Abstract
Microglia are the resident immune cells of the brain and play a crucial role in housekeeping and maintaining homeostasis of the brain microenvironment. Upon injury or disease, microglial cells become activated, at least partly, via signals initiated by injured neurons. Activated microglia, thereby, contribute to both neuroprotection and neuroinflammation. However, sustained microglial activation initiates a chronic neuroinflammatory response which can disturb neuronal health and disrupt communications between neurons and microglia. Thus, microglia-neuron crosstalk is critical in a healthy brain as well as during states of injury or disease. As most studies focus on how neurons and microglia act in isolation during neurotrauma, there is a need to understand the interplay between these cells in brain pathophysiology. This review highlights how neurons and microglia reciprocally communicate under physiological conditions and during brain injury and disease. Furthermore, the modes of microglia-neuron communication are exposed, focusing on cell-contact dependent signaling and communication by the secretion of soluble factors like cytokines and growth factors. In addition, it has been discussed that how microglia-neuron interactions could exert either beneficial neurotrophic effects or pathologic proinflammatory responses. We further explore how aberrations in microglia-neuron crosstalk may be involved in central nervous system (CNS) anomalies, namely traumatic brain injury (TBI), neurodegeneration, and ischemic stroke. A clear understanding of how the microglia-neuron crosstalk contributes to the pathogenesis of brain pathologies may offer novel therapeutic avenues of brain trauma treatment.
Keywords: CNS Injury, microglia-neuron interaction, cellular crosstalk, neuroinflammation, microglial activation, microglia phenotypes
1. INTRODUCTION
The brain microenvironment plays an important role in determining how brain cells communicate. This cellular communication is essential for normal brain functions under homeostatic conditions and can orchestrate brain responses to injury as well as other pathological conditions [1]. Homeostasis of the brain microenvironment is maintained by several structures, an example of which is the neurovascular unit, an interactive unit between brain cells, endothelial cells, and pericytes, which forms the blood-brain barrier (BBB) [2]. Aberrations of brain homeostasis lead to modifications in cellular interactions and contribute to the pathogenesis of several neurological disorders, such as traumatic brain injury (TBI), spinal cord injury (SCI), Alzheimer’s disease (AD), or Parkinson's disease (PD) [3].
Microglia are brain-resident immune cells with recently identified non-immune functions that include essential housekeeping functions in the brain. For example, microglia interact with different brain cells, thereby maintaining brain homeostasis during physiological states and responding to danger signals during brain injury or disease [4]. In this regard, microglia become activated immediately post-injury as a protective process initiated by the brain against the trauma. Indeed, microglia can go through a range of activation states depending on the brain microenvironment, leading to changes in microglia morphology, gene expression, and function [5]. In the “resting” state, microglia are characterized by a ramified morphology. It is noteworthy that despite being described as resting, microglia, during physiological states, are continuously extending and retracting their processes to contact other cells of the brain. Furthermore, microglia in the resting state have been suggested to be continuously surveilling the brain microenvironment [6, 7]. However, due to injury or a pathology, microglia become activated and assume an activated amoeboid phenotype. During their early stages of activation, microglia have been suggested to help in the restoration of brain homeostasis. However, when chronically activated, microglia develop into the classically activated phenotype, releasing pro-inflammatory molecules that cause further tissue damage and may possibly lead to neurodegeneration [5]. Hence, activated microglia could exert either pro-inflammatory or anti-inflammatory effects depending on the stage of injury and the phenotype of microglia [8].
Although microglial cells are largely implicated in the outcomes of injury or disease, they do not act in isolation. The functions and responses exhibited by microglia post-injury to the brain are a result of cellular interactions between microglia, neurons, or other glial cells [9, 10]. For instance, the recruitment of microglia towards an injury site is mediated, at least partly, by the crosstalk between injured neurons and microglial cells [11]. Injured neurons release signals which polarize the microglia towards their activation state [6]. Also, in the event of BBB disruption, peripheral immune cells infiltrate the brain and act in conjunction with the activated microglia to release inflammatory mediators that exacerbate the injury or disease [12]. Bidirectional crosstalk between neurons and microglia contributes to all these events.
In this review, we explore the modes of microglia-neuron communication focusing on cell-contact-dependent signaling and communication by the secretion of soluble factors like cytokines and growth factors. We also discuss how the microglia-neuron interaction could exert either beneficial neurotrophic effects or pathologic proinflammatory responses. We further overview and discuss how aberrations in microglia-neuron crosstalk may be involved in central nervous system (CNS) anomalies, namely TBI, neurodegeneration, and ischemic stroke.
2. MICROGLIAL ACTIVATION AND MICROGLIAL PHENOTYPES
Morphologically, resting quiescent microglia are ramified with little or no motility and possess a small cell body, along with comparatively long cytoplasmic processes which extend into the surrounding environment. In a healthy brain, microglia are not resting. Instead, their processes are continuously forming contacts with other cells of the brain. Moreover, microglia in the resting state are continually monitoring neurons’ activity and surveilling the brain microenvironment; ramified microglia are often called surveilling or homeostatic microglia [6, 7]. Contextually, microglia are kept inhibited in the resting state, partly through their crosstalk with neurons and astrocytes [13, 14]. However, this situation changes when a neuron is unhealthy or dysfunctional. This crosstalk mechanism ensures that microglia remain in “sensor” mode during healthy states and gives microglia the ability to become activated when perturbations to brain homeostasis are sensed [6, 7].
After being exposed to stimuli, such as injury, disease, or infectious agents, microglia transform into a polarized activated state. Activated microglia become motile, lose ramification and acquire an amoeboid-like shape characterized by having an enlarged cell body and the absence of processes that have retracted, allowing the microglia to adopt an amoeboid-like shape more similar to peripheral macrophages. In addition, activated microglia become robustly phagocytic, with an overtly secretory phenotype [15, 16]. In the activated state, microglia release a plethora of molecules, such as proinflammatory and anti-inflammatory cytokines, neurotrophic factors, neurotoxic proteins, and chemokines. Additionally, microglia express a unique repertoire of cell surface proteins, including Toll-like receptors (TLRs), scavenger receptors nucleotide-binding oligomerization domains (NODs), and NOD-like receptors [17].
Given the plasticity of microglial cells, it is now clear that they are not merely 'quiescent cells' within the CNS [15]. Instead, they are highly responsive to changes within the brain, and as such, either facilitate restoration of homeostasis or contribute to disease progression [7, 18-20]. While the classification of microglial phenotypes is disputable, they have been initially classified in light of the traditional method of macrophage classification. However, it is important to mention that the polarization of microglia and macrophages does not exactly follow the same mechanism [21]. The classification spectrum of microglia is more fitting with the proinflammatory 'M1-like' and the anti-inflammatory 'M2-like' phenotypes than the conventional M1 and M2 phenotypes observed in macrophages [22, 23].
In response to proinflammatory mediators, such as proinflammatory cytokines, interferon-(IFN)γ, free radicals, or damage-associated molecular patterns (DAMPs), the polarization of microglia is induced to activate the M1-like phenotype. This phenotype is mainly proinflammatory, characterized by low phagocytic activity, and is usually described as neurotoxic [24]. Mainly, M1 polarization occurs through three key pathways: LPS, IFN-γ, and Granulocyte-macrophage colony-stimulating factor (GM-CSF) [25-27]. M1 activated microglia release pro-inflammatory molecules, such as chemokines (e.g., CCL2, CXCL9) and proinflammatory cytokines (e.g., interleukin IL-1β, IL-6, IL-12, and tumor necrosis factor (TNF)-α) [28-30].
At the site of injury, the microglial activation states evolve through an inflammatory episode [31]. During this evolution, ramified microglia can transform directly into M1-like or M2-like cells, where M1-like and M2-like can transform interchangeably. Furthermore, evolution into the alternative activated microglial phenotype, 'M2-like', requires a unique inflammatory milieu comprising transforming growth factor-β (TGF β), IL-10, IL-4, and IL-13. In contrast to M1-like, the M2-like phenotype is neurotrophic, promoting an anti-inflammatory response by remarkably increasing microglial phagocytic activity to remove damaged cell debris and facilitating tissue repair [26, 32, 33]. Given the heterogeneity and plasticity of microglial phenotypes, it should be noted that, during disease or injury, microglia in affected brain areas are usually heterogeneous showing a mixture of activation phenotypes. Therefore, microglia may have either healing or harmful outcomes. These outcomes depend on factors, such as the site, extent, and nature of the insult to neurons, as well as the stage of disease progression [34, 35].
There are three subclasses of the M2 microglial phenotype, M2a, M2b, and M2c. M2a-microglia are activated by IL-4 and IL-13, and secrete the anti-inflammatory cytokine IL-10 (25). M2a microglia are characterized by activation of the STAT6/IRF4 transcriptional program and up-regulation of arginase-1 (Arg-1), RELM-α, transglutaminase-2, and YM1, which antagonize the classical M1-like phenotype [36]. Glucocorticoids and IL-10 activate the M2c phenotype of microglia that possess a phagocytic activity and can clean up cellular debris from damaged CNS areas [37]. Several phenotypic markers, such as CD206, CD163, and TGF-β, are up-regulated and distinguish the M2c phenotype [28, 38]. M2b microglia can be activated by IL-1R ligands, and secrete IL-1β, IL-10, and TNF-α. Interestingly, M2b microglia have markers for both M1 (CD86) and M2 (IL-10high) phenotypes; thus, they are considered as a mixed-phenotype exhibiting both proinflammatory and anti-inflammatory functions [37, 39, 40].
In line with this, a distinct population of activated microglia, called rod microglia, has been reported in the CNS. These cells feature a significant increase in length, narrow cell soma, and few planar side branches, with polar processes that are entirely polarized and reduced in length. In addition, they have a high proliferative capacity [41-43]. They have been reported to be involved in the phenomenon of “synaptic stripping”, where they can degrade the extracellular matrix, promote retraction axons, and destabilize synapses [44-46]. In addition, they have been implicated in the protection of uninjured axons [45]. Currently, well-established in vivo and in vitro models of rod microglia are lacking. Besides, imaging of these highly dynamic cells in injured tissues is quite challenging. Moreover, investigation regarding these rod microglial cells is currently limited by their low cell number, rare morphology, and alignment within an injured area [41-43].
It should be noted that different regions of the human brain show differential microglial heterogeneity. Such heterogeneity has been demonstrated by the presence of differential gene expression profiles of microglia from different parts of the human brain [47, 48]. However, these differences in gene expression profiles may be reflective of differences in functional differentiation rather than phenotypic diversity. Single-cell studies are expected to provide novel insights into the diversity of microglia and their roles in health and disease [34, 48-50]. Indeed, microglial heterogeneity and the regulation of microglial functional phenotypes during development, health, and disease still need to be elucidated [34, 47, 49].
Interestingly, the activation states and phenotypes of microglia are directly affected by microglia-neuron interactions. Indeed, neuron-derived soluble factors affect the activation state of microglia in the healthy brain as well as during neuroinflammation and neurodegeneration [51]. In this regard, neurotransmitters like serotonin, dopamine, or GABA can deactivate microglia, while the neurotransmitter glutamate can activate them [52]. Another example is the release of ATP by injured neurons. ATP is implicated in the activation of microglia and the resulting neuroinflammation [53]. Similarly, the different microglial phenotypes have different sensing and signaling interactions with neurons due to the difference in the secreted and cell surface protein expression profiles, which is discussed in the next section.
3. MODES OF INTERACTIONS BETWEEN NEURONS AND MICROGLIA
As mentioned earlier, microglia are the main brain-resident immune cells, yet they have functions that extend beyond their immune roles. Moreover, microglia participate in different aspects of brain homeostasis. To play their homeostatic roles, microglia are in continuous interaction with neurons, and other brain cells, allowing them to monitor neuronal activity [54]. This microglia-neuron interaction is bidirectional and often reciprocal. These bidirectional interactions take place through several modalities that include: 1) direct physical interaction with elements of the synapse; 2) secretion of soluble paracrine signaling factors; 3) microglia-neuron communication through connexin-based gap junctions and pannexin-based channels; and 4) communication via release of the extravesicular bodies, like exosomes and microvesicles. Extravesicular bodies, also known as extracellular vesicles (EVs), include exosomes, microvesicles, and apoptotic bodies. EVs are a newly discovered mode of cell-cell communication between almost all cell types [55]. EVs are a heterogeneous group of membrane vesicles that are released into the extracellular space to fuse with neighboring cells. Moreover, EVs constitute an efficient route to move bioactive cargoes between cells allowing the recipient cell to acquire new properties. In addition, EVs coordinate control across long distances. EV cargoes include lipids, proteins, miRNAs, and RNAs [55]. In the CNS, the study of this mode of cellular communication provides new insights into CNS physiology and pathobiology [35, 55-58]. Neurons and microglia were shown to communicate by the bidirectional release of EVs, thus allowing microglia and neurons to swap various molecules, including intracellular signaling molecules and messengers. Specifically, microglial cells depend on the release of the mobile EVs to disseminate proinflammatory cytokines, which mediate neuroinflammation in remote areas of the brain [59]. In addition, microglia-released EVs can increase the secretion of glutamate, stimulating excitatory transmission at synapses through enhanced metabolism of sphingolipids, such as ceramide and sphingosine [35, 60]. Importantly, the dysfunctional or aberrant release of EVs can contribute to neurological and neurodegenerative diseases [35, 56-58]. In addition, EVs cargoes were demonstrated to contain pathogenic proteins (i.e., prions) and aggregations of toxic proteins like amyloid-beta (Aβ) [61, 62]. These facts provide further evidence for the involvement of EVs in neurological diseases.
3.1. Microglia Direct Physical Interaction with Elements of Neuronal Synapses
Direct contact-dependent interactions between microglia and neurons often take place at dendritic synapses [63]; however, direct microglial contacts with axons have also been reported [64]. Recently, it was shown that microglia could contact and interact with the soma at the so-called somatic junctions [65]. At the synapse, microglia can contact the pre- or post-synaptic neurons. Also, microglia can interact with inhibitory or excitatory synapses [66-68]. The most notorious proteins that mediate this interaction include the neuronal fractalkine (CX3CL1)/microglial fractalkine receptor (CX3CR1) proteins [67], members of the immune complement cascade [66], and CD200/ CD200R [35].
CX3CR1 is a microglia-specific surface protein that is expressed by the developing and adult brain [69]. CX3CL1 is expressed by neurons as a membrane-bound chemokine and can be released to bind the CX3CR1 receptor to microglia [69]. The mechanism underlying CX3CL1-CX3CR1 signaling is still not clear, but it has been suggested that neuronally expressed CX3CL1 attracts nearby microglia by a ‘find-me’ mechanism leading to an effect on synaptic maturation through a yet undiscovered mechanism [68, 70]. Additionally, CX3CL1-CX3CR1 signaling is critical for synaptic pruning [67, 71], a process through which microglia phagocytose and eliminate inactive or functionally redundant synapses to refine synaptic circuits of the adult brain [71]. During brain development, excessive connectivity takes place between neurons, and synaptic pruning is used to refine neural connectivity in an activity-dependent manner, where active synapses are maintained while weak or inactive synapses are eliminated, mainly via microglial phagocytosis [67, 71]. Thus, mice with microglia deficient in CX3CR1 show impairment of synaptic pruning, a decrease in functional connectivity, and deficits in social and grooming behaviors [67, 72]. Thus, microglia-dependent synaptic pruning underscores the role of microglial-neuronal communication and implies that “resting” microglia continually monitor and survey neuronal activity through their extended processes [67, 73]. Importantly, malfunctional synaptic pruning can be pathological [71], implicating that the synapse pruning machinery which operates during development can be revived by certain aspects of diseases like decreased synaptic activity.
Members of the immune complement system like C1q, C3, and the receptor CR3 (CD11b/CD18) have been recently shown to participate in microglia-neuron interaction. These molecules were demonstrated to participate in microglia elimination of neurites and synapses [66, 74]. During development, retinal ganglion neuronal cells (RGC) express membrane-tethered C1q protein. C1q activates C3 to bind its receptor CR3, which is solely expressed on microglia. C1q/ C3 /CR3 activation stimulates microglia engulfment of synapses where the complement molecules mark low activity synapses for elimination by microglia [66, 74]. Genetic ablation of the C1q, C3 ligand, or its receptor CR3 causes a reduction in synapse elimination by the microglia, and mice lacking these molecules fail to undergo proper synaptic refinement and will have extra synapses in the adult CNS [66, 74].
Recently, it was shown that phosphatidylserine (PS), usually present on the inner leaflet of the plasma membrane, is locally exposed to the outer leaflet of the plasma membrane by neurites and synapses during development [75]. Locally exposed PS on neurons and synapses serves as a recognition signal for microglia by marking the neurites and synapses to be engulfed [75]. When isolated neurons were cultured with microglia, it was found that microglia-mediated synaptic elimination was dependent on PS, which binds its receptor on microglia called TREM2 (triggering receptor expressed in myeloid cells 2). It was also shown that microglia could engulf material tagged with PS [75].
Interestingly, in C1q knock-out mice, there was an increase in PS exposure at synapses along with a decrease in microglial PS engulfment [75]. In fact, a regulatory model can be envisaged, where 1) the C1q/ C3/ CR3 system acts as an “eat me signal” that mediates the activity-dependent synaptic remodeling, and 2) local exposure of PS guides microglia to the neurons and synapses to be eliminated. Of interest, DAP12 (DNAX-activation protein 12) is also expressed on microglia where it is thought to form a receptor-adaptor complex with TREM2, and this complex has been shown to regulate microglial phagocytosis as well as signaling pathways involved in the control of synaptic plasticity [35, 76]. Indeed, studies have implicated DAP12 in the remodeling of developing synapses where synaptic plasticity was increased [77], yet apoptosis of developing neurons was reduced in mice lacking DAP12 [78]. Overall, these findings identify a role for locally exposed PS in directing microglial engulfment. Importantly, complement-mediated elimination of synapses can be aberrantly reactivated in neurodegenerative disease [66, 74].
Additionally, in the CNS, CD47, a transmembrane protein member of the immunoglobulin Ig superfamily, is localized at synapses, while its receptor SIRPα, also called CD127a, is expressed on microglia and neurons. The CD47/SIRPα signaling acts as a “don’t eat me signal”, which prevents excessive phagocytosis by microglia and serves as a molecular brake to curb excessive “eat-me signals”, like C1q/C3/C3R signaling, during synaptic pruning and synaptic plasticity [35, 79].
Other protein complexes that facilitate cell-contact mediated microglia-neuron interaction include CD200/CD200R. In the CNS, CD200, a transmembrane glycoprotein, is largely expressed on the surface of neurons and astrocytes, while the expression of the CD200 receptor (CD200R) is confined to microglia [14, 35]. It is thought that CD200/ CD200R signaling has a neuroprotective role since, upon microglia-neuron interaction, CD200/ CD200R signaling operates to retain microglia in an inhibited resting state. The loss of this signaling can lead to microglia activation and the eventual elimination of neuronal synapses [14, 35]. Consequently, it is not surprising that defects in CD200/CD200R signaling are involved in a handful of neuronal conditions, such as multiple sclerosis, AD, and neurotrauma [14, 35, 80]. One study that investigated the role of CD200R in SCI found that this receptor helps in controlling excessive inflammation [81]. When the research team inhibited CD200R, the clearance of neutrophils from the site of injury was impaired, and microglial cells were driven towards a pro-inflammatory state [81]. In another research, the absence of CD200/C200R pathway in SCI caused elevated inflammation, accompanied by increased mRNA expression of TNF-α and IL1β, increased intracellular TNF-α immunoreactivity, and reduced expression levels of macrophage factors that are associated with resolution of inflammation [82]. Collectively, proper direct physical interactions between microglia and neurons are required for appropriate neuronal development and homeostasis of neuronal circuits in the adult brain. In addition, aberrations in this communication are implicated in several neuronal diseases.
3.2. Microglia and Neurons Bidirectional Interaction via Soluble Paracrine Signaling Factors
Classical paracrine signaling via a secreted soluble ligand is a predominant mode of microglia-neuron crosstalk. Microglia and neurons secrete a myriad of soluble ligands that are received by the corresponding receptors on microglia/neurons. Microglia have been shown to secrete soluble factors that can provide neurotrophic functions as well as pro-inflammatory factors that can have damaging effects on neurons [13, 35, 83]. For instance, microglia secrete IL-1 β, IL-6, IL-10, nitric oxide (NO), PGE-2 (prostaglandin E2), TNF-α, brain-derived neurotrophic factor (BDNF), and molecules that mediate purinergic (ATP), glutamatergic, or GABAergic signaling [13, 35, 84-86]. The neurotrophic secreted factors are usually released by resting microglia and have been shown to induce neurite outgrowth and modify the cytoarchitecture of the developing CNS [35, 54, 83, 87]. In contrast, pro-inflammatory factors are usually produced by activated microglia and include pro-inflammatory cytokines as well as enzymes that induce neuroinflammation and can lead to the production of reactive oxygen species (ROS) like H2O2, which have been proven to contribute to axonal damage [35, 83, 87, 88]. Microglia-secreted soluble factors bind to their cognate receptors on neurons leading to a multitude of responses in neurons, such as the alteration of neuronal integrity or activity, synaptogenesis, neurogenesis, synaptic elimination, synaptic plasticity, or remodeling of axonal processes [54, 83].
In line with this, neurons secrete several soluble factors that are received by receptors on microglia. These factors include TGF-β, CD22, ATP, UDP, NO, glycine, glutamate, neurotrophin-3 (NT-3), BDNF, nerve growth factor (NGF), IL-34, and CSF (macrophage/ colony-stimulating factor)-1 [35, 54, 89, 90]. Signaling by these neuronally secreted molecules regulates several aspects of microglia function like proliferation, differentiation/activation, motility, chemotaxis, phagocytotic capacity, MHC class II expression, and production of proinflammatory/anti-inflammatory molecules [35, 54, 89, 90]. For instance, TGF-β, secreted by both neurons and glial cells, regulates the differentiation of microglia through activation of microglial TGF-β receptors [89]. Also, TGF-β regulates microglial activation by limiting increased microglial activation. This was demonstrated in the brains of TGF-β knock-out mice that exhibited increased neuronal loss due to enhanced microglial activation and microgliosis [91]. Therefore, as previously mentioned, neurotransmitters can activate/ deactivate microglia (Table 1) [92-117].
Table 1.
Different modulators and receptors that are implicated in neuron-microglia crosstalk, and their mechanisms of action.
| Microglia | Mediators | Receptors | Mechanism of Action |
|---|---|---|---|
| Inactivation Signals | CD200 | CD200R | By binding and activation of its microglia receptor CD200R, CD200 induces an anti-inflammatory effect through the downstream activation of adaptor protein: tyrosine kinase (Dok2). Dok2 binds to and activates Ras GTPase activating protein (RasGAP) which inhibits Ras activation, with subsequent repression of inflammation-associated downstream pathways (ERK and PI3K), thereby repressing microglia activation [92-94]. |
| CX3CL1 | CX3CR1 | CX3CL1 binds to its receptor CX3CR1 on microglia to maintain the microglia in the inactivated, surveying phenotype. Upon activation, CX3CR1 represses the overproduction of inflammatory mediators, such as TNF-, IL-1 and IL-6, as well as inhibits neuronal cell death. Also, CX3CL1 induces the expression of heme oxygenase 1 (HO-1), which induces a neuroprotective effect by repressing glutamate toxicity [95, 96]. | |
| CD47 | CD172a/ SIRPα | CD47 is expressed in neuronal cells and releases inhibitory signals through its receptor CD172a. Specifically, it acts as a “don’t eat me” signal”, which functions to reduce microglia overactivation and phagocytic activity [97]. | |
| TGF | TGF | TGF is an anti-inflammatory cytokine that represses microglial activation. It functions by suppressing Interferon-gamma (IFN-)-induced expression of proinflammatory mediators IL1, IL6, and TNF, thereby repressing the proliferation of microglia. Also, TGF suppresses the activity of acid phosphatase and the production of superoxide anions [98, 99]. | |
| Serotonin | 5-HT2B | Serotonin interacts through its receptor 5-HT2B to keep the microglia in the surveillance, resting, and anti-inflammatory phenotype. The microglial 5-HT2B receptors have been reportedly implicated in the prevention of inflammation in depression-like behaviors. Also, serotonin is associated with the microglia secretion of exosomes during inflammatory responses [100-102]. | |
| GABA | GABAB | GABA, an inhibitory neurotransmitter exerts a series of anti-inflammatory effects through its microglia receptor GABAB. GABA represses the release of inflammatory cytokines IL-6 and IL-12, thereby inducing an anti-inflammatory response to microglia [103]. | |
| BDNF | TrkB | BDNF, through its receptor TrkB in the microglia, induces anti-inflammatory effects. It represses the activation of microglia through the TrkB-Erk-CREB pathway. On the other hand, BDNF has also been reported to induce microglial activation and TNF- release [104, 105]. | |
| Activation Signals | ATP | P2Y | Functioning through its P2Y receptors, ATP, which is released by damaged neurons, triggers microglia activation and phagocytic activity. It leads to increased intracellular Ca2+ in the microglia, with a concomitant release of plasminogen. Furthermore, ATP leads to the activation of NF-kB, with subsequent release of inflammatory IL-, IL-6, and TNF- [106, 107]. |
| CCL21 | CXCR3 | CCL21 is a chemokine released by injured neurons, which leads to the activation and recruitment of microglia through its receptor CXCR3. Additionally, when released by neurons, CCL21 leads to the upregulation of the microglia P2X4 receptor, a purinergic receptor that is implicated in neuropathic pain reaction during nerve injury [108, 109]. | |
| Glutamate | mGlu2 | Upon neuronal injury, excessive glutamate release, which is the major excitatory neurotransmitter of the CNS, leads to neuronal cell death. Glutamate activates its receptor mGlu2 on microglia to trigger the release of proinflammatory TNF, which induces neurotoxicity. Furthermore, glutamate leads to the release of the Fas ligand, which triggers the activation of the Fas receptor and caspase 3, thereby causing neuronal cell death by apoptosis [103, 110, 111]. | |
| CCL2 | CCR2 | CCL2, which is expressed in neurons and astrocytes, triggers microglial activation and migration, through its receptor CCR2, during pathological conditions. Specifically, CCL2-CCR2 signaling leads to the phosphorylation of STAT3, with subsequent production of IL-1, which leads to neuronal cell death [112, 113]. | |
| TREM 2/DAP12 | - | TREM 2 is expressed in microglia and elicits microglia activation and phagocytosis, aiding in the clearance of apoptotic neurons. It forms a receptor-adaptor complex with DAP12 to regulate the phagocytic activity of microglia [114, 115]. | |
| MMP 3 | - | Apoptotic neurons release matrix metalloproteinase 3 (MMP 3), which induces microglial activation, and the release of pro-inflammatory cytokines like TNF-, IL-6, and IL-1. The released cytokines elicit the activation of the NF-κB pathway. Also, MMP3 activates microglia through the phosphorylation of ERK [116, 117]. |
Abbreviations: TNF-: Tumor necrosis factor-, GABA: Gamma-aminobutyric acid, ATP: Adenosine triphosphate, TREM2: Triggering receptor expressed in myeloid cells 2, DAP12: DNAX activating protein of 12 kDa, MMP3: Matrix metalloproteinase-3, IFN-: Interferon-gamma, TGF: Transforming growth factor , TGFR: Transforming growth factor receptor, HO-1: Heme oxygenase 1, IL-6: Interleukin 6, IL-1: Interleukin 1, IL-12: Interleukin 12, ERK: extracellular-signal-regulated kinase, CNS: Central nervous system, NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells, BDNF: Brain-derived neurotrophic factor, RasGAP: Ras GTPase activating protein.
Overall, this mode of microglia-neuron interaction is critical for the proper functioning of microglia and neuron, and deficits in this mode of communication can lead to dire consequences that contribute to the pathogenesis of several neuronal diseases Fig. (1). In the next section, the consequences of aberrant microglia-neuron communication regarding the pathogenesis of brain anomalies are discussed.
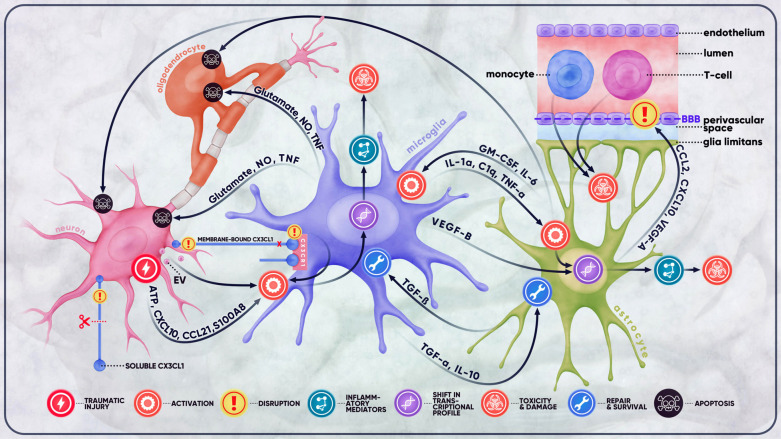
Fig. (1).
Microglial crosstalk in traumatic brain injury. Traumatic injury of the brain often results in a cascade of detrimental outcomes that involve a variety of resident and peripheral cells. At the site of injury, damaged neurons release substantial amounts of ATP, cellular debris, extracellular vesicles, and an assortment of mediators collectively responsible for the activation of microglial cells. This activation is also the result of disrupted microglia-neuron communication signals (e.g., CX3CL1-CX3CR1 signaling) between injured neurons and microglia. Damaged neurons stimulate the activation of microglia, which, once activated, secrete molecules, such as IL-1α, C1q, and TNF-α, and activate astrocytes. Reactive astrocytes, in turn, exert their effect on microglia, neurons, oligodendrocytes, and the BBB. The BBB is then rendered more permeable to such cells as monocytes, macrophages, and T-cells, which infiltrate the CNS and exacerbate the inflammatory response initially produced by microglia, thereby causing further damage and toxicity to the BBB and the brain. Depending on the extent and severity of the trauma, the complex crosstalk between the cells of the CNS may either be destructive, leading to excitotoxicity, neuroinflammation, demyelination, apoptosis, and other complications, or neuroprotective, promoting repair and survival.
4. MICROGLIA-NEURON INTERACTION IN NEURODEGENERATIVE AND PSYCHIATRIC DISORDERS
The association of microglial cells with several neurological and psychiatric disorders, such as AD, PD, brain ischemia, TBI, and schizophrenia, is well established [118-120]. Microglial activation, as discussed, is heavily dependent on proper microglia-neuron crosstalk, and aberrations in this crosstalk may lead to neurological pathologies. Indeed, disruptions in microglia-neuron communication have been extensively studied and linked to several brain pathologies, including schizophrenia [121, 122], bipolar disorder [123-125], depression [126, 127], and neurodegenerative disorders [128-131]. Further, in this review, the impact of aberrant microglia-neuron interactions on the pathogenesis of TBI, neurodegeneration, and ischemic stroke, is discussed.
4.1. Microglia-Neuron Interaction in Traumatic Brain Injury
TBI is a major public health threat and is one of the leading causes of death across all ages. Based on the severity of the injury and clinical outcomes, TBI can be classified as mild, moderate, or severe, and is a significant risk factor for other neurodegenerative disorders, such as chronic traumatic encephalopathy (CTE) and AD [132, 133].
During TBI, the primary injury introduces mechanical damage and injury to cells at the site of insult [134]. Upon injury, the brain microenvironment suffers from increased intracranial pressure, altered cerebral metabolism, vascular changes along with disruption of the BBB, and accumulation of toxic substances [135]. Notably, TBI-induced vascular changes lead to the alteration of cerebral blood flow and the disruption of the neurovascular unit [135]. Under such conditions, microglia become activated immediately post-injury as a protective process initiated against the trauma [5]. As a result of the BBB disruption, neurotoxic substances migrate into the brain, thereby altering its homeostasis [136]. At the same time, the injured neurons and astrocytes release signals, which activate and polarize the microglia towards their activation state [6]. Specifically, when neurons are injured, they release damage-associated molecular pattern (DAMP) molecules that trigger glial cells to initiate an immune response, and thus, contribute to neuroinflammation [137]. The triggered glial cells, mainly microglia, respond by releasing cytokines and chemokines [138-140]. This preliminary paracrine interaction between injured neurons and microglia initiates the inflammatory cascade implemented in brain injury. Activated microglia secrete the early players of the neuroinflammatory response, such as proinflammatory mediators like tumor TNF-α, IL-6, and IL-1β [17, 134, 141]. Once released, these inflammatory molecules stimulate neurons to release ROS, eventually leading to axonal damage. It is noteworthy that when ROS is released into the environment, microglia respond by upregulating the release of the same proinflammatory cytokines (TNF-α, IL-6, and IL-1β), making this interaction during injury a vicious loop that aggravates inflammation [134, 142, 143].
During TBI, microglia converge at injured neuronal sites in an ATP-mediated process [144]. ATP, released by astrocytes and neurons at the site of injury, forms a concentration gradient that draws microglia to the site of injury. ATP binds to microglial P2Y receptors, causing microglia to extend one process in the direction of injury, ultimately leading to the replacement of the ramified microglia by microglia, which is a long process. In confirmation, transcranial application of inhibitors of purinergic receptors (P2RY12 or P2RX4), before compression injury, prevented this phenomenon, suggesting the involvement of ATP [144]. This convergence by microglia results in phagocytosis, one of the most important interactions of microglia with neurons during an injury. Upon activation, microglia utilize phagocytosis to engulf and clear away the axonal and myelin debris from the Wallerian degeneration of the distal axonal segment, and involve TLRs, TREM-2, complement receptors 3 and 4, as well as MAC-2, for the engulfment of myelin, and the purinergic receptor P2RY6 [145]. Interestingly, rod microglia specifically have demonstrated high phagocytic activity and a dynamic emergence and resolution in the cortex in addition to the damaged neurons, suggesting that they might have a role in axonal damage and recovery post-injury [45].
Other studies have proposed a mechanism of microglial process convergence in status epilepticus. Following an injury to the brain parenchyma, there is an excessive release of glutamate, which activates NMDA receptors present on the surface of neurons [146]. This stimulation leads to the release of soluble CX3CL1, which triggers a downstream release of IL-1β by microglia, leading to an increase in the excitability of nearby neurons. This also leads to a localized release of ATP at specific dendritic locations, which attracts nearby microglial processes via P2Y12 activation to converge at the release site. Fractalkine signaling is a double-edged sword. At rest, signaling through the CX3CL1-CX3CR1 axis facilitates the communication between healthy neurons and microglia and allows the microglia to survey the integrity of neurons. At the same time, CX3CL1-CX3CR1 acts to inhibit microglial activation. However, in pathological states such as TBI, the injured neurons cannot maintain fractalkine signaling, and as a result, they can no longer keep microglia in an inhibited state. Moreover, mice knocked out for CX3CR1 and subjected to TBI had a reduction of sensorimotor deficits and less neuronal damage than wild-type injured counterparts at 4 days post-TBI, indicating that fractalkine signaling can be neurotoxic at 4 days post-TBI [147]. On the other hand, CX3CR1-/- mice showed increased cell death and sensorimotor deficits than wild-type mice at 5 weeks post-TBI, denoting that fractalkine signaling may be neuroprotective at 5 weeks post-TBI [147]. Relatedly, brains from mTBI mice have reduced levels of CX3CL1 protein [148]. Furthermore, the overexpression of CX3CR1, through lentiviral delivery, in a mouse model of radiation-induced brain injury, showed that CX3CL1-CX3CR1 signaling to be neuroprotective through a mechanism that promotes the polarization of microglia toward M2 to reduce neuroinflammation [149].
4.2. Microglia-Neuron Crosstalk in Neurodegeneration
Deficits in microglia-neuron crosstalk have been extensively investigated and linked to several brain pathologies, including neurodegenerative disorders [128-131]. For instance, the plaque-forming accumulations of amyloid-beta, a major hallmark of the AD, can be engulfed by microglia in TREM2/DAP12 dependent manner [150]. Several studies have shown that in AD, TREM-2 deficient microglia showed less effective Aβ internalization [151-154]. Consequently, it was discovered that TREM-2 is a major microglial-specific AD risk locus, and its risk is on par with that of the APOE ε4 allele [155]. Interestingly, a recent report shows that Trem-2 activation, using a Trem-2 agonistic antibody, mitigated Aβ deposition and improved cognition in a mouse model of AD [156]. These TREM-2 therapeutic effects can be explained by the recent discovery that Trem-2 triggers anti-inflammatory effects in microglia and this function is inhibited under proinflammatory states, like in neuroinflammation, due to brain injury or disease [157]. However, the TREM-2 therapeutic effects may not be straightforward since it was found that the protective effects of Trem-2 activation, in a mouse model of AD, were dependent on the activation states of existing microglia and their prior exposure to Trem-2 signaling [158].
Moreover, microglia play a significant role in the complement-mediated synapse loss observed in AD [159]. They can also aggravate tau pathology and cause neuronal injury either directly via the release of inflammatory factors, or indirectly via activating neurotoxic astrocytes [160]. Interestingly, microglial fractalkine signaling is highly dynamic in the pathogenesis of AD and plays different roles at different disease stages [161]. For instance, the neuronally-derived soluble CX3CL1 maintains microglia in a neuroprotective state, and any disruptions in this signaling dysregulate the microglial phenotype, resulting in neuronal damage [162].
Other studies have shown that the suppression of CX3CL1-CX3CR1 protected against Aβ-induced memory deficits and neurotoxicity [163, 164]. Kim et al. showed that the expression of CX3CL1 is significantly decreased during AD progression, and is inversely associated with the severity of AD [165]. Nevertheless, a recent study reports that CX3CR1 levels increase in AD through a mechanism that is regulated by the neurotransmitter noradrenaline [166]. Apparently, similar to what is observed in other neurological disorders, CX3CL1-CX3CR1 signaling can have a bimodal role depending on the context, and its details warrant extensive future investigations.
Likewise, CX3CL1-CX3CR1 communication plays a crucial role in the progression of PD. The formation of Lewy-bodies and intra-neuronal protein inclusions consisting of α-synuclein is the pathological hallmark associated with PD. α-Synuclein has been shown to activate microglia, and this activation is considered among the major contributors to neurodegeneration [167]. In different rodent models of PD, enhancing CX3CL1-CX3CR1 signaling proved to be neuroprotective via suppression of α-synuclein-mediated neurodegeneration [167-169]. These mechanisms are also observed in other forms of brain pathologies, like in the case of stroke.
4.3. Microglia-Neuron Interaction in Ischemic Stroke
Ischemic stroke, otherwise known as brain ischemia and cerebral ischemia or simply a brain attack, is a condition that occurs when there is an interruption of blood supply to the brain tissue. Tissues being supplied by this altered circulation become damaged and ultimately undergo death [170]. TBI can disrupt the vascular supply to the brain, leading to cerebral ischemia, and is associated with an increased risk of acute ischemic strokes [171]. Alteration of the physiological response of the body in the event of an ischemic stroke is dictated by the artery involved, which consequently determines the area and extent of the brain tissues affected [172]. During an ischemic stroke, microglia are activated a few hours after brain injury [173, 174] and play a vital role in safeguarding the injured brain tissue [175]. Under normal physiological conditions, the microglia are characterized by their small soma and fine elongated processes, as already discussed. In this state, microglia are characterized by a low level of expression of MHC II, CD86, CD80, CD11c, and CD45 [176, 177]. Ischemic stroke and other brain injuries cause focal inflammatory responses, which contribute to the activation of microglia [178]. Activated microglia upregulate the expression of cell surface markers, like major MHC II, CD68, and CD45 [179].
The duration and severity of ischemia determine the extent of microglial activation and morphological transformation. A reversible reduction in the length and number of microglial processes is typically observed in transient ischemia [180, 181], while severe ischemia leads to complete morphological transformation of microglia into an acquired amoeboid-like shape similar to peripheral macrophage [182]. It is traditionally believed that microglia play a detrimental role in the early stage of ischemic stroke upon activation. This is because of the findings from several studies which revealed that microglial activation is associated with ischemia-induced neuronal death [183-185] and its inhibition has been found to attenuate ischemia-induced brain injury [186].
Over the past decades, microglial detrimental effects on neurological diseases have been widely debated, as already discussed [187, 188]. On the contrary, evolving evidence shows that microglial activation following cerebral ischemic stroke provides a beneficial effect for functional recovery [189, 190]. This is because of the vital role it plays in angiogenesis, neurogenesis, and synaptic remodeling in the brain. This bimodal role of microglia following a brain injury is related to different polarization conditions of microglia and is influenced by several factors such as the pathological stage and distinct cellular context [186].
Direct communication between neurons and microglia following ischemic stroke is initiated via CD200/CD200R as well as fractalkine signaling. Several studies, using the experimental models of stroke, have revealed that microglia-neuron crosstalk via CX3CL1-CX3CR1 signaling plays neuroprotective roles [35]. In one study, enhanced recovery and reduced neuronal cell death along with decreased production of TNF-a and IL-1β have been reported in a knockout mouse model of induced focal cerebral ischemia [183]. CD200/CD200R signaling is an essential mechanism of neuroinflammation during an ischemic stroke [191]. A recent study investigated the acute effect of CD200/CD200R signaling following permanent ischemia in mice. The study revealed the expression of CD200 in neurons but its absence in microglia, and this was negatively correlated with neuronal cell death. Intraventricular injection of recombinant CD200, immediately following the induction of permanent middle cerebral artery occlusion (pMCAO), led to a reduction in microglial activation and the microglial expression of IL-10, IL-1β, and TNF-a [192]. CX3CL1-CX3CR1 signaling has also been explored in different models of brain ischemia; however, its neuroprotective role is not consistent in all studies. For instance, Denes et al. reported that, in a mice model of focal brain ischemia, knockout of CX3CR1 attenuated the production of IL-1β and TNF-α and was associated with reduced infarct size, decreased neuronal cell death, and better recovery [193]. Similarly, inhibition of CX3CR1-mediated microglial activation and neuroinflammation by Isorhynchophylline exhibited a neuroprotective effect on a mouse model of cerebral ischemia/reperfusion injury [194]. In contrast, other studies have suggested a potential neuroprotective role of CX3CL1-CX3CR1 in experimental models as well as in patients with ischemic stroke [195, 196]. In addition, an interesting recent study revealed an interaction between CX3CL1-CX3CR1 signaling and the exchange of exosomes between neurons and microglia during early brain injury after subarachnoid hemorrhage. It was concluded that CX3CL1-CX3CR1 signaling may have a protective function following subarachnoid hemorrhage by enhancing the delivery of neuronal miR-124, via exosomes, to microglia to reduce the activation of microglia and neuroinflammation [197].
Furthermore, microglial-neuronal communication through CD200/CD200R signaling has also been implicated in brain ischemia. The loss of neuronal CD200 has been shown to contribute to pro-inflammatory activation of microglia accompanied by an increased influx of inflammatory cells to the brain [192, 198]. Interestingly, Hayakawa et al. reported that treatment with the soluble form of CD200 (CD200-Fc), which inhibits CD200/CD200R signaling, led to enhanced myelination in an in vivo model of white matter ischemia [199]. It is not surprising that disruptions in microglial-neuronal communication have been found to be associated with such disorders, especially considering the significance of this communication in maintaining a normal physiological condition in the CNS.
CONCLUSION
The brain microenvironment involves a complex interplay between different cellular components. Microglia-neuron interactions are a prime component of the interactions that occur in the brain microenvironment. Microglia-neuron communication is critical for the homeostasis of the developing and the adult CNS. During injury or disease, the microglial response to injury is mediated by intricate crosstalk between neurons and glial cells. This interaction indicates the activation route of microglia and determines the functions exhibited by microglia to be either neuroprotective or neurotoxic. This is important to consider when trying to understand the events initiated during neuronal injury, whether it is TBI, ischemic stroke, or neurodegeneration. Notably, pathways involved in this dynamic communication include contact-dependent cell-to-cell communication, such as CD200/ CD200R and CX3CL1-CX3CR1 signaling, communication through secreted factors, gap junctional communication, or release of extravesicular bodies. A clear understanding of these mechanisms of communication and how their dysfunction contributes to neurological disorders is critical and requires future elucidation. It is anticipated that such an understanding will pave the way for novel therapeutic interventions that can target specific aberrations in microglia-neuron communication.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTION
FK, AS, MAH, and SI conceptualized the study. FK, AS, MS performed supervision. Original draft of the manuscript has been prepared by MAH, SI, MAR, JN, GS, YM, and RN, while manuscript review and editing have been carried out by MAH, SI, IB, and AJ. Illustrations were made by MAR.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Olah M., Biber K., Vinet J., Boddeke H.W. Microglia phenotype diversity. CNS Neurol. Disord. Drug Targets. 2011;10(1):108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- 2.Muoio V., Persson P.B., Sendeski M.M. The neurovascular unit - concept review. Acta Physiol. (Oxf.) 2014;210(4):790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 3.Weiss N., Miller F., Cazaubon S., Couraud P-O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta. 2009;1788(4):842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Mayer C.L., Huber B.R., Peskind E. Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache. 2013;53(9):1523–1530. doi: 10.1111/head.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donat C.K., Scott G., Gentleman S.M., Sastre M. Microglial activation in traumatic brain injury. Front. Aging Neurosci. 2017;9:208. doi: 10.3389/fnagi.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szepesi Z., Manouchehrian O., Bachiller S., Deierborg T. Bidirectional microglia-neuron communication in health and disease. Front. Cell. Neurosci. 2018;12(323):323. doi: 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pósfai B., Cserép C., Orsolits B., Dénes Á. New insights into microglia-neuron interactions: a neuron’s perspective. Neuroscience. 2019;405:103–117. doi: 10.1016/j.neuroscience.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A., Loane D.J. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 2012;26(8):1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Ritzel R.M., Li Y., He J., Khan N., Doran S.J., Faden A.I., Wu J. Sustained neuronal and microglial alterations are associated with diverse neurobehavioral dysfunction long after experimental brain injury. Neurobiol. Dis. 2020;136:104713. doi: 10.1016/j.nbd.2019.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinozaki Y., Shibata K., Yoshida K., Shigetomi E., Gachet C., Ikenaka K., Tanaka K.F., Koizumi S. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017;19(6):1151–1164. doi: 10.1016/j.celrep.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Fang K-M., Yang C-S., Sun S.H., Tzeng S-F. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J. Neurochem. 2009;111(5):1225–1237. doi: 10.1111/j.1471-4159.2009.06409.x. [DOI] [PubMed] [Google Scholar]
- 12.Dinet V., Petry K.G., Badaut J. Brain-immune interactions and neuroinflammation after traumatic brain injury. Front. Neurosci. 2019;13:1178. doi: 10.3389/fnins.2019.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ransohoff R.M., Cardona A.E. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 14.Hernangómez M., Mestre L., Correa F.G., Loría F., Mecha M., Iñigo P.M., Docagne F., Williams R.O., Borrell J., Guaza C. CD200-CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60(9):1437–1450. doi: 10.1002/glia.22366. [DOI] [PubMed] [Google Scholar]
- 15.Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 16.McPherson C.A., Merrick B.A., Harry G.J. In vivo molecular markers for pro-inflammatory cytokine M1 stage and resident microglia in trimethyltin-induced hippocampal injury. Neurotox. Res. 2014;25(1):45–56. doi: 10.1007/s12640-013-9422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransohoff R.M., Brown M.A. Innate immunity in the central nervous system. J. Clin. Invest. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David S., Kroner A., Greenhalgh A.D., Zarruk J.G., López-Vales R. Myeloid cell responses after spinal cord injury. J. Neuroimmunol. 2018;321:97–108. doi: 10.1016/j.jneuroim.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Filiano A.J., Gadani S.P., Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci. 2017;18(6):375–384. doi: 10.1038/nrn.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kierdorf K., Prinz M. Microglia in steady state. J. Clin. Invest. 2017;127(9):3201–3209. doi: 10.1172/JCI90602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., Koeglsperger T., Dake B., Wu P.M., Doykan C.E., Fanek Z., Liu L., Chen Z., Rothstein J.D., Ransohoff R.M., Gygi S.P., Antel J.P., Weiner H.L. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib P., Slowik A., Zendedel A., Johann S., Dang J., Beyer C. Regulation of hypoxia-induced inflammatory responses and M1-M2 phenotype switch of primary rat microglia by sex steroids. J. Mol. Neurosci. 2014;52(2):277–285. doi: 10.1007/s12031-013-0137-y. [DOI] [PubMed] [Google Scholar]
- 23.Yao X., Liu S., Ding W., Yue P., Jiang Q., Zhao M., Hu F., Zhang H. TLR4 signal ablation attenuated neurological deficits by regulat-ing microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol. 2017;310:38–45. doi: 10.1016/j.jneuroim.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Arcuri C., Mecca C., Bianchi R., Giambanco I., Donato R. The pathophysiological role of microglia in dynamic surveillance, phagocy-tosis and structural remodeling of the developing CNS. Front. Mol. Neurosci. 2017;10:191. doi: 10.3389/fnmol.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Essandoh K., Li Y., Huo J., Fan G-C. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122–131. doi: 10.1097/SHK.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173(4):649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 28.Colton C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norden D.M., Trojanowski P.J., Villanueva E., Navarro E., Godbout J.P. Sequential activation of microglia and astrocyte cytokine ex-pression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graeber M.B. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 32.Beins E., Ulas T., Ternes S., Neumann H., Schultze J.L., Zimmer A. Characterization of inflammatory markers and transcriptome pro-files of differentially activated embryonic stem cell-derived microglia. Glia. 2016;64(6):1007–1020. doi: 10.1002/glia.22979. [DOI] [PubMed] [Google Scholar]
- 33.Laffer B., Bauer D., Wasmuth S., Busch M., Jalilvand T.V., Thanos S., Hörste G.M.Z., Loser K., Langmann T., Heiligenhaus A., Kasper M. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front. Cell. Neurosci. 2019;13:430. doi: 10.3389/fncel.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond T.R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., Walker A.J., Gergits F., Segel M., Nemesh J., Marsh S.E., Saunders A., Macosko E., Ginhoux F., Chen J., Franklin R.J.M., Piao X., McCarroll S.A., Stevens B. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50(1):253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B., Wang K., Gao H.M., Mandavilli B., Wang J.Y., Hong J.S. Molecular consequences of activated microglia in the brain: overacti-vation induces apoptosis. J. Neurochem. 2001;77(1):182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh M., Xu Y., Pearse D.D. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J. Neuroinflammation. 2016;13(1):9. doi: 10.1186/s12974-015-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almolda B., de Labra C., Barrera I., Gruart A., Delgado-Garcia J.M., Villacampa N., Vilella A., Hofer M.J., Hidalgo J., Campbell I.L., González B. Alterations in microglial phenotype and hippocampal neuronal function in transgenic mice with astrocyte-targeted pro-duction of interleukin-10. Brain Behav. Immun. 2015;45:80–97. doi: 10.1016/j.bbi.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 39.Lisi L., Stigliano E., Lauriola L., Navarra P., Dello Russo C. Proinflammatory-activated glioma cells induce a switch in microglial polar-ization and activation status, from a predominant M2b phenotype to a mixture of M1 and M2a/B polarized cells. ASN Neuro. 2014;6(3):171–183. doi: 10.1042/AN20130045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chhor V., Charpentier T.L., Lebon S., Oré M. -.V.; Celador, I.L.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Sävman, K.; Mallard, C.; Gressens, P.; Fleiss, B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richard M. Ransohoff VHP. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 42.Taylor S.E., Morganti-Kossmann C., Lifshitz J., Ziebell J.M. Rod microglia: a morphological definition. PLoS One. 2014;9(5):e97096. doi: 10.1371/journal.pone.0097096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison H., Young K., Qureshi M., Rowe R.K., Lifshitz J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep. 2017;7(1):13211. doi: 10.1038/s41598-017-13581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry V.H., O’Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2(5):e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziebell J.M., Taylor S.E., Cao T., Harrison J.L., Lifshitz J. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J. Neuroinflammation. 2012;9(1):247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho B.P., Song D.Y., Sugama S., Shin D.H., Shimizu Y., Kim S.S., Kim Y.S., Joh T.H. Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia. 2006;53(1):92–102. doi: 10.1002/glia.20265. [DOI] [PubMed] [Google Scholar]
- 47.Wu S., Nguyen L.T.M., Pan H., Hassan S., Dai Y., Xu J., Wen Z. Two phenotypically and functionally distinct microglial populations in adult zebrafish. Sci. Adv. 2020;6(47):eabd1160. doi: 10.1126/sciadv.abd1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uriarte Huarte O., Richart L., Mittelbronn M., Michelucci A. Microglia in health and disease: the strength to be diverse and reactive. Front. Cell. Neurosci. 2021;15:660523. doi: 10.3389/fncel.2021.660523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geirsdottir L., David E., Keren-Shaul H., Weiner A., Bohlen S.C., Neuber J., Balic A., Giladi A., Sheban F., Dutertre C.A., Pfeifle C., Peri F., Raffo-Romero A., Vizioli J., Matiasek K., Scheiwe C., Meckel S., Mätz-Rensing K., van der Meer F., Thormodsson F.R., Stadelmann C., Zilkha N., Kimchi T., Ginhoux F., Ulitsky I., Erny D., Amit I., Prinz M. Cross-species single-cell analysis reveals di-vergence of the primate microglia program. Cell. 2019;179(7):1609–1622.e16. doi: 10.1016/j.cell.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Ochocka N., Kaminska B. Microglia diversity in healthy and diseased brain: insights from single-cell omics. Int. J. Mol. Sci. 2021;22(6):3027. doi: 10.3390/ijms22063027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramaniam S.R., Federoff H.J. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front. Aging Neurosci. 2017;9:176. doi: 10.3389/fnagi.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H., Leak R.K., Hu X. Neurotransmitter receptors on microglia. Stroke Vasc. Neurol. 2016;1(2):52–58. doi: 10.1136/svn-2016-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 54.Tian L., Ma L., Kaarela T., Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J. Neuroinflammation. 2012;9(1):155. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mondello S., Thelin E.P., Shaw G., Salzet M., Visalli C., Cizkova D., Kobeissy F., Buki A. Extracellular vesicles: pathogenetic, diag-nostic and therapeutic value in traumatic brain injury. Expert Rev. Proteomics. 2018;15(5):451–461. doi: 10.1080/14789450.2018.1464914. [DOI] [PubMed] [Google Scholar]
- 56.Paolicelli R.C., Bergamini G., Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Budnik V., Ruiz-Cañada C., Wendler F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016;17(3):160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krämer-Albers E.M., Hill A.F. Extracellular vesicles: interneural shuttles of complex messages. Curr. Opin. Neurobiol. 2016;39:101–107. doi: 10.1016/j.conb.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 59.Frühbeis C., Fröhlich D., Kuo W.P., Krämer-Albers E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antonucci F., Turola E., Riganti L., Caleo M., Gabrielli M., Perrotta C., Novellino L., Clementi E., Giussani P., Viani P., Matteoli M., Verderio C. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231–1240. doi: 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajendran L., Honsho M., Zahn T.R., Keller P., Geiger K.D., Verkade P., Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 2006;103(30):11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilette D., Courte J., Peyrin J.M., Coudert L., Schaeffer L., Andréoletti O., Leblanc P. Cellular mechanisms responsible for cell-to-cell spreading of prions. Cell. Mol. Life Sci. 2018;75(14):2557–2574. doi: 10.1007/s00018-018-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chugh D., Ekdahl C.T. Interactions between microglia and newly formed hippocampal neurons in physiological and seizure-induced inflammatory environment. Brain Plast. 2016;1(2):215–221. doi: 10.3233/BPL-150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baalman K., Marin M.A., Ho T.S., Godoy M., Cherian L., Robertson C., Rasband M.N. Axon initial segment-associated microglia. J. Neurosci. 2015;35(5):2283–2292. doi: 10.1523/JNEUROSCI.3751-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cserép C., Pósfai B., Lénárt N., Fekete R., László Z.I., Lele Z., Orsolits B., Molnár G., Heindl S., Schwarcz A.D., Ujvári K., Környei Z., Tóth K., Szabadits E., Sperlágh B., Baranyi M., Csiba L., Hortobágyi T., Maglóczky Z., Martinecz B., Szabó G., Erdélyi F. Szipőcs, R.; Tamkun, M.M.; Gesierich, B.; Duering, M.; Katona, I.; Liesz, A.; Tamás, G.; Dénes, Á. Microglia monitor and protect neu-ronal function through specialized somatic purinergic junctions. Science. 2020;367(6477):528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 66.Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y., Dissing-Olesen L., MacVicar B.A., Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36(10):605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrison J.K., Jiang Y., Chen S., Xia Y., Maciejewski D., McNamara R.K., Streit W.J., Salafranca M.N., Adhikari S., Thompson D.A., Botti P., Bacon K.B., Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ransohoff R.M., Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science. 2011;333(6048):1391–1392. doi: 10.1126/science.1212112. [DOI] [PubMed] [Google Scholar]
- 71.Sakai J. Core Concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc. Natl. Acad. Sci. USA. 2020;117(28):16096–16099. doi: 10.1073/pnas.2010281117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., Gross C.T. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 73.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 74.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W., Barres B.A. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 75.Scott-Hewitt N., Perrucci F., Morini R., Erreni M., Mahoney M., Witkowska A., Carey A., Faggiani E., Schuetz L.T., Mason S., Tamborini M., Bizzotto M., Passoni L., Filipello F., Jahn R., Stevens B., Matteoli M. Local externalization of phosphatidylserine me-diates developmental synaptic pruning by microglia. EMBO J. 2020;39(16):e105380. doi: 10.15252/embj.2020105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiialainen A., Hovanes K., Paloneva J., Kopra O., Peltonen L. Dap12 and Trem2, molecules involved in innate immunity and neuro-degeneration, are co-expressed in the CNS. Neurobiol. Dis. 2005;18(2):314–322. doi: 10.1016/j.nbd.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Roumier A., Béchade C., Poncer J.C., Smalla K.H., Tomasello E., Vivier E., Gundelfinger E.D., Triller A., Bessis A. Impaired synap-tic function in the microglial KARAP/DAP12-deficient mouse. J. Neurosci. 2004;24(50):11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakselman S., Béchade C., Roumier A., Bernard D., Triller A., Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008;28(32):8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lehrman E.K., Wilton D.K., Litvina E.Y., Welsh C.A., Chang S.T., Frouin A., Walker A.J., Heller M.D., Umemori H., Chen C., Ste-vens B. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100(1):120–134.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varnum M.M., Kiyota T., Ingraham K.L., Ikezu S., Ikezu T. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2015;36(11):2995–3007. doi: 10.1016/j.neurobiolaging.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lago N., Pannunzio B., Amo-Aparicio J., López-Vales R., Peluffo H. CD200 modulates spinal cord injury neuroinflammation and outcome through CD200R1. Brain Behav. Immun. 2018;73:416–426. doi: 10.1016/j.bbi.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Cohen M., Ben-Yehuda H., Porat Z., Raposo C., Gordon S., Schwartz M. Newly formed endothelial cells regulate myeloid cell activity following spinal cord injury via expression of CD200 Ligand. J. Neurosci. 2017;37(4):972–985. doi: 10.1523/JNEUROSCI.2199-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincenti J.E., Murphy L., Grabert K., McColl B.W., Cancellotti E., Freeman T.C., Manson J.C., Caughey B. Defining the microglia response during the time course of chronic neurodegeneration. J. Virol. 2015;90(6):3003–3017. doi: 10.1128/JVI.02613-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawasaki Y., Zhang L., Cheng J.K., Ji R.R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim S.H., Park E., You B., Jung Y., Park A.R., Park S.G., Lee J.R. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One. 2013;8(11):e81218. doi: 10.1371/journal.pone.0081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., III, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia pro-mote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schafer D.P., Jha S., Liu F., Akella T., McCullough L.D., Rasband M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009;29(42):13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar A., Barrett J.P., Alvarez-Croda D.M., Stoica B.A., Faden A.I., Loane D.J. NOX2 drives M1-like microglial/macrophage activa-tion and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 2016;58:291–309. doi: 10.1016/j.bbi.2016.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paglinawan R., Malipiero U., Schlapbach R., Frei K., Reith W., Fontana A. TGFbeta directs gene expression of activated microglia to an anti-inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia. 2003;44(3):219–231. doi: 10.1002/glia.10286. [DOI] [PubMed] [Google Scholar]
- 90.Mott R.T., Ait-Ghezala G., Town T., Mori T., Vendrame M., Zeng J., Ehrhart J., Mullan M., Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46(4):369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- 91.Brionne T.C., Tesseur I., Masliah E., Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–1145. doi: 10.1016/S0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 92.Hoek R.M., Ruuls S.R., Murphy C.A., Wright G.J., Goddard R., Zurawski S.M., Blom B., Homola M.E., Streit W.J., Brown M.H., Barclay A.N., Sedgwick J.D. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 93.Walker D.G., Lue L-F. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflamma-tion in human brains? Future Neurol. 2013;8(3):321–332. doi: 10.2217/fnl.13.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mihrshahi R., Brown M.H. Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J. Immunol. 2010;185(12):7216–7222. doi: 10.4049/jimmunol.1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizuno T., Kawanokuchi J., Numata K., Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1-2):65–70. doi: 10.1016/S0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 96.Noda M., Doi Y., Liang J., Kawanokuchi J., Sonobe Y., Takeuchi H., Mizuno T., Suzumura A. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J. Biol. Chem. 2011;286(3):2308–2319. doi: 10.1074/jbc.M110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biber K., Neumann H., Inoue K., Boddeke H.W.G.M. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Suzumura A., Sawada M., Yamamoto H., Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J. Immunol. 1993;151(4):2150–2158. [PubMed] [Google Scholar]
- 99.Shrikant P., Lee S.J., Kalvakolanu I., Ransohoff R.M., Benveniste E.N. Stimulus-specific inhibition of intracellular adhesion molecule-1 gene expression by TGF-beta. J. Immunol. 1996;157(2):892–900. [PubMed] [Google Scholar]
- 100.Turkin A., Tuchina O., Klempin F. Microglia function on precursor cells in the adult hippocampus and their responsiveness to serotonin signaling. Front. Cell Dev. Biol. 2021;9(1131):665739. doi: 10.3389/fcell.2021.665739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D’Andrea I., Béchade C., Maroteaux L. Chapter 34 - Serotonin and 5-HT2B receptors in microglia control of behavior. In: Handbook of Behavioral Neuroscience; Müller, C.P.; Cunningham, K.A., Eds.; Elsevier, 2020;31:589–599. doi: 10.1016/B978-0-444-64125-0.00034-7. [DOI] [Google Scholar]
- 102.Glebov K., Löchner M., Jabs R., Lau T., Merkel O., Schloss P., Steinhäuser C., Walter J. Serotonin stimulates secretion of exosomes from microglia cells. Glia. 2015;63(4):626–634. doi: 10.1002/glia.22772. [DOI] [PubMed] [Google Scholar]
- 103.Pocock J.M., Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 104.Wu S-Y., Pan B-S., Tsai S-F., Chiang Y-T., Huang B-M., Mo F-E., Kuo Y.M. BDNF reverses aging-related microglial activation. J. Neuroinflammation. 2020;17(1):210. doi: 10.1186/s12974-020-01887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X., Zeng L., Yu T., Xu Y., Pu S., Du D., Jiang W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell. Physiol. Biochem. 2014;34(3):715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- 106.Illes P., Rubini P., Ulrich H., Zhao Y., Tang Y. Regulation of microglial functions by purinergic mechanisms in the healthy and dis-eased CNS. Cells. 2020;9(5):1108. doi: 10.3390/cells9051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40(2):156–163. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- 108.de Jong E.K., Dijkstra I.M., Hensens M., Brouwer N., van Amerongen M., Liem R.S.B., Boddeke H.W., Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J. Neurosci. 2005;25(33):7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biber K., Tsuda M., Tozaki-Saitoh H., Tsukamoto K., Toyomitsu E., Masuda T., Boddeke H., Inoue K. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30(9):1864–1873. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor D.L., Jones F., Kubota E.S., Pocock J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necro-sis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005;25(11):2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Domercq M., Vázquez-Villoldo N., Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 2013;7(49):49. doi: 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conductier G., Blondeau N., Guyon A., Nahon J.L., Rovère C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroin-flammatory diseases. J. Neuroimmunol. 2010;224(1-2):93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Tian D.S., Peng J., Murugan M., Feng L.J., Liu J.L., Eyo U.B., Zhou L.J., Mogilevsky R., Wang W., Wu L.J. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1β production after status epilepticus. J. Neurosci. 2017;37(33):7878–7892. doi: 10.1523/JNEUROSCI.0315-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yao H., Coppola K., Schweig J.E., Crawford F., Mullan M., Paris D. Distinct signaling pathways regulate TREM2 phagocytic and NFκB antagonistic activities. Front. Cell. Neurosci. 2019;13(457):457. doi: 10.3389/fncel.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsieh C.L., Koike M., Spusta S.C., Niemi E.C., Yenari M., Nakamura M.C., Seaman W.E. A role for TREM2 ligands in the phagocy-tosis of apoptotic neuronal cells by microglia. J. Neurochem. 2009;109(4):1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim Y.S., Kim S.S., Cho J.J., Choi D.H., Hwang O., Shin D.H., Chun H.S., Beal M.F., Joh T.H. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J. Neurosci. 2005;25(14):3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Connolly C., Magnusson-Lind A., Lu G., Wagner P.K., Southwell A.L., Hayden M.R., Björkqvist M., Leavitt B.R. Enhanced immune response to MMP3 stimulation in microglia expressing mutant huntingtin. Neuroscience. 2016;325:74–88. doi: 10.1016/j.neuroscience.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 118.Wohleb E.S. Neuron-microglia interactions in mental health disorders: “for better, and for worse”. Front. Immunol. 2016;7:544. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joe E.H., Choi D.J., An J., Eun J.H., Jou I., Park S. Astrocytes, microglia, and Parkinson’s disease. Exp. Neurobiol. 2018;27(2):77–87. doi: 10.5607/en.2018.27.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perea J.R., Llorens-Martín M., Ávila J., Bolós M. The role of microglia in the spread of tau: relevance for tauopathies. Front. Cell. Neurosci. 2018;12:172. doi: 10.3389/fncel.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribeiro B.M., do Carmo M.R., Freire R.S., Rocha N.F., Borella V.C., de Menezes A.T., Monte A.S., Gomes P.X., de Sousa F.C., Vale M.L., de Lucena D.F., Gama C.S., Macêdo D. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophr. Res. 2013;151(1-3):12–19. doi: 10.1016/j.schres.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 122.Ishizuka K., Fujita Y., Kawabata T., Kimura H., Iwayama Y., Inada T., Okahisa Y., Egawa J., Usami M., Kushima I., Uno Y., Oka-da T., Ikeda M., Aleksic B., Mori D., Someya T., Yoshikawa T., Iwata N., Nakamura H., Yamashita T., Ozaki N. Rare genetic vari-ants in CX3CR1 and their contribution to the increased risk of schizophrenia and autism spectrum disorders. Transl. Psychiatry. 2017;7(8):e1184. doi: 10.1038/tp.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haarman B.C., Riemersma-Van der Lek R.F., de Groot J.C., Ruhé H.G., Klein H.C., Zandstra T.E., Burger H., Schoevers R.A., de Vries E.F., Drexhage H.A., Nolen W.A., Doorduin J. Neuroinflammation in bipolar disorder - A [(11)C]-(R)-PK11195 positron emis-sion tomography study. Brain Behav. Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 124.Réus G.Z., Fries G.R., Stertz L., Badawy M., Passos I.C., Barichello T., Kapczinski F., Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 125.Pinto J.V., Passos I.C., Librenza-Garcia D., Marcon G., Schneider M.A., Conte J.H., da Silva J.P.A., Lima L.P., Quincozes-Santos A., Kauer-Sant Anna M., Kapczinski F. Neuron-glia interaction as a possible pathophysiological mechanism of bipolar disorder. Curr. Neuropharmacol. 2018;16(5):519–532. doi: 10.2174/1570159X15666170828170921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yirmiya R., Rimmerman N., Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Hellwig S., Brioschi S., Dieni S., Frings L., Masuch A., Blank T., Biber K. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 2016;55:126–137. doi: 10.1016/j.bbi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Du L., Zhang Y., Chen Y., Zhu J., Yang Y., Zhang H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017;54(10):7567–7584. doi: 10.1007/s12035-016-0245-0. [DOI] [PubMed] [Google Scholar]
- 129.Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., Császár E., Fekete R., West B.L., Katona G., Rózsa B., Dénes Á. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016;7(1):11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loane D.J., Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol. 2016;275 Pt 3(0 3):316-327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu X., Li P., Guo Y., Wang H., Leak R.K., Chen S., Gao Y., Chen J. Microglia/macrophage polarization dynamics reveal novel mech-anism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 132.Gupta R., Sen N. Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev. Neurosci. 2016;27(1):93–100. doi: 10.1515/revneuro-2015-0017. [DOI] [PubMed] [Google Scholar]
- 133.Maas A.I.R. Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; Citerio, G.; Coburn, M.; Cooper, D.J.; Crowder, A.T.; Czeiter, E.; Czosnyka, M.; Diaz-Arrastia, R.; Dreier, J.P.; Duhaime, A.C.; Ercole, A.; van Es-sen, T.A.; Feigin, V.L.; Gao, G.; Giacino, J.; Gonzalez-Lara, L.E.; Gruen, R.L.; Gupta, D.; Hartings, J.A.; Hill, S.; Jiang, J.Y.; Ketharana-than, N.; Kompanje, E.J.O.; Lanyon, L.; Laureys, S.; Lecky, F.; Levin, H.; Lingsma, H.F.; Maegele, M.; Majdan, M.; Manley, G.; Marsteller, J.; Mascia, L.; McFadyen, C.; Mondello, S.; Newcombe, V.; Palotie, A.; Parizel, P.M.; Peul, W.; Piercy, J.; Polinder, S.; Puybasset, L.; Rasmussen, T.E.; Rossaint, R.; Smielewski, P.; Söderberg, J.; Stanworth, S.J.; Stein, M.B.; von Steinbüchel, N.; Stewart, W.; Steyerberg, E.W.; Stocchetti, N.; Synnot, A.; Te Ao, B.; Tenovuo, O.; Theadom, A.; Tibboel, D.; Videtta, W.; Wang, K.K.W.; Williams, W.H.; Wilson, L.; Yaffe, K.; Adams, H.; Agnoletti, V.; Allanson, J.; Amrein, K.; Andaluz, N.; Anke, A.; Antoni, A.; van As, A.B.; Audi-bert, G.; Azaševac, A.; Azouvi, P.; Azzolini, M.L.; Baciu, C.; Badenes, R.; Barlow, K.M.; Bartels, R.; Bauerfeind, U.; Beauchamp, M.; Beer, D.; Beer, R.; Belda, F.J.; Bellander, B-M.; Bellier, R.; Benali, H.; Benard, T.; Beqiri, V.; Beretta, L.; Bernard, F.; Bertolini, G.; Bilotta, F.; Blaabjerg, M.; den Boogert, H.; Boutis, K.; Bouzat, P.; Brooks, B.; Brorsson, C.; Bullinger, M.; Burns, E.; Calappi, E.; Cameron, P.; Carise, E.; Castaño-León, A.M.; Causin, F.; Chevallard, G.; Chieregato, A.; Christie, B.; Cnossen, M.; Coles, J.; Collett, J.; Della Corte, F.; Craig, W.; Csato, G.; Csomos, A.; Curry, N.; Dahyot-Fizelier, C.; Dawes, H.; DeMatteo, C.; Depreitere, B.; Dewey, D.; van Dijck, J.; Đilvesi, Đ.; Dippel, D.; Dizdarevic, K.; Donoghue, E.; Duek, O.; Dulière, G-L.; Dzeko, A.; Eapen, G.; Emery, C.A.; English, S.; Esser, P.; Ezer, E.; Fabricius, M.; Feng, J.; Fergusson, D.; Figaji, A.; Fleming, J.; Foks, K.; Francony, G.; Freedman, S.; Freo, U.; Frisvold, S.K.; Gagnon, I.; Galanaud, D.; Gantner, D.; Giraud, B.; Glocker, B.; Golubovic, J.; Gómez López, P.A.; Gordon, W.A.; Gradisek, P.; Gravel, J.; Griesdale, D.; Grossi, F.; Haagsma, J.A.; Håberg, A.K.; Haitsma, I.; Van Hecke, W.; Helbok, R.; Helseth, E.; van Heugten, C.; Hoedemaekers, C.; Höfer, S.; Horton, L.; Hui, J.; Huijben, J.A.; Hutchinson, P.J.; Jacobs, B.; van der Jagt, M.; Jankowski, S.; Janssens, K.; Jelaca, B.; Jones, K.M.; Kamnitsas, K.; Kaps, R.; Karan, M.; Katila, A.; Kaukonen, K-M.; De Keyser, V.; Kivisaari, R.; Kolias, A.G.; Kolumbán, B.; Kolundžija, K.; Kondziella, D.; Koskinen, L-O.; Kovács, N.; Kramer, A.; Kutsogiannis, D.; Kyprianou, T.; Lagares, A.; Lamontagne, F.; Latini, R.; Lauzier, F.; Lazar, I.; Ledig, C.; Lefering, R.; Legrand, V.; Levi, L.; Lightfoot, R.; Lozano, A.; MacDonald, S.; Major, S.; Mana-ra, A.; Manhes, P.; Maréchal, H.; Martino, C.; Masala, A.; Masson, S.; Mattern, J.; McFadyen, B.; McMahon, C.; Meade, M.; Melegh, B.; Menovsky, T.; Moore, L.; Morgado Correia, M.; Morganti-Kossmann, M.C.; Muehlan, H.; Mukherjee, P.; Murray, L.; van der Naalt, J.; Negru, A.; Nelson, D.; Nieboer, D.; Noirhomme, Q.; Nyirádi, J.; Oddo, M.; Okonkwo, D.O.; Oldenbeuving, A.W.; Ortolano, F.; Osmond, M.; Payen, J-F.; Perlbarg, V.; Persona, P.; Pichon, N.; Piippo-Karjalainen, A.; Pili-Floury, S.; Pirinen, M.; Ple, H.; Poca, M.A.; Posti, J.; Van Praag, D.; Ptito, A.; Radoi, A.; Ragauskas, A.; Raj, R.; Real, R.G.L.; Reed, N.; Rhodes, J.; Robertson, C.; Rocka, S.; Røe, C.; Røise, O.; Roks, G.; Rosand, J.; Rosenfeld, J.V.; Rosenlund, C.; Rosenthal, G.; Rossi, S.; Rueckert, D.; de Ruiter, G.C.W.; Sacchi, M.; Sahakian, B.J.; Sahuquillo, J.; Sakowitz, O.; Salvato, G.; Sánchez-Porras, R.; Sándor, J.; Sangha, G.; Schäfer, N.; Schmidt, S.; Schneider, K.J.; Schnyer, D.; Schöhl, H.; Schoonman, G.G.; Schou, R.F.; Sir, Ö.; Skandsen, T.; Smeets, D.; Sorinola, A.; Stamatakis, E.; Stevanovic, A.; Stevens, R.D.; Sundström, N.; Taccone, F.S.; Takala, R.; Tanskanen, P.; Taylor, M.S.; Telgmann, R.; Temkin, N.; Teodorani, G.; Thomas, M.; Tolias, C.M.; Trapani, T.; Turgeon, A.; Vajkoczy, P.; Valadka, A.B.; Valeinis, E.; Vallance, S.; Vámos, Z.; Vargiolu, A.; Vega, E.; Verheyden, J.; Vik, A.; Vilcinis, R.; Vleggeert-Lankamp, C.; Vogt, L.; Volovici, V.; Voormolen, D.C.; Vulekovic, P.; Vande Vyvere, T.; Van Waesberghe, J.; Wessels, L.; Wildschut, E.; Williams, G.; Winkler, M.K.L.; Wolf, S.; Wood, G.; Xirouchaki, N.; Younsi, A.; Zaaroor, M.; Zelinkova, V.; Zemek, R.; Zumbo, F. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 134.Simon D.W., McGeachy M.J. Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kan E.M., Ling E.A., Lu J. Microenvironment changes in mild traumatic brain injury. Brain Res. Bull. 2012;87(4-5):359–372. doi: 10.1016/j.brainresbull.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Lu J., Moochhala S., Kaur C., Ling E.A. Cellular inflammatory response associated with breakdown of the blood-brain barrier after closed head injury in rats. J. Neurotrauma. 2001;18(4):399–408. doi: 10.1089/089771501750170976. [DOI] [PubMed] [Google Scholar]
- 137.Wofford K.L., Loane D.J., Cullen D.K. Acute drivers of neuroinflammation in traumatic brain injury. Neural Regen. Res. 2019;14(9):1481–1489. doi: 10.4103/1673-5374.255958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139(Suppl. 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lafrenaye A.D., Todani M., Walker S.A., Povlishock J.T. Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation. 2015;12(1):186. doi: 10.1186/s12974-015-0405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alam A., Thelin E.P., Tajsic T., Khan D.Z., Khellaf A., Patani R., Helmy A. Cellular infiltration in traumatic brain injury. J. Neuroinflammation. 2020;17(1):328. doi: 10.1186/s12974-020-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaelber S., Pantcheva P., Borlongan C.V. Drug- and cell-based therapies for targeting neuroinflammation in traumatic brain injury. Neural Regen. Res. 2016;11(10):1575–1576. doi: 10.4103/1673-5374.193231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Witcher K.G., Eiferman D.S., Godbout J.P. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015;38(10):609–620. doi: 10.1016/j.tins.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olmos G., Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roth T.L., Nayak D., Atanasijevic T., Koretsky A.P., Latour L.L., McGavern D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benusa S.D., Lafrenaye A.D. Microglial process convergence on axonal segments in health and disease. Neuroimmunol. Neuroinflamm. 2020;7(23):23–39. doi: 10.20517/2347-8659.2019.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eyo U.B., Peng J., Murugan M., Mo M., Lalani A., Xie P., Xu P., Margolis D.J., Wu L-.J. Regulation of physical microglianeuron interactions by fractalkine signaling after status epilepticus. eNeuro. 2017;3(6):ENEURO.0209-16.2016. doi: 10.1523/ENEURO.0209-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zanier E.R., Marchesi F., Ortolano F., Perego C., Arabian M., Zoerle T., Sammali E., Pischiutta F., De Simoni M.G. Fractalkine re-ceptor deficiency is associated with early protection but late worsening of outcome following brain trauma in mice. J. Neurotrauma. 2016;33(11):1060–1072. doi: 10.1089/neu.2015.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tweedie D., Karnati H.K., Mullins R., Pick C.G., Hoffer B.J., Goetzl E.J., Kapogiannis D., Greig N.H. Time-dependent cytokine and chemokine changes in mouse cerebral cortex following a mild traumatic brain injury. eLife. 2020;9:9. doi: 10.7554/eLife.55827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang J., Pan H., Lin Z., Xiong C., Wei C., Li H., Tong F., Dong X. Neuroprotective effect of fractalkine on radiation-induced brain injury through promoting the M2 polarization of microglia. Mol. Neurobiol. 2021;58(3):1074–1087. doi: 10.1007/s12035-020-02138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mecca C., Giambanco I., Donato R., Arcuri C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018;19(1):E318. doi: 10.3390/ijms19010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yuan P., Condello C., Keene C.D., Wang Y., Bird T.D., Paul S.M., Luo W., Colonna M., Baddeley D., Grutzendler J. TREM2 hap-lodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dys-trophy. Neuron. 2016;90(4):724–739. doi: 10.1016/j.neuron.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y., Ulland T.K., Ulrich J.D., Song W., Tzaferis J.A., Hole J.T., Yuan P., Mahan T.E., Shi Y., Gilfillan S., Cella M., Grutzendler J., DeMattos R.B., Cirrito J.R., Holtzman D.M., Colonna M. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 2016;213(5):667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao Y., Wu X., Li X., Jiang L.L., Gui X., Liu Y., Sun Y., Zhu B., Piña-Crespo J.C., Zhang M., Zhang N., Chen X., Bu G., An Z., Huang T.Y., Xu H. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron. 2018;97(5):1023–1031.e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ulrich J.D., Holtzman D.M. TREM2 function in Alzheimer’s disease and neurodegeneration. ACS Chem. Neurosci. 2016;7(4):420–427. doi: 10.1021/acschemneuro.5b00313. [DOI] [PubMed] [Google Scholar]
- 155.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., Rujescu D., Hampel H., Giegling I., Andreassen O.A., Engedal K., Ulstein I., Djurovic S., Ibrahim-Verbaas C., Hofman A., Ikram M.A., van Duijn C.M., Thorsteinsdottir U., Kong A., Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Price B.R., Sudduth T.L., Weekman E.M., Johnson S., Hawthorne D., Woolums A., Wilcock D.M. Therapeutic Trem2 activation ame-liorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J. Neuroinflammation. 2020;17(1):238. doi: 10.1186/s12974-020-01915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu W., Taso O., Wang R., Bayram S., Graham A.C., Garcia-Reitboeck P., Mallach A., Andrews W.D., Piers T.M., Botia J.A., Po-cock J.M., Cummings D.M., Hardy J., Edwards F.A., Salih D.A. Trem2 promotes anti-inflammatory responses in microglia and is sup-pressed under pro-inflammatory conditions. Hum. Mol. Genet. 2020;29(19):3224–3248. doi: 10.1093/hmg/ddaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ellwanger D.C., Wang S., Brioschi S., Shao Z., Green L., Case R., Yoo D., Weishuhn D., Rathanaswami P., Bradley J., Rao S., Cha D., Luan P., Sambashivan S., Gilfillan S., Hasson S.A., Foltz I.N., van Lookeren Campagne M., Colonna M. Prior activation state shapes the microglia response to antihuman TREM2 in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2021;118(3):e2017742118. doi: 10.1073/pnas.2017742118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jiang S., Bhaskar K. Dynamics of the complement, cytokine, and chemokine systems in the regulation of synaptic function and dysfunc-tion relevant to Alzheimer’s disease. J. Alzheimers Dis. 2017;57(4):1123–1135. doi: 10.3233/JAD-161123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217(2):459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang L., Xu J., Gao J., Wu Y., Yin M., Zhao W. CD200-, CX3CL1-, and TREM2-mediated neuron-microglia interactions and their involvements in Alzheimer’s disease. Rev. Neurosci. 2018;29(8):837–848. doi: 10.1515/revneuro-2017-0084. [DOI] [PubMed] [Google Scholar]
- 162.Febinger H.Y., Thomasy H.E., Pavlova M.N., Ringgold K.M., Barf P.R., George A.M., Grillo J.N., Bachstetter A.D., Garcia J.A., Cardona A.E., Opp M.R., Gemma C. Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J. Neuroinflammation. 2015;12(1):154. doi: 10.1186/s12974-015-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu J., Bie B., Yang H., Xu J.J., Brown D.L., Naguib M. Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiol. Aging. 2013;34(12):2843–2852. doi: 10.1016/j.neurobiolaging.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 164.Dworzak J., Renvoisé B., Habchi J., Yates E.V., Combadière C., Knowles T.P., Dobson C.M., Blackstone C., Paulsen O., Murphy P.M. Neuronal Cx3cr1 deficiency protects against amyloid β-induced neurotoxicity. PLoS One. 2015;10(6):e0127730. doi: 10.1371/journal.pone.0127730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim T-S., Lim H-K., Lee J.Y., Kim D-J., Park S., Lee C., Lee C.U. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2008;436(2):196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 166.González-Prieto M., Gutiérrez I.L., García-Bueno B., Caso J.R., Leza J.C., Ortega-Hernández A., Gómez-Garre D., Madrigal J.L.M. Microglial CX3CR1 production increases in Alzheimer’s disease and is regulated by noradrenaline. Glia. 2021;69(1):73–90. doi: 10.1002/glia.23885. [DOI] [PubMed] [Google Scholar]
- 167.Nash K.R., Moran P., Finneran D.J., Hudson C., Robinson J., Morgan D., Bickford P.C. Fractalkine over expression suppresses α-synuclein-mediated neurodegeneration. Mol. Ther. 2015;23(1):17–23. doi: 10.1038/mt.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morganti J.M., Nash K.R., Grimmig B.A., Ranjit S., Small B., Bickford P.C., Gemma C. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J. Neurosci. 2012;32(42):14592–14601. doi: 10.1523/JNEUROSCI.0539-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pabon M.M., Bachstetter A.D., Hudson C.E., Gemma C., Bickford P.C. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflammation. 2011;8(1):9. doi: 10.1186/1742-2094-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. CDC. Stroke 2020 (cited 2021 7th february, 2021). Available from: https://www.cdc.gov/stroke/about.htm. [Google Scholar]
- 171.Kowalski R.G., Haarbauer-Krupa J.K., Bell J.M., Corrigan J.D., Hammond F.M., Torbey M.T., Hofmann M.C., Dams-O’Connor K., Miller A.C., Whiteneck G.G. Acute ischemic stroke after moderate to severe traumatic brain injury: incidence and impact on outcome. Stroke. 2017;48(7):1802–1809. doi: 10.1161/STROKEAHA.117.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflammation. 2019;16(1):142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen Y., Won S.J., Xu Y., Swanson R.A. Targeting microglial activation in stroke therapy: pharmacological tools and gender effects. Curr. Med. Chem. 2014;21(19):2146–2155. doi: 10.2174/0929867321666131228203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kawabori M., Kacimi R., Kauppinen T., Calosing C., Kim J.Y., Hsieh C.L., Nakamura M.C., Yenari M.A. Triggering receptor ex-pressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experi-mental stroke. J. Neurosci. 2015;35(8):3384–3396. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gülke E., Gelderblom M., Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018;11:1756286418774254. doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ponomarev E.D., Shriver L.P., Maresz K., Dittel B.N. Microglial cell activation and proliferation precedes the onset of CNS autoim-munity. J. Neurosci. Res. 2005;81(3):374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 177.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 178.Heindl S., Gesierich B., Benakis C., Llovera G., Duering M., Liesz A. Automated morphological analysis of microglia after stroke. Front. Cell. Neurosci. 2018;12:106. doi: 10.3389/fncel.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27(1):119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 180.Ju F., Ran Y., Zhu L., Cheng X., Gao H., Xi X., Yang Z., Zhang S. Increased BBB permeability enhances activation of microglia and exacerbates loss of dendritic spines after transient global cerebral ischemia. Front. Cell. Neurosci. 2018;12:236. doi: 10.3389/fncel.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Masuda T., Croom D., Hida H., Kirov S.A. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59(11):1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li T., Pang S., Yu Y., Wu X., Guo J., Zhang S. Proliferation of parenchymal microglia is the main source of microgliosis after is-chaemic stroke. Brain. 2013;136(Pt 12):3578–3588. doi: 10.1093/brain/awt287. [DOI] [PubMed] [Google Scholar]
- 183.Denes A., McColl B.W., Leow-Dyke S.F., Chapman K.Z., Humphreys N.E., Grencis R.K., Allan S.M., Rothwell N.J. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J. Cereb. Blood Flow Metab. 2011;31(4):1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Franco E.C., Cardoso M.M., Gouvêia A., Pereira A., Gomes-Leal W. Modulation of microglial activation enhances neuroprotection and functional recovery derived from bone marrow mononuclear cell transplantation after cortical ischemia. Neurosci. Res. 2012;73(2):122–132. doi: 10.1016/j.neures.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 185.Neher J.J., Emmrich J.V., Fricker M., Mander P.K., Théry C., Brown G.C. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl. Acad. Sci. USA. 2013;110(43):E4098–E4107. doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma Y., Wang J., Wang Y., Yang G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 187.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 188.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 189.Neumann J., Gunzer M., Gutzeit H.O., Ullrich O., Reymann K.G., Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20(6):714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- 190.Neumann J., Sauerzweig S., Rönicke R., Gunzer F., Dinkel K., Ullrich O., Gunzer M., Reymann K.G. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J. Neurosci. 2008;28(23):5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neumann H. Control of glial immune function by neurons. Glia. 2001;36(2):191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- 192.Yang Y., Zhang X.J., Zhang C., Chen R., Li L., He J., Xie Y., Chen Y. Loss of neuronal CD200 contributed to microglial activation after acute cerebral ischemia in mice. Neurosci. Lett. 2018;678:48–54. doi: 10.1016/j.neulet.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 193.Dénes A., Ferenczi S., Halász J., Környei Z., Kovács K.J. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J. Cereb. Blood Flow Metab. 2008;28(10):1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 194.Deng Y., Tan R., Li F., Liu Y., Shi J., Gong Q. Isorhynchophylline ameliorates cerebral ischemia/reperfusion injury by inhibiting CX3CR1-mediated microglial activation and neuroinflammation. Front. Pharmacol. 2021;12:574793. doi: 10.3389/fphar.2021.574793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cipriani R., Villa P., Chece G., Lauro C., Paladini A., Micotti E., Perego C., De Simoni M.G., Fredholm B.B., Eusebi F., Limatola C. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J. Neurosci. 2011;31(45):16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Donohue M.M., Cain K., Zierath D., Shibata D., Tanzi P.M., Becker K.J. Higher plasma fractalkine is associated with better 6-month outcome from ischemic stroke. Stroke. 2012;43(9):2300–2306. doi: 10.1161/STROKEAHA.112.657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen X., Jiang M., Li H., Wang Y., Shen H., Li X., Zhang Y., Wu J., Yu Z., Chen G. CX3CL1/CX3CR1 axis attenuates early brain injury via promoting the delivery of exosomal microRNA-124 from neuron to microglia after subarachnoid hemorrhage. J. Neuroinflammation. 2020;17(1):209. doi: 10.1186/s12974-020-01882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Denieffe S., Kelly R.J., McDonald C., Lyons A., Lynch M.A. Classical activation of microglia in CD200-deficient mice is a conse-quence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav. Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- 199.Hayakawa K., Pham L-D.D., Seo J.H., Miyamoto N., Maki T., Terasaki Y. Sakadžić S.; Boas, D.; van Leyen, K.; Waeber, C.; Kim, K.W.; Arai, K.; Lo, E.H. CD200 restrains macrophage attack on oligodendrocyte precursors via toll-like receptor 4 downregulation. J. Cereb. Blood Flow Metab. 2016;36(4):781–793. doi: 10.1177/0271678X15606148. [DOI] [PMC free article] [PubMed] [Google Scholar]