deficient serum) (Fig. 1A), suggesting that both the alternative and the classical and/or lectin pathways were capable of mediating ingestion of M. avium. To specifically examine the activation of the classical complement pathway by mycobacteria, purified C1, C4, C2, and C3 were incubated with mycobacteria, and complement activation was determined by an ELISA-based assay to measure C3 cleavage. We found that the classical complement pathway is activated by the surfaces of pathogenic (M. avium) and nonpathogenic (M. phlei and M. smegmatis) mycobacteria but not by the gram-negative bacterium E. coli or the gram-positive bacterium B. cereus (Fig. 1B). This classical pathway activation occurred in the absence of specific antibody. However, trace amounts of immunoglobulin M are known to copurify with C1, so we cannot rule out the possibility that nonspecific antibody mediated the classical pathway activation on the mycobacterial surface, as has been shown for Mycobacterium leprae (23). It is interesting that mycobacteria are more potent activators of the classical pathway than other bacteria are, suggesting that complement opsonization may be an important process in mycobacterial pathogenesis.
FIG. 1.
Mycobacteria activate the classical complement pathway in the absence of specific antibody. M. avium 724 was opsonized with 10% heat-inactivated normal human serum (HI-NHS), normal human serum (NHS), human C2-deficient serum, or human factor B-deficient serum for 2 h at 37°C and then incubated for an additional 2 h at 37°C with J774 cells at a ratio of bacteria to cells of 10:1. The mycobacteria associated with macrophages were visualized by fluorescence microscopy. At least 100 cells were counted for each sample. (A) Representative results of three experiments in which107 bacteria were incubated with purified C1, C2, C3, and C4 for 2 h at 37°C and C3 cleavage was assessed by ELISA for C3a. The solid bars indicate the means and the error bars indicate the standard deviations based on triplicate experimental samples. (B) Optical densities at 450 nm (OD 450 nm) of different preparations.
It has been well established that mycobacteria activate the alternative complement pathway (for a review see reference 19). Mycobacteria have also been shown to use a C2a-specific pathway (24). Finally, M. avium is known to bind the mannose binding lectin (17), the recognition component of the lectin pathway. Therefore, mycobacteria have evolved multiple mechanisms for complement activation, and we hypothesized that complement opsonization in vivo is an important component in the uptake and subsequent survival of macrophage-ingested mycobacteria. Mice genetically deficient in complement component C3 were infected with M. avium to test this hypothesis.
C3 deficiency does not alter susceptibility to infection with M. avium.
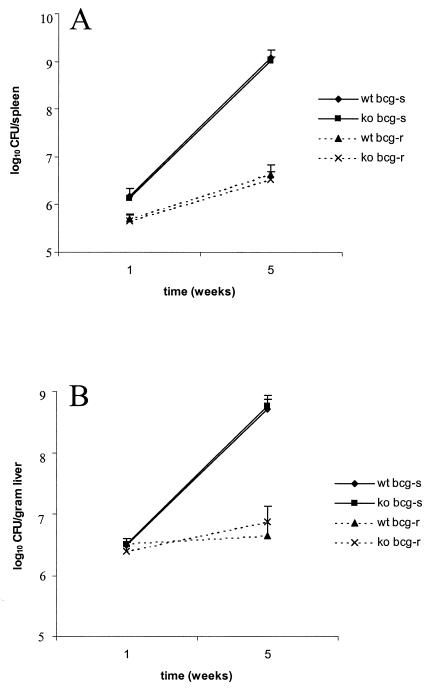
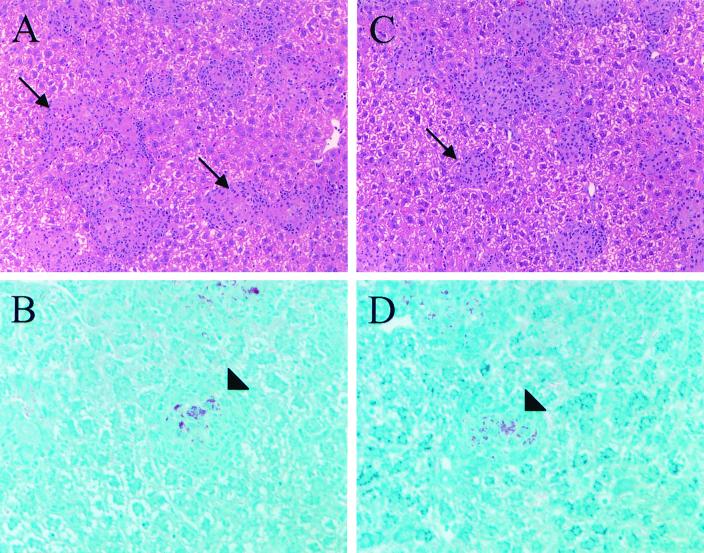
C3-deficient mice were backcrossed into the susceptible C57BL/6 background or the resistant 129/J background. The susceptible and resistant phenotypes were based on the genotypes at the bcg locus, bcg ss and bcg rr, respectively. The bcg locus encodes natural resistance-associated membrane protein-1, a protein known to confer susceptibility to numerous intracellular pathogens (1). M. avium 724 is known to be virulent in mice, and the virulence is enhanced in mice that are homozygous for the susceptible natural resistance-associated membrane protein-1 allele (3). Therefore, we initiated our studies using M. avium 724, an organism capable of engaging an aggressive host response. C3-deficient mice and littermate control mice were infected intravenously with 106 CFU of M. avium 724, and mice were sacrificed 1 and 5 weeks after challenge. The colony counts recovered from spleens and livers were similar for the C3-deficient mice and the littermate control mice, indicating that C3 deficiency did not alter mycobacterial survival within the host (Fig. 2). Liver sections were obtained from the infected mice, and histological examination revealed similar pathologies in C3-deficient mice and littermate control mice. As expected, acid-fast mycobacteria were found associated with leukocytes in granulomas, and larger numbers of granulomas and acid-fast bacteria were found at later times and in susceptible mice (Fig. 3).
FIG. 2.
Bacterial loads in spleens (A) and livers (B) of C3-sufficient and C3-deficient mice were similar after 1 or 5 weeks of infection. C3-sufficient and C3-deficient mice with both resistant (bcg-r) and susceptible (bcg-s) backgrounds were retroorbitally infected with 106 M. avium 724 cells, and the mice were maintained for 1 or 5 weeks. The mice were sacrificed, spleens and livers were homogenized, and serial dilutions were plated onto Middlebrook 7H10 agar to determine the numbers of CFU per organ. The data are representative of at least two experiments, and each data point represents the data from four to seven mice; the error bars indicate standard deviations. wt, wild type; ko, C3 knockout.
FIG. 3.
C3 deficiency does not alter granuloma formation in response to M. avium 724 infection. Liver sections from infected mice were processed and stained with hematoxylin and eosin (A and C) and Ziehl-Neelsen (B and D). C3-deficient mice (C and D) contained numbers of granulomas and acid-fast organisms within granulomas similar to the numbers in wild-type controls (A and B). Liver sections from bcg ss mice following 5 weeks of infection with M. avium 724 are shown. Hematoxylin- and eosin-stained sections were visualized by using a magnification of ×200, and Ziehl-Neelsen-stained sections were visualized by using a magnification of ×400. The arrows indicate hematoxylin- and eosin-stained granulomas, and the arrowheads indicate granulomas containing acid-fast bacilli.
We obtained similar findings when 8 × 104 CFU of M. avium 724 were used, showing that the results were not specific to a particular infectious dose. Furthermore, a 5-week infection experiment with M. avium 2-151 also resulted in no difference in susceptibility to M. avium infection between C3-deficient mice and C3-sufficient mice, indicating that the results were not dependent on the strain of M. avium used (data not shown).
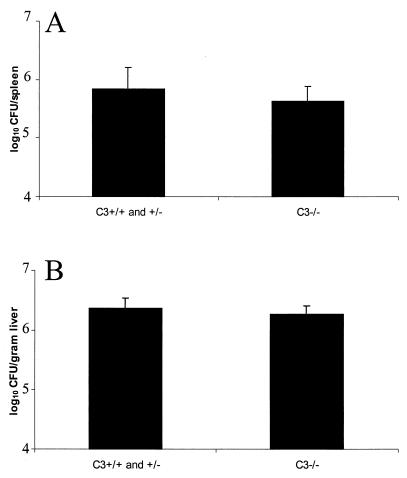
Complement-opsonized particles in circulation are transported to the liver and spleen either directly or through binding to CR1 on erythrocytes, which traffic the particles to acceptor phagocytes (reviewed in reference 16). Therefore, we tested the efficiency of mycobacterial localization to spleens and livers 24 h postinoculation. Similar levels of bacteria were found in livers and spleens from C3-sufficient and C3-deficient mice, suggesting that complement does not increase the efficiency of clearance of mycobacteria from the circulation (Fig. 4). The detection assay was not sensitive enough to deduce the number of bacteria in blood even 1 h postinoculation; therefore, only bacteria in spleens and livers were quantitated. Based on these studies, we concluded that C3 deficiency does not alter susceptibility to a systemic M. avium infection in the mouse.
FIG. 4.
Presence of C3 does not alter the efficiency of mycobacterial localization to spleens and livers 24 h after infection. C3-sufficient and C3-deficient bcg rr mice were infected with 107 CFU M. avium 724 cells. At 24 h after challenge the mice were sacrificed, and the bacterial loads in the organs were assessed as described in the legend to Fig. 2. The data are representative of three experiments. The solid bars indicate the means and the error bars indicate the standard deviations based on at least three mice.
With increased incubation times, nonopsonized M. avium is efficiently ingested by mouse bone marrow-derived macrophages.
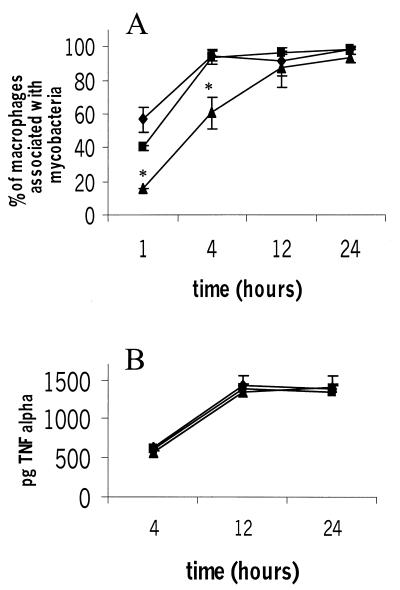
Complement is clearly important in mediating uptake of mycobacteria by macrophages in vitro, so we sought to understand why similar findings were not apparent in our in vivo infection model by finding an in vitro correlate. Serum and bone marrow-derived macrophages were obtained from C3-sufficient and C3-deficient mice, and infections with M. avium 724 were carried out in vitro. We found that although complement-opsonized mycobacteria were ingested with increased kinetics, the nonopsonized M. avium cells were eventually ingested, and by 12 h after challenge equal numbers of macrophages were associated with mycobacteria in the presence and in the absence of C3 (Fig. 5A). A similar ingestion mechanism may occur during an in vivo infection in which the time course of exposure to bacteria is not necessarily limited.
FIG. 5.
Bone marrow-derived macrophages ingest mycobacteria and produce TNF-α in response to mycobacteria in the absence of C3. Bone marrow-derived macrophages and serum were recovered from C3-sufficient and C3-deficient mice, and macrophages were exposed to serum-opsonized mycobacteria for 1, 4, 12, and 24 h; this was followed by extensive washing and fixation. Mycobacteria associated with macrophages were visualized by fluorescence staining and subsequent microscopy. The data are representative of two experiments. Each data point represents the mean based on triplicate samples, and the error bars indicate standard deviations. Statistical differences were determined for macrophages in the absence of C3 compared to macrophages in the presence of C3. Symbols: ⧫, C3+/+ macrophages and serum; ▪, C3−/− macrophages and C3+/+ serum; ▴, C3−/− macrophages and serum. (A) An asterisk indicates that the P value was <0.05, as determined by an unpaired t test. (B) Supernatants from infected macrophages assayed for the presence of TNF-α by ELISA.
Macrophage TNF-α production does not require C3.
Aside from ingesting mycobacteria, macrophages also mount a cytokine response which serves to alert the immune system to the invading pathogen. TNF-α, produced by the activated macrophages, is required to control mouse infections with M. avium 724, and mice lacking TNF receptor p55 succumb to progressive and fatal granulomatous necrosis when they are intravenously infected with M. avium 724 (5). Therefore, we sought to determine if the macrophage TNF-α response was affected by the absence of C3 in vitro. Even at 4 h, when fewer bacteria were associated with macrophages in the absence of C3 (Fig. 5A), similar TNF-α levels were produced by the macrophages (Fig. 5B). These results show that complement protein C3 does not appear to be absolutely required for TNF-α production from macrophages in vitro. We hypothesize that mycobacterial components such as lipoarabinomannan which come into contact with the macrophages during the 1- to 24-h incubation period are responsible for macrophage production of TNF-α.
DISCUSSION
Our studies indicate that mycobacteria can activate the alternative and classical complement pathways independent of specific antibody. Using purified classical complement components, we also demonstrated that both pathogenic and nonpathogenic mycobacteria could initiate the formation of a classical pathway C3 convertase in the absence of any additional serum proteins. Classical pathway activation was specific for mycobacteria since no C3 cleavage was observed for either E. coli or B. cereus under identical conditions. The results of these in vitro experiments and the results obtained in other laboratories (20–24) indicate that complement plays a central role in mediating mycobacterial invasion of macrophages. Therefore, studies were initiated to determine if complement activation and subsequent opsonization of mycobacteria were critical components in mediating mycobacterial infection in vivo. Surprisingly, C3-deficient mice, which are deficient in many effector functions of the complement system (e.g., opsonization with fragments of C3b), were as susceptible to infection with M. avium 724 as wild-type controls were, as assessed by bacterial loads in spleens and livers and the extent of the granulomatous response. Furthermore, like mycobacteria in control animals, mycobacteria in C3-deficient mice were associated with leukocytes inside granulomas. Interestingly, C3 deficiency did not alter susceptibility to M. avium infection, even in mice which were highly susceptible to infection due to their genetic background. Even under conditions in which the infection was progressive, a role for C3 in dictating pathology and host response was not observed. We also found that C3 deficiency failed to alter susceptibility to mycobacterial infection when different strains or different concentrations of M. avium were used.
To help discern our contradicting in vitro and in vivo findings, we performed an in vitro kinetic analysis of the mycobacterial invasion process in the presence and in the absence of C3. Our results were in agreement with the results of others who described a role for complement in enhancing mycobacterial invasion of mouse macrophages in vitro at early time points (20–23). The more recent in vitro studies of Hu et al. indicated that the kinetics of M. tuberculosis infection were delayed in macrophages lacking CR3 (10). However, these studies were complicated by the ability of macrophages to make the various complement components and the other complement receptors (CR1 and CR4) on the macrophage surface. Our results indicate that the initial deficiency in mycobacterial invasion in the absence of C3 is largely reversible with increased exposure time between macrophages and M. avium. This is not due to the ability of macrophages to make complement components since the macrophages used in these experiments were isolated from C3-deficient mice. This indicates that M. avium can use other attachment mechanisms, albeit less efficient ones, to gain access to the host macrophages. Candidates for these alternative invasion pathways include the mannose receptor and the lectin-binding site on CR3. Both receptors have been shown to mediate mycobacterial attachment to macrophages and subsequent invasion (4, 12). Furthermore, activation of the classical complement pathway by mycobacteria could result in C4b opsonization and subsequent entry of the mycobacteria through the macrophage CR1. This mechanism of complement-mediated ingestion would remain in C3-deficient mice.
The length of time to which the mycobacteria are exposed to macrophages in vivo remains unclear. Perhaps exposure is not a limiting factor during an intravenous infection. Extrapolation of our in vitro results suggests that M. avium does not require C3 to gain access to macrophages in vivo. The association of mycobacteria in granulomas of C3-deficient mice and the similar quantities of M. avium isolated from tissues of C3-sufficient and C3-deficient mice support this conclusion. Our results confirm and extend the results of previous studies which indicated that there was no difference in susceptibility to M. avium and M. tuberculosis in mice deficient in CD18 and CD11b, respectively (2, 10, 15). In these studies the authors argued that mycobacteria do not require these specific complement receptors to mediate invasion of macrophages in vivo. However, previous studies have also indicated that CR1 can mediate ingestion of C3b-opsonized mycobacteria (22, 24), and it is not known whether CR1 functions in this capacity in CD18 or CD11b knockout mice. Our experiments with C3-deficient mice, which are not capable of mediating opsonization of mycobacteria with C3b and fragments of C3b, clearly indicate that the major effector functions of complement are not required to initiate or control an M. avium infection. What other invasion mechanisms are responsible for mediating mycobacterial invasion in vivo and what role complement plays in mediating mycobacterial entry via the gastrointestinal tract or lungs require further investigation. This is particularly important since the route of entry for pathogenic mycobacteria can have important effects on the host's immune response. This has been illustrated in various experiments performed with cytokine-deficient mice which show diverse immune responses after aerosolized M. tuberculosis infection compared to those of mice infected intravenously (for a review, see reference 7).
It is interesting that CR3 contains both a C3bi site and a lectin-binding site and that both sites have been shown to mediate mycobacterial attachment and ingestion (4, 20–23). Perhaps multiple invasion mechanisms can enhance mycobacterial invasion in vivo and the absence of one pathway can be compensated for by other pathways. It would be interesting to determine what effect a CD11b-C3 double knockout would have on mycobacterial infection in vivo since such a mouse would lack both the CR3-lectin interaction and complement-mediated entry.
ACKNOWLEDGMENTS
We thank Eric Brown (University of California, San Francisco), in whose laboratory these studies were initiated, and Andrea Cooper (Colorado State University) for her helpful suggestions. We also thank Harvey Colten (Northwester University) for providing the C3-deficient mice.
This work was supported by grants from the American Lung Association and the American Heart Association. J.S.S. is a Parker B. Francis Fellow.
REFERENCES
- 1.Bellamy R. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1999;1:23–27. doi: 10.1016/s1286-4579(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E, Goodman J, Petrofsky M. Role of complement receptors in uptake of Mycobacterium avium by macrophages in vivo: evidence from studies using CD18-deficient mice. Infect Immun. 1999;67:4912–4916. doi: 10.1128/iai.67.9.4912-4916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins F M, Stokes R W. Mycobacterium avium-complex infections in normal and immunodeficient mice. Tubercle. 1987;68:127–136. doi: 10.1016/0041-3879(87)90028-6. [DOI] [PubMed] [Google Scholar]
- 4.Cywes C, Hoppe H C, Daffe M, Ehlers M R. Nonopsonic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect Immun. 1997;65:4258–4266. doi: 10.1128/iai.65.10.4258-4266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel E T, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst J D. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn J L, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Gomes M S, Florido M, Pais T F, Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J Immunol. 1999;162:6734–6739. [PubMed] [Google Scholar]
- 9.Griffith D E. Mycobacteria as pathogens of respiratory infection. Infect Dis Clin N Am. 1998;12:593–611. doi: 10.1016/s0891-5520(05)70200-2. [DOI] [PubMed] [Google Scholar]
- 10.Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol. 2000;165:2596–2602. doi: 10.4049/jimmunol.165.5.2596. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath C, Hoffmann H, Sepulveda E, Actor J K, Wetsel R A, Hunter R L. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5) Scand J Immunol. 2000;52:369–379. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang B K, Schlesinger L S. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect Immun. 1998;66:2769–2777. doi: 10.1128/iai.66.6.2769-2777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy D W, Abkowitz J L. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci USA. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina E, Rogerson B J, North R J. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–481. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melo M D, Catchpole I R, Haggar G, Stokes R W. Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell Immunol. 2000;205:13–23. doi: 10.1006/cimm.2000.1710. [DOI] [PubMed] [Google Scholar]
- 16.Nardin A, Lindorfer M A, Taylor R P. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol Immunol. 1999;36:827–835. doi: 10.1016/s0161-5890(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 17.Polotsky V Y, Belisle J T, Mikusova K, Ezekowitz R A, Joiner K A. Interaction of human mannose-binding protein with Mycobacterium avium. J Infect Dis. 1997;175:1159–1168. doi: 10.1086/520354. [DOI] [PubMed] [Google Scholar]
- 18.Roach T, Slater S, Koval M, White L, McFarland E C, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 19.Schlesinger L S. Mycobacterium tuberculosis and the complement system. Trends Microbiol. 1998;6:47–49. doi: 10.1016/S0966-842X(97)01203-1. . (Discussion, 6:49–50.) [DOI] [PubMed] [Google Scholar]
- 20.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 21.Schlesinger L S, Horwitz M A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement componenet C3 in serum. J Clin Investig. 1990;85:1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlesinger L S, Horwitz M A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 23.Schlesinger L S, Horwitz M A. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect Immun. 1994;62:280–289. doi: 10.1128/iai.62.1.280-289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schorey J S, Carroll M C, Brown E J. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277:1091–1093. doi: 10.1126/science.277.5329.1091. [DOI] [PubMed] [Google Scholar]