Abstract
Cocaine Use Disorder (CUD) is one of the diseases with the greatest social and health impact, due to the high cost of rehabilitation management and the high risk of dangerous behavior and relapse. This pathology frequently leads to unsuccessful attempts to interrupt the consumption, resulting in relapses and a vicious cycle of binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation (craving). The alternation of these phases in addiction was well illustrated by Koob and colleagues in the so-called “addictive cycle”, which nowadays represents a landmark in the addiction field. Recently, there has been a surge of interest in the worldwide literature for biomarkers that might explain the different stages of addiction, and one of the most studied biomarkers is, without a doubt, Brain-derived Neurotrophic Factor (BDNF). In this perspective article, we discuss the potential role of BDNF as biomarker of the CUD phases described in the “Addictive Cycle”, speculating about the close relationship between BDNF fluctuations and the clinical course of CUD. We also discuss BDNF’s potential role as “staging” biomarker, predicting the progression of the disease. Finding valuable biomarkers of CUD severity and disease stage could shift clinicians' focus away from behavioral symptomatic treatment and toward a novel brain-based approach, allowing for the development of more effective and targeted therapeutic strategies, thus determining major benefits for CUD patients.
Keywords: BDNF, biomarker, addiction, staging, craving, relapse, cocaine use disorder, neurotrophic factor
1. COCAINE USE DISORDER: A “BRAIN DISEASE” SEEKING FOR POTENTIAL BIOMARKERS
Cocaine Use Disorder (CUD) represents a chronic and relapsing disease, characterized by compulsive drug intake, frequent drug-seeking behaviors and loss of control capacity over substance consumption [1]. CUD is an important contributor to global disease burden, and currently available diagnostic tools and treatments are often inadequate to control the course of this disorder, leading to the development of a vicious cycle of binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation (craving). These phases of the “addictive cycle” have been described by Koob and colleagues, representing a landmark in the addiction field [2]. While drug addicts’ assessment is largely based on the evaluation of drug use (i.e., cocaine daily use, days of heavy use, relapse rates, etc.) and cocaine craving intensity, increasing research has recently been directed toward the development of valuable biomarkers to monitor disease severity and to predict treatment response. Moreover, the addiction cycle laid the groundwork to explore the neurobiological changes, that corroborate the “brain disease model” of addiction [3]. This concept is well supported by the increasing evidence of the leading role of neurodegeneration in CUD [4]. Chronic consumption, in fact, seems to be related to oxidative injury and consequently to the activation of inflammatory pathways. The “hormetic response”, a biphasic cellular activity induced by redox-active agents, exposure, and its impact on inflammatory/antinflammatory pathways [5, 6] have recently received much attention. The hormetic response appears to impact brain pathophysiology and stress resistance mechanisms to oxidative, inflammatory insult, and neurodegenerative damage as in chronic cocaine use. Due to its involvement in neurogenesis, neuroplastic and neurodegenerative processes, the brain derived neurotrophic factor (BDNF) has been extensively investigated as a potential biomarker in several mental disorders [7], including CUD. Some studies [8-10] reported lower BDNF levels in CUD subjects compared to healthy controls, while other findings showed higher levels in these subjects [11]. Based on these potential consistent inconsistencies, here we discuss the potential role of BDNF as a candidate biomarker of CUD, across its different phases, severity levels and disease progression.
2. BDNF FLUCTUATIONS DURING THE “ADDICTIVE CYCLE”
We propose that discrepancies in BDNF levels found in CUD subjects across the studies may be primarily related to fluctuations detected in different phases of the “addictive cycle”. Animal model studies emphasized that a single dose of cocaine can trigger a rapid, but transient, increase in BDNF levels [12-14]. This raise seems to determine the activation of reward circuit, with a subsequent potentiation of cocaine-induced reinforcement. Nevertheless, some studies describe a persistent reduction of BDNF when moving from occasional use to chronic abuse [8, 10, 15], in the so-called binge/intoxication phase. Perpetuation of substance abuse leads to a sustained hyperactivation of the reward system and may induce an allostatic neuroadaptive phenomenon at the dopaminergic mesolimbic circuit [16, 17], resulting in significant reductions in BDNF levels [15]. During this phase, decrease of BDNF levels appears to be deeply influenced by physiological and pathological factors, including years of substance use and the amount usually ingested [9].
Previous findings have described higher BDNF levels during the withdrawal phase [18] and others indicate a clear transition from lower to higher BDNF levels when moving from intoxication to withdrawal phase [10, 18]. In the withdrawal/negative affect phase of the addiction cycle, clinical features (loss of motivation for natural rewards, dysphoria, alexithymia, chronic irritability) are associated with neurobiological hypoactivation of the reward circuit in response to drug-independent stimuli, increased stress sensitivity and HPA axis hyperactivity; in this perspective, the increase in BDNF levels could be related to both HPA axis hyperactivation [19, 20] as well as to the allostatic dysregulation of reward circuits induced by chronic cocaine use [12, 21].
Drug withdrawal leads to increased substance craving and risky behaviors over times, corresponding to preoccupation/anticipation phase of the addiction cycle. During this phase, BDNF levels could potentially predict the risk of dangerous behaviors and relapse: higher levels seem to be associated with craving and an increased occurrence of relapse episodes as well as with higher drug intake [11].
Taken together, these evidence support the hypothesis that BDNF levels fluctuates along with the clinical course of CUD, in line with the results of previous preclinical investigations [12, 13, 22]. In fact, in these preclinical studies, even if in a simplified manner compared to our hypothesis, a fluctuation to high levels of BDNF was detected after cocaine administration. In addition, preclinical evidences supports the close relationship between BDNF and the behavioral aspects of CUD, observing the craving and self-administration of cocaine after direct BDNF injection into the brain. The opposite effect is obtained by blocking the function of BDNF by antagonizing the TrkB receptor, resulting in reduction of self-administration [23]. These findings could generate important translational hypotheses on the crucial role of the BDNF and its stabilization on addictive behaviors even in a clinical setting.
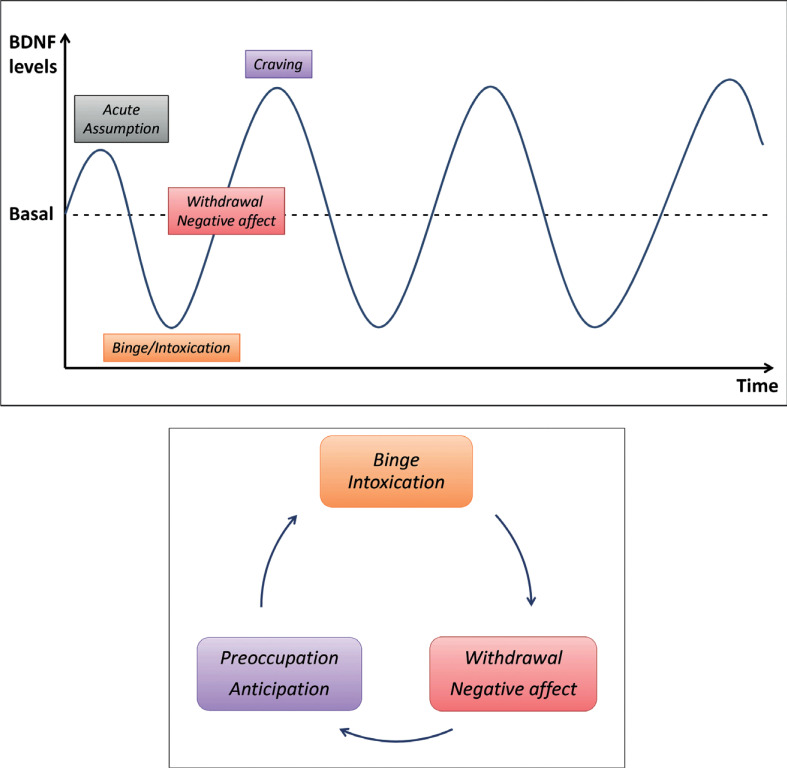
From the transiently elevated levels during the acute intake [12-14], BDNF move to a significant reduction in the intoxication phase [8, 10, 15], until a subsequent renewed increase in the withdrawal phase [10, 18], with a peak related to the rise of craving levels and to higher risk for relapse [11].
BDNF fluctuations are reproduced in Fig. (1), where it is shown the periodic trend in which variations appear closely associated with the different phases of CUD disease.
Fig. (1).
The “Addictive” period: a representation of periodic BDNF levels fluctuation between lower levels and higher ones, consistently with the three phases of Koob cycle. Adapted from Koob, G.F., Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry, 2016.
Although previous works have suggested a possible variation of BDNF in CUD phases [10, 18], this is the first article, in our knowledge, to theorize a relationship between clinical presentation and BDNF variation in CUD, conceptualizing a novel hypothesis of an “Addictive period”. As the different phases of disease appear to repeat over the time, BDNF levels fluctuate, reproducing a “periodic” oscillation. In this context, different CUD phase, on a biochemical level, could generate this “addictive period”, in which the periodic oscillations of BDNF levels may underpin the occurrence of various clinical phenomena (acute ingestion, chronic abuse and intoxication, abstinence and craving).
3. BDNF AS POTENTIAL BIOMARKER OF THE “ADDICTIVE PERIOD” AND DISEASE WORSENING
Considering the fluctuations of BDNF levels as a potential biomarkers of CUD clinical course, future studies may explore their predictive value in terms of relapse risk and treatment response. Although this speculation needs further confirmation, this conceptual framework could stimulate future research in the field to design well-powered prospective studies. If this “addictive period” hypothesis is confirmed, it could represent a valuable tool for clinicians. In fact, in real-world clinical practice objective markers for CUD are lacking and their development could have a deep impact in terms of disease monitoring and relapse prevention. Furthermore, since changes in mesolimbic reward circuitry are the result of allostatic neuroadaptation mechanisms and cocaine-induced damage [24], it could be hypothesized that chronic drug use could lead to a persistent reduction in BDNF over time, in a manner related to the severity of CUD.
In this context, BDNF levels could be interpreted as a potential “indicator” of disease worsening over the years, mainly related to the reduction of neuroplasticity potential along with disease progression. Supporting this hypothesis, the increased rate of BDNF during abstinence was observed to be inversely related to the severity of dependence, intended as the amount of substance taken and the years of use [10, 18]. This mechanism has also been observed in individuals with recurrent depression, where lower BDNF levels are associated with longer depressive episodes and neurotrophin levels remain lower during intercritical periods than in healthy controls [25, 26].
When studying the “addictive period”, not only BDNF but also its metabolism should be considered, as neurotrophin production is finely regulated by numerous convergent epigenetic regulatory pathways that seems to be deeply influenced by cocaine ingestion [27, 28]. In fact, the epigenetic modulation of BDNF following acute cocaine intake is controlled by the acetylation and deacetylation of histones of various gene promoters [27, 29] and the regulation of the Bdnf gene via micro-RNA [28]. In this framework, the BDNF precursor (proBDNF) should also be considered, as it represents the bioavailability pool of neurotrophin and its concentration in combination with BDNF provides crucial information about the metabolic and neurotrophic state of the nervous system as observed in major depression, bipolar disorder [30] and other substance use disorders [31].
Overall, studying the temporal changes related to the persistent cocaine use in BDNF and in its metabolic pathways could lead to a better “staging” of this pathology, able to predict the impairment degree and the prognosis of patients based on the substance’s brain tissue damage and thus the possible outcomes of a “targeted” therapeutic approach.
4. BDNF AS A POTENTIAL TARGET FOR NEW THERAPEUTIC STRATEGIES
As previously mentioned, preclinical findings have shown the close relationship between BDNF regulation and the clinical presentation of CUD [23]. In this perspective, future studies could contribute to determining a possible role for BDNF as a potential mediator for novel therapeutic tools in CUD.
Several pharmacological agents [32] and non-invasive brain stimulation techniques [33-36] have been tested as potential treatment of CUD. Future investigations should explore their potential in terms of BDNF levels “stabilization”, in order to prevent periodic fluctuations that potentially drive disease progression across the phases. The concept of stabilization of BDNF underlies several regulatory mechanisms that involve proBDNF and the endogenous modulation of the metabolism of these neurotrophins. Some recent evidence in the psychiatric field already supports this hypothesis; in this respect, Wu and collaborators have found that the hypermetabolism of BDNF is related to an increase in BDNF/ proBDNF ratio only in patients with severe manifestation of depression [37].
Drugs that act on glutamatergic transmissions, like ketamine and esketamine, both selective NMDA receptor antagonists are currently able to modulate BDNF production. Ketamine has been observed to increase BDNF production along with its specific receptor TrkB, triggering phenomena of neuroplasticity and neurogenesis that support the drug's rapid antidepressant effect. Furthermore, the regulation of glutamatergic neurotransmission by ketamine and esketamine has been proposed as a potential intervention in substance use disorders [38]. In addition, other pharmacological agents displayed efficacy in modulate serum BDNF levels like, for instance, Serotonin Selective Reuptake Inhibitors and Serotonin and Norepinephrine Reuptake Inhibitors: these agents are able to trigger a rapid increase of serum BDNF [39] and, although this increase seems not to be directly related to changes in depression levels, BDNF stabilization has emerged as a facilitatory role in the mechanism of action of these antidepressants, although it does not seem to be directly related to changes in depression levels [40].
BDNF levels could also be affected by various brain stimulation techniques such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). It has been observed that selective stimulation of the dorsolateral prefrontal cortex (DLPFC) induces an increase in BDNF levels and a significant reduction in craving in patients with addictive disorders [34, 35]. Furthermore, genetic-determined variability in BDNF levels seems to highly influence treatment response: studies evaluating efficacy of rTMS in treatment-resistant depression have shown how Single Nucleotide Polymorphisms are able to predict treatment response, with the BDNF Val66Met polymorphism particularly related to better outcomes in patients treated with rTMS [41].
CONCLUSION
In conclusion, a better understanding of the “addictive period” here hypothesized could lead to targeted, patient-tailored, well-timed treatment interventions.
Furthermore, valuable biomarkers of CUD severity and disease stage could shift the attention of clinicians from the perspective of a behavioral symptomatic treatment, towards a novel brain-oriented approach. Translating the addiction brain disease model into clinical practice may allow to develop more effective therapeutic strategies, thus determining great benefits for CUD patients.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.APA. Diagnostic and Statistical Manual. Arlington, USA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Koob G.F., Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59(1):29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.Koob G.F., Volkow N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch G.E., Jaskulski M., Hamerski H.M., Porto F.G., da Silva B., Aita C.A.M., Kroker K., de Bem Silveira G., Silveira P.C.L., Santos G.T., Klafke J.Z., Viecili P.R.N. Evaluation of oxidative stress and brain-derived neurotrophic factor levels related to crack-use detoxification. Neurosci. Lett. 2018;670:62–68. doi: 10.1016/j.neulet.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress re-sponses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dattilo S., Mancuso C., Koverech G., Di Mauro P., Ontario M.L., Petralia C.C., Petralia A., Ma-iolino L., Serra A., Calabrese E.J., Calabrese V. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing. 2015;12(1):20. doi: 10.1186/s12979-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C-C., Huang T-L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020;43(2):134–142. doi: 10.1016/j.bj.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelucci F., Ricci V., Pomponi M., Conte G., Mathé A.A., Attilio Tonali P., Bria P. Chronic hero-in and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J. Psychopharmacol. 2007;21(8):820–825. doi: 10.1177/0269881107078491. [DOI] [PubMed] [Google Scholar]
- 9.Pianca T.G., Rosa R.L., Ceresér K.M.M., de Aguiar B.W., de Abrahão R.C., Lazzari P.M., Kapczinski F., Pechansky F., Rohde L.A., Szobot C.M. Differences in biomarkers of crack-cocaine adolescent users before/after abstinence. Drug Alcohol Depend. 2017;177:207–213. doi: 10.1016/j.drugalcdep.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Corominas-Roso M., Roncero C., Eiroa-Orosa F.J., Gonzalvo B., Grau-Lopez L., Ribases M., Rodriguez-Cintas L., Sánchez-Mora C., Ramos-Quiroga J-A., Casas M. Brain-derived neurotrophic factor serum levels in cocaine-dependent patients during early abstinence. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuro-psychopharmacol. 2013;23(9):1078–1084. doi: 10.1016/j.euroneuro.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 11.D’Sa C., Fox H.C., Hong A.K., Dileone R.J., Sinha R. Increased serum brain-derived neurotrophic factor is predictive of cocaine relapse outcomes: a prospective study. Biol. Psychiatry. 2011;70(8):706–711. doi: 10.1016/j.biopsych.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham D.L., Edwards S., Bachtell R.K., DiLeone R.J., Rios M., Self D.W. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 2007;10(8):1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Wolf M.E. Multiple faces of BDNF in cocaine addiction. Behav. Brain Res. 2015;279:240–254. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logrip M.L., Barak S., Warnault V., Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628(Pt A):60-67. doi: 10.1016/j.brainres.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornell F., Hansen F., Schuch F.B., Pezzini Rebelatto F., Tavares A.L., Scherer J.N., Valerio A.G., Pechansky F., Paim Kessler F.H., von Diemen L. Brain-derived neurotrophic factor in substance use disor-ders: A systematic review and meta-analysis. Drug Alcohol Depend. 2018;193:91–103. doi: 10.1016/j.drugalcdep.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Kapczinski F., Vieta E., Andreazza A.C., Frey B.N., Gomes F.A., Tramontina J., Kauer-Sant’anna M., Grassi-Oliveira R., Post R.M. Allostatic load in bipolar disorder: Implications for pathophysiology and treatment. Neurosci. Biobehav. Rev. 2008;32(4):675–692. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 17.McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43(1):2–15. doi: 10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 18.von Diemen L., Kapczinski F., Sordi A.O., de Magalhães Narvaez J.C., Guimarães L.S.P., Kess-ler F.H.P., Pfaffenseller B., de Aguiar B.W., de Moura Gubert C., Pechansky F. Increase in brain-derived neurotrophic factor expression in early crack cocaine withdrawal. Int. J. Neuropsychopharmacol. 2014;17(1):33–40. doi: 10.1017/S146114571300103X. [DOI] [PubMed] [Google Scholar]
- 19.Smith M.A., Makino S., Kvetnansky R., Post R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fumagalli F., Di Pasquale L., Caffino L., Racagni G., Riva M.A. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur. J. Neurosci. 2007;26(10):2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadri-Vakili G., Kumaresan V., Schmidt H.D., Famous K.R., Chawla P., Vassoler F.M., Over-land R.P., Xia E., Bass C.E., Terwilliger E.F., Pierce R.C., Cha J-H.J. Cocaine-induced chromatin remodel-ing increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of co-caine. J. Neurosci. 2010;30(35):11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu L., Dempsey J., Liu S.Y., Bossert J.M., Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J. Neurosci. 2004;24(7):1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham D.L., Krishnan V., Larson E.B., Graham A., Edwards S., Bachtell R.K., Simmons D., Gent L.M., Berton O., Bolanos C.A., DiLeone R.J., Parada L.F., Nestler E.J., Self D.W. Tropo-myosin-related kinase B in the mesolimbic dopamine system: Region-specific effects on cocaine reward. Biol. Psychiatry. 2009;65(8):696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha-Oliveira T., Rego A.C., Oliveira C.R. Cellular and molecular mechanisms involved in the neurotoxicity of opi-oid and psychostimulant drugs. Brain Res. Brain Res. Rev. 2008;58(1):192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Birkenhäger T.K., Geldermans S., Van den Broek W.W., van Beveren N., Fekkes D. Serum brain-derived neurotrophic factor level in relation to illness severity and episode duration in patients with major depression. J. Psychiatr. Res. 2012;46(3):285–289. doi: 10.1016/j.jpsychires.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Takebayashi N., Maeshima H., Baba H., Nakano Y., Satomura E., Kita Y., Namekawa Y., Nomoto H., Suzuki T., Arai H. Duration of last depressive episode may influence serum BDNF levels in remitted patients with major depression. Depress. Anxiety. 2012;29(9):775–779. doi: 10.1002/da.21933. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt H.D., McGinty J.F., West A.E., Sadri-Vakili G. Epigenetics and psychostimulant addiction. Cold Spring Harb. Perspect. Med. 2013;3(3):a012047. doi: 10.1101/cshperspect.a012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Im H-I., Hollander J.A., Bali P., Kenny P.J. MeCP2 controls BDNF expression and cocaine intake through homeostat-ic interactions with microRNA-212. Nat. Neurosci. 2010;13(9):1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan J-S., Haggarty S.J., Giacometti E., Dannenberg J-H., Joseph N., Gao J., Nieland T.J.F., Zhou Y., Wang X., Mazitschek R., Bradner J.E., DePinho R.A., Jaenisch R., Tsai L-H. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G., Zhang C., Chen J., Su Y., Zhou R., Wang F., Xia W., Huang J., Wang Z., Hu Y., Cao L., Guo X., Yuan C., Wang Y., Yi Z., Lu W., Wu Y., Wu Z., Hong W., Peng D., Fang Y. Ratio of mBDNF to proBDNF for Differential Diagnosis of Major Depressive Disorder and Bipolar Depression. Mol. Neurobiol. 2017;54(7):5573–5582. doi: 10.1007/s12035-016-0098-6. [DOI] [PubMed] [Google Scholar]
- 31.Bachis A., Campbell L.A., Jenkins K., Wenzel E., Mocchetti I. Morphine withdrawal increases brain-derived neurotrophic factor precursor. Neurotox. Res. 2017;32(3):509–517. doi: 10.1007/s12640-017-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castrén E., Monteggia L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry. 2021;90(2):128–136. doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Valiuliene G., Valiulis V., Dapsys K., Vitkeviciene A., Gerulskis G., Navakauskiene R., Ger-manavicius A. Brain stimulation effects on serum BDNF, VEGF, and TNFα in treatment-resistant psychiatric disorders. Eur. J. Neurosci. 2021;53(11):3791–3802. doi: 10.1111/ejn.15232. [DOI] [PubMed] [Google Scholar]
- 34.Eskandari Z., Dadashi M., Mostafavi H., Armani Kia A., Pirzeh R. Comparing the efficacy of anodal, ca-thodal, and sham transcranial direct current stimulation on brain-derived neurotrophic factor and psychological symptoms in opioid-addicted patients. Basic Clin. Neurosci. 2019;10(6):641–650. doi: 10.32598/BCN.10.6.1710.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinotti G., Lupi M., Montemitro C., Miuli A., Di Natale C., Spano M.C., Mancini V., Lorus-so M., Stigliano G., Tambelli A., Di Carlo F., Di Caprio L., Fraticelli S., Chillemi E., Pettorruso M., Sepede G., di Giannantonio M. Transcranial direct current stimulation reduces craving in substance use disorders: A double-blind, placebo-controlled study. J. ECT. 2019;35(3):207–211. doi: 10.1097/YCT.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 36.Pettorruso M., Martinotti G., Santacroce R., Montemitro C., Fanella F., di Giannantonio M. rTMS reduces psychopathological burden and cocaine consumption in treatment-seeking subjects with cocaine use disorder: An open label, fea-sibility study. Front. Psychiatry. 2019;10:621. doi: 10.3389/fpsyt.2019.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Lu J., Lu S., Huang M., Xu Y. Increased ratio of mature BDNF to precursor-BDNF in patients with major de-pressive disorder with severe anhedonia. J. Psychiatr. Res. 2020;126:92–97. doi: 10.1016/j.jpsychires.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Martinotti G., Chiappini S., Pettorruso M., Mosca A., Miuli A., Di Carlo F., D’Andrea G., Collevecchio R., Di Muzio I., Sensi S.L., Di Giannantonio M. Therapeutic potentials of ketamine and esketa-mine in obsessive-compulsive disorder (OCD), substance use disorders (SUD) and eating disorders (ED): A review of the current litera-ture. Brain Sci. 2021;11(7):856. doi: 10.3390/brainsci11070856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C., Zhong J., Zou B., Fang L., Chen J., Deng X., Zhang L., Zhao X., Qu Z., Lei Y., Lei T. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS One. 2017;12(2):e0172270. doi: 10.1371/journal.pone.0172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolkowitz O.M., Wolf J., Shelly W., Rosser R., Burke H.M., Lerner G.K., Reus V.I., Nelson J.C., Epel E.S., Mellon S.H. Serum BDNF levels before treatment predict SSRI response in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(7):1623–1630. doi: 10.1016/j.pnpbp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kar S.K. Predictors of response to repetitive transcranial magnetic stimulation in depression: A review of recent updates. Clin. Psychopharmacol. Neurosci. 2019;17(1):25–33. doi: 10.9758/cpn.2019.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]