Abstract
Depression is a prevalent disease worldwide, limiting psychosocial functioning and thequality of life. Linalool is the main constituent of some essential oils from aromatic plants, representing about 70% of these volatile concentrates. Evidence of the linalool activity on the central nervous system, mainly acting as an antidepressant agent, is increasingly abundant. This review aimed to extend the knowledge of linalool's antidepressant action mechanisms, which is fundamental for future research, intending to highlight this natural compound as a new antidepressant phytomedication. A critical analysis is proposed here with probable hypotheses of the synergic mechanisms that support the evidence of antidepressant effects of the linalool. The literature search has been conducted in databases for published scientific articles before December 2020, using relevant keywords. Several pieces of evidence point to the anticonvulsant, sedative, and anxiolytic actions. In addition to these activities, other studies have revealed that linalool acts on the monoaminergic and neuroendocrine systems, inflammatory process, oxidative stress, and neurotrophic factors, such as BDNF, resulting in considerable advances in the knowledge of the etiology of depression. In this context, linalool emerges as a promising bioactive compound in the therapeutic arsenal, capable of interacting with numerous pathophysiological factors and acting on several targets. This review claims to contribute to future studies, highlighting the gaps in the linalool knowledge, such as its kinetics, doses, routes of administration, and multiple targets of interaction, to clarify its antidepressant activity.
Keywords: Linalool, neuropharmacological effects, therapeutic effects, antidepressant bioactive compound, depression, essential oils
1. INTRODUCTION
Depression is a prevalent disease worldwide, limiting psychosocial functioning and the quality of life. The disease is a mood disorder that displays repeated depressive episodes, characterized by several symptoms, including depressed mood, loss of interest and anhedonia (loss of pleasure), anxiety symptoms, disturbed sleep and appetite, guilt and low self-esteem, and poor concentration [1].
The pathophysiological mechanism of depression is not yet fully elucidated. However, the involvement of decreased monoaminergic neurotransmitters or modified monoaminergic receptors, hypothalamic-pituitary-adrenal (HPA) axis dysregulation, inflammatory process, oxidative stress, and neuroplasticity dysfunction have been historically confirmed. Furthermore, the potential indirect involvement of the glutamatergic pathway in the etiology of this psychiatric disease has also been pointed [2, 3].
Clinical and pre-clinical studies that include aromatherapy with essential oils rich in linalool and their potential interaction with the various factors involved in the pathophysiology of depressive disorders have been reported. Evidence of the action of linalool in the central nervous system (CNS), mainly acting as an antidepressant agent, is increasingly abundant [4-6]. Several studies have shown that aromatherapy based on linalool-rich essential oils, such as lavender (Lavandula angustifolia Mill.), bergamot (Citrus bergamia Risso), and orange (Citrus sinensis L.) improves mood and reduces symptoms of stress, anxiety, and depression of patients submitted to surgeries, invasive methods, endoscopic exams, hemodialysis, and dental procedures [7]. Besides, in human studies, linalool and its enantiomers have been investigated according to the physiological and mood-altering effects of essential oils fragrances. The data revealed that both ylang ylang essential oil (Cananga odorata Hook.) and linalool have “harmonizing” effects. The oils of peppermint (Mentha piperita L.) and spearmint (Mentha spicata L.), which presents lower concentrations of linalool, also displayed this same effect [8]. Thus, the knowledge of linalool's antidepressant action mechanisms is fundamental for future research, intending to highlight this natural compound as a new antidepressant phytomedication [9].
2. MATERIALS AND METHODS
Scientific information about depression, the pathophysiological factors and the action of linalool on these factors, other biological activities of linalool, and its natural sources were retrieved from the online bibliographic databases, including Web of Science, Scopus, Springer Link, Google Scholar, PubMed, and Science Direct. The keywords used include linalool, depression, pathophysiological factors, monoaminergic system, stress, HPA axis, neuroendocrine system, inflammatory process, oxidative stress, Brain-Derived Neurotrophic Factor (BDNF), biological activities, natural sources, and other related words. Several literature articles published before December 2020 were consulted. Secondary resources were also analyzed, like books and proceedings.
3. MOLECULAR ASPECTS OF DEPRESSION
The pathogenesis of depression may present multifactorial causes, mainly dysregulation of numerous neurotransmitters and metabolic systems. In this context, monoaminergic neurotransmitters, HPA axis, inflammatory process, oxidative stress, and neurotrophic factors appear in a prominent position in the etiology of the disease and continue to be the focus of several studies [3, 10].
Concerning the monoamines theory, serotonin and norepinephrine play a pivotal role in the pathophysiology of this psychiatric disorder. There is clear evidence of reduced availability of tryptophan, the precursor to serotonin, and changes in healthy physiological metabolism and serotonin level receptors may elicit depression. In addition to serotonin, noradrenergic dysfunction has been closely related to depression disease. Thus, several drugs currently used to treat depressive states present the pharmacological objective of increasing the monoamines levels on the synaptic cleft through the norepinephrine reuptake inhibitors, with minimal effect on serotonin receptors [11], or acting on both receptors with similar affinity [3]. Evidence of dopaminergic system involvement in depression is less noted compared to the serotonergic/noradrenergic pathway. However, dopamine effects have been related to mood regulation, reward system, and motivational circuitry. Thus, in clinical practice, specific antidepressant agents increase dopamine levels in the brain by inhibiting dopamine reuptake or acting as a dopaminergic receptor agonist [12].
The involvement of environmental stress and severe life events in the development of depression is also well documented [13]. Stress and depression share several mediators and circuitry that interfere with the clinical course of the disease [14]. Thus, protection against the onset of depression can occur with the absence of stress. Therefore, deregulation of the stress response, displaying morphofunctional changes in the CNS, can trigger depression [14]. Stress information is recognized in the cerebral cortex, which follows the hypothalamus, where the HPA axis activation occurs, regulating the stress response [3]. The corticotropin-releasing factor (CRF) regulates HPA axis activation by triggering adrenocorticotropic hormone (ACTH) secretion and consequent cortisol release from adrenal glands, characterizing stress conditions [15].
In this regard, there is extensive literature that aims to understand the close link between depression and stress. Depressed patients present cortisol hypersecretion, dysfunctional glucocorticoid response mechanisms, increased ACTH secretion, inefficient HPA axis suppression in response to exogenous glucocorticoid administration, impaired corticosteroid receptor signaling, and alteration of the levels of CRF [15]. However, contrasting results have been found for CRF [16]. Conflicting data were also described for drugs that act on the HPA axis [17]. Thus, further studies are needed to elucidate the role of the CRF activity on depression, considering the complexity of the pathophysiology of this disorder.
In addition to these factors, several studies confirm the involvement of positive regulation of inflammation in major depression, including in experimental results [18]. According to Gimeno and coworkers (2009) [19], depression occurs before inflammation, rather than as a result of it. However, depressed patients show all chemical signs of the inflammatory response, such as the increased concentration of acute phase mediators and expression of proinflammatory cytokines and their receptors, and high levels of chemokines and adhesion molecules found in blood-cerebrospinal fluid (CSF) [3]. Thus, anti-inflammatory therapy becomes a potential treatment, since the correlation between the pathogenesis of depression and immune activation and cytokine secretion, such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), interferon-γ (IFN-γ), leukotrienes (LTs) and prostaglandins (PGs) have been demonstrated [18, 20]. This robust finding indicates IL-6 and TNF-α as the most frequent cytokines involved in the pathogenesis of depression. Furthermore, antibodies targeting IL-6, interleukin-12/23, and TNF-α displayed a positive impact on the main depressive symptoms [21].
Exacerbated central activation of the inflammatory response may interfere with several known processes in the pathophysiology of depression, including neurotrophic support, oxidative stress, neurogenesis, and apoptosis [22, 23]. Thus, the scientific literature has reported the antidepressant efficacy of anti-inflammatory drugs [20]. However, the use of the anti-inflammatory therapeutic strategy in the clinical practice of primary depressive disorder patients is still incipient, despite the positive effects found in experimental studies. Thus, the most effective mechanism of anti-inflammatory action for the treatment of depression must be well outlined to expand the arsenal of antidepressant therapy, especially for patient’s refractory to conventional treatment.
The involvement of oxidative stress in depressive disorders has also been the focus of studies, highlighting that oxidative unbalance plays a pivotal role in the etiology of the disease. Depression has been related to increased free radical production and low antioxidant concentration [24]. Some studies have shown that extracellular antioxidant chemical compounds are affected by depression. Depressed patients present reduced serum levels of vitamin E, uric acid, albumin, and glutathione. In addition to the nonenzymatic antioxidants, enzymatic antioxidants are also essential for maintaining oxidative balance, in which catalase and superoxide dismutase activity appear to be altered by depression disorder [24].
Furthermore, the BDNF supports the neurotrophic hypothesis of depression. The relationship between neuroplasticity and stress stimuli has been explored. Stress conditions can lead to changes in neural processes and the number of neurons [10]. In neuroimaging studies with depressive patients, selective structural changes in different limbic and non-limbic brain regions, such as the prefrontal and cingulate cortex, were identified [25, 26]. Impairment of structural plasticity and cellular resilience may be closely related to depressive disorders, with deficiency of neurotrophic factors, such as BDNF and neurotrophin-3 [26, 27]. The reduction of BDNF compromises synaptic transmission on mood-controlling structures, such as the hippocampus and prefrontal cortex, and decreases neurogenesis [28-30]. According to experimental studies of depressive episodes, BDNF is directly related to stress, neurogenesis, and hippocampal atrophy [3]. Therefore, it should be noted that the increase of monoamine concentrations in the synaptic cleft by antidepressant treatments currently used in clinical practice stimulates, through adaptive mechanisms, the positive regulation of BDNF [28-30]. For example, agomelatine and vortioxetine treatments increased BDNF levels in patients affected by depressive disorders [31, 32]. Thereby, BDNF is closely linked to the other factors related to depression, according to the monoaminergic and inflammatory process theory [3]. The pathophysiological factors of depression are summarized in Table 1.
Table 1.
Pathophysiological factors of depression.
| Pathophysiological Theory | Relevant Features |
|---|---|
| Monoaminergic neurotransmitters | Decreased levels of serotonin, norepinephrine, and dopamine |
| HPA axis | Cortisol hypersecretion, dysfunctional glucocorticoid response mechanisms, increased ACTH secretion, and alteration of the CRF levels |
| Inflammatory process | Increased levels of IL-1, IL-6, TNF-α, IFN-γ, LTs, and PGs |
| Oxidative stress | Reduced levels of vitamin E, uric acid, albumin, glutathione, and altered levels of catalase and superoxide dismutase |
| Neurotrophic factors | Deficiency of neurotrophic factors, such as BDNF and neurotrophin-3 |
4. LINALOOL AS A THERAPEUTIC TOOL IN DEPRESSION
The potential activity of linalool in the CNS has been investigated for some time, and studies on its mechanism of antidepressant action have become of greater interest in recent years. Thus, new studies have been stimulated to expand knowledge about the interaction of linalool with the targets of monoaminergic pathways, using other types of antagonists and different pathways, in addition to treatments and behavioral models, including their kinetics. Although still scarce, existing research points to linalool as a promising natural compound in treating depression due to its interaction with the monoaminergic system's different targets and classic antidepressant drugs (Fig. 1).
Fig. (1).
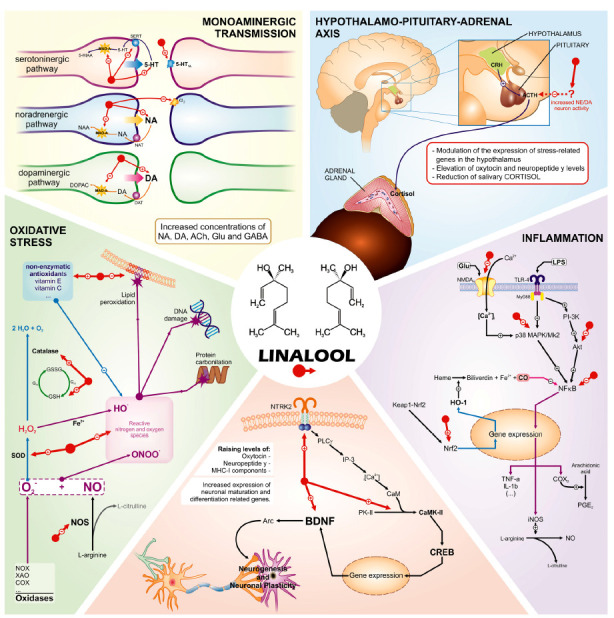
Hypothetical representation of potential linalool interaction targets on several pathophysiological factors of depressive disorders.
Abbreviations: 5-HT1A: serotonergic receptor, α2: noragrenergic receptor, α1: noragrenergic receptor, ABTS: 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), ACTH: adrenocorticotropic hormone, AKT: protein kinase B, ASC: apoptosis-associated speck-like protein containing a caspase-recruitment domain, BDNF: brain-derived neurotrophic factor, BrdU: bromodeoxyuridine, CALNN: Cys-Ala-Leu-Asn-Asn, CaMKII: calcium-calmodulin-dependent protein kinase II, cAMP: cyclic adenosine monophosphate, CNS: Central Nervous System, COX-2: cyclooxygenase 2, CRF: corticotropin-releasing factor, CSF: cerebrospinal fluid, DNA: deoxyribonucleic acid, HO-1: heme oxygenase-1, HPA: Hypothalamic-Pituitary-Adrenal, IFN-γ: interferon-γ, IL-1: interleukin-1, IL-1α: interleukin-1α, IL-1β: interleukin-1β, IL-6: interleukin-6, iNOS: inducible nitric oxide synthase, LPS: lipopolysaccharides, LTs: leukotrienes, MAPK: mitogen-activated protein kinase, NF-κB: nuclear factor kappa B, NLRP3: Nod-like receptor family, pyrin domain containing 3, NMDA: N-methyl-d-aspartate, NO: nitric oxide, Nos2: nitric oxide synthase 2, Nrf2: nuclear factor erythroid-2-related factor 2, PGE2: prostaglandins E2, PGs: prostaglandins, TLR4: toll-like receptor 4, TNF-α: tumor necrosis factor α, TrkB: tropomyosin receptor kinase B, UVB: ultraviolet –B.
4.1. Linalool Action on the Pathophysiological Factors of Depression
Our research group has studied linalool as an antidepressant agent since 2016. The potential for interaction of linalool with the CNS is significant, allowing it to be a new source of natural compounds capable of intervening in the pathways of neurodegenerative and behavioral disorders, including depression. In a work entitled “Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents,” some aromatic plants’ essential oils from Brazilian Amazon, rich in linalool, showed antidepressant activity on mice behavioral models [33]. Our study suggested the interaction of linalool with one or more pathophysiological factors of depression.
4.2. Linalool Action in Monoaminergic System
Studies of the potential involvement of the mechanism of action of linalool on the pathways of the monoaminergic system in antidepressant activity are still scarce. The first evidence emerges from the study by Guzmán-Gutiérrez and co-workers (2012) [34], showing the antidepressant activity of the essential oil and main constituents of Litsea glaucescens Kunth, as linalool and β-pinene, on the forced swimming model in mice. The antidepressant-like activity of linalool was subsequently ratified through the same behavioral model [9]. Additionally, the study evaluated the antidepressant mechanism of action of linalool using serotonergic, dopaminergic, and noradrenergic receptor antagonist drugs. The results suggest that prior administration of a serotonergic receptor antagonist (5-HT1A) blocks the antidepressant activity of linalool. However, using a serotonin synthesis inhibitor did not reverse this activity, suggesting the interaction of linalool with postsynaptic 5-HT1A receptors. In addition to these receptors, pretreatment with an α2 adrenergic receptor antagonist also reversed the effect of linalool. Therefore, the study suggests that the mechanism of linalool antidepressant action is due to its interaction with the serotoninergic and noradrenergic pathways [9].
In another study, lavender (Lavandula angustifolia Mill.) essential oil and linalool, its primary constituent, were evaluated for pharmacological targets involved in antidepressant effects. The results showed that both lavender oil and linalool inhibit serotonin reuptake. These findings may explain the antidepressant pharmacological activity shared by Lavender essential oil and linalool [35]. In a later investigation, the anti-stress effect of linalool was evaluated in mice subjected to sleep deprivation. Treatment with linalool decreased the animals' immobility time in the forced swim test and increased serotonin levels in the hippocampus. Also, linalool attenuated the reduction in sleep deprivation-induced plasma serotonin levels. Thus, the study suggests that linalool positively affects behavioral changes and memory consolidation through modulation of the serotonergic pathway [36]. These studies reveal the possibility of linalool interacting with different targets of the monoaminergic system.
Postsynaptic 5-HT1A receptors' involvement with the antidepressant activity has also been demonstrated by using a serotonergic agonist of this receptor subtype [37]. Besides, there was progressive sensitization of dorsal hippocampal postsynaptic 5-HT1A receptors following chronic use of a tricyclic antidepressant [38]. The scientific literature also describes the interaction of noradrenergic receptors (α1 and α2) with antidepressant drugs [39].
In another study, clonidine, a high-affinity α2-adrenergic agonist, had similar effects to antidepressants in the forced swimming test in mice [40]. On the other hand, these effects of clonidine were reversed by yohimbine, an α2 receptor antagonist, which may have occurred in postsynaptic noradrenergic receptors. According to the same study, increased stimulation of postsynaptic noradrenergic receptors relieves depressive symptoms [41]. Also, these findings corroborate the results demonstrated by Guzmán-Gutiérrez et al. [9].
Essential oils composed of primary alcohols and monoterpene hydrocarbons, belonging to the same plant biosynthetic pathways of linalool, also showed antidepressant activity by monoaminergic modulation, primarily of the serotoninergic pathway, through interaction with the 5-HT1A receptor [42-44]. Together, these findings indicate the need for further investigation on the mechanism of antidepressant action of linalool and similar compounds, particularly on the monoaminergic hypothesis of depression.
4.3. Linalool Action on Stress, HPA Axis Modulation, and the Neuroendocrine System
The interaction of linalool with monoamines and HPA axis modulation during stress induction was the study's objective in experimental models. In one of these studies, inhalation of linalool by menopausal rats restored noradrenaline and dopamine levels, which are reduced after stress induction due to high CNS catecholamine turnover [45]. According to study data, inhalation of linalool increased catecholamine levels to the standard index. Thus, the data suggest that linalool inhibits the accelerated turnover of catecholamine metabolism, increasing the activity of adrenergic/dopaminergic neurons, indirectly inducing the concomitant reduction of ACTH in animals. Despite controversial evidence, some studies indicate the potential inhibitory effect of catecholamines on the HPA axis through the regulation of ACTH secretion [46].
Another linalool interaction pathway has also been proposed by Yamamoto and co-workers (2013) [47] in which (+)-linalool modulates the genic expression on the hypothalamus in stressed rats, reflecting one of the mechanisms for reducing cortisol during stress. The gene expression profile revealed that treatment with (+)-linalool significantly modified 316 genes related to cell-to-cell signaling and nervous system development in the restrained rats. However, the authors failed to demonstrate the differences in ACTH, corticosterone, and leukocyte concentrations. Later, in a similar study, linalool exposure altered the expression of genes on the hypothalamic region related to synaptic transmission [48]. A comparative analysis of transcription levels demonstrated that the expression of 560 stress-induced probe sets was restored to a normal state. The neurotransmitters involved include oxytocin and neuropeptide Y, downregulated by stress and upregulated after linalool inhalation. Primary histocompatibility complex class I molecules, which are critical to neural development and plasticity, were also involved in altering the gene expression of the hypothalamus. Moreover, linalool restored dopamine, acetylcholine, and glutamate levels, as well as GABAergic transmission.
Investigation of the effects of (-)-linalool inhalation on stress hormones, blood cells, and gene expression has also been performed in rats subjected to a combination of physical and psychological stressors. Genetic ontology analysis revealed that stress-induced gene changes were suppressed by (-)-linalool. The treatment prevented alterations in 109 genes, although increased changes in 6 genes were detected in DNA microarray analysis. Besides, the suppression of changes in blood cell profiles has also occurred. A strong relationship was observed between the (-)-linalool-caused modulation of gene and immune system cell distributions. However, the treatment did not suppress plasma ACTH and corticosterone levels [49]. Probably, this occurred because chirality influences the effects of linalool on the physiological parameters of stress [50]. In another study, lavender essential oil and linalool increased social interaction behaviors and reversed the social aversion of stressed animals, similarly to antidepressant agents [51]. These data suggest that linalool may interact with different targets to modulate the HPA axis.
Evidence of linalool's influence on stress modulation in humans was also reported in a 24 volunteers' study of an experimental stress model, where linalool inhalation modulated stress response through changes in physiological parameters, such as reduced blood pressure levels, heart rate variation, and salivary cortisol [50]. In a similar study, inhalation of linalool-rich lavender essential oil also reduced cortisol levels and daytime blood pressure in 83 stressed prehypertensive subjects [52]. Also, lavender oil reduced cortisol and relieved stress symptoms in students who performed arithmetic tasks, such as a mental stressor [53] and stressed women suffering from urinary incontinence [54]. Cortisol reduction was also detected in healthy volunteers' saliva after lavender oil inhalation [55].
In a recent study, linalool-rich lavender essential oil showed a positive effect on depressive-type behaviors, increased neurogenesis, and dendritic complexity of rats in a model of depression, anxiety, and reduced neurogenesis induced by chronic corticosterone administration [56]. The study revealed that lavender essential oil reversed corticosterone-induced weight loss of the adrenal gland and normalized serum hormone levels 2-3 days after the treatment. Therefore, essential oil improved negative HPA axis feedback caused by exogenous corticosterone administration. In addition, lavender essential oil also upregulated the BDNF index and increased peripheral oxytocin levels, which had a protective effect against dorsal hippocampal stress damage [56, 57].
In a previous study, essential oil inhalation of the basil (Ocimum basilicum L.), which presents linalool as the main constituent, showed antidepressant effect by positive modulation of glucocorticoid receptor and BDNF gene, mRNA, and protein expressions in an equal manner than the standard drug fluoxetine. Also, the oil reduced the serum corticosterone levels and the hippocampal nerve cell atrophy, restoring the number of astrocytes and hippocampal nerve and normalizing glial apoptotic cells in stressed animals [58].
The linalool action on the HPA axis is still not very well understood. In animal studies, conflicting evidence of linalool activity on ACTH and corticosterone concentrations has been presented, while in humans, robust evidence points to its action in reducing cortisol levels. However, in preclinical studies, linalool under stressful conditions had effects on stress-related biological responses through modulation of the expression of hypothalamic genes [45, 47, 50].
Morphofunctional changes in CNS caused by dysregulation of the HPA axis are closely associated with the onset of depressive symptoms [15]. In experimental models, linalool prevented or restored stress-induced genes changes related to neural development, plasticity, and synaptic transmission [47, 48]. It is also possible that other mechanisms would be involved, such as the interaction with catecholamines and BDNF. Thus, linalool becomes promising for the treatment of various diseases that are related to HPA axis dysregulation. Currently, linalool is used mainly in aromatherapy, producing beneficial effects for treating depression, anxiety, and stress [4, 7].
4.4. Linalool Action on the Inflammatory Process
The anti-inflammatory activity of linalool is already well established. Several studies describe such activity through in vitro and in vivo models. In experimental models of inflammation, one of the enantiomers, the racemic mixture or undetermined enantiomers, inhibits the inflammatory response and reverses the main signs of inflammation [59]. CNS studies have demonstrated the importance of cytokines role, such as TNF-α, interleukin-1β (IL-1β), interleukin-1α (IL-1α), IFN-γ, in the inflammatory process [60]. According to this study, (-)-linalool inhibited the behavioral nociceptive responses of mice in a model of inflammatory hypersensitivity induced by IL-1β and TNF-α, blocking N-methyl-d-aspartate (NMDA) receptors.
In a recent study, the expression of IL-1β, nitric oxide synthase 2 (NOS2), TNF-α, and cyclooxygenase 2 (COX-2) genes were evaluated to determine whether they were involved in the linalool protective effect after NMDA induced excitotoxicity in the mice hippocampus. The results suggest that NMDA increased COX-2 gene expression while linalool treatment reversed the elevation of this inflammatory mediator. Linalool reduced NOS2 gene levels, while the results showed no efficiency to IL-1β and TNF-α [61]. In a model of inflammation induced by lipopolysaccharides (LPS), linalool inhibited the synthesis of TNF-α, IL-1β, nitric oxide (NO), and prostaglandins E2 (PGE2) through the activation of the nuclear factor erythroid-2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway. Also, LPS-induced nuclear factor kappa B (NF-κB) activation was inhibited by linalool in a dose-dependent manner [62]. Lately, daily use of intranasal linalool has produced an anti-inflammatory effect through the reduction of IL-1β and COX-2 levels in vivo and in vitro excitotoxicity models. It has also shown microglial protective activity in supporting neurological and cognitive recovery after an ischemia process [63].
Similarly, linalool reversed the neuropathological and behavioral deficiencies in a model of Alzheimer's disease in mice through an anti-inflammatory effect [64]. According to the results, linalool treatment reduced the inflammatory markers up-regulated in animals affected by Alzheimer's disease, such as IL-1β, p38 mitogen-activated protein kinase (MAPK), COX-2, and inducible nitric oxide synthase (iNOS). Besides, (-)-linalool prevented and recovered the inflammatory response of astrocytes induced by the combination of LPS and tryptase in an in vitro study [65]. In another inflammatory challenge study, (-)-linalool attenuated the carrageenan-induced inflammatory hyperalgesia and inhibited the development of L-glutamate and PGE2-induced inflammatory hyperalgesia, possibly through central mechanisms [66]. These studies ratify linalool as an anti-inflammatory agent, revealing its action on a wide range of cytokines and inflammatory mediators.
Peripherally, there is also evidence of linalool anti-inflammatory activity. In a recent study, linalool attenuated pulmonary inflammation and exacerbated ovalbumin-induced mucus production, and suppressed the recruitment of inflammatory cells and molecules in mice [67]. Also, linalool blocked primary inflammatory targets related to the inflammatory course, such as protein kinase B (AKT), MAPKs, and NF-κB. Thus, the study suggests that linalool presents an anti-inflammatory protective effect to asthmatic symptoms. In another study, oral administration of linalool prevented endotoxin-induced systemic inflammation by inhibiting NF-κB and suppressing the expression of toll-like receptor 4 (TLR4) pathway signaling molecules. Besides, linalool also suppressed the expression of key molecules of the Nod-like receptor family, pyrin domain containing 3 (NLRP3) signaling pathway such as apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), NLRP3, and caspase-1 [68].
In addition to its effects on stress conditions, linalool's anti-inflammatory properties have been well documented. The central mechanisms of anti-inflammatory action elicit the linalool as a useful agent in treating depression, also acting on this significant pathophysiological factor.
4.5. Linalool Action on Oxidative Stress
The ability of free radical scavenging is an antioxidant feature that plays a crucial role in reducing cell damage. Linalool has been reported for its antioxidant activity on the oxidative stress related to depression in numerous works of literature, which confers the possible contribution in such pathophysiological factors [24, 69].
Linalool isolated from the essential oil of ginger (Zingiber officinale Roscoe) rhizomes showed a significant antioxidant effect, displaying a medium inhibitory effect on erythrocyte hemolysis and a slight inhibitory effect on the 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) method [70]. According to this study, ginger oil and linalool can produce natural antioxidants and flavoring agents. In a posterior study, linalool on the surface of modified glutathione gold nanoparticles, or linked to the Cys-Ala-Leu and lipoic acid, displayed a protective effect on oxidative stress induced by hydrogen peroxide in guinea pig brain tissue [71, 72]. The provided neuroprotection was mediated by eliminating peroxyl radicals and increased levels of superoxide dismutase and catalase, reducing oxidative deoxyribonucleic acid (DNA) damage [73]. Also, linalool presented a neuroprotective effect against the acrylamide-induced neurotoxicity model in rats. According to the study, administration of (-)-linalool increased glutathione content in the cerebral cortex, and reduced malondialdehyde concentration, and the abnormalities caused by acrylamide [74]. Such results have been confirmed by other neuronal injury models induced by oxygen and glucose deprivation, in which (-)-linalool showed a neuroprotective effect through the decrease of oxidative stress due to its ability to eliminate free radicals, such as peroxyl radicals, restoring the reduced activities of superoxide dismutase and catalase associated to the minimization of reactive species oxygen [75].
In another study, linalool antioxidant activity has been identified as a potential mechanism of action to attenuate uremia-induced vascular calcification in rats. In the same study, linalool showed moderate superoxide radical scavenging ability and low hydrogen peroxide scavenging ability [76]. Thus, linalool negatively regulated oxidative stress in uremic animals due to its moderate antioxidant action, and this compound directly reduced the synthesis of superoxide radicals. Also, linalool showed a protective effect in oxidative stress conditions by preserving and restoring mitochondrial function [61].
The protective and therapeutic effects of linalool in other body systems were also determined by its antioxidant capacity on doxorubicin-induced cardiotoxicity in rats through histopathological and biochemical methods. Linalool treatment significantly increased glutathione, superoxide dismutase, and catalase levels, relieving the symptoms of doxorubicin-induced cardiomyopathy [77]. Also, linalool prevented the synthesis of reactive oxygen species, depletion of endogenous antioxidant compounds, and lipid peroxidation while eliminated free radicals in human epithelial cells exposed to ultraviolet-B (UVB) [78]. Additionally, inhalation of linalool increased the antioxidant activity in patients with carpal tunnel syndrome [79]. In a Pasteurella multocida-induced lung inflammation, linalool showed a protective effect by oxidative stress modulatory activity [80]. According to the results, linalool increased superoxide dismutase activity, reduced the accumulation of reactive oxygen species, and increased Nrf2 expression. Thus, an alternative mechanism of modulation of linalool antioxidant activity would be activating the Nrf2 signaling pathway. Subsequently, coriander (Coriandrum sativum L.) oil and linalool displayed antioxidant activity by eliminating free radicals and significantly inhibiting lipid peroxidation [81, 82]. These varieties of results suggest different mechanisms for linalool antioxidant action, focused on enzymatic antioxidant modulation, as well as free radicals scavenging activities.
In an innovative study, a simple, fast, and inexpensive method was proposed to determine and compare the antioxidant activity of different monoterpenes. Monoterpenes' ability to prevent oxidation of a dye in the presence of a strong oxidant was evaluated. The results suggest an intense antioxidant activity of linalool in a lower concentration than the other monoterpenes [83]. In another study, linalool showed antioxidant activity by its co-oxidation with cumene, used as a reaction substrate [84].
Although the involvement of oxidative stress with depression is not well elucidated, studies have correlated such a possible relationship. Thus, a significant antioxidant activity of linalool already described in several studies may act synergistically with its effects on the other pathophysiological factors of the disease, converging to general antidepressant activity. It is also important to note that linalool antioxidant activity can eliminate free radicals and elevate antioxidant levels.
4.6. Linalool Action in BDNF and Neurotrophic Theory of Depression
In general, the potential interaction of linalool with neurotrophic factors, especially BDNF and neuroplasticity, still has little evidence. However, recent studies have reported a close relationship. In a model of Alzheimer's disease in mice, the lavender oil and linalool improved animals' cognitive function through various mechanisms, highlighting the neuroprotective effect against oxidative stress. The treatment restored the reduced expression of synaptic plasticity-related proteins, such as calcium-calmodulin-dependent protein kinase II (CaMKII), BDNF, and tropomyosin receptor kinase B (TrkB), in the hippocampus [85].
In another study, gene expression in the hypothalamus of rats exposed to linalool after restriction stress induction was evaluated by analyzing genetic ontology. Findings revealed that linalool inhalation regulated the expression of genes related exclusively to synaptic transmission, nerve impulse transmission, and the peptide antigens’ processing and presentation [48]. Thus, linalool restored expression of genes encoding neurotransmitters, such as oxytocin and neuropeptide Y, and primary histocompatibility complex class I molecules. Therefore, linalool promoted positive effects on synaptic connectivity, development, and neuronal plasticity. According to Leuner and co-workers (2012) [57], oxytocin improves plasticity, increases cell proliferation and hippocampal neurogenesis.
In a similar study, inhalation of (-)-linalool increased the expression of genes related to neuron differentiation, associated with the increase of genes expression related to neurite development, and transcriptional regulatory factors, which are fundamental for neuronal maturation [86]. This study showed that (-)-linalool positively affects neuron maturation by regulating neuronal connectivity and synaptic formation in the hypothalamus. These findings suggest different mechanisms of action of linalool on neuroplasticity.
In addition to linalool isolated studies, linalool-rich essential oils works have also been performed. In one of these studies, inhaling linalool-rich lavender essential oil increased the number of positive bromodeoxyuridine (BrdU) cells (neuronal cell proliferation marker) in the hippocampus and subventricular zone, as reversed corticosterone-induced suppression of neurogenesis in rats. The improvement in hippocampus dendritic complexity and increase of immature cells in the subventricular zone were also found. Besides, there was an increased concentration of BDNF and oxytocin. Thus, the study suggests the positive effect of linalool-rich lavender essential oil on depressive-like behaviors, neurogenesis, and dendritic complexity [56].
The attenuating effect of inhaling the basil essential oil, which has linalool as its primary constituent, was evaluated for changes associated with unpredictable moderate stress through an animal model of depression [58]. According to the study, the basil essential oil reduced hippocampal nerve cell atrophy and restored the reduced number of stress-induced astrocytes. In addition, the essential oil reduced nerve and glial apoptotic cells in the hippocampus of stressed animals. The authors postulated that the linalool antidepressant mechanism of action relies on the positive modulation of gene and protein expression of BDNF and glucocorticoid receptors [58].
The development of studies on neuroplasticity has identified its involvement with significant depression. Besides, it has identified the positive activity of traditional antidepressants on this etiological factor [3]. Additionally, clinically practiced drugs have evidenced the interaction of natural compounds on neuroplasticity. Recent investigations indicate the beneficial effects of linalool by increasing neurotrophic factors, especially BDNF, triggering neuronal differentiation and neuroplasticity in limbic regions [85, 86]. Besides, linalool's regulatory action on the HPA axis would be one of the consequences of this mechanism. All pathophysiological factors of depression are closely related. Thus, linalool could interact directly with such factors by different mechanisms of action or act on a specific pathway, which may display a cascade of indirect effects on the other depressant molecular mechanisms. Only through advanced new studies, these questions will be answered.
4.7. New Approaches on the Pathogenesis of Depression
4.7.1. Linalool Versus the Microbiota-gut-brain Axis
In recent years, significant progress on the knowledge of bidirectional communication between the CNS and the gastrointestinal tract has been postulated. Several pieces of evidence have also emerged revealing the role of the gut microbiota on the gut-brain relationship [87, 88]. The hypothesis of the microbiota-gut-brain axis imbalance has been linked to the pathogenesis of depression [89]. Alterations in gut microbiota can lead to several conditions, such as increased gut barrier permeability, modulation of release and efficacy of monoamines, alteration of the HPA axis activity, activation of the systemic inflammation and immune response, as well as the BDNF levels modification [90]. Thus, the imbalance of the microbiota-gut-brain axis has been closely associated with several pathophysiological factors of depression.
In this scenario, the linalool antimicrobial activity against pathogens on the digestive system has been a pivotal role related to the potential beneficial effects on the regulation of the gut microbiota, with contributions on the depressive symptoms. Studies revealed that linalool was effective in inhibiting pathogenic strains of E. coli, P. aeruginosa, S. typhimurium, S. aureus, C. botulinum, C. perfringens, S. enterica, B. fragilis, S. enteritidis, L. monocytogenes, and B. thetaiotaomicron [91-93]. In addition, linalool presented moderate activity against six other harmful intestinal bacteria [94, 95].
Importantly, preclinical evidence of the positive effect of linalool-rich essential oils on gut microflora emphasizes that intestinal pathogen reduction has been associated with the resident microbiota increase or preservation (i.e., Lactobacillus spp) [96-99]. An additional mechanism of the regulatory and stabilization of the microbiota may be attributed to the increase of digestive enzymes activity and positive histomorphological changes of the intestinal tissue, such as increase of the villus height and crypt depth, as well as reduction of goblet cells and epithelial thickness [96, 97, 100]. These positive changes in the structure of intestinal mucosa indicate the improvement of intestinal health [100]. in vitro assays have reinforced the antibacterial effect against E. coli of linalool-rich essential oils, due to which these natural products have been suggested as good candidates for the treatment of irritable bowel syndrome, in which one of the causative factors relies on the alteration of gut microbiota followed by infection [101].
The advancement of research on the dysfunction of the microbiota-gut-brain axis has elicited several pieces of evidence linking the imbalance of gut microbiota on the pathophysiological factors of depression. The linalool may interact with several pathophysiological factors of the disease, as previously discussed. In addition, studies have shown the positive impact of linalool and linalool-rich essential oils on the modulation of the gut microbiota, which opens the possibilities to investigate the relationship of the linalool bioactive compound versus depression disorder. This finding consists of an additional element that reinforces linalool as a probable new candidate for the treatment of depression.
4.7.2. Linalool Action in the Glutamatergic System
Due to the critical limitations of conventional antidepressants, numerous studies have focused on the glutamatergic system, especially the NMDA receptor, to find therapeutic alternatives for the treatment of depression [102]. Several pieces of evidence support the involvement of glutamate-NMDA receptor overstimulation on the etiopathology of depression through the animal models of depression, postmortem tissues of suicide victims, and detection of changes in NMDA receptor activity after chronic administration of antidepressants [103]. The findings suggest that the blockade of NMDA receptors elicits improvement or remission of symptoms in the depressed state [102].
Recently, considerable advancement in antidepressant therapy has occurred with the approval of esketamine, an NMDA receptor antagonist, to treat resistant depression in adults [102]. However, the approval of this ketamine isomer follows significant restrictions due to adverse effects. Dissociation and delirium may occur in low doses shortly after the start of the infusion, which tends to disappear before the antidepressant response [102]. Our research group has evaluated several intermittent ketamine exposure implications, in which anxiogenic- and depressive-type behaviors, as well as memory impairment related to hippocampal and systemic oxidative damage, were found in female adolescent rats [104].
Despite dual evidence and a still poorly understood mechanism, the potential antidepressant activity of NMDA receptor blocker compounds highlights a possible new target for linalool's antidepressant effect. The properties of linalool as an antagonist of NMDA receptors have been proposed through the antinociceptive, analgesic, sedative, and anticonvulsant challenges [5, 105]. Finally, linalool showed anxiolytic and antidepressant effects by NMDA receptor blockade and inhibiting the reuptake of serotonin [35].
These findings on the glutamatergic system modulatory effects reflect an additional target for the linalool antidepressant action. However, the antidepressant effect cannot be explained exclusively by the interaction with NMDA receptors. We suggest that the pleiotropic property of linalool confers the antidepressant response due to direct mechanisms, such as the involvement of several neurotransmitters, and indirect mechanisms, such as adaptive responses.
4.8. Other Biological Activities of Linalool
In addition to the action on the pathophysiological factors of depression, linalool has several other biological activities well documented in the scientific literature. In one of these studies, anticonvulsant activity of enantiomers and the racemic mixture of linalool was demonstrated through the pentylenetetrazole- and picrotoxin-induced challenge in mice [106]. Previously, linalool also showed anticonvulsant activity in glutamate-related seizure models [107]. According to Brum et al., the direct interaction with NMDA receptors consists of the mechanism of anticonvulsant action elicited by linalool [108]. In another investigation, linalool-rich rosewood (Aniba rosaeodora Ducke) oil, (-)-linalool, and its racemate inhibited the activity of adenylate cyclase in the retina of chicks, preventing cell accumulation of cyclic adenosine monophosphate (cAMP), which has been implicated in the pathophysiology of epilepsy. Thus, the study suggests that the enzyme's inhibition would be a secondary mechanism to these constituents' relaxing and anticonvulsant action [109]. Vago-vagal bradycardia and depressor reflex induced by linalool-rich rosewood oil have also been reported in rats [110].
Sedation is another well-established biological activity of linalool. In a mice model, inhaled linalool produced sedative effects, eliciting hypothermia, reducing locomotion, and increasing the sleep time induced by pentobarbital [111]. The essential oil of Litsea glaucescens Kunth, constituted by linalool and β-pinene as active principles, was administered intraperitoneally in mice, exhibiting sedative effects without affecting muscle strength and motor coordination of the animals [34]. In another experimental protocol, coriander (Coriandrum sativum L.) seed oil and linalool showed similar sedative effects after intracerebroventricular administration in neonatal chicks [112]. The linalool-rich rosewood (Aniba rosaeodora Ducke) essential oil also elicited a sedative effect and depressed the excitability of the sciatic nerve of mice [113].
Addressing other activities of linalool on the CNS, there are pieces of evidence of its potential anxiolytic action. In a recent study, linalool inhalation induced a significant anxiolytic-type effect in mice classic behavioral tests. There was no effect in anosmic mice, indicating that it occurs through the olfactory input of linalool. The anxiolytic-type effects of linalool were antagonized by flumazenil, demonstrating the interaction with GABAergic transmission [114]. Another work revealed that lavender (Lavandula angustifolia Mill.) essential oil and linalool neutralized the social defeat-induced social aversion in mice tests, promoting sedative effects. Thus, according to the authors, both oil and linalool per se may be a valuable tool to treat anxiety and social disorders caused by social stress [51]. Linalool inhalation displayed a mice anxiolytic-type activity, increasing social interaction and decreasing the animals' aggressive behavior. The study suggested that intranasal administration of linalool-rich essential oils promotes relaxation and anxiolytic activity [115]. A variety of experimental studies have revealed anxiolytic-type effects derived from linalool-rich essential oils (i.e., Cinnamomum osmophloeum Kaneh.), linalool enantiomers (i.e., (+)-linalool), and linalool racemic mixture. The results showed a reduction in serotonin, dopamine, and norepinephrine released by the frontal cortex and hippocampus, while there was also an increase of dopamine in the mice striatum [116]. All the studies mentioned above reveal the broad spectrum of a promising bioactive chemical compound and biological activities of linalool on the CNS and improve its importance as a potential therapeutic tool for neurological and psychiatry disorders. Table 2 describes the experimental model, extract, dose, and treatment of the literature related to the linalool activities on the pathophysiological factors of depression and other activities on the CNS.
Table 2.
Summary of studies of the linalool action on the pathophysiological factors of depression and other activities on the central nervous system.
| Linalool Action on Monoaminergic System | ||||
| References | Experimental Model | Extract/Constituent | Concentration/Dose | Duration of Treatment |
| Guzman-Gutierrez et al., 2015 | Pre-treatment in vivo |
β-pinene and linalool | β-pinene i.p. and linalool i.p. (100 mg/kg) | 3 times (24, 18 and 1 h before forced swimming test) |
| Guzman-Gutierrez et al., 2012 | Pre-treatment in vivo |
Litsea glaucescens essential oil (linalool 3.64%), β-pinene and linalool | Litsea glaucescens essential oil i.p. (100 and 300 mg/kg), β-pinene i.p. and linalool i.p. (100 mg/kg) | 3 times (24, 18 and 1 h before behavioral tests) |
| López et al., 2017 | Pre-, co-treatment in vitro |
Lavender essential oil (linalool 37.4%), linalool and linalyl acetate | Lavender oil (0.8, 4 and 8 µl/ml) and linalool (8, 0.8, 0.08 and 0.008 µl/ml); lavender oil – cell viability (0.05, 0.1, 0.5 and 1 µl/ml) | Incubation for 30 min (MAO-A); 2 h (SERT); 40 min (GABAA); 1 h (NMDA receptor); 0, 2 and 24 h before hydrogen peroxide, malonate and Aβ25–35 |
| Lee et al., 2018 | Post-treatment in vivo |
Linalool | Linalool i.p. (0.3, 1 and 3 mg/kg) | 24 h after sleep deprivation model |
| Yamada et al., 2005 | Pre-, post-treatment in vivo |
Lavender essential oil and linalool | Inhalation of lavender oil and linalool – 0.3 and 0.7 ml in a plastic cage (32 cm × 21 cm × 13 cm) | Twice a day/3 days one month after ovariectomy and before inhalation ether vapor |
| Yamamoto et al., 2013 | Co-treatment in vivo |
(+)-linalool | Inhalation of (+)-linalool – 20 µl in a 40 l box | During restraint stress |
| Yoshida et al., 2017 | Co-treatment in vivo |
Linalool | Inhalation of linalool – 20 µl in a 40 l box | During restraint stress |
| Nakamura et al., 2009 | Co-treatment in vivo |
(-)-linalool | Inhalation of (-)-linalool – 20 µl in a 40 l box | During restraint stress |
| Höferl et al., 2006 | Co-treatment in vivo |
(-)-linalool and (+)-linalool | Inhalation of (-)-linalool (2.7 mg/m3) and (+)-linalool (9.8 mg/m3) | During standardised stress task |
| Caputo et al., 2018 | Pre-, post-treatment in vivo/co-treatment in vitro |
Lavender essential oil (linalool 33.1%) and linalool | Lavender oil i.p. (200 mg/kg) and linalool i.p. (100 mg/kg)/lavender oil (100-400 µg/ml) and linalool (100-200 µg/ml) | 30 min before experiment 1; 10 min after stress and 30 min before experiment 2; 17 days after acute or a chronic administration (once a day/10 days), 30 min before stress and 1 h 10 min before behavioral tests/cells were treated for 24 h |
| Kim et al., 2012 | Post-treatment in vivo |
Essential oils mixed – lavender, ylang-ylang, marjoram and neroli | Inhalation of lavender, ylang-ylang, marjoram and neroli oils – the oils were blended at a 20:15: 10:2 ratio |
Immediate effects (2 min); four weeks (24 h) |
| Toda and Morimoto, 2008 | Post-treatment in vivo |
Lavender essential oil | Inhalation of lavender oil – 150 µl infiltrated into filter paper to 10 cm from the nose | After the arithmetic task |
| Seol et al., 2013 | Co-treatment in vivo |
Lavender (linalool 33.3%) and clary (linalool 17.7%) essential oils | Inhalation of lavender oil (5%); Inhalation of clary oil (5%) | 1 single-dose inhalation during urodynamic examination |
| Atsumi and Tonosaki, 2007 | Pre-treatment in vivo |
Lavender and rosemary essential oils | Inhalation of essential oils – 1000 times dilution (low-concentration solution) and 10 times dilution (high-concentration solution) | Natural breathing for 5 min |
| Sánchez-Vidaña et al., 2019 | Co-treatment in vivo |
Lavender essential oil | Inhalation of lavender oil - cotton impregnated with 1 ml (2.5%) in an acrylic fiber box (42 × 30 × 29 cm) | Exposure of 1 h daily for 14 consecutive days (with corticosterone administration) |
| Ayuob et al., 2017 | Co-treatment in vivo |
Basil essential oil (linalool 35.9%) | Inhalation of basil oil - 1 ml (1, 2.5 and 5%) on cotton inside a odor-isolated chamber (32 × 24 × 32 cm) | Once daily for 15 min in the last 2 weeks of exposure to chronic unpredictable mild stress (4 weeks) |
| Huo et al., 2013 | Pre-treatment in vivo/in vitro |
Linalool | Linalool i.p. (25 mg/kg)/linalool (40, 80 and 120 µg/ml) | 1 h before LPS-induced acute lung injury/1 h before LPS stimulation |
| Batista et al., 2010 | Pre-, post-treatment in vivo |
(-)-linalool | Acute treatment: (-)-linalool i.p. (50 and 200 mg/kg); chronic treatment: (-)-linalool i.p. (50 mg/kg) | A single injection 30 minutes before the inflammatory induction by cytokines, 24 h after the CFA or 7 days after surgery; twice a day (every 12 h) for a period of 7 days for both methods |
| Sabogal-Guáqueta et al., 2019 | Co-treatment in vitro |
Linalool | Linalool 100 μM | Incubated with glutamate or NMDA |
| Li et al., 2015 | Pre-treatment in vitro |
Linalool | Linalool (25, 50 and 100 µg/ml) or (162, 324, 648 µM) | 1 h before LPS treatment |
| Barrera-Sandoval et al., 2019 | Post-treatment in vivo/in vitro |
Linalool | Bioavailability study: single-dose of linalool v.o., i.p. and i.n. (25 mg/kg); other tests: linalool i.n. (25 mg/kg)/linalool (100 nM) | Once a day for one month after focal ischemia/cells treated for 24 h after glutamate |
| Sabogal-Guáqueta et al., 2016 | Post-treatment in vivo |
Linalool | Linalool p.o. (25 mg/kg) | Every 48 h for 3 months on aged mice with a triple transgenic model of Alzheimer's disease |
| Hansson et al., 2016 | Pre-, co-, post-treatment in vitro |
Cocktail of naloxone, (-)-linalool and levetiracetam | Naloxone (10-12 M), (-)-linalool (10-6 M) and levetiracetam (10-4 M) | Cocktail for 30 min, then, cocktail and LPS + tryptase for 24 h; LPS + tryptase for 24 h, then, LPS + tryptase and cocktail for an additional 24 h; LPS for 24 h, and then the cocktail 3.5 min before glutamate stimulation |
| Peana et al., 2004 | Pre-treatment in vivo |
(-)-linalool | (-)-linalool s.c. (50, 100, 150 and 200 mg/kg) | 30 min before carrageenan, L-glutamate and PGE2 |
| Kim et al., 2019 | Co-, post-treatment in vivo/pre-treatment in vitro |
Linalool | Linalool p.o. (15 and 30 mg/kg)/ linalool (2.5, 5, 10, 20, 40 and 80 µg/ml) | After ovalbumin and alum, linalool for 5 consecutive days; ovalbumin was also administered in the last 3 days of linalool/1 h before incubation with LPS for 24 h |
| Lee et al., 2018 | Pre-treatment in vivo |
Cinnamomum osmophloeum Kanehira essential oil (linalool 40.24%), cinnamaldehyde and linalool | C. osmophloeum essential oil p.o. (13 mg/kg), cinnamaldehyde p.o. (0.45 and 0.9 mg/kg) and linalool p.o. (2.6 and 5.2 mg/kg) | Every other day for 2 weeks before endotoxin (24 h after) |
| Elgendy and Semeih, 2019 | Co-treatment in vitro |
Linalool | Linalool (1.56, 3.125, 6.25, 12.5, 25, 50 and 100 µg/ml) | - |
| Jabir et al., 2019 | Pre-treatment in vivo/co-treatment in vitro |
Linalool, GNPs, Linalool-GNPs and linalool-GNPs-CALNN | All the compounds i.p. (500 µg/kg)/linalool (10 µg/ml), GNPs (10 µg/ml), LIN-GNPs (10 µg/ml - 10µg/ml), LIN-GNPs-CALNN (10 µg/ml - 10 µg/ml - 1 µg/ml) | in vivo toxicity: once before monitorization for 4 weeks/antioxidant activity (25 min of incubation); anticancer activity (48 h) |
| Celik and Ozkaya, 2002 | Co-treatment in vivo |
Linalool, lipoic acid and vitamin E | Lipoic acid i.p. (3 mg/kg), vitamin E i.p. (24 mg/kg) and linalool i.p. (120 mg/kg) | Every other day for 4 weeks during H2O2-induced oxidative stress |
| Thapa et al., 2019 | Pre-, co-treatment in vitro |
Linalool, nerolidol, thymol, geraniol, methylisoeugenol and agolin | Nerolidol (62.5 ppm), thymol (12.5 ppm), geraniol (125 ppm), methylisoeugenol (125 ppm), linalool (250 ppm) and Agolin (250 ppm) | Incubation for 1 and 24 h (cytotoxicity); 24 h (genotoxicity); 24 h (genoprotection) before exposition genotoxic agents |
| Mehri et al., 2015 | Pre-, co-treatment in vivo |
(-)-linalool | (-)-linalool i.p. (12.5, 25, 50 and 100 mg/kg) | Simultaneously (all doses), 3 days before (12.5 mg/kg) or 3 days after (12.5 mg/kg) starting acrylamide (daily for 11 days) and continued during treatment |
| Park et al., 2016 | Pre-, co-treatment in vitro |
(-)-linalool | (-)-linalool (10 μM) | Immediately before the initiation of oxygen-glucose deprivation and maintained throughout oxygen-glucose deprivation/reoxygenation |
| Kaur et al., 2018 | Co-treatment in vivo/in vitro |
Linalool | Linalool p.o. (100 and 150 mg/kg)/linalool (50, 100, 150 and 200 µg/ml) | Daily for 4 weeks with phosphate diet after adenine diet for 4 weeks |
| Sabogal-Guáqueta et al., 2019 | Co-treatment in vitro |
Linalool | Linalool 100 μM | Incubation with glutamate or NMDA |
| Oner et al., 2019 | Pre-, post-treatment in vivo |
Linalool | Linalool i.p. (50 and 100 mg/kg) | Daily for 5 days before and after single dose of doxorubicin (treatments in different groups) |
| Gunaseelan et al., 2017 | Pre-treatment in vitro |
Linalool | Linalool (30 μM) | Incubation for 1 h before exposition of UVB-radiation |
| Seol et al., 2016 | Post-treatment in vivo |
Linalool | Inhalation of linalool (1%) – 0.5 ml placed onto a gauze pad (3 × 2 cm2) | Gauze pad positioned 5 cm from the nose for 10 min in patients with carpal tunnel syndrome |
| Wu et al., 2014 | Post-treatment in vivo/co-, post-treatment in vitro |
Linalool | Linalool s.c. (5, 15 and 25 mg/kg)/ linalool (40, 80 and 120 μg/ml) | 2 h after infection and then at 12 h intervals/treatment for 6 h; after cells are challenged by P. multocida |
| Duarte et al., 2016 | Co-treatment in vitro |
Coriander essential oil (linalool 64.38%) and linalool | Oil of coriander and linalool (0.1-32 µl/ml) | Incubation for 48 h with the Campylobacter strains; 24 h with Chromobacterium violaceum; 90 min for the DPPH and 2 h for β-carotene bleaching test |
| Samojlik et al., 2010 | Pre-treatment in vivo/co-treatment in vitro |
Essential oil of coriander (linalool 74.6%), caraway and linalool | Oil of coriander p.o. (0.03 g/kg) and oil of caraway p.o. (0.13 g/kg)/ both oils (2.5, 5, 7.5, 10 and 12.5 μl/ml); oil of coriander (12.5, 18.75, 25, 50, 75, 100 and 125 μl/ml); linalool (unreported) |
Daily for 5 consecutive days before a single dose of CCl4 |
| Noacco et al., 2018 | Co-treatment in vitro |
linalool, geraniol and 1,8-cineole | Linalool, geraniol and 1,8-cineole (6.48 x 10-3-648 mM) | Incubation in the darkness for 5 min |
| Baschieri et al., 2017 | Co-treatment in vitro |
linalool, (+)-limonene and citral | Linalool (28-2800 mM), (+)-limonene (30-1500 mM) and citral (0.15-90 mM) | - |
| Xu et al., 2017 | Co-, post-treatment in vivo |
Lavender essential oil (linalool 37.96%) and linalool | Lavender oil i.p. and linalool i.p. (50 and 100 mg/kg) | Daily for 4 weeks (with D-galactose and AlCl3) and 9 days |
| Yoshida et al., 2017 | Co-treatment in vivo |
Linalool | Inhalation of linalool – 20 µl in a 40 l box | During restraint stress |
| Nakamura et al., 2010 | Co-treatment in vivo |
(-)-linalool | Inhalation of (-)-linalool – 20 µl in a 40 l box | During restraint stress |
| Sánchez-Vidaña et al., 2019 | Co-treatment in vivo |
Lavender essential oil | Inhalation of lavender oil - cotton impregnated with 1 ml (2.5%) in an acrylic fiber box (42 × 30 × 29 cm) | Exposure of 1 h daily for 14 consecutive days (with corticosterone administration) |
| Ayuob et al., 2017 | Co-treatment in vivo |
Basil essential oil (linalool 35.9%) | Inhalation of basil oil - 1 ml (1, 2.5 and 5%) on cotton inside a odor-isolated chamber (32 × 24 × 32 cm) | Once daily for 15 min in the last 2 weeks of exposure to chronic unpredictable mild stress (4 weeks) |
| Sousa et al., 2010 | Pre-treatment in vivo |
(-)-linalool, (+)-linalool and racemate of linalool | (-)-linalool i.p., (+)-linalool i.p. and linalool i.p. (100, 200 and 300 mg/kg) | 30 min before administration of pentylenetetrazol, picrotoxin and electroconvulsive shock |
| Elisabetsky et al., 1999 | Pre-, co-treatment in vivo/co-treatment in vitro |
Linalool | Linalool i.p. (350 mg/kg); linalool i.c.v. infusion (15, 30, and 45 mM); linalool p.o. (2.2 and 2.5g/kg)/linalool (0.1, 0.3, 1, 3 and 5 mM). | 30 min before NMDA; 5 min before quinolinic acid; treatments repeated once every 3 days (6 treatments) – linalool 30 min before pentylenetetrazol/incubation for 15 min with glutamate |
| Brum et al., 2001 | Co-treatment in vitro |
Linalool | Linalool (0.1, 0.3, 1, 3 and 5 mM) | Incubation for 1 h with glutamate, glycine and NMDA antagonist; 30 min with GABAA agonist |
| Sampaio et al., 2012 | Pre-, co-treatment in vitro |
Rosewood essential oil (linalool 84.8%), (-)-linalool and racemate of linalool | Rosewood oil, (-)-linalool and linalool (40 µM-17.5 mM) | Incubation for 10 min before and additional 20 min with forskolin |
| Siqueira et al., 2014 | Pre-, post-treatment in vivo/co-treatment in vitro |
Rosewood essential oil (linalool 87.7%) | Rosewood oil i.v. (1, 5, 10 and 20 mg/kg)/rosewood oil (0.15-771.25 μg/ml) | Before bivagotomy and capsaicin; after capsazepine, ondansetron and HC030031 |
| Linck et al., 2009 | Pre-treatment in vivo |
Linalool | Inhalation of linalool (1% and 3%) – sealed cylindrical (internal diameter 15 cm) glass chamber | 1 h of inhalation before the evaluation of pentobarbital-induced sleep time and other tests |
| Guzman-Gutierrez et al., 2012 | Pre-treatment in vivo |
Litsea glaucescens essential oil (linalool 3.64%), β-pinene and linalool | Litsea glaucescens essential oil i.p. (100 and 300 mg/kg), β-pinene i.p. and linalool i.p. (100 mg/kg) | 3 times (24, 18 and 1 h before behavioral tests) |
| Gastón et al., 2016 | Pre-treatment in vivo |
Coriander essential oil (linalool 81.7%) and linalool | Coriander oil i.c.v. and linalool i.c.v. (0.86, 8.6 and 86 μg/chick) | Immediately before open field test |
| Almeida et al., 2009 | Pre-, co-treatment in vivo/co-treatment in vitro |
Rosewood essential oil (linalool 87.7%) | Rosewood oil i.p. (100, 200 and 300 mg/kg)/rosewood oil (2, 5, 10, 50 and 100 μg/ml) | Before behavioral studies and together with pentobarbital to same tests/ incubation for 30 min |
| Harada et al., 2018 | Pre-, post-treatment in vivo |
Linalool | Inhalation of linalool – 0, 20, 200 and 2.000 µl in filter paper placed at each of the 4 corners of an acryl box (25 × 25 × 25 cm) | Inhalation for 30 min before behavioral tests; after flumazenil and WAY100635 to same tests; 2 weeks after anosmia |
| Caputo et al., 2018 | Pre-, post-treatment in vivo/co-treatment in vitro |
Lavender essential oil (linalool 33.1%) and linalool | Lavender oil i.p. (200 mg/kg) and linalool i.p. (100 mg/kg)/lavender oil (100-400 µg/ml) and linalool (100-200 µg/ml) | 30 min before experiment 1; 10 min after stress and 30 min before experiment 2; 17 days after acute or a chronic administration (once a day/10 days) 30 min before stress and 1 h 10 min before behavioral tests/cells were treated for 24 h |
| Linck et al., 2010 | Pre-treatment in vivo |
Linalool | Inhalation of linalool (1% and 3%) – sealed cylindrical (internal diameter 15 cm) glass chamber | 1h of inhalation before behavioral tests |
| Cheng et al., 2015 | Pre-treatment in vivo |
Essential oil of C. osmophloeum ct. linalool ((+)-linalool 90%), (-)-linalool and (+)-linalool | Essential oil p.o. (250 and 500 mg/kg), (-)-linalool p.o. and (+)-linalool p.o. (500 mg/kg) | Daily for 14 days before behavioral tests |
5. LINALOOL NATURAL SOURCES
Linalool is one of the constituents most found in aromatic plants' volatile concentrates, accounting for about 70% content in one thousand investigated aromatic plants [117]. Some species of fungi are also linalool producers [118]. The essential oil of rosewood (Aniba rosaeodora Ducke, Lauraceae) is one of the leading natural linalool sources. The species is found in the Amazon region of Brazil, Guyana, Suriname, Peru, Colombia, and Venezuela [119, 120]. In addition to rosewood, macacaporanga (Aniba parviflora [Meiss.] Mez), another Amazon Lauraceous species, is also a linalool source although with a lower content, ranging from 32% to 40% [120-122]. Oil of caatinga-de-mulata (Aeollanthus suaveolens Matt. ex Spreng., Lamiaceae), a medicinal plant cultivated in Belém, PA, Brazil, displayed linalool as a primary constituent, varying from 31.6% to 49.3% [123]. Essential oils rich in linalool (80-97%) are of great economic importance, especially for the aroma and cosmetics industry. For this reason, rosewood oil from the Amazon has been commercially explored for over 100 years [124].
Oil of coriander (Coriandrum sativum L., Apiaceae), an annual herbaceous from the Mediterranean and the Middle East, is another important natural source of linalool, with about 70% content [125]. It is cultivated mainly in India, North Africa, China, Europe, and Thailand and is used as a food flavoring agent [126, 127]. Besides, coriander oil has significant commercial value for its importance to the aroma industry [128]. Chemotype oils of basil (Ocimum basilicum L., Lamiaceae), an annual herb grown all over the world, also stands out for their high content of (-)-linalool, about 70%, which is widely used in the markets of food, pharmaceutical, cosmetic and aromatherapy [129]. The cultivation of different basil chemotypes generates various oils used in international trade [130]. Oil of lavender (Lavandula angustifolia Mill., Lamiaceae), an aromatic and medicinal plant originating in the Mediterranean and southern Europe, is also of great commercial importance, which presents linalool (25-38%) and linalyl acetate (25-45%) as its primary constituents [131, 132]. It is widely used in perfumes, cosmetics, food processing, and aromatherapy products [133]. The species Cinnamomum camphora (L.) J. Presl and C. osmophloeum Kaneh, natives to Southern China and Japan, have presented chemotype oils with a high linalool content (58-92%) and other chemotype oils with different primary constituents. These oils have been used as a raw material for the cosmetic and pharmaceutical industries [116, 134-137]. The oil of Cinnamomum osmophloeum ct. linalool, which occurs in Taiwan, has about 90% content of (+)-linalool, a significant source for aromatherapy uses [116, 138].
Linalool has long been produced in large volumes, whether from natural precursors or through chemical synthesis. For example, in 2000, half of the estimated amount of linalool was produced synthetically, while the other half was originated from aromatic plants [118]. Presently, there is a significant growth in several scientific areas with linalool as the protagonist. This fact highlights the importance of this monoterpene tertiary alcohol in the international market, in traditional medicine, in aromatherapy, and, more recently, for treating various diseases, such as depression.
CONCLUSION
In recent years, there has been considerable advancement in the knowledge of the etiology of depression. Previously, all attention was focused on the monoaminergic hypothesis of the disease. However, with scientific evolution, other pathophysiological factors started to play a prominent role. The most current hypotheses involve the close relationship between depression and environmental factors, endocrine, inflammatory, immunological, metabolic, and oxidative stress mediators, and cellular, molecular and epigenetic components of plasticity. In disease-related studies, the broader scope extends to the possibility of using natural products, interacting with different targets to become new antidepressant drugs.
In this context, linalool appears as a promising bioactive compound in the therapeutic arsenal, capable of treating depressive disorders. Studies have revealed that linalool positively interacts with a variety of pathophysiological factors involved in depression disorder. In addition, linalool potentially acts on different targets within the same etiological factor. These findings indicated several possible mechanisms of antidepressant action of linalool, a well-known natural aromatic compound. This profile of acting on multiple mechanisms on the pathophysiology of depression highlights its advantage over the conventional antidepressant treatment.
The traditional antidepressants currently used act directly on the monoaminergic pathway, mainly by inhibiting serotonin and norepinephrine reuptake. The linalool effect on several other pathways related to the pathogenesis of depression highlights its importance as an excellent candidate to integrate the medicines of clinical practice in the future. In addition, the broad spectrum of linalool mechanisms may reduce the dose required for the antidepressant response.
In this context, new studies on the mechanism of antidepressant action of linalool may focus on its pharmacokinetic profile, the use of different doses and routes of administration, and the application of different antagonists in the possible targets of the interaction with this monoterpene. Thus, it will be possible to intensify the knowledge about linalool's antidepressant activity and ultimately elucidate its pleiotropic mechanism of biological activity.
ACKNOWLEDGEMENTS
The authors acknowledge the support by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-FINANCE CODE 001). This work was also supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq/ Brazil through the Research Productivity Grant (number 311335/2019-5 to CSFM). José Guilherme Maia is also research fellowship funded by CNPq.
LIST OF ABBREVIATIONS
- 5-HT1A
Serotonergic receptor
- α2
Noragrenergic receptor
- α1
Noragrenergic receptor
- ABTS
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ACTH
Adrenocorticotropic hormone
- AKT
Protein kinase B
- ASC
Apoptosis-associated speck-like protein containing a caspase-recruitment domain
- BDNF
Brain-derived neurotrophic factor
- BrdU
Bromodeoxyuridine
- CALNN
Cys-Ala-Leu-Asn-Asn
- CaMKII
Calcium-calmodulin-dependent protein kinase II
- cAMP
Cyclic adenosine monophosphate
- CNS
Central nervous system
- COX-2
Cyclooxygenase 2
- CRF
Corticotropin-releasing factor
- CSF
Cerebrospinal fluid
- DNA
Deoxyribonucleic acid
- HO-1
Heme oxygenase-1
- HPA
Hypothalamic-pituitary-adrenal
- IFN-γ
Interferon-γ
- IL-1
Interleukin-1
- IL-1α
Interleukin-1α
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharides
- LTs
Leukotrienes
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa B
- NLRP3
Nod-like receptor family, pyrin domain containing 3
- NMDA
N-methyl-d-aspartate
- NO
Nitric oxide
- Nos2
Nitric oxide synthase 2
- Nrf2
Nuclear factor erythroid-2-related factor 2
- PGE2
Prostaglandins E2
- PGs
Prostaglandins
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor α
- TrkB
Tropomyosin receptor kinase B
- UVB
Ultraviolet -B
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-FINANCE CODE 001). This work was also supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq/ Brazil through the Research Productivity Grant (number 311335/2019-5 to CSFM). José Guilherme Maia is also research fellowship funded by CNPq.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.World Health Organization. Fact Sheets: Depression. Available from: https://www.who.int/en/news-room/fact-sheets/detail/depression (Accessed August 10, 2020).
- 2.Leonard B.E. Impact of inflammation on neurotransmitter changes in major depression: An insight into the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:261–267. doi: 10.1016/j.pnpbp.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Villas Boas G.R., Boerngen de Lacerda R., Paes M.M., Gubert P., Almeida W.L.D.C., Rescia V.C., de Carvalho P.M.G., de Carvalho A.A.V., Oesterreich S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019;851:99–121. doi: 10.1016/j.ejphar.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Aprotosoaie A.C., Hăncianu M., Costache I., Miron A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragrance J. 2014;29(4):193–219. doi: 10.1002/ffj.3197. [DOI] [Google Scholar]
- 5.Pereira I., Severino P., Santos A.C., Silva A.M., Souto E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces. 2018;171:566–578. doi: 10.1016/j.colsurfb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Perry N., Perry E. Aromatherapy in the management of psychiatric disorders: clinical and neuropharmacological perspectives. CNS Drugs. 2006;20(4):257–280. doi: 10.2165/00023210-200620040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Agatonovic-Kustrin S., Kustrin E., Gegechkori V., Morton D.W. Anxiolytic Terpenoids and Aromatherapy for Anxiety and Depression. In: Guest P.C., editor. Reviews on New Drug Targets in Age-Related Disorders. Cham: Springer; 2020. pp. 283–296. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara Y., Shigetho A., Yoneda M., Tuchiya T., Matumura T., Hirano M. Relationship between mood change, odour and its physiological effects in humans while inhaling the fragrances of essential oils as well as linalool and its enantiomers. Molecules. 2013;18(3):3312–3338. doi: 10.3390/molecules18033312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzmán-Gutiérrez S.L., Bonilla-Jaime H., Gómez-Cansino R., Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari F., Villa R.F. The neurobiology of depression: An integrated overview from biological theories to clinical evidence. Mol. Neurobiol. 2017;54(7):4847–4865. doi: 10.1007/s12035-016-0032-y. [DOI] [PubMed] [Google Scholar]
- 11.Cleare J.A. Biological Models of Unipolar Depression. In: Power M., editor. Mood Disorders: A Handbook of Science and Practice. Chichester: Wiley & Sons; 2004. pp. 29–46. [DOI] [Google Scholar]
- 12.Camardese G., Di Giuda D., Di Nicola M., Cocciolillo F., Giordano A., Janiri L., Guglielmo R. Imaging studies on dopamine transporter and depression: A review of literature and suggestions for future research. J. Psychiatr. Res. 2014;51:7–18. doi: 10.1016/j.jpsychires.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Paykel E.S. The evolution of life events research in psychiatry. J. Affect. Disord. 2001;62(3):141–149. doi: 10.1016/S0165-0327(00)00174-9. [DOI] [PubMed] [Google Scholar]
- 14.Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015:581976. doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesulola E., Micalos P., Baguley I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav. Brain Res. 2018;341:79–90. doi: 10.1016/j.bbr.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Paez-Pereda M., Hausch F., Holsboer F. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert Opin. Investig. Drugs. 2011;20(4):519–535. doi: 10.1517/13543784.2011.565330. [DOI] [PubMed] [Google Scholar]
- 17.Maric N.P., Adzic M. Pharmacological modulation of HPA axis in depression - new avenues for potential therapeutic benefits. Psychiatr. Danub. 2013;25(3):299–305. [PubMed] [Google Scholar]
- 18.Paudel Y.N., Shaikh M.F., Shah S., Kumari Y., Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur. J. Pharmacol. 2018;837:145–155. doi: 10.1016/j.ejphar.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Gimeno D., Kivimäki M., Brunner E.J., Elovainio M., De Vogli R., Steptoe A., Kumari M., Lowe G.D., Rumley A., Marmot M.G., Ferrie J.E. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittenberg G.M., Stylianou A., Zhang Y., Sun Y., Gupta A., Jagannatha P.S., Wang D., Hsu B., Curran M.E., Khan S., Chen G., Bullmore E.T., Drevets W.C., Drevets W.C. Effects of immunomodulatory drugs on depressive symptoms: A mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol. Psychiatry. 2020;25(6):1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goshen I., Kreisel T., Ounallah-Saad H., Renbaum P., Zalzstein Y., Ben-Hur T., Levy-Lahad E., Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8-10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Ben Menachem-Zidon O., Goshen I., Kreisel T., Ben Menahem Y., Reinhartz E., Ben Hur T., Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33(9):2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- 24.Wigner P., Czarny P., Galecki P., Su K-P., Sliwinski T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018;262:566–574. doi: 10.1016/j.psychres.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Gould E., Tanapat P., Rydel T., Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48(8):715–720. doi: 10.1016/S0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 26.Manji H.K., Drevets W.C., Charney D.S. The cellular neurobiology of depression. Nat. Med. 2001;7(5):541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A.N., da Costa e Silva B.F., Soares J.C., Carvalho A.F., Quevedo J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J. Affect. Disord. 2016;197:9–20. doi: 10.1016/j.jad.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobb J.A., Simpson J., Mahajan G.J., Overholser J.C., Jurjus G.J., Dieter L., Herbst N., May W., Rajkowska G., Stockmeier C.A. Hippocampal volume and total cell numbers in major depressive disorder. J. Psychiatr. Res. 2013;47(3):299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duman R.S. Pathophysiology of depression: the concept of synaptic plasticity. Eur. Psychiatry. 2002;17(S3) Suppl. 3:306–310. doi: 10.1016/S0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 30.Duman R.S., Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1601):2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinotti G., Pettorruso M., De Berardis D., Varasano P.A., Lucidi P.G., De Remigis V., Valchera A., Ricci V., Di Nicola M., Janiri L., Biggio G., Di Giannantonio M. Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int. J. Neuropsychopharmacol. 2016;19(5):pyw003. doi: 10.1093/ijnp/pyw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvojkovic A., Nikolac P.M., Sagud M., Nedic Erjavec G., Mihaljevic Peles A., Svob Strac D., Vuksan Cusa B., Tudor L., Kusevic Z., Konjevod M., Zivkovic M., Jevtovic S., Pivac N. Effect of vortioxetine vs. escitalopram on plasma BDNF and platelet serotonin in depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;105:110016. doi: 10.1016/j.pnpbp.2020.110016. [DOI] [PubMed] [Google Scholar]
- 33.Dos Santos É.R.Q., Maia C.S.F., Fontes Junior E.A., Melo A.S., Pinheiro B.G., Maia J.G.S. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 2018;212:43–49. doi: 10.1016/j.jep.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Guzmán-Gutiérrez S.L., Gómez-Cansino R., García-Zebadúa J.C., Jiménez-Pérez N.C., Reyes-Chilpa R. Antidepressant activity of Litsea glaucescens essential oil: identification of β-pinene and linalool as active principles. J. Ethnopharmacol. 2012;143(2):673–679. doi: 10.1016/j.jep.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 35.López V., Nielsen B., Solas M., Ramírez M.J., Jäger A.K. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017;8:280. doi: 10.3389/fphar.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B.K., Jung A.N., Jung Y-S. Linalool ameliorates memory loss and behavioral impairment induced by REM-sleep deprivation through the serotonergic pathway. Biomol. Ther. (Seoul) 2018;26(4):368–373. doi: 10.4062/biomolther.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luscombe G.P., Martin K.F., Hutchins L.J., Gosden J., Heal D.J. Mediation of the antidepressant-like effect of 8-OH-DPAT in mice by postsynaptic 5-HT1A receptors. Br. J. Pharmacol. 1993;108(3):669–677. doi: 10.1111/j.1476-5381.1993.tb12859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddjeri N., Blier P., de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998;18(23):10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millan M.J. The role of monoamines in the actions of established and “novel” antidepressant agents: A critical review. Eur. J. Pharmacol. 2004;500(1-3):371–384. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Bourin M., Colombel M.C., Malinge M., Bradwejn J. Clonidine as a sensitizing agent in the forced swimming test for revealing antidepressant activity. J. Psychiatry Neurosci. 1991;16(4):199–203. [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill M.F., Osborne D.J., Woodhouse S.M., Conway M.W. Selective imidazoline I2 ligands do not show antidepressant-like activity in the forced swim test in mice. J. Psychopharmacol. 2001;15(1):18–22. doi: 10.1177/026988110101500104. [DOI] [PubMed] [Google Scholar]
- 42.Abbasi-Maleki S., Kadkhoda Z., Taghizad-Farid R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J. Tradit. Complement. Med. 2019;10(4):327–335. doi: 10.1016/j.jtcme.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao C-W., Lai W-S., Ho C-T., Sheen L-Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: use of the tail suspension test. J. Funct. Foods. 2013;5(1):370–379. doi: 10.1016/j.jff.2012.11.008. [DOI] [Google Scholar]
- 44.Komiya M., Takeuchi T., Harada E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006;172(2):240–249. doi: 10.1016/j.bbr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Yamada K., Mimaki Y., Sashida Y. Effects of inhaling the vapor of Lavandula burnatii super-derived essential oil and linalool on plasma adrenocorticotropic hormone (ACTH), catecholamine and gonadotropin levels in experimental menopausal female rats. Biol. Pharm. Bull. 2005;28(2):378–379. doi: 10.1248/bpb.28.378. [DOI] [PubMed] [Google Scholar]
- 46.Jasnic N., Djordjevic J., Djurasevic S., Lakic I., Vujovic P., Spasojevic N., Cvijic G. Specific regulation of ACTH secretion under the influence of low and high ambient temperature—The role of catecholamines and vasopressin. J. Therm. Biol. 2012;37(7):469–474. doi: 10.1016/j.jtherbio.2012.04.003. [DOI] [Google Scholar]
- 47.Yamamoto N., Fujiwara S., Saito-Iizumi K., Kamei A., Shinozaki F., Watanabe Y., Abe K., Nakamura A. Effects of inhaled (S)-linalool on hypothalamic gene expression in rats under restraint stress. Biosci. Biotechnol. Biochem. 2013;77(12):2413–2418. doi: 10.1271/bbb.130524. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida K., Yamamoto N., Fujiwara S., Kamei A., Abe K., Nakamura A. Inhalation of a racemic mixture (R,S)-linalool by rats experiencing restraint stress alters neuropeptide and MHC class I gene expression in the hypothalamus. Neurosci. Lett. 2017;653:314–319. doi: 10.1016/j.neulet.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura A., Fujiwara S., Matsumoto I., Abe K. Stress repression in restrained rats by (R)-(-)-linalool inhalation and gene expression profiling of their whole blood cells. J. Agric. Food Chem. 2009;57(12):5480–5485. doi: 10.1021/jf900420g. [DOI] [PubMed] [Google Scholar]
- 50.Höferl M., Krist S., Buchbauer G. Chirality influences the effects of linalool on physiological parameters of stress. Planta Med. 2006;72(13):1188–1192. doi: 10.1055/s-2006-947202. [DOI] [PubMed] [Google Scholar]
- 51.Caputo L., Reguilon M.D., Mińarro J., De Feo V., Rodriguez-Arias M. Lavandula angustifolia essential oil and linalool counteract social aversion induced by social defeat. Molecules. 2018;23(10):2694. doi: 10.3390/molecules23102694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim I-H., Kim C., Seong K., Hur M-H., Lim H.M., Lee M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid. Based Complement. Alternat. Med. 2012;2012:984203. doi: 10.1155/2012/984203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toda M., Morimoto K. Effect of lavender aroma on salivary endocrinological stress markers. Arch. Oral Biol. 2008;53(10):964–968. doi: 10.1016/j.archoralbio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Seol G.H., Lee Y.H., Kang P., You J.H., Park M., Min S.S. Randomized controlled trial for Salvia sclarea or Lavandula angustifolia: differential effects on blood pressure in female patients with urinary incontinence undergoing urodynamic examination. J. Altern. Complement. Med. 2013;19(7):664–670. doi: 10.1089/acm.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atsumi T., Tonosaki K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007;150(1):89–96. doi: 10.1016/j.psychres.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Sánchez-Vidaña D.I., Po K.K-T., Fung T.K-H., Chow J.K-W., Lau W.K-W., So P-K., Lau B.W-M., Tsang H.W-H. Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats. Neurosci. Lett. 2019;701:180–192. doi: 10.1016/j.neulet.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 57.Leuner B., Caponiti J.M., Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayuob N.N., Firgany A.E.L., El-Mansy A.A., Ali S. Can Ocimum basilicum relieve chronic unpredictable mild stress-induced depression in mice? Exp. Mol. Pathol. 2017;103(2):153–161. doi: 10.1016/j.yexmp.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Huo M., Cui X., Xue J., Chi G., Gao R., Deng X., Guan S., Wei J., Soromou L.W., Feng H., Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180(1):e47–e54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 60.Batista P.A., Werner M.F., Oliveira E.C., Burgos L., Pereira P., Brum L.F.S., Story G.M., Santos A.R.S. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain. 2010;11(11):1222–1229. doi: 10.1016/j.jpain.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Sabogal-Guáqueta A.M., Hobbie F., Keerthi A., Oun A., Kortholt A., Boddeke E., Dolga A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019;118:109295. doi: 10.1016/j.biopha.2019.109295. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Lv O., Zhou F., Li Q., Wu Z., Zheng Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 2015;40(7):1520–1525. doi: 10.1007/s11064-015-1629-7. [DOI] [PubMed] [Google Scholar]
- 63.Barrera-Sandoval A.M., Osorio E., Cardona-Gómez G.P. Microglial-targeting induced by intranasal linalool during neurological protection postischemia. Eur. J. Pharmacol. 2019;857:172420. doi: 10.1016/j.ejphar.2019.172420. [DOI] [PubMed] [Google Scholar]
- 64.Sabogal-Guáqueta A.M., Osorio E., Cardona-Gómez G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansson E., Werner T., Björklund U., Skiöldebrand E. Therapeutic innovation: Inflammatory-reactive astrocytes as targets of inflammation. IBRO Rep. 2016;1:1–9. doi: 10.1016/j.ibror.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peana A.T., De Montis M.G., Sechi S., Sircana G., D’Aquila P.S., Pippia P. Effects of (-)-linalool in the acute hyperalgesia induced by carrageenan, L-glutamate and prostaglandin E2. Eur. J. Pharmacol. 2004;497(3):279–284. doi: 10.1016/j.ejphar.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Kim M-G., Kim S-M., Min J-H., Kwon O-K., Park M-H., Park J-W., Ahn H.I., Hwang J.Y., Oh S.R., Lee J.W., Ahn K.S. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019;74:105706. doi: 10.1016/j.intimp.2019.105706. [DOI] [PubMed] [Google Scholar]
- 68.Lee S-C., Wang S-Y., Li C-C., Liu C-T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018;26(1):211–220. doi: 10.1016/j.jfda.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elgendy E.M. Photooxygenation of natural ã-terpinene. Boll. Chim. Farm. 2004;143(9):337–339. [PubMed] [Google Scholar]
- 70.Elgendy E.M., Semeih M.Y. Phyto-Monoterpene linalool as precursor to synthesis epoxides and hydroperoxides as anti carcinogenic agents via thermal and photo chemical oxidation reactions. Arab. J. Chem. 2019;12(7):966–973. doi: 10.1016/j.arabjc.2018.09.008. [DOI] [Google Scholar]
- 71.Jabir M.S., Taha A.A., Sahib U.I., Taqi Z.J., Al-Shammari A.M., Salman A.S. Novel of nano delivery system for Linalool loaded on gold nanoparticles conjugated with CALNN peptide for application in drug uptake and induction of cell death on breast cancer cell line. Mater. Sci. Eng. C. 2019;94:949–964. doi: 10.1016/j.msec.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 72.Celik S., Ozkaya A. Effects of intraperitoneally administered lipoic acid, vitamin E, and linalool on the level of total lipid and fatty acids in guinea pig brain with oxidative stress induced by H2O2. J. Biochem. Mol. Biol. 2002;35(6):547–552. doi: 10.5483/bmbrep.2002.35.6.547. [DOI] [PubMed] [Google Scholar]
- 73.Thapa D., Richardson A.J., Zweifel B., Wallace R.J., Gratz S.W. Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. J. Food Sci. 2019;84(7):1979–1985. doi: 10.1111/1750-3841.14665. [DOI] [PubMed] [Google Scholar]
- 74.Mehri S., Meshki M.A., Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem. Toxicol. 2015;38(2):162–166. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- 75.Park H., Seol G.H., Ryu S., Choi I-Y. Neuroprotective effects of (-)-linalool against oxygen-glucose deprivation-induced neuronal injury. Arch. Pharm. Res. 2016;39(4):555–564. doi: 10.1007/s12272-016-0714-z. [DOI] [PubMed] [Google Scholar]
- 76.Kaur T., Kaul S., Bhardwaj A. Efficacy of linalool to ameliorate uremia induced vascular calcification in wistar rats. Phytomedicine. 2018;51:191–195. doi: 10.1016/j.phymed.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Oner Z., Altınoz E., Elbe H., Ekinci N. The protective and therapeutic effects of linalool against doxorubicin-induced cardiotoxicity in Wistar albino rats. Hum. Exp. Toxicol. 2019;38(7):803–813. doi: 10.1177/0960327119842634. [DOI] [PubMed] [Google Scholar]
- 78.Gunaseelan S., Balupillai A., Govindasamy K., Ramasamy K., Muthusamy G., Shanmugam M., Thangaiyan R., Robert B.M., Prasad N.R., Ponniresan V.K., Rathinaraj P. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12(5):e0176699. doi: 10.1371/journal.pone.0176699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seol G-H., Kang P., Lee H.S., Seol G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016;16:17. doi: 10.1186/s12883-016-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Q., Yu L., Qiu J., Shen B., Wang D., Soromou L.W., Feng H. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway. Int. Immunopharmacol. 2014;21(2):456–463. doi: 10.1016/j.intimp.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 81.Duarte A., Luís Â., Oleastro M., Domingues F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control. 2016;61:115–122. doi: 10.1016/j.foodcont.2015.09.033. [DOI] [Google Scholar]
- 82.Samojlik I., Lakić N., Mimica-Dukić N., Daković-Svajcer K., Božin B. Antioxidant and hepatoprotective potential of essential oils of coriander (Coriandrum sativum L.) and caraway (Carum carvi L.) (Apiaceae). J. Agric. Food Chem. 2010;58(15):8848–8853. doi: 10.1021/jf101645n. [DOI] [PubMed] [Google Scholar]
- 83.Noacco N., Rodenak-Kladniew B., de Bravo M.G., Castro G.R., Islan G.A. Simple colorimetric method to determine the in vitro antioxidant activity of different monoterpenes. Anal. Biochem. 2018;555:59–66. doi: 10.1016/j.ab.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Baschieri A., Ajvazi M.D., Tonfack J.L.F., Valgimigli L., Amorati R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017;232:656–663. doi: 10.1016/j.foodchem.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 85.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Yang Y., Liu X. The protective effect of lavender essential oil and its main component linalool against the cognitive deficits induced by D-galactose and aluminum trichloride in mice. Evid. Based Complement. Alternat. Med. 2017;2017:7426538. doi: 10.1155/2017/7426538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura A., Fujiwara S., Ishijima T., Okada S., Nakai Y., Matsumoto I., Misaka T., Abe K. Neuron differentiation-related genes are up-regulated in the hypothalamus of odorant-inhaling rats subjected to acute restraint stress. J. Agric. Food Chem. 2010;58(13):7922–7929. doi: 10.1021/jf101200p. [DOI] [PubMed] [Google Scholar]
- 87.Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster J.A., McVey Neufeld K-A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Limbana T., Khan F., Eskander N. Gut microbiome and depression: how microbes affect the way we think. Cureus. 2020;12(8):e9966. doi: 10.7759/cureus.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du Y., Gao X-R., Peng L., Ge J-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon. 2020;6(6):e04097. doi: 10.1016/j.heliyon.2020.e04097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cabuk M., Alcicek A., Bozkurt M., Imre N. Antimicrobial Properties of the Essential Oils Isolated from Aromatic Plants and Using Possibility as Alternative Feed Additives. ; Proceedings of the 2nd National Animal Nutrition Congress, Konya, Turquia; September 8-20; 2003. pp. 184–187. [Google Scholar]
- 92.Friedman M., Henika P.R., Levin C.E., Mandrell R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004;52(19):6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- 93.Soković M., Glamočlija J., Marin P.D., Brkić D., van Griensven L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15(11):7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ngan L.T.M., Moon J.-K., Kim J.-H., Shibamoto T., Ahn Y.-J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 2012;28(4):1575–1583. doi: 10.1007/s11274-011-0961-6. [DOI] [PubMed] [Google Scholar]
- 95.Wang L., Zhang Y., Fan G., Ren J.N., Zhang L.L., Pan S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019;99(8):4019–4028. doi: 10.1002/jsfa.9629. [DOI] [PubMed] [Google Scholar]
- 96.Ghazanfari S., Moradi M.A., Bardzardi M.M. Intestinal morphology and microbiology of broiler chicken fed diets containing myrtle (Myrtus communis) essential oil supplementation. Iran. J. Appl. Anim. Sci. 2014;4(3):549–554. [Google Scholar]
- 97.Ghazanfari S., Mohammadi Z., Moradi M.A. Effects of coriander essential oil on the performance, blood characteristics, intestinal microbiota and histological of broilers. Braz. J. Poult. Sci. 2015;17(4):419–426. doi: 10.1590/1516-635X1704419-426. [DOI] [Google Scholar]
- 98.Adaszyńska-Skwirzyńska M., Szczerbińska D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2019;98(1):358–365. doi: 10.3382/ps/pey385. [DOI] [PubMed] [Google Scholar]
- 99.Al-Okbi S.Y., Amin M.A., Mohamed A.E.A., Edris A.E., Sharaf O.M., Mabrok H.B., Ramadan A.A. Basil essential oil and its nanoemulsion mitigate non alcoholic steatohepatitis in rat model with special reference to gut microbiota. J. Oleo Sci. 2020;69(8):913–927. doi: 10.5650/jos.ess20067. [DOI] [PubMed] [Google Scholar]
- 100.Barbarestani S.Y., Jazi V., Mohebodini H., Ashayerizadeh A., Shabani A., Toghyani M. Effects of dietary lavender essential oil on growth performance, intestinal function, and antioxidant status of broiler chickens. Livest. Sci. 2020;233:103958. doi: 10.1016/j.livsci.2020.103958. [DOI] [Google Scholar]
- 101.Thompson A., Meah D., Ahmed N., Conniff-Jenkins R., Chileshe E., Phillips C.O., Claypole T.C., Forman D.W., Row P.E. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complement. Altern. Med. 2013;13:338. doi: 10.1186/1472-6882-13-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adell A. Brain NMDA receptors in schizophrenia and depression. Biomolecules. 2020;10(6):947. doi: 10.3390/biom10060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newport D.J., Carpenter L.L., McDonald W.M., Potash J.B., Tohen M., Nemeroff C.B. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry. 2015;172(10):950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 104.de Carvalho Cartágenes S., Fernandes L.M.P., Carvalheiro T.C.V.S., de Sousa T.M., Gomes A.R.Q., Monteiro M.C., de Oliveira Paraense R.S., Crespo-López M.E., Lima R.R., Fontes-Júnior E.A., Prediger R.D., Maia C.S.F. “Special K” drug on adolescent rats: oxidative damage and neurobehavioral impairments. Oxid. Med. Cell. Longev. 2019;2019:5452727. doi: 10.1155/2019/5452727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elisabetsky E., Brum L.F.S. Linalool as active component of traditional remedies: Anticonvulsant properties and mechanisms of action. Curare. 2003;26:45–52. [Google Scholar]
- 106.de Sousa D.P., Nóbrega F.F.F., Santos C.C.M.P., de Almeida R.N. Anticonvulsant activity of the linalool enantiomers and racemate: investigation of chiral influence. Nat. Prod. Commun. 2010;5(12):1847–1851. doi: 10.1177/1934578X1000501201. [DOI] [PubMed] [Google Scholar]
- 107.Elisabetsky E., Brum L.F.S., Souza D.O. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 1999;6(2):107–113. doi: 10.1016/S0944-7113(99)80044-0. [DOI] [PubMed] [Google Scholar]
- 108.Brum L.F.S., Elisabetsky E., Souza D. Effects of linalool on [(3)H]MK801 and [(3)H] muscimol binding in mouse cortical membranes. Phytother. Res. 2001;15(5):422–425. doi: 10.1002/ptr.973. [DOI] [PubMed] [Google Scholar]
- 109.Sampaio Lde.F., Maia J.G.S., de Parijós A.M., de Souza R.Z., Barata L.E.S. Linalool from rosewood (Aniba rosaeodora Ducke) oil inhibits adenylate cyclase in the retina, contributing to understanding its biological activity. Phytother. Res. 2012;26(1):73–77. doi: 10.1002/ptr.3518. [DOI] [PubMed] [Google Scholar]
- 110.de Siqueira R.J., Rodrigues K.M.S., da Silva M.T., Correia Junior C.A., Duarte G.P., Magalhães P.J.C., dos Santos A.A., Maia J.G.S., da Cunha P.J., Lahlou S. Linalool-rich rosewood oil induces vago-vagal bradycardic and depressor reflex in rats. Phytother. Res. 2014;28(1):42–48. doi: 10.1002/ptr.4953. [DOI] [PubMed] [Google Scholar]
- 111.Linck V.M., da Silva A.L., Figueiró M., Piato A.L., Herrmann A.P., Dupont Birck F., Caramão E.B., Nunes D.S., Moreno P.R.H., Elisabetsky E. Inhaled linalool-induced sedation in mice. Phytomedicine. 2009;16(4):303–307. doi: 10.1016/j.phymed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Gastón M.S., Cid M.P., Vázquez A.M., Decarlini M.F., Demmel G.I., Rossi L.I., Aimar M.L., Salvatierra N.A. Sedative effect of central administration of Coriandrum sativum essential oil and its major component linalool in neonatal chicks. Pharm. Biol. 2016;54(10):1954–1961. doi: 10.3109/13880209.2015.1137602. [DOI] [PubMed] [Google Scholar]
- 113.de Almeida R.N., Araújo D.A.M., Gonçalves J.C.R., Montenegro F.C., de Sousa D.P., Leite J.R., Mattei R., Benedito M.A.C., de Carvalho J.G., Cruz J.S., Maia J.G.S. Rosewood oil induces sedation and inhibits compound action potential in rodents. J. Ethnopharmacol. 2009;124(3):440–443. doi: 10.1016/j.jep.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 114.Harada H., Kashiwadani H., Kanmura Y., Kuwaki T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018;12:241. doi: 10.3389/fnbeh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Linck V.M., da Silva A.L., Figueiró M., Caramão E.B., Moreno P.R.H., Elisabetsky E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17(8-9):679–683. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Cheng B-H., Sheen L-Y., Chang S-T. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2014;5(1):27–34. doi: 10.1016/j.jtcme.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knudsen J.T., Eriksson R., Gershenzon J., Stahl B. Diversity and distribution of floral scent. Bot. Rev. 2006;72(1):1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- 118.Organisation for Economic Co-operation and Development Screening Information Data Set. Linalool, CAS No. 78-70-6. Paris: UNEP Publications; 2002. [Google Scholar]
- 119.Maia J.G.S., Andrade E.H.A., Couto H.A.R., Silva A.C.M., Marx F., Henke C. Plant sources of Amazon rosewood oil. Quim. Nova. 2007;30(8):1906–1910. doi: 10.1590/S0100-40422007000800021. [DOI] [Google Scholar]
- 120.Maia J.G.S., Mourão R.H.V. Amazon Rosewood (Aniba rosaeodora Ducke) Oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Cambridge: Academic Press; 2016. pp. 193–201. [DOI] [Google Scholar]
- 121.Maia J.G.S., Zoghbi M.G.B., Andrade E.H.A. Plantas Aromáticas na Amazônia e Seus Óleos Essenciais. Belém: Museu Paraense Emílio Goeldi; 2001. [Google Scholar]
- 122.Tranchida P.Q., Souza R.C.Z., Barata L.E.S., Mondello M., Dugo P., Dugo G., Mondello L. Analysis of macacaporanga (Aniba parviflora) leaf essential oil by using comprehensive two-dimensional gas chromatography combined with rapid-scanning quadrupole mass spectrometry. Chromatogr. Today. 2008;95:5–9. [Google Scholar]
- 123.Maia J.G.S., Zoghbi M.G.B., Andrade E.H.A. Essential oils of Aeollanthus suaveolens Matt. ex Spreng. J. Essent. Oil Res. 2003;15(2):86–87. doi: 10.1080/10412905.2003.9712074. [DOI] [Google Scholar]
- 124.Lawless J. The Encyclopedia of Essential Oils. London: Thorsons; 2002. [Google Scholar]
- 125.Cioanca O., Hritcu L., Mihasan M., Trifan A., Hancianu M. Inhalation of coriander volatile oil increased anxiolytic-antidepressant-like behaviors and decreased oxidative status in beta-amyloid (1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2014;131:68–74. doi: 10.1016/j.physbeh.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 126.Sriti J., Bettaieb I., Bachrouch O., Talou T., Marzouk B. Chemical composition and antioxidant activity of the coriander cake obtained by extrusion. Arab. J. Chem. 2019;12(7):1765–1773. doi: 10.1016/j.arabjc.2014.11.043. [DOI] [Google Scholar]
- 127.Tulsani N.J., Hamid R., Jacob F., Umretiya N.G., Nandha A.K., Tomar R.S., Golakiya B.A. Transcriptome landscaping for gene mining and SSR marker development in Coriander (Coriandrum sativum L.). Genomics. 2020;112(2):1545–1553. doi: 10.1016/j.ygeno.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 128.López P.A., Widrlechner M.P., Simon P.W., Rai S., Boylston T.D., Isbell T.A., Bailey T.B., Gardner C.A., Wilson L.A. Assessing phenotypic, biochemical, and molecular diversity in coriander (Coriandrum sativum L.) germplasm. Genet. Resour. Crop Evol. 2008;55(2):247–275. doi: 10.1007/s10722-007-9232-7. [DOI] [Google Scholar]
- 129.Zheljazkov V.D., Callahan A., Cantrell C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2008;56(1):241–245. doi: 10.1021/jf072447y. [DOI] [PubMed] [Google Scholar]
- 130.Simon J.E., Morales M.R., Phippen W.B., Vieira R.F., Hao Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In: Janick J., editor. Perspectives on New Crops and New Uses. Alexandria: ASHS Press; 1999. pp. 499–505. [Google Scholar]
- 131.Lesage-Meessen L., Bou M., Sigoillot J-C., Faulds C.B., Lomascolo A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015;99(8):3375–3385. doi: 10.1007/s00253-015-6511-7. [DOI] [PubMed] [Google Scholar]
- 132.Lis-Balchin M. Lavender Essential Oil. In: Lis-Balchin M., editor. Lavender, the Genus Lavandula. London, New York: Taylor and Francis; 2002. p. 117. [DOI] [Google Scholar]
- 133.Denny E.F.K. Distillation of the Lavender Type Oils: Theory and Practice. In: Lis-Balchin M., editor. Lavender, the Genus Lavandula. London, New York: Taylor and Francis; 2002. pp. 100–116. [Google Scholar]
- 134.Babu K.N., Sajina A., Minoo D., John C.Z., Mini P.M., Tushar K.V., Rema J., Ravindran P.N. Micropropagation of camphor tree (Cinnamomum camphora). Plant Cell Tissue Organ Cult. 2003;74(2):179–183. doi: 10.1023/A:1023988110064. [DOI] [Google Scholar]
- 135.Chen C., Zheng Y., Zhong Y., Wu Y., Li Z., Xu L-A., Xu M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genomics. 2018;19(1):550. doi: 10.1186/s12864-018-4941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo X., Cui M., Deng M., Liu X., Huang X., Zhang X., Luo L. Molecular differentiation of five Cinnamomum camphora chemotypes using desorption atmospheric pressure chemical ionization mass spectrometry of raw leaves. Sci. Rep. 2017;7:46579. doi: 10.1038/srep46579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi W.Y., He W., Wen G-Y., Guo D-X., Long G-Y., Lin Y-G. Study on chemical constituents of the essential oil and classification of types from Cinnamomum camphora. J. Integr. Plant Biol. 1989;31(3):209–214. [Google Scholar]
- 138.Cheng B.H., Lin C.Y., Yeh T.F., Cheng S.S., Chang S.T. Potential source of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaf: essential oil profile and enantiomeric purity. J. Agric. Food Chem. 2012;60(31):7623–7628. doi: 10.1021/jf302248w. [DOI] [PubMed] [Google Scholar]


