Abstract
The metabolic peptide hormone amylin, in concert with other metabolic peptides like insulin and leptin, has an important role in metabolic homeostasis and has been intimately linked to Alzheimer’s disease (AD). Interestingly, this pancreatic amyloid peptide is known to self-aggregate much like amyloid-beta and has been reported to be a source of pathogenesis in both Type II diabetes mellitus (T2DM) and Alzheimer’s disease. The traditional “gain of toxic function” properties assigned to amyloid proteins are, however, contrasted by several reports highlighting neuroprotective effects of amylin and a recombinant analog, pramlintide, in the context of these two diseases. This suggests that pharmacological therapies aimed at modulating the amylin receptor may be therapeutically beneficial for AD development, as they already are for T2DMM. However, the nature of amylin receptor signaling is highly complex and not well studied in the context of CNS function. Therefore, to begin to address this pharmacological paradox in amylin research, the goal of this review is to summarize the current research on amylin signaling and CNS functions and critically address the paradoxical nature of this hormone's signaling in the context of AD pathogenesis.
Keywords: Alzheimer’s disease, amylin, therapy, diabetes, metabolism, amyloid, neuroprotection
1. INTRODUCTION
Alzheimer’s disease (AD) is a form of dementia characterized by progressive memory loss and changes in cognitive and neuropsychiatric behaviors that lead to the inability to perform everyday tasks and death [1, 2]. There are two classifications of AD, familial and sporadic. Familial AD, representing only <1% of all cases, is inherited through mutations in 3 genes which include: presenilin (PSEN-1 and -2) or amyloid precursor protein (APP) genes. These cause the overexpression and cleavage of APP, producing excess amyloid beta (A β) peptide accumulation. Sporadic AD, also known as late onset AD, is a multifactorial form of neurodegeneration where the cause is currently unknown.
The main cellular hallmarks of AD include neurodegeneration of hippocampal neurons that progressively spread to other regions and two main pathological entities, extracellular Aβ plaques and intracellular tangles made of hyper-phosphorylated tau protein [3-7]. The progressive accumulation of these hallmarks is thought to lead to the progressive cognitive and neuropsychiatric decline observed in these patients [5]. However, despite both tau and Aβ pathology being hallmarks of AD, it is not yet fully clear whether in late-onset AD, pathology is the driver of cellular dysregulation or rather a result of dyshomeostasis of more fundamental cellular processes. The latter suggests a more complex AD development, likely associated with multiple independent insults to which we are exposed throughout our lifetime [8-10].
1.1. The Exposome in Late Onset AD
Although aging is the number one risk factor for the development of sporadic AD [11], AD is not a normal consequence of aging [1, 12]. A growing list of genes, the most prominent of which being APOE [13], are also being implicated in late onset AD. However, several environmental exposures throughout one’s lifespan can independently, or in combination with aging, drive AD development. These range from traumatic brain injury [14-18], viruses [19, 20] or toxins [21-24] to socioeconomic aspects such as low education levels [25], or lifestyle choices such as a sedentary lifestyle [26, 27]. In fact, clinical outcomes associated with lack of exercise and poor diet, namely high blood pressure, cardiovascular disease and particularly Type II Diabetes Mellitus (T2DM), have all been linked to AD development [28-36].
The exact mechanisms that underlie the relationship between AD and T2DM remain unknown, however, both diseases share multiple commonalities as detailed previously [37]. T2DM is characterized by the presence of hyperglycemia, hyperinsulinemia, and insulin resistance [38], both systemically and centrally. In fact, a consequence of systemic hyperglycemia and hyperinsulinemia is the reduction of insulin receptors within the blood-brain barrier (BBB), which in turn lead to decreased insulin and glucose signaling within the brain [39-42].
Importantly, clinical reports show that diabetic patients have reduced thickness of brain regions, or atrophy, in regions affected in AD, such as the hippocampus [43-45]. Thus, not surprisingly, 70% of T2DM patients report cognitive impairment [46, 47]. Like AD, T2DM is also associated with exacerbated reactive oxygen species (ROS) production linked with increased mitochondrial and ER stress [48-50], and activation of inflammatory cascades [51-54] within the brain. Also, of note, is the fact that T2DM and AD, albeit different amyloid proteins (amylin in T2DM and A β in AD) share amyloidogenesis and amyloid processing, clearing and aggregation changes as potential common pathogenic mechanisms, one in the pancreas and the other within the brain.
The assessment of metabolic hormone levels throughout normal aging and during disease states and their impact on neuronal processes may offer new biomarkers and novel directions to target therapeutics for AD. In this regard, the common pathogenic mechanism between the two pathologies and the potential relationship between the two amyloids (amylin and A β) in the initiation of both diseases has been the focus of increasing research in the last decade as will be discussed throughout this review. Interestingly, data supports both a pathogenic and therapeutic role of amylin for AD pathogenesis [55, 56]. This paradox highlights an incomplete understanding of mechanisms underlying this relationship. This is further exacerbated by the complex physiology of amyloids and receptor signaling system through which amyloids - like amylin - signal [57-59]. The latter is a potential source for further understanding the pathophysiology of both diseases as well as a fruitful pathway for novel pharmacology development. Thus, here we summarize the current research in the area of amylin and CNS function and AD, provide an up-to-date review of its receptor signaling mechanisms, and critically discuss the pharmacological paradox associated with amylin pharmacological therapy in the context of AD pathogenesis.
Although peripheral mechanisms have been suggested, satiety, energy homeostasis, and newer signaling mechanisms associated with amylin action are thought to be largely regulated via the central nervous system (CNS), as will be detailed in the sections to follow.
2. AMYLIN AND THE AMYLIN RECEPTOR
Amylin is 37 amino acid (aa) peptide, a part of the calcitonin family alongside calcitonin (Calc), two Calc related genes (aCGRP and bCGRP) and adrenomedullin (AM) [60]. Amylin is packaged and co-released with insulin in 1:100 (15:1, insulin: amylin molar) ratio from β-islet cells of the pancreas after a meal consumption [61, 62].
Amylin has an important role in energy homeostasis systemically and centrally and acts as a satiety hormone [63-66]. For example, amylin serves an important glucoregulatory role by limiting insulin release from the pancreas [64, 67]. Similarly, amylin inhibits local glucagon secretion in the liver, stomach, and intestine, slowing gastric emptying and, thus, nutrient absorption [63, 64, 68-70]. In the CNS, amylin is known to sensitize leptin signaling [71, 72], thus, serving an important role in energy homeostasis acutely, during meals, and longer-term, through the hypothalamic processes. These findings have been further validated in animal models in which amylin is genetically deleted [72-75].
2.1. Amylin Receptor Expression & Signaling
Amylin does not have a cognate receptor but rather signals through the native calcitonin receptor (CalcR), a class B, seven transmembrane spanning G-coupled protein receptor (GPCR). Specificity to amylin is conferred by the heterodimerization of CalcR with one of three receptor activity modifying protein (RAMPs 1-3) [76]. Thus, the amylin receptor (AMYR) is accepted to be CalcR complexed with a RAMP [59, 76-81]. There are two isoforms of CalcR, alpha and beta [82, 83], as well as three known isoforms of RAMPs, named RAMP1, -2 and -3, providing a highly complex signaling network through AMYR subtypes: AMYR1a, AMYR1b, AMYR2a, AMYR2b, AMYR3a and AMYR3b [79, 83, 84].
The different components that make up the AMYR have been reported in both the periphery, namely, the kidney, testes, skeletal muscle, pancreas, liver, stomach, small intestine, osteoclasts, and in dorsal root ganglia [85, 86], as well as the CNS. Radioligand binding studies suggest the brain to be a region of the highest level of amylin binding within the body [87]. Of note, early studies found amylin binding sites within the CNS in 1993, roughly six years before AMYR was fully characterized [59, 81]. To this end, dense binding sites were originally characterized within the area postrema (AP), nucleus accumbens (NAc) and the hypothalamus. These brain regions are linked to amylin’s regulation of satiety. Additional sites of amylin of binding as well as amylin uptake, confirmed amylin-labeled radioactivity include: ventral tegmental area (VTA), hypothalamus, NAc, subfornical organ (SFO), amygdala, bed nucleus of the stria terminalis (BNST), locus coeruleus, thalamus pons, medulla, hippocampus, striatum, as well as frontal, occipital and parietal lobes [59, 85, 88-96].
CalcR and all three RAMPS have been shown to co-localize in hindbrain and midbrain areas such the Nucleus Tractus Solitarii (NTS) within the brain stem and the lateral parabrachial nucleus (LBPN), part of the pons within the midbrain [59, 90-94]. Additionally, different RAMP subtypes are known to localize to different areas. For example, along with CalcR, RAMP1 has been found in the area AP, ventromedial hypothalamus (VMH) and the NAc, caudate putamen and olfactory tubercles [91, 92, 97-99]. On the other hand, RAMP3 has been reported within the AP, dorsal thalamus, and SFO. In humans, RAMP2 is primarily expressed in the vasculature, and knockout of RAMP2 is lethal [77], thus, it is less commonly studied. Together, these data suggest that RAMPs may confer regional and signaling specificity. Importantly, the study of AMYR signaling in vivo is further complicated by the fact that no AMYR conformation is 100% specific to amylin binding [81, 82], and the fact that RAMPs are known to complex with nine different receptors, not just CalcR [100, 101]. Similarly, since CalcR signals for its native ligand calcitonin, unless it is coupled to RAMPs, knockout strategies for either RAMPs or CalcR are not useful to determine AMYR signaling mechanisms [58, 102].
In vitro studies, which confer most of our understanding of AMYR signaling, demonstrate that ligand binding to the AMYR activates adenyl cyclase and phospholipase C pathways (G ás and G áq, respectively) [80, 103-105]. Either cascade is known to drive ERK phosphorylation (pERK), which has been established as a key signaling molecule in AMYR action [103, 106, 107]. When comparing-the main AMYR subtypes, AMYR1 and AMYR3 have been reported to have similar binding affinity and dose-dependent response signaling for amylin [108]. However, some studies have shown a more diverse signaling pattern depending on the cascade activated and the cell type studied. For example, both AMYR1 and AMYR3 were shown to increase second messenger cAMP twenty-fold in Cos7 cells at similar doses. However, activation of these two receptor subtypes only led to a three to five-fold increase in intracellular Ca2+ and ERK phosphorylation (pERK) in Cos7 and HEK293 [105]. Moreover, this study also reported that AMYR3 preferentially signaled for through Gq, driving an increase of Ca2+ and pERK, over AMYR1 in Cos7 cells, but not HEK293 cells. Such studies highlight the complex nature of AMYR signaling, even in vitro.
Antagonists for the members of the calcitonin family (i.e., AC413, AC66, CGRP8-37, AC187, AC253) have been widely used to address the action of the AMYR. These antagonists are typically N-truncated isoforms of the native agonists that competitively inhibit receptor activation [109]. The most commonly used antagonist within this family, AC187, is modeled after salmon CT (sCT), where aa 35-37 are homologous to rat amylin. sCT is known to activate AMYR second to CalcR (64); additionally, sCT and amylin share a 30% aa sequence similarity [110]. However, AC187 is missing the disulfide bridge found in amylin that is thought to be the biologically active portion of these peptides within the calcitonin family [111]. AC187 is found to be equally potent as an antagonist of AMYR1a and AMYR3a receptor subtypes, suggesting it cannot discriminate between the two [58, 102, 112]. Together, these data emphasize the need for developing new AMY receptor pharmacology to deepen the mechanistic understanding of this hormone peptide.
3. AMYLIN CENTRAL NERVOUS SYSTEM FUNCTION
3.1. Hindbrain Amylin Function
Canonical amylin signaling within the CNS was first linked to the AP [103, 113, 114], a nucleus within the medulla oblongata, critical in the integration of neural inputs from the peripheral nervous system that allows larger peptides, like amylin, to access the CNS. AMYR activation within the AP reduces feeding behavior via the initiation of meal ending signals [115, 116]. This effect is reversed by antagonist delivery [117, 118]. Amylin also serves as a modulator of energy intake through the regulation of glucagon secretion, processes that are reversed by delivery of the AC187 antagonist, particularly as they pertain to glucagon secretion [119], food consumption [120], and inhibition of gastric emptying largely suggested to be mediated through vagal stimulation from AP outputs [63, 65, 121].
The CalcR and RAMP3 subtype most prominently co-localize within the AP to form the AMYR3 [93, 122]. AMYR3 has been suggested to play a key role in amylin-mediated effects on glucose regulation and satiety signaling, shown by altered glucose sensitization and decreased inter-meal duration in the RAMP3 KO mouse [104]. These findings seem to be restricted to AMYR3, as Zhang and colleagues (2011) showed that over-expression of RAMP1 does not alter feeding behaviors in mice [123]. However, as mentioned before, these results need to be weighed with caution since knockout or over-expression RAMPs may be driving changes beyond amylin signaling.
3.2. Hypothalamic Amylin Function
Amylin effects on long-term energy homeostasis, as well as additional regulation of satiety mechanisms, are also mediated through AMYR signaling in the hypothalamus [124]. A transporter-mediated mechanism enables amylin crossing across the BBB [96, 125] and allows amylin effects in brain regions independently of AP mechanisms.
A key area in long-term energy regulation and body weight is the VMH. These aspects are primarily regulated via peripheral adiposity input signals. Similarly, amylin shows positive effects on overall energy balance and increases energy expenditure [120, 126, 127]. In this regard, amylin is proposed to regulate energy expenditure by increasing brown adipose tissue activity mediated through the sympathetic nervous system. Mediation of sympathetic output in combination with amylin ability to reduce meal size is suggested to be the key component of amylin signaling ability to reduce adiposity [127]. This effect is blocked by AMYR antagonist administration, which markedly increases body adiposity [120]. Such changes are also seen in a human RAMP1 overexpression mouse model [128]. Specifically, Coester et al. (2020) reported that RAMP1 over-expressor male mice showed increased fat mass deposits, despite displaying similar body weights to controls. Female RAMP1 KO did not show changes in fat mass; however, they showed altered plasma leptin levels compared to controls. This work suggests that AMYR1 may be important for these amylin mechanisms [129].
In fact, it has been hypothesized that functional amylin and leptin signaling are both required for these actions, suggesting a synergistic relationship. To this end, leptin sensitivity is lost during the obese and diabetic states, leading to the loss of satiation and accumulation of fat mass over time; however, amylin administration in combination with leptin in obese mice shows additive results on fat-specific weight loss over amylin or leptin therapy alone [72, 114, 130]. Furthermore, AMYR knockdown within the VMH results in reduced pSTAT3 signaling, whereas amylin gene knockdown mice have significantly less LepR mRNA within the VMH, as well as overall leptin insensitivity [71, 72], collectively elucidating important amylin sensitizing effect on leptin.
Another important downstream target of leptin and amylin activation within the hypothalamus is proopiomelanocortin (POMC), a precursor protein important for satiety and body weight management that counteracts the orexigenic actions of agouti related peptide/ neuropeptide Y (AgRP/NPY) neurons [124, 131, 132]. The activation of POMC neurons within the ARC, causes POMC to be converted to one of several end products, including melanocyte-stimulating hormones (MSHs), corticotrophin (ACTH), and endorphins (i.e., β-endorphin). MSH subtype alpha ( áMSH) activates the melanocortin receptor subtype 4 (MC4R) receptors to mediate satiety [133, 134]. A functional MC4R circuit has been suggested for amylin actions on satiety as MC4RK314X/K314X mice, a receptor loss-of-function model, showed a loss of responsiveness on feeding behaviors upon AMYR agonist therapy compared to WT mice [135]. More specifically, both LepR and AMYR have been suggested to trigger JAK/STAT3 or ERK signaling cascades within POMC neurons, respectively, to drive such effects [71, 124].
Altogether this body of work highlights the amylin actions in mediating metabolic homeostasis processes. Importantly, however, amylin’s actions extend beyond metabolic regulation to other processes that are relevant to aging and AD development, discussed in the section below.
4. AMYLIN LEVELS AND ALZHEIMER’S DISEASE
The current literature on amylin signaling is conflicting regarding its role in AD pathogenesis. On one end, amylin has been hypothesized to drive AD pathogenesis. As the name suggests, amylin is an amyloid peptide that aggregates in vivo during pathological states, thus, it is not surprising that its discovery was in aggregates in the pancreas of T2DM patients as well as diabetic cats [61, 136, 137]. Interestingly, amylin oligomers and plaques have been reported to be present in temporal lobes and within arteriole walls of both diabetic and non-diabetic patients with AD, as well as cognitively unimpaired patients. These aggregates have been reported to be mixed amylin- A β plaques or amylin-only plaques [138-140]. Amylin fibrils, like A β, are toxic to β-islet cells in late stage T2DM, as well as neurons in vitro [141-143]. Recent work suggests that amylin may be serving as a seeding mechanism for amyloid beta in diabetic patients, thus further linking T2DM to AD and supporting the traditional view of amyloid aggregation as a ‘gain of toxic function’ mechanism in disease etiology [138, 144].
The above evidence, however, is contested by studies showing negative correlations between amylin levels and disease pathogenesis. For example, Adler et al. (2014) showed that plasma amylin levels were negatively correlated with cognitive impairment [145]. Both mild cognitive impairment (MCI) and AD patients showed significantly lower levels of circulating amylin than age-matched control subjects. These findings were confirmed by others [146] (after adjusting for APOE4 allele, diabetes, stroke, kidney function and lipid profile [147]. An additional study from Zhu and colleagues [148] found that plasma amylin levels and AD risk might fall on an inverted U-shaped curve, where lower and extremely high plasma amylin concentrations were associated with increased AD risk, whereas high plasma amylin did not show this relationship. Interestingly, this study also reported that plasma amylin concentrations shared a positive correlation with temporal gray matter volume [149]. Collectively, this work suggests that, at least at the level of circulating amylin, the relationship is one that is beneficial, not pathogenetic, thus supporting a “loss of native function” mechanism of pathogenesis in conditions such as T2DM, as well as AD.
4.1. Amylin Receptor Modulation in AD Models: Cognition
Several animal studies using native amylin preparations or non-aggregating forms of amylin (pramlintide acetate) [150], which shows similar pharmacokinetics and pharmacodynamics as human amylin [108], support these conclusions. Of note, pramlintide acetate therapy has shown beneficial outcomes in T2DM patients receiving insulin therapy, such as improving glycemic index, increasing weight loss in obese patients, and improving cognitive decline [151]. Of importance, and in addition, pramlintide lowers postprandial glucagon in T2DM patients [152] without inducing hypoglycemia [153]. These benefits also extend to cognition and mitigating AD-related pathogenesis.
Adler et al. (2014) found that a five-week chronic infusion of pramlintide in SAMP8 mice (a model of accelerated aging) improved novel object recognition, a hippocampal formation dependent memory test. These benefits were linked to increased expression of antioxidant enzymes and synaptic markers, including synapsin I and CDK5 [145]. This group extended this work to an APP/PS1 AD mouse model [154], where they showed that pramlintide therapy rescued hippocampal spatial memory deficits [155]. Additional studies have confirmed these findings in other mouse AD-mouse models. Both human amylin and pramlintide, in 5XFAD and Tg2576 mice, improved Y maze and MWM performance [156], an aspect that was demonstrated to be AMYR dependent [157].
As initially discussed, however, whether amylin receptor activation is beneficial or detrimental in AD is controversial. This is evidenced by data demonstrating that blocking receptor activation, thus inhibiting amylin function, is beneficial in AD pathogenesis. AC253 or cyclic AC253(cAC253) antagonists reportedly improved T-maze and MWM in 8 mos. TgCRND8 AD mice [56, 158]. AC253 and cAC253 were suggested to improve memory deficits through increased synaptic markers, synapsin I and synaptophysin [56, 158]. Kimura and colleagues (2016) demonstrated that high doses of (50nM) human amylin induce long-term depression (LTD) in CA1 hippocampal slices of older TgCRND8 mice, suggesting that amylin receptor antagonism would result in benefits [159]. However, it is important to note that the high dose and advanced stage of pathology in these mice could have confounded the resulting conclusions. Interestingly, the same authors reported that pramlintide application to CA1 in hippocampal slices induced long-term potentiation (LTP), important for memory formation, compared to controls, and suggested that pramlintide served an antagonist function, an aspect that is not well supported, at least in vitro [108].
Genetic manipulation of amylin receptor components in AD models suggests that amylin receptor activation may be detrimental in AD models. For example, a recent study from Patel et al. (2020) [160] demonstrated that depletion of amylin function via a 50% hemizygous CalcR knockdown in a TgCRND8 or 5XFAD rescued LTD and cognitive deficits in an MWM task observed in this mouse. However, it is important to note that the knockdown of the CalcR is not specific to amylin action.
4.2. Amylin Receptor Modulation in AD Models: AD Pathology
Amylin and A β, both being amyloids, possess similar secondary beta-pleated sheet structures and are both degraded by insulin-degrading enzyme (IDE) [143, 161]; because of this, it has been suggested that A β can bind to and signal through AMYR, specifically, AMYR3 [143, 162]. A β binding to AMYR has been proposed to be detrimental by 1) toxic intracellular signaling and 2) inhibiting amylin binding to its cognate receptor and driving accumulation of amylin extracellularly [162, 163]. Furthermore, Mousa et al. (2020) proposed that amylin and pramlintide alter ã-secretase subunits, increasing their translocation to lipid rafts and increasing total A β in TgSwDI mice [55]. However, it is important to note that while in vitro and ex-vivo work suggest benefits of AMYR blockade on amyloid-related parameters, in vivo studies using amylin receptor antagonists [56, 158] reported the lack of changes in A β burden nor APP processing.
Contrary to the above studies, others have reported that amylin and pramlintide administration reduce A β plaque burden, again supporting a neuroprotective action of amylin within the CNS. Patrick et al. (2019) saw that chronic pramlintide reduced plaque burden and formic acid-soluble fraction (fibrillar A β) A β1-40 and A β1-42 compared to APP/PS1 saline controls [155]. The same study showed that hippocampal and cortical ADAM10 protein expression was increased in pramlintide-treated mice compared to saline controls, concluding that mediation of alpha secretase could be a potential mechanism of action of pramlintide to reduce amyloidosis [155]. In parallel, Zhu et al. (2015) saw that both human amylin and pramlintide decreased A β plaque size and burden, and suggested decreased BACE1 activity as a mechanism due to findings that amylin treated Tg2576 mice had reduced CTF β cleavage products, confirmed through decreased BACE1 activity [156]. Overall alteration of APP-processing enzymes may speak to amylin modulation of APP enzyme trafficking or availability, a theory that remains to be carefully tested.
Amylin administration has also been suggested to regulate A β clearance from the brain [156]. To this end, a single injection of either human amylin or pramlintide via IP or ICV leads to increased serum A β1-40 and A β 1-42 24 hours later in both mouse models, suggesting changes in A β efflux by amylin [156]. This mechanism of A β clearance has been proposed to be via AMYR activation within cerebral arteries, causing vasodilation, increasing cerebral blood flow, thus increasing A β efflux from the brain [164, 165].
Recent reports also suggest the ability of amylin to regulate tau pathology. For example, amylin has been shown to interact, via co-localization, with MAP2 and tau in hippocampal cells of individuals with AD, implicating negative protein-protein alterations that promote tau aggregation [166]. Conversely, Zhu et al. (2017) reported that amylin significantly decreased phosphorylated Ser396/Ser 404 Tau (PHF-1) and p25 in 3XFAD mice, compared to controls, thus reducing pathology. Additionally, this study also found that AC253 blockade of AMYR blocked these effects [157]. However, the relationship between amylin and tau and the impact of amylin receptor agonism or antagonism on tau pathology remains fully explored. Importantly, how such regulation or even which receptor subtype mediates A β and tau protein burden or clearance remains unknown.
4.3. Amylin Receptor Modulation in AD Models: Oxidative Stress & Inflammation
As mentioned previously, oxidative stress and inflammation commonly seen in T2DM and AD are detrimental to neuronal function, contributing to cellular damage and synaptic dysfunction [167, 168]. In vitro data also supports a potential antioxidant function for amylin [155]. Therefore, a mechanism of action of amylin/pramlintide associated with cognitive benefits in AD could also involve such a mechanism. To this end, within the CNS, pramlintide treated SAMP8 [145] and APP/PS1 mice [155] showed a profound impact on stress-related enzymes including hemoxigenase-1 (HO1) and glutathione (GPx) and manganese superoxide dismutase (MnSOD) in vivo and in vitro.
A well-known source of oxidative stress stems from inflammatory processes. In connection with this, Fu et al. (2017) demonstrated that AMYR activation may be involved in microglial activation. Both CalcR and RAMP3 were shown to be expressed in human fetal microglia, and their activation results in increased intracellular Ca2+, an action associated with microglial activation. This effect was diminished by AMYR antagonism using cAC253. Nevertheless, like the findings for cognition and pathology, the above findings have also been contradicted. To this end, Wang et al. (2015) demonstrated that amylin attenuated LPS induction of CD68, a proinflammatory marker, in a microglia BV2 cell line. Additionally, knockdown of RAMP3, via siRNA, abolished amylin-mediated inhibition of CD68, suggesting that AMYR3 may be responsible for this relationship. In vivo work in the 5XFAD mouse showed lower expression of ionized calcium-binding adaptor molecule 1 (IBA-1) and diminished microglial activation. Importantly, co-administration of AC253 + amylin blocked this reduction of neuroinflammation, suggesting that AMYR activation may mediate anti-inflammatory pathways [157]. Together, while the data are conflicting, the presence of amylin AMYR in microglia suggests a direct inflammation regulatory role for this peptide that should be further explored.
CONCLUSION
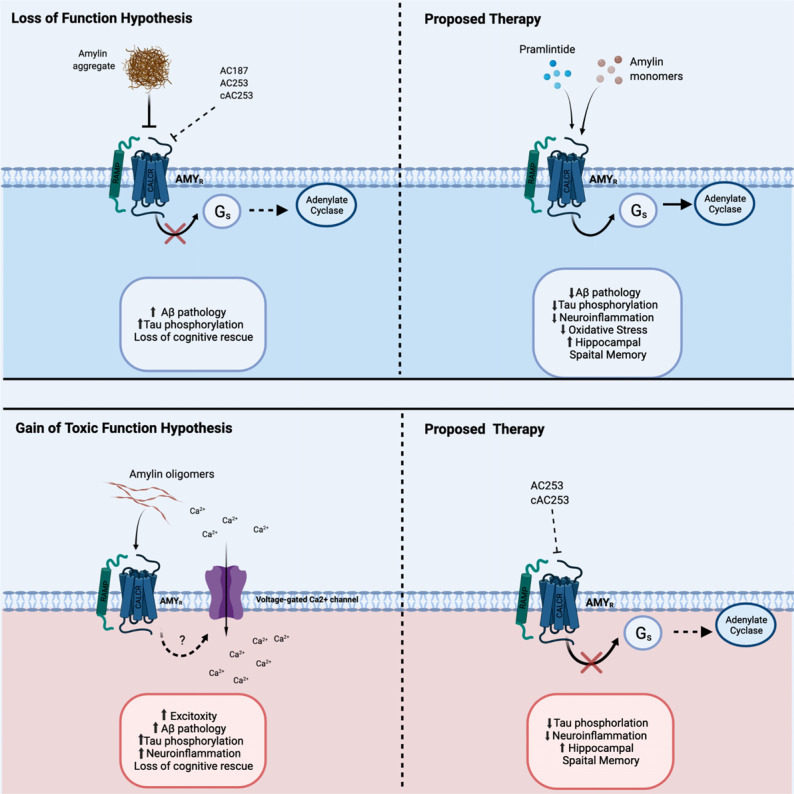
The work described above supports a much more complex role for the peptide amylin than previously thought. Functions for this peptide expand beyond the traditional peripheral metabolic regulatory roles to include several CNS functions, such as long-term energy balance, reward functions, and, reviewed in detail here, cognitive functions and cellular endpoints associated with AD development (Table 1). The most attention-grabbing aspect of amylin research in AD is the directly conflicting reports that amylin agonism and antagonism are therapeutically beneficial in the disease. To this end, studies support both a ‘gain-of-toxic-function’ by amylin aggregation, either by providing a seed for Aβ [55, 56], or driving toxicity through its receptor [159, 162], as well as a beneficial effect of amylin or analog therapy [155-157, 164]. The latter supports the hypothesis that aggregation-mediated depletion of free amylin may lead to a “loss of native function”, an aspect that is supported by negative correlations between free amylin and AD in human studies [149, 155] (Fig. 1).
Table 1.
A condensed list of studies that evaluated amylin impact on cognition, amyloid and tau pathology, OS, inflammation, and signaling that utilized AD rodent and cellular models discussed within this review.
| Amylin Findings in Alzheimer’s Disease Literature | |||
|---|---|---|---|
| Study | Model | Treatment | Findings |
| Lim et al., 2010 | SHSY5Y | Fibular hAMY or A β | Increased cytotoxicity LDH Reduced mitochondrial oxygen consumption |
| Adler et al., 2014 | Human SAMP8 mice |
---------- PRAM |
MCI and AD patients have significantly decreased plasma amylin compared to normal aging individuals PRAM increased Novel Object Recognition task PRAM increased synaptic markers PRAM increased Cdk5 |
| Qui et al., 2014 | Human | ---------- | Amylin plasma positively correlated with verbal memory and vasoconstriction tasks |
| Zhu et al., 2015 | 5XFAD & Tg2576 Human |
hAMY or PRAM AD & Cognitively unimpaired |
Reduced A β plaque size Increased CSF A β1-42 Improved Y maze & MWM performance Tg2576 + hAMY only- decreased CTF β Plasma amylin positively correlates with A β1-40 and A β1-42 |
| Soudy et al., 2016 | TgCRND8 HEK293 |
AC253 AC253, cAC253 |
AC253 improved T Maze and MWM performance No differences in A β pathology AC253 increased synaptic markers AC253 decreased microglia activation Blocked hAMY-induced cAMP (dose dependent manner) |
| Kimura et al., 2016 | TgCRND8 | Fibular hAMY or A β PRAM | Depressed hippocampal LTP Increased hippocampal LTP |
| Verma et al., 2016 | Humans (T2DM + AD) HIP rats C57BL6/J |
--------- --------- Daily hAMY Daily hAMY |
Neuronal amylin aggregates + decreased membrane integrity Neuronal amylin aggregates + decreased membrane integrity Neuronal amylin aggregates + decreased membrane integrity |
| Tao et al., 2018 | Healthy elderly | PRAM | Single PRAM dose increased A β1-42 efflux |
| Wang et al., 2017 | BV2 (microglia line) 5XFAD |
Amylin Amylin |
Amylin reduced LPS-induced Iba-1 & CD68 phagocytic microglial marker Amylin reduced amyloid and tau pathology Amylin “corrected” mitochondrial gene expression (microarray) |
| Zhu et al., 2017 | 5XFAD 3xTgAD |
hAMY or hAMY + AC253 hAMY or hAMY + AC253 |
hAMY reduced A β pathology; AC253 attenuated these results reduced tauopathy hAMY decreased p25 both models- hAMY reduced microglia activation, AC253 attenuated hAMY improved Y maze and MWM |
| Fu et al., 2017 | 5XFAD Human fetal microglia |
cAC253, hAMY, AB cAC253, hAMY, AB |
cAC253 reduced A β pathology hAMY & A β increased inflammation AC253 inhibited oligomeric hAMY and AB induced Ca+2 influx |
| Zhu et al., 2019 | Human | ---------- | Plasma amylin correlated with brain volume Plasma amylin concentration was correlated with AD-incidence on a U-shaped curve |
| Patrick et al., 2019 | APP/PS1 SHSY5Y |
PRAM PRAM |
PRAM improved MWM PRAM reduced A β pathology PRAM increased OS enzymes PRAM increased ADAM10 (CTX & HIPP), increased BACE1 (HIPP) Reduced OS-induced toxicity |
| Patel et al., 2020 | 5XFAD TgCRND8 |
50% hemizygous CalcR KD | (both strains) Reduced A β pathology Improved MWM task |
| Mousa et al., 2020 | TgSwDI | Amylin, PRAM | Amylin and PRAM alter y-secretase subunits, increasing transportation to lipid rafts |
Fig. (1).
The proposed signaling relationships between amylin, pramlintide (PRAM), and AMYR in AD as discussed throughout this review of the current literature. Top Left: Amylin aggregates (also mimicked by AMYR antagonist) may serve as a “loss of function hypothesis” of normal AMYR downstream signaling during Alzheimer’s disease (AD) or metabolic dysregulation by blocking the receptor. It is proposed that due to this loss of amylin, there will be toxic consequences such as increased A β pathology, Tau phosphorylation and disruption of cognitive processes. Top Right: Proposed therapeutic approach signaling of amylin, PRAM and amylin as monomers activating AMYR in the brain leads to downstream adenylate cyclase activation to increase ERK signaling that leads to increased neuroprotective effects. Bottom Left: “Gain of Toxic Function Hypothesis”, higher concentrations or amylin oligomers/fibrils activating AMYR may cause the activation of Voltage-gated Ca2+ ion channels, to open leading to an excitotoxicity state due to chronic intracellular Ca2+ in state of disease or pathology, such as AD or Type II Diabetes. Bottom Right: Proposed therapy for this rationale is to block AMYR with antagonists that inhibit potential toxic downstream signaling. Created with BioRender.com.
The key to resolving some of these conflicts could lie in delving deeper into the complex amylin signaling pharmacology. This is particularly critical, given that amylin does not have its own receptor but rather signals through two subtypes of the calcitonin receptors when coupled to three potential modulating receptor proteins. Additional complexity is added to this already complicated system by the differential expression of these combinations across regions within the CNS, all of which could have slightly different affinities for the ligand. Because of this complex receptor anatomy and the lack of a unique amylin receptor, the use of modern genetic tools to knock out a receptor becomes limited. Under genetic knockdown of calcitonin receptor or RAMPS one inherently disrupts both calcitonin and amylin signaling or any of the receptors that are modulated through RAMP binding, i.e., calcitonin-like receptor and adrenomedullin receptor.
Together, the above highlights a key needed for “traditional” pharmacological studies, including detailed pharmacokinetics and pharmacodynamic studies of the native hormone, analogs, and existing antagonist in relation to each receptor subtype and CNS cell type. It also spawns for the development of novel pharmacology development in this area, one which utilizes modern computational modeling and high throughput techniques to understand how these peptides bind to each receptor subtype and activate or inhibit it. The development of specific antagonist for each receptor subtype based would allow for a much deeper understanding of amylin signaling in vivo.
Lastly, in relation to AD treatment, it will also become critical to determine the effects of such molecules under carefully controlled Aβ levels as well as carefully controlled measurements of aggregation, amyloid-beta processing, and degradation since one can impact the levels of the other. Together, however, the study of this pharmacological paradox is an opportunity to foster a deeper understanding of physiological amyloid functions and novel therapies in disease that is unfortunately devoid of pharmacological interventions beyond those aimed at lowering amyloid-beta.
ACKNOWLEDGEMENTS
R.R.C. wrote the review article and created the figures and table. H.P. edited the article. G.C. wrote and edited the article.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work related to this manuscript was funded by the NIA (R15AG050292-01; R21AG064479-01).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1. Alzheimer’s & Dementia | Alzheimer’s Association. [cited 2021 Mar 26]. Available from: https://www.alz.org/alzheimer_s_dementia.
- 2.James B.D., Leurgans S.E., Hebert L.E., Scherr P.A., Yaffe K., Bennett D.A. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045–1050. doi: 10.1212/WNL.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K., Liu F., Gong C-X., Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010;7(8):656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serpell L.C. Alzheimer’s amyloid fibrils: structure and assembly. Biochimica et Biophysica Acta (BBA) -. Biochim. Biophys. Acta Mol. Basis Dis. 2000;1502(1):16–30. doi: 10.1016/S0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 7.Mandelkow E., Mandelkow E.M. Microtubules and microtubule-associated proteins. Curr. Opin. Cell Biol. 1995;7(1):72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 8.Dumont M., Stack C., Elipenahli C., Jainuddin S., Gerges M., Starkova N.N., Yang L., Starkov A.A., Beal F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J. 2011;25(11):4063–4072. doi: 10.1096/fj.11-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M.K., Sudol K.L., Janelsins M.C., Mastrangelo M.A., Frazer M.E., Bowers W.J. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo L., Hemmelgarn B.T., Chuang C.C., Best T.M. The role of oxidative stress-induced epigenetic alterations in amyloid-β production in Alzheimer’s disease. Oxid. Med. Cell. Longev. 2015;2015:604658. doi: 10.1155/2015/604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H., Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging. 1997;18(4):351–357. doi: 10.1016/S0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 12. FastStats - Leading Causes of Death. [cited 2021 Mar 26]. Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- 13.Bertram L., Tanzi R.E. Genome-wide association studies in Alzheimer’s disease. Hum. Mol. Genet. 2009;18(R2):R137–R145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellinger K.A., Paulus W., Wrocklage C., Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayeux R., Stern Y., Ottman R., Tatemichi T.K., Tang M-X., Maestre G., Ngai C., Tycko B., Ginsberg H. The apolipoprotein ε 4 allele in patients with Alzheimer’s disease. Ann. Neurol. 1993;34(5):752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- 16.O’Meara E.S., Kukull W.A., Sheppard L., Bowen J.D., McCormick W.C., Teri L., Pfanschmidt M., Thompson J.D., Schellenberg G.D., Larson E.B. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am. J. Epidemiol. 1997;146(5):373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 17.Graham N.S.N., Sharp D.J. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry. 2019;90(11):1221–1233. doi: 10.1136/jnnp-2017-317557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon D.W., McGeachy M.J., Baylr H., Clark R.S.B., Loane D.J., Kochanek P.M. Neuroinflammation in the evolution of secondary injury, repair, and chronic neurodegeneration after traumatic brain injury. Nat. Rev. Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin W.J. Stealth Adapted Viruses – Possible Drivers of Major Neuropsychiatric Illnesses Including Alzheimer’s Disease. J Neurol Stroke. 2015;2(3):00057. doi: 10.15406/jnsk.2015.02.00057. [DOI] [Google Scholar]
- 20.Wozniak M.A., Shipley S.J., Combrinck M., Wilcock G.K., Itzhaki R.F. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J. Med. Virol. 2005;75(2):300–306. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- 21.Fulop T., Witkowski J.M., Bourgade K., Khalil A., Zerif E., Larbi A., Hirokawa K., Pawelec G., Bocti C., Lacombe G., Dupuis G., Frost E.H. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front. Aging Neurosci. 2018;10:224. doi: 10.3389/fnagi.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soscia S.J., Kirby J.E., Washicosky K.J., Tucker S.M., Ingelsson M., Hyman B., Burton M.A., Goldstein L.E., Duong S., Tanzi R.E., Moir R.D. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One. 2010;5(3):e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamez P., Caballero A.B. Copper in Alzheimer’s disease: Implications in amyloid aggregation and neurotoxicity. AIP Adv. 2015;5(9):92503. doi: 10.1063/1.4921314. [DOI] [Google Scholar]
- 24.Curtain C.C., Ali F., Volitakis I., Cherny R.A., Norton R.S., Beyreuther K., Barrow C.J., Masters C.L., Bush A.I., Barnham K.J. Alzheimer’s disease amyloid-β binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 2001;276(23):20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- 25.Evans D.A., Hebert L.E., Beckett L.A., Scherr P.A., Albert M.S., Chown M.J., Pilgrim D.M., Taylor J.O. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch. Neurol. 1997;54(11):1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 26.Whitmer R.A., Gunderson E.P., Quesenberry C.P., Jr, Zhou J., Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr. Alzheimer Res. 2007;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 27.Whitmer R.A., Gunderson E.P., Barrett-Connor E., Quesenberry C.P., Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibson C.L., Rocca W.A., Hanson V.A., Cha R., Kokmen E., O’Brien P.C., Palumbo P.J. The risk of dementia among persons with diabetes mellitus: A population-based cohort study. Ann. N. Y. Acad. Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Song D., Leng S.X. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin. Interv. Aging. 2015;10:549–560. doi: 10.2147/CIA.S74042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C-C., Chung C-M., Leu H-B., Lin L-Y., Chiu C-C., Hsu C-Y., Chiang C.H., Huang P.H., Chen T.J., Lin S.J., Chen J.W., Chan W.L. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS One. 2014;9(1):e87095. doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvanitakis Z., Wilson R.S., Bienias J.L., Evans D.A., Bennett D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 32.Yassine H.N., Solomon V., Thakral A., Sheikh-Bahaei N., Chui H.C., Braskie M.N., Schneider L.S., Talbot K. Brain energy failure in dementia syndromes: Opportunities and challenges for glucagon-like peptide-1 receptor agonists. Alzheimers Dement. 2021 doi: 10.1002/alz.12474. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Więckowska-Gacek A., Mietelska-Porowska A., Wydrych M., Wojda U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021;70(Nov):101397. doi: 10.1016/j.arr.2021.101397. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen T.T., Ta Q.T.H., Nguyen T.K.O., Nguyen T.T.D., Giau V.V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020;21(9):E3165. doi: 10.3390/ijms21093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes-Canteli M., Iadecola C. Alzheimer’s disease and vascular aging: JACC focus seminar. J. Am. Coll. Cardiol. 2020;75(8):942–951. doi: 10.1016/j.jacc.2019.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poor S.R., Ettcheto M., Cano A., Sanchez-Lopez E., Manzine P.R., Olloquequi J., Camins A., Javan M. Metformin a potential pharmacological strategy in late onset Alzheimer’s disease treatment. Pharmaceuticals (Basel) 2021;14(9):890. doi: 10.3390/ph14090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grizzanti J., Lee H.G., Camins A., Pallas M., Casadesus G. The therapeutic potential of metabolic hormones in the treatment of age-related cognitive decline and Alzheimer’s disease. Nutr. Res. 2016;36(12):1305–1315. doi: 10.1016/j.nutres.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneto H., Katakami N., Matsuhisa M., Matsuoka T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Back S.H., Kaufman R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz R.S. Exercise training in treatment of diabetes mellitus in elderly patients. Diabetes Care. 1990;13(Suppl. 2):77–85. doi: 10.2337/diacare.13.2.S77. [DOI] [Google Scholar]
- 43.Moran C., Beare R., Phan T.G., Bruce D.G., Callisaya M.L., Srikanth V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85(13):1123–1130. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez-González R., Alvira-Botero M.X., Robayo O., Antequera D., Garzón M., Martín-Moreno A.M., Brera B., de Ceballos M.L., Carro E. Leptin gene therapy attenuates neuronal damages evoked by amyloid-β and rescues memory deficits in APP/PS1 mice. Gene Ther. 2014;21(3):298–308. doi: 10.1038/gt.2013.85. [DOI] [PubMed] [Google Scholar]
- 45.Wennberg A.M.V., Spira A.P., Pettigrew C., Soldan A., Zipunnikov V., Rebok G.W., Roses A.D., Lutz M.W., Miller M.M., Thambisetty M., Albert M.S. Blood glucose levels and cortical thinning in cognitively normal, middle-aged adults. J. Neurol. Sci. 2016;365:89–95. doi: 10.1016/j.jns.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dash S.K. Cognitive impairment and diabetes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013;7(2):155–165. doi: 10.2174/1872214811307020009. [DOI] [PubMed] [Google Scholar]
- 47.Biessels G.J., Strachan M.W.J., Visseren F.L.J., Kappelle L.J., Whitmer R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 48.Kelley D.E., He J., Menshikova E.V., Ritov V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 49.Mootha V.K., Lindgren C.M., Eriksson K-F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M.J., Patterson N., Mesirov J.P., Golub T.R., Tamayo P., Spiegelman B., Lander E.S., Hirschhorn J.N., Altshuler D., Groop L.C. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 50.Patti M.E., Butte A.J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., Landaker E.J., Goldfine A.B., Mun E., DeFronzo R., Finlayson J., Kahn C.R., Mandarino L.J. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wieser V., Moschen A.R., Tilg H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Ther. Exp. (Warsz.) 2013;61(2):119–125. doi: 10.1007/s00005-012-0210-1. [DOI] [PubMed] [Google Scholar]
- 52.Burgos-Morón E., Abad-Jiménez Z., Marañón A.M., Iannantuoni F., Escribano-López I., López-Domènech S., Salom C., Jover A., Mora V., Roldan I., Solá E., Rocha M., Víctor V.M. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019;8(9):1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasnain M., Vieweg W.V.R., Hollett B. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: a review for primary care physicians. Postgrad. Med. 2012;124(4):154–167. doi: 10.3810/pgm.2012.07.2577. [DOI] [PubMed] [Google Scholar]
- 54.Eizirik D.L., Miani M., Cardozo A.K. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56(2):234–241. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- 55.Mousa Y.M., Abdallah I.M., Hwang M., Martin D.R., Kaddoumi A. Amylin and pramlintide modulate γ-secretase level and APP processing in lipid rafts. Sci. Rep. 2020;10(1):3751. doi: 10.1038/s41598-020-60664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soudy R., Kimura R., Patel A., Fu W., Kaur K., Westaway D., Yang J., Jhamandas J. Short amylin receptor antagonist peptides improve memory deficits in Alzheimer’s disease mouse model. Sci. Rep. 2019;9(1):10942. doi: 10.1038/s41598-019-47255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archbold J.K., Flanagan J.U., Watkins H.A., Gingell J.J., Hay D.L. Structural insights into RAMP modification of secretin family G protein-coupled receptors: implications for drug development. Trends Pharmacol. Sci. 2011;32(10):591–600. doi: 10.1016/j.tips.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Hay D.L., Christopoulos G., Christopoulos A., Sexton P.M. Amylin receptors: molecular composition and pharmacology. Biochem. Soc. Trans. 2004;32(Pt 5):865–867. doi: 10.1042/BST0320865. [DOI] [PubMed] [Google Scholar]
- 59.Sexton P.M., Paxinos G., Kenney M.A., Wookey P.J., Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. . Neuroscience. 1994;62(2):553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 60.Naot D., Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43(5):813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Commun. 1986;140(3):827–831. doi: 10.1016/0006-291X(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 62.Sanke T., Hanabusa T., Nakano Y., Oki C., Okai K., Nishimura S. Plasma islet amyloid polypeptide (Amylin) levels and their responses to oral glucose in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):129–132. doi: 10.1007/BF00500385. [DOI] [PubMed] [Google Scholar]
- 63.Young A., Pittner R., Gedulin B., Vine W., Rink T. Amylin regulation of carbohydrate metabolism. Biochem. Soc. Trans. 1995;23(2):325–331. doi: 10.1042/bst0230325. [DOI] [PubMed] [Google Scholar]
- 64.Young A. Inhibition of food intake. Adv. Pharmacol. 2005;52:79–98. doi: 10.1016/S1054-3589(05)52005-2. [DOI] [PubMed] [Google Scholar]
- 65.Gedulin B.R., Rink T.J., Young A.A. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46(1):67–70. doi: 10.1016/S0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 66.Reidelberger R.D., Kelsey L., Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282(5):R1395–R1404. doi: 10.1152/ajpregu.00597.2001. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z-L., Bennet W.M., Ghatei M.A., Byfield P.G.H., Smith D.M., Bloom S.R. Influence of islet amyloid polypeptide and the 8-37 fragment of islet amyloid polypeptide on insulin release from perifused rat islets. Diabetes. 1993;42(2):330–335. doi: 10.2337/diab.42.2.330. [DOI] [PubMed] [Google Scholar]
- 68.Chance W.T., Balasubramaniam A., Zhang F.S., Wimalawansa S.J., Fischer J.E. Anorexia following the intrahypothalamic administration of amylin. Brain Res. 1991;539(2):352–354. doi: 10.1016/0006-8993(91)91644-G. [DOI] [PubMed] [Google Scholar]
- 69.Mesaros A., Koralov S.B., Rother E., Wunderlich F.T., Ernst M.B., Barsh G.S., Rajewsky K., Brüning J.C. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7(3):236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Turek V.F., Trevaskis J.L., Levin B.E., Dunn-Meynell A.A., Irani B., Gu G., Wittmer C., Griffin P.S., Vu C., Parkes D.G., Roth J.D. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151(1):143–152. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 71.Olsson M., Herrington M.K., Reidelberger R.D., Permert J., Arnelo U. Comparison of the effects of chronic central administration and chronic peripheral administration of islet amyloid polypeptide on food intake and meal pattern in the rat. Peptides. 2007;28(7):1416–1423. doi: 10.1016/j.peptides.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Mollet A., Meier S., Riediger T., Lutz T.A. Histamine H1 receptors in the ventromedial hypothalamus mediate the anorectic action of the pancreatic hormone amylin. Peptides. 2003;24(1):155–158. doi: 10.1016/S0196-9781(02)00288-7. [DOI] [PubMed] [Google Scholar]
- 73.Gebre-Medhin S., Mulder H., Pekny M., Westermark G., Törnell J., Westermark P., Sundler F., Ahrén B., Betsholtz C. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin). Biochem. Biophys. Res. Commun. 1998;250(2):271–277. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- 74.Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J., Main M.J., Foord S.M., Sexton P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56(1):235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 75.Barwell J., Wootten D., Simms J., Hay D.L., Poyner D.R. RAMPs and CGRP receptors. Adv. Exp. Med. Biol. 2012;744:13–24. doi: 10.1007/978-1-4614-2364-5_2. [DOI] [PubMed] [Google Scholar]
- 76.Muff R., Bühlmann N., Fischer J.A., Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140(6):2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 77.Leuthäuser K., Gujer R., Aldecoa A., McKinney R.A., Muff R., Fischer J.A., Born W. Receptor-activity-modifying protein 1 forms heterodimers with two G-protein-coupled receptors to define ligand recognition. Biochem. J. 2000;351(Pt 2):347–351. [PMC free article] [PubMed] [Google Scholar]
- 78.Hay D.L., Christopoulos G., Christopoulos A., Sexton P.M. Determinants of BIBN4096BS affinity for CGRP and amylin receptors; the role of RAMP1. Mol. Pharmacol. Fast Forward; 2006. [DOI] [PubMed] [Google Scholar]
- 79.Beaumont K., Kenney M.A., Young A.A., Rink T.J. High affinity amylin binding sites in rat brain. Mol. Pharmacol. 1993;44(3):493–497. [PubMed] [Google Scholar]
- 80.Hilton J.M., Chai S.Y., Sexton P.M. In vitro autoradiographic localization of the calcitonin receptor isoforms, C1a and C1b, in rat brain. . Neuroscience. 1995;69(4):1223–1237. doi: 10.1016/0306-4522(95)00322-A. [DOI] [PubMed] [Google Scholar]
- 81.Poyner D.R., Sexton P.M., Marshall I., Smith D.M., Quirion R., Born W., Muff R., Fischer J.A., Foord S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54(2):233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 82.Husmann K., Sexton P.M., Fischer J.A., Born W. Mouse receptor-activity-modifying proteins 1, -2 and -3: amino acid sequence, expression and function. Mol. Cell. Endocrinol. 2000;162(1-2):35–43. doi: 10.1016/S0303-7207(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 83.Christopoulos G., Paxinos G., Huang X.F., Beaumont K., Toga A.W., Sexton P.M. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can. J. Physiol. Pharmacol. 1995;73(7):1037–1041. doi: 10.1139/y95-146. [DOI] [PubMed] [Google Scholar]
- 84.Flahaut M., Rossier B.C., Firsov D. Respective roles of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMP) in cell surface expression of CRLR/RAMP heterodimeric receptors. J. Biol. Chem. 2002;277(17):14731–14737. doi: 10.1074/jbc.M112084200. [DOI] [PubMed] [Google Scholar]
- 85.Bhogal R., Smith D.M., Bloom S.R. Investigation and characterization of binding sites for islet amyloid polypeptide in rat membranes. Endocrinology. 1992;130(2):906–913. doi: 10.1210/endo.130.2.1310282. [DOI] [PubMed] [Google Scholar]
- 86.Olgiati V.R., Guidobono F., Netti C., Pecile A. Localization of calcitonin binding sites in rat central nervous system: evidence of its neuroactivity. Brain Res. 1983;265(2):209–215. doi: 10.1016/0006-8993(83)90334-7. [DOI] [PubMed] [Google Scholar]
- 87.Nakamoto H., Suzuki N., Roy S.K. Constitutive expression of a small heat-shock protein confers cellular thermotolerance and thermal protection to the photosynthetic apparatus in cyanobacteria. FEBS Lett. 2000;483(2-3):169–174. doi: 10.1016/S0014-5793(00)02097-4. [DOI] [PubMed] [Google Scholar]
- 88.Ueda T., Ugawa S., Saishin Y., Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res. Mol. Brain Res. 2001;93(1):36–45. doi: 10.1016/S0169-328X(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 89.Stachniak T.J.E., Krukoff T.L. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. J. Neuroendocrinol. 2003;15(9):840–850. doi: 10.1046/j.1365-2826.2003.01064.x. [DOI] [PubMed] [Google Scholar]
- 90.Barth S.W., Riediger T., Lutz T.A., Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004;997(1):97–102. doi: 10.1016/j.brainres.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 91.Becskei C., Riediger T., Zünd D., Wookey P., Lutz T.A. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030(2):221–233. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 92.Banks W.A., Kastin A.J., Maness L.M., Huang W., Jaspan J.B. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57(22):1993–2001. doi: 10.1016/0024-3205(95)02197-Q. [DOI] [PubMed] [Google Scholar]
- 93.Le Foll C., Johnson M.D., Dunn-Meynell A.A., Boyle C.N., Lutz T.A., Levin B.E. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes. 2015;64(5):1621–1631. doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mietlicki-Baase E.G., Rupprecht L.E., Olivos D.R., Zimmer D.J., Alter M.D., Pierce R.C., Schmidt H.D., Hayes M.R. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013;38(9):1685–1697. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bower R.L., Hay D.L. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br. J. Pharmacol. 2016;173(12):1883–1898. doi: 10.1111/bph.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christopoulos A., Christopoulos G., Morfis M., Udawela M., Laburthe M., Couvineau A., Kuwasako K., Tilakaratne N., Sexton P.M. Novel receptor partners and function of receptor activity-modifying proteins. J. Biol. Chem. 2003;278(5):3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- 97.Bailey R.J., Bradley J.W.I., Poyner D.R., Rathbone D.L., Hay D.L. Functional characterization of two human receptor activity-modifying protein 3 variants. Peptides. 2010;31(4):579–584. doi: 10.1016/j.peptides.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Potes C.S., Boyle C.N., Wookey P.J., Riediger T., Lutz T.A. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin’s eating inhibitory effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(3):R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- 99.Coester B., Pence S.W., Arrigoni S., Boyle C.N., Le Foll C., Lutz T.A. RAMP1 and RAMP3 differentially control amylin’s effects on food intake, glucose and energy balance in male and female mice. Neuroscience. 2020;447:74–93. doi: 10.1016/j.neuroscience.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 100.Morfis M., Tilakaratne N., Furness S.G.B., Christopoulos G., Werry T.D., Christopoulos A., Sexton P.M. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149(11):5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- 101.Dacquin R., Davey R.A., Laplace C., Levasseur R., Morris H.A., Goldring S.R., Gebre-Medhin S., Galson D.L., Zajac J.D., Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo . . J. Cell Biol. 2004;164(4):509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qi R., Luo Y., Ma B., Nussinov R., Wei G. Conformational distribution and α-helix to β-sheet transition of human amylin fragment dimer. Biomacromolecules. 2014;15(1):122–131. doi: 10.1021/bm401406e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gingell J.J., Burns E.R., Hay D.L. Activity of pramlintide, rat and human amylin but not Aβ1-42 at human amylin receptors. Endocrinology. 2014;155(1):21–26. doi: 10.1210/en.2013-1658. [DOI] [PubMed] [Google Scholar]
- 104.Hay D.L., Garelja M.L., Poyner D.R., Walker C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018;175(1):3–17. doi: 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Amylin: Physiology and Pharmacology - Andrew Young - Google Books. Available from: https://books.google.com/books?id=25NS2UArEoQC&pg=PA47&source=gbs_toc_r&cad=4#v=onepage&q&f=false.
- 106.Banks W.A., Willoughby L.M., Thomas D.R., Morley J.E. Insulin resistance syndrome in the elderly: assessment of functional, biochemical, metabolic, and inflammatory status. Diabetes Care. 2007;30(9):2369–2373. doi: 10.2337/dc07-0649. [DOI] [PubMed] [Google Scholar]
- 107.Bailey R.J., Walker C.S., Ferner A.H., Loomes K.M., Prijic G., Halim A., Whiting L., Phillips A.R., Hay D.L. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br. J. Pharmacol. 2012;166(1):151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lutz T.A. Control of food intake and energy expenditure by amylin-therapeutic implications. Int. J. Obes. 2009;33(S1) Suppl. 1:S24–S27. doi: 10.1038/ijo.2009.13. [DOI] [PubMed] [Google Scholar]
- 109.Trevaskis J.L., Parkes D.G., Roth J.D. Insights into amylin-leptin synergy. Trends Endocrinol. Metab. 2010;21(8):473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 110.Lutz T.A., Tschudy S., Mollet A., Geary N., Scharrer E. Dopamine D(2) receptors mediate amylin’s acute satiety effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(6):R1697–R1703. doi: 10.1152/ajpregu.2001.280.6.R1697. [DOI] [PubMed] [Google Scholar]
- 111.Roth J.D. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20(1):8–13. doi: 10.1097/MED.0b013e32835b896f. [DOI] [PubMed] [Google Scholar]
- 112.Geary N. A new way of looking at eating. Am. J. Physiol. 2005 doi: 10.1152/ajpregu.00066.2005. [DOI] [Google Scholar]
- 113.Mollet A., Gilg S., Riediger T., Lutz T.A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol. Behav. 2004;81(1):149–155. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 114.Gedulin B.R., Jodka C.M., Herrmann K., Young A.A. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Pept. 2006;137(3):121–127. doi: 10.1016/j.regpep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 115.Rushing P.A., Hagan M.M., Seeley R.J., Lutz T.A., D’Alessio D.A., Air E.L., Woods S.C. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142(11):5035–5038. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- 116.Clementi G., Valerio C., Emmi I., Prato A., Drago F. Behavioral effects of amylin injected intracerebroventricularly in the rat. Peptides. 1996;17(4):589–591. doi: 10.1016/0196-9781(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 117.Liberini C.G., Boyle C.N., Cifani C., Venniro M., Hope B.T., Lutz T.A. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur. J. Neurosci. 2016;43(5):653–661. doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Z., Liu X., Morgan D.A., Kuburas A., Thedens D.R., Russo A.F., Rahmouni K. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011;60(4):1063–1071. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lutz T.A., Coester B., Whiting L., Dunn-Meynell A.A., Boyle C.N., Bouret S.G., Levin B.E., Le Foll C. Amylin Selectively Signals Onto POMC Neurons in the Arcuate Nucleus of the Hypothalamus. Diabetes. 2018;67(5):805–817. doi: 10.2337/db17-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Banks W.A., Farr S.A., Butt W., Kumar V.B., Franko M.W., Morley J.E. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J. Pharmacol. Exp. Ther. 2001;297(3):1113–1121. [PubMed] [Google Scholar]
- 121.Roth J.D., Hughes H., Kendall E., Baron A.D., Anderson C.M. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147(12):5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 122.Mack C., Wilson J., Athanacio J., Reynolds J., Laugero K., Guss S., Vu C., Roth J., Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293(5):R1855–R1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- 123.Riddle M., Frias J., Zhang B., Maier H., Brown C., Lutz K., Kolterman O. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care. 2007;30(11):2794–2799. doi: 10.2337/dc07-0589. [DOI] [PubMed] [Google Scholar]
- 124.Coester B., Koester-Hegmann C., Lutz T.A., Le Foll C. Amylin/Calcitonin Receptor-Mediated Signaling in POMC Neurons Influences Energy Balance and Locomotor Activity in Chow-Fed Male Mice. Diabetes. 2020;69(6):1110–1125. doi: 10.2337/db19-0849. [DOI] [PubMed] [Google Scholar]
- 125.Roth J.D., Trevaskis J.L., Turek V.F., Parkes D.G. “Weighing in” on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res. 2010;1350:86–94. doi: 10.1016/j.brainres.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 126.Ernst M.B., Wunderlich C.M., Hess S., Paehler M., Mesaros A., Koralov S.B., Kleinridders A., Husch A., Münzberg H., Hampel B., Alber J., Kloppenburg P., Brüning J.C., Wunderlich F.T. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J. Neurosci. 2009;29(37):11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schiöth H.B., Chhajlani V., Muceniece R., Klusa V., Wikberg J.E.S. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59(10):797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 128.Adan R.A.H., Gispen W.H. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18(8):1279–1287. doi: 10.1016/S0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 129.Eiden S., Daniel C., Steinbrueck A., Schmidt I., Simon E. Salmon calcitonin - a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J. Physiol. 2002;541(Pt 3):1041–1048. doi: 10.1113/jphysiol.2002.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cooper G.J., Willis A.C., Clark A., Turner R.C., Sim R.B., Reid K.B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA. 1987;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Westermark P., Wernstedt C., O’Brien T.D., Hayden D.W., Johnson K.H. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am. J. Pathol. 1987;127(3):414–417. [PMC free article] [PubMed] [Google Scholar]
- 132.Verma N., Ly H., Liu M., Chen J., Zhu H., Chow M., Hersh L.B., Despa F. Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1β synthesis in brains of Alzheimer’s disease patients with type-2 diabetes and in diabetic HIP rats. J. Alzheimers Dis. 2016;53(1):259–272. doi: 10.3233/JAD-160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ly H., Verma N., Sharma S., Kotiya D., Despa S., Abner E.L., Nelson P.T., Jicha G.A., Wilcock D.M., Goldstein L.B., Guerreiro R., Brás J., Hanson A.J., Craft S., Murray A.J., Biessels G.J., Troakes C., Zetterberg H., Hardy J., Lashley T. Aesg; Despa, F. The association of circulating amylin with β-amyloid in familial Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2021;7(1):e12130. doi: 10.1002/trc2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jackson K., Barisone G.A., Diaz E., Jin L.W., DeCarli C., Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann. Neurol. 2013;74(4):517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lorenzo A., Razzaboni B., Weir G.C., Yankner B.A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 136.Bharadwaj P., Solomon T., Sahoo B.R., Ignasiak K., Gaskin S., Rowles J., Verdile G., Howard M.J., Bond C.S., Ramamoorthy A., Martins R.N., Newsholme P. Amylin and beta amyloid proteins interact to form amorphous heterocomplexes with enhanced toxicity in neuronal cells. Sci. Rep. 2020:10. doi: 10.1038/s41598-020-66602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim Y.A., Ittner L.M., Lim Y.L., Götz J. Human but not rat amylin shares neurotoxic properties with Abeta42 in long-term hippocampal and cortical cultures. FEBS Lett. 2008;582(15):2188–2194. doi: 10.1016/j.febslet.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 138.Lim S., Paterson B.M., Fodero-Tavoletti M.T., O’Keefe G.J., Cappai R., Barnham K.J., Villemagne V.L., Donnelly P.S. A copper radiopharmaceutical for diagnostic imaging of Alzheimer’s disease: a bis(thiosemicarbazonato)copper(II) complex that binds to amyloid-β plaques. Chem. Commun. (Camb.) 2010;46(30):5437–5439. doi: 10.1039/c0cc01175d. [DOI] [PubMed] [Google Scholar]
- 139.Adler B.L., Yarchoan M., Hwang H.M., Louneva N., Blair J.A., Palm R., Smith M.A., Lee H.G., Arnold S.E., Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol. Aging. 2014;35(4):793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 140.Qiu W.Q., Au R., Zhu H., Wallack M., Liebson E., Li H., Rosenzweig J., Mwamburi M., Stern R.A. Positive association between plasma amylin and cognition in a homebound elderly population. J. Alzheimers Dis. 2014;42(2):555–563. doi: 10.3233/JAD-140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiu W.Q., Zhu H. Amylin and its analogs: a friend or foe for the treatment of Alzheimer’s disease? Front. Aging Neurosci. 2014;6(JUL):186. doi: 10.3389/fnagi.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu H., Tao Q., Ang T.F.A., Massaro J., Gan Q., Salim S., Zhu R.Y., Kolachalama V.B., Zhang X., Devine S., Auerbach S.H., DeCarli C., Au R., Qiu W.Q. Association of plasma amylin concentration with Alzheimer disease and brain structure in older adults. JAMA Netw. Open. 2019;2(8):e199826–e199826. doi: 10.1001/jamanetworkopen.2019.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.May P.C., Boggs L.N., Fuson K.S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer’s disease amyloid-β neurotoxicity. J. Neurochem. 1993;61(6):2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- 144.Ryan G.J., Jobe L.J., Martin R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther. 2005;27(10):1500–1512. doi: 10.1016/j.clinthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 145.Nyholm B., Ørskov L., Hove K.Y., Gravholt C.H., Møller N., Alberti K.G., Moyses C., Kolterman O., Schmitz O. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism. 1999;48(7):935–941. doi: 10.1016/S0026-0495(99)90232-9. [DOI] [PubMed] [Google Scholar]
- 146.Riddle M., Pencek R., Charenkavanich S., Lutz K., Wilhelm K., Porter L. Randomized comparison of pramlintide or mealtime insulin added to basal insulin treatment for patients with type 2 diabetes. Diabetes Care. 2009;32(9):1577–1582. doi: 10.2337/dc09-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garcia-Alloza M., Robbins E.M., Zhang-Nunes S.X., Purcell S.M., Betensky R.A., Raju S., Prada C., Greenberg S.M., Bacskai B.J., Frosch M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006;24(3):516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 148.Patrick S., Corrigan R., Grizzanti J., Mey M., Blair J., Pallas M., Camins A., Lee H.G., Casadesus G. Neuroprotective effects of the amylin analog, pramlintide, on Alzheimer’s disease are associated with oxidative stress regulation mechanisms. J. Alzheimers Dis. 2019;69(1):157–168. doi: 10.3233/JAD-180421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu H., Wang X., Wallack M., Li H., Carreras I., Dedeoglu A., Hur J.Y., Zheng H., Li H., Fine R., Mwamburi M., Sun X., Kowall N., Stern R.A., Qiu W.Q. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol. Psychiatry. 2015;20(2):252–262. doi: 10.1038/mp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu H., Xue X., Wang E., Wallack M., Na H., Hooker J.M., Kowall N., Tao Q., Stein T.D., Wolozin B., Qiu W.Q. Amylin receptor ligands reduce the pathological cascade of Alzheimer’s disease. Neuropharmacology. 2017;119:170–181. doi: 10.1016/j.neuropharm.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Soudy R., Patel A., Fu W., Kaur K., MacTavish D., Westaway D., Davey R., Zajac J., Jhamandas J. Cyclic AC253, a novel amylin receptor antagonist, improves cognitive deficits in a mouse model of Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2016;3(1):44–56. doi: 10.1016/j.trci.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kimura R., MacTavish D., Yang J., Westaway D., Jhamandas J.H. Pramlintide antagonizes beta amyloid (Aβ)- and human amylin-induced depression of hippocampal long-term potentiation. Mol. Neurobiol. 2017;54(1):748–754. doi: 10.1007/s12035-016-9684-x. [DOI] [PubMed] [Google Scholar]
- 153.Patel A., Shiritsu S., Tokyo Y., Daigaku R., Fu W., Soudy R. Genetic depletion of amylin/calcitonin receptors improves memory and learning in transgenic Alzheimer’s disease mouse models. Mol. Neurobiol. 2021 doi: 10.21203/rs.3.rs-515476/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bennett R.G., Hamel F.G., Duckworth W.C. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52(9):2315–2320. doi: 10.2337/diabetes.52.9.2315. [DOI] [PubMed] [Google Scholar]
- 155.Fu W., Ruangkittisakul A., MacTavish D., Shi J.Y., Ballanyi K., Jhamandas J.H. Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J. Biol. Chem. 2012;287(22):18820–18830. doi: 10.1074/jbc.M111.331181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Andreetto E., Yan L. -.M.; Caporale, A.; Kapurniotu, A. Dissecting the role of single regions of an IAPP mimic and IAPP in inhibition of Aβ40 amyloid formation and cytotoxicity. ChemBioChem. 2011;12(9):1313–1322. doi: 10.1002/cbic.201100192. [DOI] [PubMed] [Google Scholar]
- 157.Tao Q., Zhu H., Chen X., Stern R.A., Kowall N., Au R., Blusztajn J.K., Qiu W.Q. Pramlintide: The effects of a single drug injection on blood phosphatidylcholine profile for Alzheimer’s disease. J. Alzheimers Dis. 2018;62(2):597–609. doi: 10.3233/JAD-170948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Edvinsson L., Goadsby P.J., Uddman R. Amylin: localization, effects on cerebral arteries and on local cerebral blood flow in the cat. ScientificWorldJournal. 2001;1:168–180. doi: 10.1100/tsw.2001.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martinez-Valbuena I., Valenti-Azcarate R., Amat-Villegas I., Riverol M., Marcilla I., de Andrea C.E., Sánchez-Arias J.A., Del Mar Carmona-Abellan M., Marti G., Erro M.E., Martínez-Vila E., Tuñon M.T., Luquin M.R. Amylin as a potential link between type 2 diabetes and alzheimer disease. Ann. Neurol. 2019;86(4):539–551. doi: 10.1002/ana.25570. [DOI] [PubMed] [Google Scholar]
- 160.Cavallucci V., Ferraina C., D’Amelio M. Key role of mitochondria in Alzheimer’s disease synaptic dysfunction. Curr. Pharm. Des. 2013;19(36):6440–6450. doi: 10.2174/1381612811319360005. [DOI] [PubMed] [Google Scholar]
- 161.Yu Q., Du F., Douglas J.T., Yu H., Yan S.S., Yan S.F. Mitochondrial dysfunction triggers synaptic deficits via activation of p38 MAP kinase signaling in differentiated Alzheimer’s disease trans-mitochondrial cybrid cells. . J. Alzheimers Dis. 2017;59(1):223–239. doi: 10.3233/JAD-170283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., Yan S.S. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller K.E., Sheetz M.P. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117(Pt 13):2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 164.Swerdlow R.H., Khan S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp. Neurol. 2009;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fu W., Patel A., Kimura R., Soudy R., Jhamandas J.H. Amylin receptor: A potential therapeutic target for Alzheimer’s disease. Trends Mol. Med. 2017;23(8):709–720. doi: 10.1016/j.molmed.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 166.Wang X., Wang W., Li L., Perry G., Lee H. -.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohamed L.A., Zhu H., Mousa Y.M., Wang E., Qiu W.Q., Kaddoumi A. Amylin enhances amyloid-β peptide brain to blood efflux across the blood-brain barrier. J. Alzheimers Dis. 2017;56(3):1087–1099. doi: 10.3233/JAD-160800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiu W.Q., Wallack M., Dean M., Liebson E., Mwamburi M., Zhu H. Association between Amylin and Amyloid-β Peptides in Plasma in the Context of Apolipoprotein E4 Allele. PLoS One. 2014;9(2):e88063. doi: 10.1371/journal.pone.0088063. [DOI] [PMC free article] [PubMed] [Google Scholar]