Abstract
Background: Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic infection caused by John Cunningham virus (JCV) reactivation, potentially associated with natalizumab (NTZ) treatment for Multiple Sclerosis (MS). The anti-JCV antibodies titre (JCV index) increases during NTZ treatment; however, the effects of other disease-modifying therapies (DMTs) on the JCV index have not been fully explored.
Objective: The aim of the study was to evaluate changes in the JCV index during treatment with several DMTs.
Methods: This longitudinal study evaluated the JCV index before starting DMT (T0) and during treatment with DMT (T1).
Results: A total of 260 participants (65.4% females, mean age 43 ± 11.3) were enrolled: 68 (26.2%) treated with fingolimod (FTY), 65 (25%) rituximab or ocrelizumab (RTX/OCR), 37 (14.2%) dimethyl-fumarate (DMF), 29 (11.2%) cladribine (CLD), 23 (8.8%) teriflunomide (TFM), 20 (7.7%) interferon or glatiramer acetate (IFN/GA), and 18 (6.9%) alemtuzumab (ALM). At T1, the percentage of patients with JCV index <0.90 was found to be significantly increased in the ALM group (16.7% versus 66.7%, p = 0.05), while the percentage of patients with JCV index >1.51 was found to be significantly reduced in the RTX/OCR group (51.6% versus 37.5%, p = 0.04). In the FTY group, a significant reduction in the percentage of patients with JCV index <0.90 was also found (23.5% versus 1.4%, p = 0.0006). The mean JCV index was reduced in the RTX/OCR and ALM groups, while a significant increase was observed in the FTY group.
Conclusion: DMTs with a T and/or B depleting mechanism of action induced a significant reduction in the JCV index. These results may suggest new possible sequencing strategies potentially maximizing disease control while reducing the PML risk.
Keywords: Multiple sclerosis, JCV index, disease-modifying therapies, T cells depleting drugs, B cells depleting drugs, PML risk, treatment strategy
1. INTRODUCTION
Treatment options for multiple sclerosis (MS) have changed over the last two decades, ranging between categories of drugs with a heterogeneous mechanism of action, in particular B or T depleting profile [1].
However, with the development of highly effective therapeutic options for MS, several safety issues have been encountered. As a consequence, the MS management has required comprehensive knowledge of each treatment’s mechanism of action and of the increasingly complex array of adverse events, especially related to the long-term effects of chronic immune therapy on the immune system [2].
One of the most fearsome viral complications associated with the reduced immune surveillance of the tissues is the Progressive Multifocal Leukoencephalopathy (PML), an opportunistic infection caused by John Cunningham virus (JCV) reactivation [3, 4], which has a severe impact on patients’ disability course, functional outcome, and quality of life [5].
PML is a life-threatening disease caused by a failure of the immune system to control a brain infection with JCV, a polyomavirus, exclusively found in humans [3]. The clinical manifestations of PML are related to the location and extent of damage in the CNS. Common symptoms are progressive weakness, impaired visual acuity, alteration of speech, and psychiatric disorders that can rapidly progress within days [6]. The diagnosis of PML is based on clinical findings, evidence of typical lesions of the white matter at the magnetic resonance imaging (MRI), and the detection of JCV-DNA in cerebrospinal fluid (CSF) via polymerase chain reaction (PCR) or histological examination of brain biopsies [6, 7]. The prognosis, in the majority of cases, remains poor, with a mortality rate of 30-50% in the first few months [8].
In immunocompetent people, after primary infection, JCV reaches the kidneys, persistently replicates, and is secreted by the urine [9]. In these patients, the disease course is typically asymptomatic and the JCV is rarely detected in the bloodstream. On the contrary, symptomatic JCV infections are associated with a compromised adaptive immune system. Indeed, especially in the past, PML mainly occurred in patients with hematological malignancies and HIV infection [6]. More recently, PML has been associated with the use of immunosuppressive or immunomodulating drugs [5]. Currently, PML secondary to treatment with natalizumab (NTZ) accounts for the majority of all the cases of MS patients. To date, a few cases have also been observed with dimethyl-fumarate (DMF) and fingolimod (FTY) [5]. Moreover, PML is listed as a potential side effect also in patients suffering from diseases other than MS and treated with rituximab (RTX) with an adjusted odds ratio of 3.22 [10, 11], while 10 confirmed cases (9 of the patients previously treated with NTZ or FTY) have been reported in patients using OCR [12, 13]. No cases of PML attributable to teriflunomide (TFM) or cladribine (CLD) have been described yet, and a single case has been reported during alemtuzumab (ALM) therapy after switching from NTZ [14-16].
The pathogenesis of PML is far from being fully understood. CD4+ and CD8+ cytotoxic T-cell recognition of viral antigens may probably play an important role in limiting the spreadiof JCV infection and is directly related to the prognosis of PML [17]. Particularly, B cells represent latent sites of JCV, playing a significant role in viral transmission, replication and coordination of the expression of transcription factors [5, 18]. Furthermore, B lymphocytes induce T-cell responses through cytokine production, contributing to JCV control. Brain-resident memory T cells also seem to be involved, relying on help from the peripheral re-circulating CD4+ T cells [5, 19].
PML risk in MS patients is also typically associated with the previous use of immunosuppressant drugs (IM) and the presence of a high titre of anti-JCV antibody (JCV index) [20-23]. It has been demonstrated that the high JCV index reflects a high risk for PML, as it is associated with a large viral reservoir, which in turn may increase the risk for viral reactivation [24].
Therefore, the determination of antibodies against JCV is considered an important tool for risk stratification and an algorithm based on the JCV index is usually applied as a screening tool prior to start MS therapy and during treatment in MS patients [22].
In our previous study, NTZ treatment increased the JCV index and its suspension seemed not to be able to interfere in the JCV status for a long time period [25]. Moreover, according to our data, FTY used as an exit strategy after NTZ suspension was able to cause a progressive increase in the JCV index. However, no sufficient data regarding JCV status modification during other disease-modifying therapies (DMTs) are currently available and the effects of approved DMTs on JCV status have not been fully explored yet.
The aim of our study was to evaluate JCV status modification during treatment with currently used DMTs in order to identify possible alternative therapeutic strategies to minimize the risk of PML in MS patients.
2. MATERIALS AND METHODS
2.1. Study Population
This longitudinal observational study screened all MS patients treated with several DMTs and followed at the MS Centre of Catania University Hospital in the period between January 2010 and February 2021. Data regarding patients were obtained retrospectively from the database iMED®, a computerized medical record collecting demographic, clinical and laboratory data.
This study protocol was approved by the local Ethical Committee of the University of Catania (Catania 1). Each patient participating in the study signed an informed consent specifically designed for the study.
Patients with a diagnosis of MS according to the Mc Donald criteria 2017 [26] and treated for at least six months with interferons or glatiramer acetate (IFN/GA), RTX or OCR, DMF, TFN, ALM, CLD or FTY were included in the study. We excluded patients with a diagnosis of clinically isolated syndrome, radiologically isolated syndrome or neuromyelitis optica, and treated with steroids within 3 months before the JCV status evaluation.
In order to evaluate anti-JCV antibodies status (JCV index) changes during the follow-up, the JCV status was evaluated at baseline before treatment (T0) and after at least 12 months of treatment (T1).
At each time point, patients were divided into two subgroups based on their JCV status: negative JCV index (<0.90) and positive JCV index, with a JCV value between 0.91 and 1.50 (low positive) or > 1.51 (high positive), respectively. Blood samples were collected by peripheral venous puncture. JCV index was determined only through a qualitative result (positive or negative) for patients screened before 2011 (STRATIFY JCV Dx Select) and by a two-step enzyme-linked immunosorbent assay (STRATIFY II) for patients screened after 2011 [21, 27]. The analysis was centrally performed at Unilabs® in Copenhagen, Denmark. The STRATIFY test uses virus-like particles (VLPs) that consist of the VP1 capsid protein of the MAD-1 JCV reference strain (genotype 1) to capture antibodies. The STRATIFY test provides the level of antibodies as an index value and not as a titre. The antibody index is the ratio between the signal derived from antibodies in the serum and the signal from a JCV antibody-positive calibrator sample used in the assay [28]. Qualitative (negative/positive) and, for anti-JCV antibody positive patients, semi-quantitative results were obtained. An index value of less than 0.90 was considered as negative and equal to or greater than 0.91 as positive. Seroconversion was defined as changing the status of serum JCV antibody.
In order to simplify the categorization of DMTs, we decided to include in the same group patients treated with RTX and OCR because of the similar mechanism of action.
2.2. Statistical Analysis
Statistical analysis was performed using Stata 16.0 software (StataCorp LP, College Station, US). In descriptive analyses, continuous variables were summarized as mean and standard deviation (SD) or median and interquartile range (IQR), while categorical variables were expressed as percentages. Nonparametric statistics were used if the distribution of data deviated from normality. Shapiro-Wilk test was used for the assessment of normal distribution. To calculate mean differences, a series of ANOVAs were performed for the JCV values. Post-hoc analyses were performed with Bonferroni corrections for p-values. To calculate median differences, a series of Kruskal-Wallis-tests were performed for the EDSS score variable. Post-hoc analyses (Mann-Whitney U-tests) were performed with Bonferroni corrections for p-values. The association between two quantitative variables was evaluated through Pearson correlation coefficient or Spearman correlation coefficient (depending on the data distribution). A two-sided p-value of <.05 was considered as statistically significant.
3. RESULTS
Out of 319 patients screened, 260 met inclusion criteria and were finally enrolled. The mean age was 43 ± 11.3 years, and 170 (65.4%) were females.
A total of 68 (26.2%) patients were treated with FTY, 65 (25%) RTX/OCR, 37 (14.2%) DMF, 29 (11.2%) CLD, 23 (8.8%) TFM, 20 (7.7%) IFN/GA, and 18 (6.9%) ALM. Overall, the mean duration of treatment was 24.7 ± 7.2 months (median 22.3, range 6.4-33.6). The JCV index re-evaluation was performed after 23.3 ± 13.4 months (median 24, range 3-33). Demographic and clinical characteristics are summarized in (Table 1).
Table 1.
Demographic and clinical characteristics of the whole cohort of patients.
|
Drug
N (%) |
FTY
68 (26.2) |
RTX/OCRE
65 (25) |
DMF
37 (14.2) |
CLD
29 (11.2) |
TFM
23 (8.8) |
IFN/GA
20 (7.7) |
ALM
18 (6.9) |
ANOVA Test after Bonferroni Correction p-Value |
|---|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 44.6 ± 9.8 | 44.8 ± 12.5 | 44.6 ± 11.7 | 36.9 ± 9.6 | 44.7 ± 11.1 | 39.2 ± 11 | 38.8 ± 10 | CLD vs FTY = 0.03 CLD vs RTX/OCRE = 0.03 |
| Female (%) |
43 (63.2) |
39 (60) |
24 (64.9) |
24 (82.7) |
14 (60.9) |
15 (75) |
11 (61.1) |
CLD vs RTX/OCRE = 0.01 CLD vs TFM = 0.03 CLD vs ALM = 0.03 CLD vs FTY = 0.04 |
| Disease duration* (mean ± SD) | 17.9 ± 7.8 | 10 ± 7.6 | 10.1 ± 7.6 | 9.3 ± 6.2 | 12.1 ± 8.4 | 7.9 ± 6.7 | 15.3 ± 5.9 | FTY vs IFN/GA = 0.001 FTY vs CLD = 0.01 FTY vs RTX/OCRE = 0.02 |
| Patients with previous use of IM (%) |
21 (30.9%) | 10 (15.6%) | 5 (13.5%) | 4 (13.8%) | 5 (21.7%) | 0 | 5 (27.8%) | FTY vs CLD = 0.01 FTY vs DMF = 0.01 FTY vs RTX/OCRE = 0.03 |
| Patients with previous use of NTZ (%) |
41 (60.3%) | 19 (29.2%) | 7 (18.9%) | 10 (34.5%) | 2 (8.7%) | 0 | 13 (72.2%) | TFM vs ALM = 0.001 TFM vs FTY = 0.01 TFM vs CLD = 0.02 TFM vs RTX/OCRE = 0.03 |
| EDSS at T0 (mean ± SD) |
3.3 ± 2.1 | 4.0 ± 2.1 | 3.0 ± 1.9 | 2.7 ± 1.7 | 2.5 ± 2.1 | 2.0 ± 1.4 | 3.5 ± 1.8 | IFN/CA vs RTX/OCRE = 0.001 |
| EDSS at T1 (mean ± SD) |
3.7 ± 2.4 | 3.8 ± 2.5 | 3.2 ± 2.2 | 2.6 ± 1.9 | 3.1 ± 2.6 | 2.2 ± 1.7 | 3.4 ± 2.1 | n.s. |
| DMT duration** (mean ± SD) |
48.6 ± 23.6 | 25.9 ± 15.0 | 36.9 ± 21.3 | 18.7 ± 7.4 | 22.3 ± 9.5 | 28.8 ± 13.4 | 43.7 ± 14.5 | FTY vs CLD = 0.001 FTY vs RTX/OCRE = 0.001 FTY vs IFN/GA = 0.001 FTY vs TFM = 0.03 DMF vs CLD = 0.001 |
| Time between T0-T1 (mean ± SD)** |
26.2 ± 16.5 | 21.8 ± 14.0 | 25.7 ± 15.7 | 15.3 ± 7.3 | 17.9 ± 9.1 | 26 ± 13.7 | 30.1 ± 17.7 | ALM vs CLD = 0.0001 ALM vs TFM = 0.0001 FTY vs CLD = 0.001 FTY vs TFM = 0.001 IFN/GA vs CLD = 0.01 |
Note: *in years
**in months
Abbreviations: DMT: disease-modifying therapy; FTY; fingolimod; RTX/OCRE: rituximab or ocrelizumab; DMF: dimethyl-fumarate; CLD: cladribine; TFM: teriflunomide; IFN/GA: interferons or glatiramer acetate; ALM: alemtuzumab; IM: immunosuppressant drugs; EDSS: Expanded disability status scale; SD: standard deviation; n.s.: not significant.
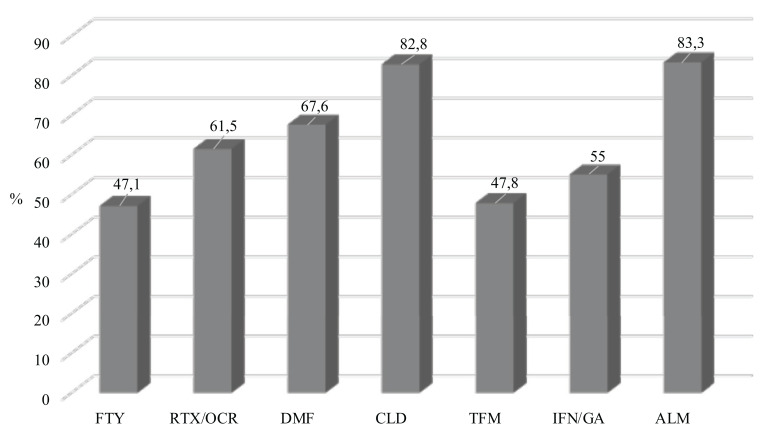
Data regarding the seroprevalence of anti-JCV antibodies at baseline for each DMT are illustrated in (Fig. 1).
Fig. (1).
Seroprevalence of positive JCV status at T0 for each DMT. Abbreviations: FTY; fingolimod; RTX/OCRE: rituximab or ocrelizumab; DMF: dimethyl-fumarate; CLD: cladribine; TFM: teriflunomide; IFN/GA: interferons or glatiramer acetate; ALM: alemtuzumab; DMT: disease-modifying therapy; JCV: John-Cunningham virus.
At T1, the percentage of patients with JCV index <0.9 was found to be significantly increased in the ALM group (66.7% versus 16.7%, p = 0.05), while the percentage of patients with JCV index >1.51 was significantly reduced in the RTX/OCR group (37.5% versus 51.6%, p = 0.04), and a trend was observed in the ALM group (27.8% versus 77.7%, p = 0.09). In the FTY group, a significant reduction in the percentage of patients with negative JCV status was also found (1.4% versus 23.5%, p = 0.0006) (Table 2).
Table 2.
Percentage of patients with negative and positive status at T0 and at T1.
|
DMT
N (%) |
JCV Index value |
T0
N (%) |
T1
N (%) |
p-Value |
|---|---|---|---|---|
| FTY 68 (26.2) |
≥ 0.9 | 16 (23.5) | 1 (1.4) | 0.0006 |
| ≥ 0.91 ≤ 1.5 | 8 (11.8) | 8 (11.8) | 1.0 | |
| > 1.51 | 44 (64.7) | 59 (86.7) | 0.3 | |
| RTX/OCRE 65 (25) |
≥ 0.9 | 24 (37.5) | 39 (47.7) | 0.1 |
| ≥ 0.91 ≤ 1.5 | 7 (10.9) | 10 (15.6) | 0.5 | |
| > 1.51 | 33 (51.6) | 16 (37.5) | 0.04 | |
| DMF 37 (14.2) |
≥ 0.9 | 12 (32.4) | 11 (29.7) | 0.9 |
| ≥ 0.91 ≤ 1.5 | 8 (21.6) | 7 (18.9) | 0.9 | |
| > 1.51 | 17 (46) | 19 (51.4) | 0.9 | |
| CLD 29 (11.2) |
≥ 0.9 | 5 (17.2) | 11 (37.9) | 0.2 |
| ≥ 0.91 ≤ 1.5 | 5 (17.2) | 4 (13.8) | 0.8 | |
| > 1.51 | 19 (65.6) | 14 (48.3) | 0.5 | |
| TFM 23 (8.8) |
≥ 0.9 | 12 (52.2) | 11 (47.85) | 0.9 |
| ≥ 0.91 ≤ 1.5 | 1 (4.3) | 1 (4.3) | 1.0 | |
| > 1.51 | 10 (43.5) | 11 (47.9) | 0.9 | |
| IFN/GA 20 (7.7) |
≥ 0.9 | 9 (45) | 9 (45) | 1.0 |
| ≥ 0.91 ≤ 1.5 | 3 (15) | 3 (15) | 1.0 | |
| > 1.51 | 8 (40) | 8 (40) | 1.0 | |
| ALM 18 (6.9) |
≥ 0.9 | 3 (16.7) | 12 (66.7) | 0.05 |
| ≥ 0.91 ≤ 1.5 | 1 (5.6) | 1 (5.6) | 1.0 | |
| > 1.51 | 14 (77.7) | 5 (27.8) | 0.09 |
Abbreviations: DMT: disease-modifying therapy; JCV: John-Cunningham virus; FTY; fingolimod; RTX/OCRE: rituximab or ocrelizumab; DMF: dimethyl-fumarate; CLD: cladribine; TFM: teriflunomide; IFN/GA: interferons or glatiramer acetate; ALM: alemtuzumab.
A significant reduction of the mean JCV index value in patients treated with RTX/OCR (1.87 ± 1.54 vs. 1.57 ± 1.16, p = 0.0032) and in those on ALM (2.68 ± 1.15 vs. 1.50 ± 1.24, p = 0.0053) was observed. A trend in reduction of the mean JCV index value, even if not significant, was also associated with CLD treatment (2.16 ± 1.26 vs. 1.57 ± 1.12, p = 0.06). Conversely, a significant increase in JCV index was observed in the patients treated with FTY (2.11 ± 1.29 vs. 2.58 ± 1.40, p = 0.04) (Table 3). Adjusting for the covariates, DMT duration and previous use of IM or NTZ, similar results were obtained.
Table 3.
Differences in term of JCV index values between T0 and T1 for each DMT.
|
DMT
N (%) |
JCV Index at T0
(mean ± SD) |
JCV Index at T1
(mean ± SD) |
p-Value |
|---|---|---|---|
| FTY 68 (26.2) |
2.11 ± 1.29 | 2.58 ± 1.40 | 0.04 |
| RTX/OCRE 65 (25) |
1.87 ± 1.54 | 1.57 ± 1.16 | 0.003 |
| DMF 37 (14.2) |
1.54 ± 1.34 | 1.85 ± 1.43 | 0.34 |
| CLD 29 (11.2) |
2.16 ± 1.26 | 1.57 ± 1.12 | 0.06 |
| TFM 23 (8.8) |
1.57 ± 1.61 | 1.67 ± 1.61 | 0.82 |
| IFN/GA 20 (7.7) |
1.35 ± 1.27 | 1.45 ± 1.47 | 0.83 |
| ALM 18 (6.9) |
2.68 ± 1.15 | 1.50 ± 1.24 | 0.005 |
Abbreviations: FTY; fingolimod; RTX/OCRE: rituximab or ocrelizumab; DMF: dimethyl-fumarate; CLD: cladribine; TFM: teriflunomide; IFN/GA: interferons or glatiramer acetate; ALM: alemtuzumab; DMT: disease-modifying therapy; JCV: John-Cunningham virus.
Moreover, a significant percentage of patients treated with therapies with a T and/or B depleting mechanism of action seroconverted to a negative JCV status, in particular 14.6% of patients treated with RTX/OCR, 26.7% of those treated with ALM, and 29.2% of those treated with CLD. A total of 31% of patients treated with FTY showed a statistically significant rate of seroconversion to a positive status (Fig. 2).
Fig. (2).
Percentage of patients seroconverted to positive or negative JCV status between T0 and T1 for each DMT. Abbreviations: IFN/GA: interferons or glatiramer acetate; RTX/OCRE: rituximab or ocrelizumab; DMF: dimethyl-fumarate; TFM: teriflunomide; ALM: alemtuzumab; CLD: cladribine; FTY; fingolimod; DMT: disease-modifying therapy; JCV: John-Cunningham virus.
Stratifying according to the previous treatment, 92 (35.4% of 260) were previously treated with NTZ and 168 (64.6% of 260) patients were naïve to NTZ. In both groups, variations of mean JCV index value between T0 and T1 were not statistically significant (2.32 ± 1.44 vs. 2.14 ± 1.26, p = 0.4; 1.68 ± 1.43 vs. 1.55 ± 1.37, p = 0.4). A total of 50 (19.2% of 260) patients were previously treated with IM and 210 (80.8% of 260) were naïve to IMs. Mean JCV index values were found to be similar between T0 and T1 in both groups (1.72 ± 1.36 vs. 1.60 ± 1.37, p = 0.7; 1.96 ± 1.42 vs. 1.80 ± 1.45, p = 0.3) (Supplementary material).
4. DISCUSSION
Our study demonstrated that the percentage of patients with negative JCV index increased after ALM, while the percentage of patients with positive JCV index decreased after ALM and RTX/OCR treatments. Moreover, the percentage of patients with negative JCV status was reduced in the FTY group. It further showed that drugs with a T and/or B depleting mechanism of action, as ALM and RTX/OCR, induced a statistically significant reduction of the JCV index. Even if not statistically significant, also treatment with CLD is associated with a reduction in the anti-JCV antibodies titre.
It is well known that B cells can directly contribute to the development and progression of MS, both being the source of antibody-producing plasma cells and acting as potent Antigen-Presenting Cells (APC) [29]. Indeed, it has been demonstrated that B lymphocytes, in peripheral blood and CNS, exhibit signs of chronic inflammation along with a shift towards antigen-experienced memory B cells due to the antigen-mediated activation of B cells [29, 30]. In MS patients, B lymphocytes express a higher level of co-stimulatory molecules that potentially facilitate the pro-inflammatory differentiation of responding T cells [31]. For these reasons, in the last years, several drugs targeting B/T cells have been developed [1, 32].
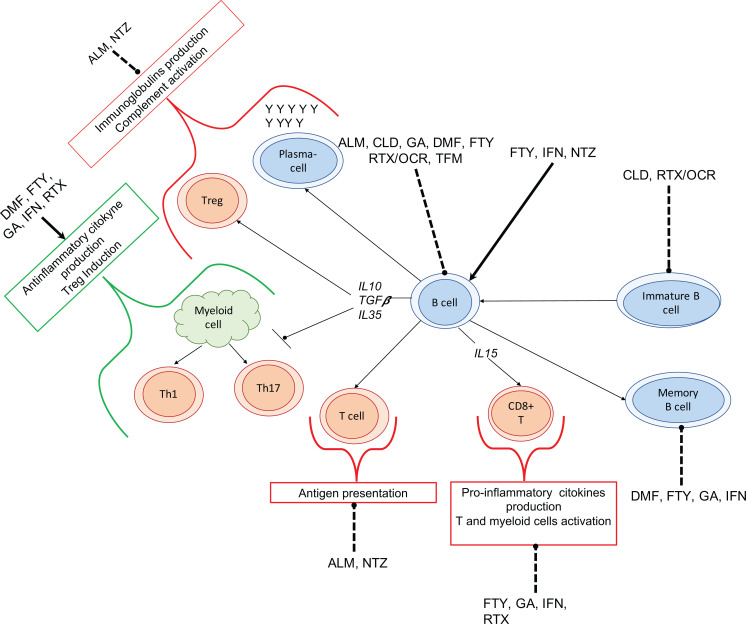
Figure (3) summarizes the role of B cells in MS pathogenesis and the influence of MS therapeutic agents on the effector and regulatory B cells.
Fig. (3).
Role of B cells in Multiple Sclerosis pathogenesis and the effects of disease modifying therapies on B cells. B cells are able to differentiate into plasma-cells, activating complement and causing tissue damage. B cells also activate T cells by presentation of antigen through the major histocompatibility complex (MHC) class II, increased secretion of pro-inflammatory cytokines inducing the differentiation and activation of pathogenic T cell subsets and myeloid cells through. Alemtuzumab (ALM) induces a rapid reduction in circulating T cells, B cells and monocytes. ALM also increases the levels of serum B cell activating factor (BAFF) and of autoantibodies (especially against the thyroid), while inhibiting IgG production. Cladribine (CLD) depletes peripheral T and B lymphocytes, in particular memory B cells. Dimethyl-fumarate (DMF) decreases the circulating pool of lymphocytes, especially CD8 T cells and B cells; in contrast DMF increases the ratio of naïve to memory B cells and levels of transitional and IL-10+ B cells. Fingolimod (FTY) induces a reduction of total circulating B cells, especially of memory B cells, while increasing transitional and plasma-cells. FTY also increase IL-10+, CD25+, CD86+, CD5+ B cells levels, with an overall increase in anti-inflammatory cytokine to pro-inflammatory ratio. Glatiramer acetate (GA) induces Tregs activation through the binding to MHC class II on B cells. GA also decreases percentages of B cells, especially plasma-cells and memory B cells. GA has no effect on Ig serum level and on B cell proliferation. Interferon-beta (IFN) induces a short-term reduction of B cells, decreasing B cell-induced proliferative response of CD4 T cells, the levels of CD40+ and CD80+ B cells, and the antigen presenting capacity. IFN also reduces the percentage of memory B cells, while increasing the transitional B cells. Natalizumab (NTZ) induces a reduction of B cells, plasma-cells, and levels of IgM and IgG, while increasing levels of immature CD10+ pre-B cells and memory B cells. Rituximab and Ocrelizumab (RTX/OCR) reduce naïve and memory B cells with slight effect of tissue-resident B cells. RTX/OCR slightly decrease circulating IgG. Teriflunomide (TFM) inhibits the proliferation of activated T and B cells, also reducing circulating leukocytes. The arrows indicate the induction of cells differentiation, the interrupted line means the reduction of the cells differentiation. Bold arrows represent an increasing effect; dashes arrows represent a decreasing effect. Abbreviations: ALM: alemtuzumab; CLD: cladribine; DMF: dimethyl-fumarate; FTY; fingolimod; GA: glatiramer acetate; IFN: interferons; RTX/OCRE: rituximab or ocrelizumab; TFM: teriflunomide.
ALM is directed against CD52, a molecule highly expressed on B and T surfaces, resulting in a rapid and profound depletion of T and B cells [33]. RTX and OCR are monoclonal antibodies directed against CD20, a molecule expressed on B cells from the late pro-B cell through the memory cell stages, thus inducing cell apoptosis via complement-dependent cytotoxicity (CDC) or antibody-dependent cellular cytotoxicity (ADCC) [34]. As a consequence, RTX and OCR treatments are characterized by a marked depletion of CD20 + B cells, while the CD4 and CD8 T cells populations are partially depleted by about 10-25% in the peripheral blood [35]. CLD is a purine nucleoside analogue that selectively depletes peripheral T and B lymphocytes with a particular predilection for memory B cells [36].
According to our results, the reduction of JCV index in patients treated with ALM, RTX/OCR and CLD could be explained by the rapid depletion in B circulating cells, consequently responsible for the decrease in CD4+ T cells. It has been demonstrated that CD4+ T cells have a major role in developing PML because they are required for the maintenance of CD8+ T cells, which are the most important player in the immune control of JCV infection [37]. Therefore, it could be hypothesized that in patients treated with B depleting drugs the rapid decrease in B circulating cells and the associated reduction of JCV index could lead to a lower risk of PML. In line with this hypothesis, few and no cases of PML attributable to ALM and CLD, respectively, have been described in MS [14, 38]. Even considering the last updates regarding PML cases after OCR, it should be noted that most of them may be considered as carryover PML, while only one case could be related to OCR treatment [12, 13]. In this last case (a 78-year-old man with progressive MS, who have received OCR as only DMT), the age-related reduction in the proliferation or of the activity of the lymphocytes, resulting in an increased susceptibility to infections (also called “immunosenescence”), likely played a role in the PML pathogenesis [7].
It is well known that DMTs with B and T cell profiles cause only a partial and transitory depletion of immunocompetent cells, with a complete cell repopulation over time with different timing for each treatment [39, 40]. After ALM treatment, a complete B cell recovery has been usually shown within 6 months, while T lymphocytes recover more slowly and generally do not return to baseline by 12-18 months post-treatment [41]. The reduction of JCV index after ALM treatment in our study could be explained by the fact that the rapid B lymphocyte recovery consists mainly of restored immature B cells, preferentially confined in the lymphoid organs and potentially reacting to a new JCV presentation with a low immunogenic profile [39, 42]. Otherwise, a possible resetting of the whole immune system with the production of new B circulating cells with a different immuno-pattern may play a role in it [43].
Assuming that the anti-JCV antibody response is similar to other viral infections, in RTX/OCR treated patients, the lower production of anti-JCV antibody titre and the decrease of the mean JCV index value might be explained by the reduction in the B circulating cells. This is in line with a previous study in which patients treated with RTX showed a lower production of immunoglobulins, as well as of antibodies selected against anti-JCV [44]. Moreover, it could be hypothesized that the functional preservation of residual T cells could preserve the maintenance of immunocompetence [45].
According to its mechanism of action, CLD’s selective depletion on memory B cells might explain the reduction of mean JCV index and consequently the reduced risk of PML without predisposing to immunosuppression-related complications [36]. Indeed, patients treated with this drug rarely develop severe lymphopenia [46].
Our results also confirmed the statistically significant progressive increase of the mean JCV index in the group of patients treated with FTY, as shown in our previous study [25]. FTY acts as a functional antagonist of sphingosine 1-phosphate (S1P), mainly causing egress inhibition of central memory T cells and naïve T cells from lymph nodes, leading to presumably reduced migration of those T cells to the central nervous system [47]. FTY also plays a role in the modification of B cell subsets, reducing circulating memory B cells, while increasing the proportions of transitional B cells and B regulatory cells (B-regs) [47, 48]. With promoting lymphocyte homing, FTY might accelerate this phenomenon, expanding B cell pool due to the presence of new circulating transitional B cells and B-regs and enhancing the role of antibody-producing B cells [49]. These immunological modifications might be responsible for the increase of JCV index found in our study.
JCV index did not significantly change in the groups of patients treated with DMT, TFM and IFN/GA. These DMTs act as anti-inflammatory agents downregulating the expression of proinflammatory cytokines; however, their direct impact on B cells is still unclear [50].
This study involves several limitations. Firstly, the timing for the acquisition of the blood samples for JCV status detection may have potentially impacted our results as treatment with several depleting drugs, such as RTX/OCRE, CLD, and ALM, is associated with different rates of B and T cells repopulation. Second, we included patients treated with first-line injectable therapies (IFN and GA) in the same group, despite the different mechanisms of action. More importantly, whether the changes in the JCV index observed in our study could be potentially associated with a modification of the PML risk has to be proven yet.
CONCLUSION
This is the first study evaluating the JCV index during treatment with all the currently approved MS drugs other than NTZ. In particular, the finding of a reduction in anti-JCV antibodies titre in those patients treated with B depleting drugs, as ALM and RTX/OCR, may have important implications in clinical practice. Indeed, it is known that NTZ is one of the most efficacious drugs for treating MS and the decision of suspending NTZ is mainly driven by the PML risk stratification [51, 52]. If confirmed by longitudinal studies, future therapeutic scenarios might foresee the possibility of using B cell profile drugs as a “therapeutic bridge” in NTZ-treated patients with high anti-JCV antibodies titre who have to discontinue treatment due to the high risk of NTZ driven-PML. Once a reduction of JCV index below 1.50 or seroconversion to a negative status has been obtained, clinicians may consider re-starting NTZ treatment. Indeed, PML risk with other available and emerging DMTs is much lower than the risk associated with NTZ [24]. Hence, our data suggest that the temporary replacement of NTZ with DMTs with comparable effectiveness and safety profile may be done to reduce the risk of PML; however, this needs to be confirmed in future studies.
Furthermore, the finding of an increase in JCV index in patients treated with FTY may raise the question of whether the use of this kind of treatment as an exit strategy after NTZ discontinuation would represent the safest choice.
Thus, our results could open new treatments sequencing scenarios in which switching to different highly-effective DMTs could maximize disease control and minimize PML risk based on the mechanism of action.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee (Catania 1) of University of Catania, Italy approved this study (37/2015/PO).
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All human procedures were followed in accordance with the World Medical Association Declaration of Helsinki.
CONSENT FOR PUBLICATION
All the participants or their legal surrogate gave written consent for their personal or clinical details to be published in this study.
STANDARDS OF REPORTING
STROBE guidelines were followed for the study.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Hauser S.L., Cree B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020;133(12):1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cree B.A.C., Mares J., Hartung H.P. Current therapeutic landscape in multiple sclerosis: An evolving treatment paradigm. Curr. Opin. Neurol. 2019;32(3):365–377. doi: 10.1097/WCO.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin K.J., Hogg J.P. Progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Curr. Opin. Neurol. 2013;26(3):318–323. doi: 10.1097/WCO.0b013e328360279f. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen P.S., Bertolotto A., Edan G., Giovannoni G., Gold R., Havrdova E., Kappos L., Kieseier B.C., Montalban X., Olsson T. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult. Scler. 2012;18(2):143–152. doi: 10.1177/1352458511435105. [DOI] [PubMed] [Google Scholar]
- 5.Major E.O., Yousry T.A., Clifford D.B. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: A decade of lessons learned. Lancet Neurol. 2018;17(5):467–480. doi: 10.1016/S1474-4422(18)30040-1. [DOI] [PubMed] [Google Scholar]
- 6.Kartau M., Sipilä J.O., Auvinen E., Palomäki M., Verkkoniemi-Ahola A. Progressive Multifocal Leukoencephalopathy: Current Insights. Degener. Neurol. Neuromuscul. Dis. 2019;9:109–121. doi: 10.2147/DNND.S203405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger J.R., Aksamit A.J., Clifford D.B., Davis L., Koralnik I.J., Sejvar J.J., Bartt R., Major E.O., Nath A. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koralnik I.J. Progressive multifocal leukoencephalopathy revisited: Has the disease outgrown its name? Ann. Neurol. 2006;60(2):162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 9.Wollebo H.S., White M.K., Gordon J., Berger J.R., Khalili K. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann. Neurol. 2015;77(4):560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner I., Berger J.R. Update on progressive multifocal leukoencephalopathy. Curr. Neurol. Neurosci. Rep. 2012;12(6):680–686. doi: 10.1007/s11910-012-0313-4. [DOI] [PubMed] [Google Scholar]
- 11.Oshima Y., Tanimoto T., Yuji K., Tojo A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult. Scler. 2019;25(8):1141–1149. doi: 10.1177/1352458518786075. [DOI] [PubMed] [Google Scholar]
- 12.Patel A., Sul J., Gordon M.L., Steinklein J., Sanguinetti S., Pramanik B., Purohit D., Haroutunian V., Williamson A., Koralnik I., Harel A. Progressive Multifocal Leukoencephalopathy in a Patient With Progressive Multiple Sclerosis Treated With Ocrelizumab Monotherapy. JAMA Neurol. 2021;78(6):736–740. doi: 10.1001/jamaneurol.2021.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toorop A.A., van Lierop Z.Y.G., Strijbis E.E.M., Teunissen C.E., Petzold A., Wattjes M.P., Barkhof F., de Jong B.A., van Kempen Z.L.E., Killestein J. Mild progressive multifocal leukoencephalopathy after switching from natalizumab to ocrelizumab. Neurol. Neuroimmunol. Neuroinflamm. 2020;8(1):e904. doi: 10.1212/NXI.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerevini S., Capra R., Bertoli D., Sottini A., Imberti L. Immune profiling of a patient with alemtuzumab-associated progressive multifocal leukoencephalopathy. Mult. Scler. 2019;25(8):1196–1201. doi: 10.1177/1352458519832259. [DOI] [PubMed] [Google Scholar]
- 15.Jalkh G., Abi Nahed R., Macaron G., Rensel M. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines (Basel) 2020;9(1):12. doi: 10.3390/vaccines9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisari C.G., Toscano S., D’Amico E., Lo Fermo S., Zanghì A., Arena S., Zappia M., Patti F. An update on the safety of treating relapsing-remitting multiple sclerosis. Expert Opin. Drug Saf. 2019;18(10):925–948. doi: 10.1080/14740338.2019.1658741. [DOI] [PubMed] [Google Scholar]
- 17.Pavlovic D., Patel M.A., Patera A.C., Peterson I. T cell deficiencies as a common risk factor for drug associated progressive multifocal leukoencephalopathy. Immunobiology. 2018;223(6-7):508–517. doi: 10.1016/j.imbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Monaco M.C., Major E.O. Immune system involvement in the pathogenesis of JC virus induced PML: What is learned from studies of patients with underlying diseases and therapies as risk factors. Front. Immunol. 2015;6:159. doi: 10.3389/fimmu.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoy K., Mariotte D., Defer G., Petit G., Toutirais O., Le Mauff B. Natalizumab in multiple sclerosis treatment: From biological effects to immune monitoring. Front. Immunol. 2020;11:549842. doi: 10.3389/fimmu.2020.549842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomgren G., Richman S., Hotermans C., Subramanyam M., Goelz S., Natarajan A., Lee S., Plavina T., Scanlon J.V., Sandrock A., Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012;366(20):1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 21.Plavina T., Subramanyam M., Bloomgren G., Richman S., Pace A., Lee S., Schlain B., Campagnolo D., Belachew S., Ticho B. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann. Neurol. 2014;76(6):802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho P.R., Koendgen H., Campbell N., Haddock B., Richman S., Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: A retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16(11):925–933. doi: 10.1016/S1474-4422(17)30282-X. [DOI] [PubMed] [Google Scholar]
- 23.Chisari C.G., Grimaldi L.M., Salemi G., Ragonese P., Iaffaldano P., Bonavita S., Sparaco M., Rovaris M., D’Arma A., Lugaresi A., Ferrò M.T., Grossi P., Di Sapio A., Cocco E., Granella F., Curti E., Lepore V., Trojano M., Patti F., Italian M.S.R.S.G. Clinical effectiveness of different natalizumab interval dosing schedules in a large Italian population of patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2020;91(12):1297–1303. doi: 10.1136/jnnp-2020-323472. [DOI] [PubMed] [Google Scholar]
- 24.Berger J.R. Classifying PML risk with disease modifying therapies. Mult. Scler. Relat. Disord. 2017;12:59–63. doi: 10.1016/j.msard.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Sgarlata E., Chisari C.G., D’Amico E., Millefiorini E., Patti F. Changes in anti-JCV antibody status in a large population of multiple sclerosis patients treated with natalizumab. CNS Drugs. 2020;34(5):535–543. doi: 10.1007/s40263-020-00716-6. [DOI] [PubMed] [Google Scholar]
- 26.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee P., Plavina T., Castro A., Berman M., Jaiswal D., Rivas S., Schlain B., Subramanyam M. A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J. Clin. Virol. 2013;57(2):141–146. doi: 10.1016/j.jcv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Reuwer A.Q., Heron M., van der Dussen D., Schneider-Hohendorf T., Murk J.L. The clinical utility of JC virus antibody index measurements in the context of progressive multifocal leukoencephalopathy. Acta Neurol. Scand. 2017;136(Suppl. 201):37–44. doi: 10.1111/ane.12840. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann-Horn K., Kronsbein H.C., Weber M.S. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther. Adv. Neurol. Disord. 2013;6(3):161–173. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duddy M., Niino M., Adatia F., Hebert S., Freedman M., Atkins H., Kim H.J., Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007;178(10):6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 31.Harp C.T., Ireland S., Davis L.S., Remington G., Cassidy B., Cravens P.D., Stuve O., Lovett-Racke A.E., Eagar T.N., Greenberg B.M., Racke M.K., Cowell L.G., Karandikar N.J., Frohman E.M., Monson N.L. Memory B cells from a subset of treatment-naïve relapsing-remitting multiple sclerosis patients elicit CD4(+) T-cell proliferation and IFN-γ production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 2010;40(10):2942–2956. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker D., Marta M., Pryce G., Giovannoni G., Schmierer K., Memory B., Memory B. Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis. EBioMedicine. 2017;16:41–50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havrdova E., Horakova D., Kovarova I. Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther. Adv. Neurol. Disord. 2015;8(1):31–45. doi: 10.1177/1756285614563522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chisari C.G., Sgarlata E., Arena S., Toscano S., Luca M., Patti F. Rituximab for the treatment of multiple sclerosis: A review. J. Neurol. 2022;269(1):159–183. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palanichamy A., Jahn S., Nickles D., Derstine M., Abounasr A., Hauser S.L., Baranzini S.E., Leppert D., von Büdingen H.C. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J. Immunol. 2014;193(2):580–586. doi: 10.4049/jimmunol.1400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceronie B., Jacobs B.M., Baker D., Dubuisson N., Mao Z., Ammoscato F., Lock H., Longhurst H.J., Giovannoni G., Schmierer K. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J. Neurol. 2018;265(5):1199–1209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelcic I., Jelcic I., Kempf C., Largey F., Planas R., Schippling S., Budka H., Sospedra M., Martin R. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann. Neurol. 2016;79(3):404–418. doi: 10.1002/ana.24574. [DOI] [PubMed] [Google Scholar]
- 38.Scarpazza C., De Rossi N., Tabiadon G., Turrini M.V., Gerevini S., Capra R. Four cases of natalizumab-related PML: A less severe course in extended interval dosing? Neurol. Sci. 2019;40(10):2119–2124. doi: 10.1007/s10072-019-03959-4. [DOI] [PubMed] [Google Scholar]
- 39.Rolla S., Maglione A., De Mercanti S.F., Clerico M. The meaning of immune reconstitution after alemtuzumab therapy in multiple sclerosis. Cells. 2020;9(6):E1396. doi: 10.3390/cells9061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rommer P.S., Milo R., Han M.H., Satyanarayan S., Sellner J., Hauer L., Illes Z., Warnke C., Laurent S., Weber M.S., Zhang Y., Stuve O. Immunological aspects of approved MS therapeutics. Front. Immunol. 2019;10:1564. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z., Richards S., Surks H.K., Jacobs A., Panzara M.A. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin. Exp. Immunol. 2018;194(3):295–314. doi: 10.1111/cei.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durali D., de Goër de Herve M.G., Gasnault J., Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front. Immunol. 2015;6:241. doi: 10.3389/fimmu.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meltzer E., Campbell S., Ehrenfeld B., Cruz R.A., Steinman L., Parsons M.S., Zamvil S.S., Frohman E.M., Frohman T.C. Mitigating alemtuzumab-associated autoimmunity in MS: A “whack-a-mole” B-cell depletion strategy. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(6):e868. doi: 10.1212/NXI.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baber U., Bouley A., Egnor E., Sloane J.A. Anti-JC virus antibody index changes in rituximab-treated multiple sclerosis patients. J. Neurol. 2018;265(10):2342–2345. doi: 10.1007/s00415-018-8996-3. [DOI] [PubMed] [Google Scholar]
- 45.Focosi D., Tuccori M., Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev. Med. Virol. 2019;29(6):e2077. doi: 10.1002/rmv.2077. [DOI] [PubMed] [Google Scholar]
- 46.Hermann R., Karlsson M.O., Novakovic A.M., Terranova N., Fluck M., Munafo A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin. Pharmacokinet. 2019;58(3):283–297. doi: 10.1007/s40262-018-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subei A.M., Cohen J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29(7):565–575. doi: 10.1007/s40263-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blumenfeld-Kan S., Staun-Ram E., Miller A. Fingolimod reduces CXCR4-mediated B cell migration and induces regulatory B cells-mediated anti-inflammatory immune repertoire. Mult. Scler. Relat. Disord. 2019;34:29–37. doi: 10.1016/j.msard.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 50.Longbrake E.E., Cross A.H. Effect of multiple sclerosis disease-modifying therapies on B cells and humoral immunity. JAMA Neurol. 2016;73(2):219–225. doi: 10.1001/jamaneurol.2015.3977. [DOI] [PubMed] [Google Scholar]
- 51.Montalban X., Gold R., Thompson A.J., Otero-Romero S., Amato M.P., Chandraratna D., Clanet M., Comi G., Derfuss T., Fazekas F., Hartung H.P., Havrdova E., Hemmer B., Kappos L., Liblau R., Lubetzki C., Marcus E., Miller D.H., Olsson T., Pilling S., Selmaj K., Siva A., Sorensen P.S., Sormani M.P., Thalheim C., Wiendl H., Zipp F. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018;24(2):96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 52.Chisari C.G., Comi G., Filippi M., Paolicelli D., Iaffaldano P., Zaffaroni M., Brescia M.V., Cocco E., Marfia G.A., Grimaldi L.M., Inglese M., Bonavita S., Lugaresi A., Salemi G., De Luca G., Cottone S., Conte A., Sola P., Aguglia U., Maniscalco G.T., Gasperini C., Ferrò M.T., Pesci I., Amato M.P., Rovaris M., Solaro C., Lus G., Maimone D., Bergamaschi R., Granella F., Di Sapio A., Bertolotto A., Totaro R., Vianello M., Cavalla P., Bellantonio P., Lepore V., Patti F. PML risk is the main factor driving the choice of discontinuing natalizumab in a large multiple sclerosis population: results from an Italian multicenter retrospective study. J. Neurol. 2022;269(2):933–944. doi: 10.1007/s00415-021-10676-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.