1. INTRODUCTION
Intestinal microflora involving the inhabiting microorganisms in the human gastrointestinal tract plays a vital role in health and diseases. It affects the local environment and the immunological, metabolic and neurological systems regulating a variety of disease pathways in the body. The latest research has suggested the association of gut microbiome dysbiosis with leading neurodegenerative diseases (NDDs), including Parkinson's disease (PD), Alzheimer's disease (AD), Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS), and Autism spectrum disorder (ASD). These pathologies appear to manifest through bidirectional communication across the gut-brain axis, involving neuroendocrine, neuroimmune, and direct neuronal routes such as the vagus nerve. Even Eran Blacher, the winner of NOSTER & Science Microbiome Prize in 2021, has suggested that the microbiome and its metabolite can greatly impact human health and physiology. Various environmental factors such as nutrition, hygiene, daily rhythms, physical activity, and exposure to pollutants or drugs can severely impact the composition and function of the gut-microflora. Interestingly, preclinical as well as clinical studies have demonstrated attractive prospects of prebiotics, probiotics, and synbiotics in the therapeutic regulation of NDDs.
2. TARGETING THE GUT-BRAIN AXIS
The microbiota-gut-brain (MGB) axis has been better understood and characterized over the past decade. It has answered pertinent questions about the etiology, pathophysiology, and developing clinical therapies for NDDs. Moreover, it has revealed the association of gut-microbiome with several neurodevelopment processes, such as neurogenesis, myelination, microglial maturation, and blood-brain barrier formation [1], where any microbial alterations are associated with NDD's progression. For instance, a reduction in Bifidobacterium, Firmicutes, and an increase in Bacteroidetes are reported in AD [2]. In another study, two bacterial strains- Enterococcus faecium and Lactobacillus rhamnosus are found to reduce oxidative stress via decreasing TNF-α production in the brain [3]. In fact, several studies have suggested the therapeutic potential of Lactobacilli and Bifidobacteria in curbing neuroinflammation-based memory deficits in AD [4]. On the other hand, a reduction in Abeta amyloid pathology is also observed in the absence of certain gut microbiota [5].
Likewise, gut-microbiome alterations also lead to motor deficits and neuroinflammation in PD [6]. There is an increase in Lactobacillaceae and Verrucomicrobiaceae, while a reduction in Prevotellaceae have been observed in PD. Instead, enteric infections by Helicobacter pylori and small intestinal bacterial overgrowth may be linked with motor fluctuations in PD [7]. A lower abundance of Akkermansiaceae, Firmicutes, and Lachnospiraceae are reported in HD gene expansion carriers, signifying their association in gut-barrier maintenance and inflammatory regulation. Moreover, symptomatic HD patients who exhibit a lower abundance of Eubacterium hallii tend to show more severe motor symptoms associated with the metabolism of certain amino acids such as methionine linking with oxidative stress and neuroinflammation in the brain [8]. Similarly, gut-microbiome such as Ruminococcus torques and Parabacteroides distasonis have been identified to exacerbate, and Akkermansia muciniphila has been shown to alleviate symptoms in ALS [9]. Particularly, the reduction in short-chain fatty acid (SCFA)-producing bacteria have been observed in most of the diseases causing inflammation [10].
Interestingly, SCFAs, one of the major products of the microbiome, exerts neuroprotective effects by increasing nerve growth factors and reducing inflammation in the brain. Still, some SCFA such as butyric acid, has also shown a controversial role in human health. For instance, butyric acid regulates synaptic activity and inflammation through GPCR signaling, and the epigenetic mechanism underlying HDAC inhibition. On the other hand, it alters glycolipid metabolism, thereby promoting obesity, diabetes, and other metabolic syndromes [11]. Similarly, kynurenine metabolites such as kynurenic acid and quinolinic acid act differently in the central nervous system, impacting neurons' function and survival. For instance, kynurenic acid has long been reported as a neuroprotective NMDA and α7 nicotinic antagonist. In contrast, quinolinic acid is an excitotoxic NMDA agonist involved in regulating synaptic transmission in the brain [12, 13].
Therefore, dietary interventions and selective prebiotic or probiotic administrations could be a supplemental therapeutic approach for ameliorating neurodegenerative processes while considering the complexity of the pathogenesis of NDDs (Fig. 1). There is increasing evidence that supports this notion. For instance, long-term probiotic interventions are reported to mitigate memory dysfunctions in lead-exposed rats through epigenetic inflammation–hippocampal pathway (IL-6-EZH2-H3K27me3) [14]. In another instance, the administration of prebiotics (oligofructose-enriched inulin) modulated peripheral immune responses and ameliorated age-related neuroinflammatory pathologies and brain functions [15]. Likewise, kynurenic acid imparted protection against quinolinic acid-induced oxidative imbalance and mitochondrial dysfunction by restoring Nrf2 levels in the rat striatum [16]. Importantly, the potentials of prebiotics, probiotics, and synbiotics are emerging in the clinical trials for NDDs like AD and PD (Table 1). In summary, immunomodulation through gut metabolites explained ameliorating neurodegenerative symptoms, but the direct interplay of gut metabolites on neuronal and glial cells needs to be addressed.
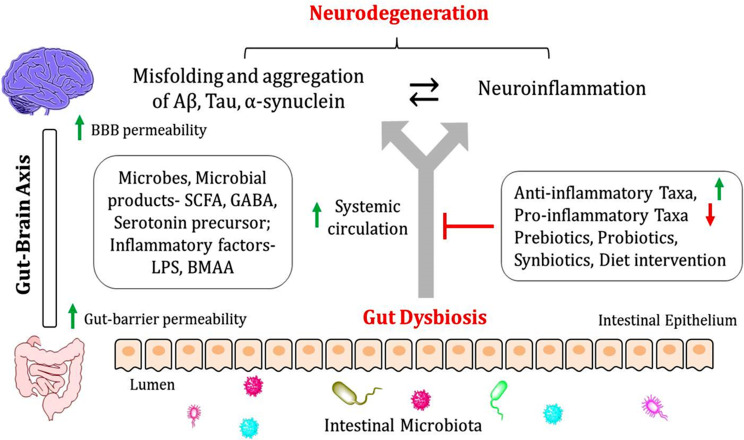
Fig. (1).
Gut-microbiome intervention technology- Gut dysbiosis is responsible for an abundance of pro-inflammatory cytokines, LPS and BMAA, destroying intestinal permeability and increasing the systemic circulation of microorganisms, microbial products (SCFA, GABA), and inflammatory factors (LPS, BMAA), which in turn disrupts the blood-brain barrier (BBB) and cause neuroinflammation and neurodegeneration. Therefore, administration of anti-inflammatory taxa (anti-inflammatory cytokines producing microorganisms) and restricting pro-inflammatory taxa (pro-inflammatory cytokine producing microorganisms), Prebiotics, Probiotics, and Synbiotics is an emerging strategy to target NDDs, including AD, PD, HD, and ALS.
Table 1.
Clinical trials highlighting therapeutic prospects of gut-microbiome in NDDs.
| Therapeutic Candidate | Intervention | Phase | Enrolled Patients | Clinical outcome | NDDs | References |
|---|---|---|---|---|---|---|
| L. acidophilus, L. casei, B. bifidum, and L. fermentum | Probiotics | II | 60 | Improved cognition and beta cell function, and decreased malondialdehyde levels, CRP, insulin resistance, and triglycerides | AD | [21] |
| L. acidophilus, B. bifidum, and B. longum | Probiotics | II | 79 | Improved cognition, total antioxidant capacity and glutathione, and decreased CRP, cholesterol, insulin, and triglycerides | AD | [22] |
| A. aceti, L. delbrueckii, L. fermentum, L. fructivorans, E. faecium, Leuconostoc sp., L. kefiranofaciens, C. famata, C. krusei, and Kefir | Synbiotics | _ | 13 | Improved cognition, inflammation, and oxidative stress, and blood cell damage | AD | [23] |
| S. salivarius thermophilus, E. faecium, L. rhamnosus GG, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii bulgaricus, and B. breve, and B. animalis lactis | Synbiotics | _ | 120 | Increased bowel movements in patients with constipation | PD | [24] |
| L. acidophilus, B. bifidum, L. reuteri, and L. fermentum | Probiotics | _ | 50 | Downregulation of inflammation (IL-1 and IL-8), insulin, and upregulated TGF-β and PPAR-γ | PD | [25] |
| L. acidophilus, B. bifidum, L. reuteri, and L. fermentum | Probiotics | II | 60 | Improved movement, glutathione levels and decreased CRP, malondialdehyde, insulin, and insulin resistance | PD | [26] |
A.- Acetobacter; B.- Bifidobacterium; C.- Candida; E.- Enterococcus; IL- Interleukin; L.- Lactobacillus; PPAR-γ- Peroxisome proliferator-activated receptor gamma; S.- Streptococcus.
3. DEVELOPMENT OF MICROBIOME-BASED MEDICINES
Though there are nearly 200 private and public firms working on microbiome-based treatments, only a few are dedicated to therapeutic intervention in NDDs. Seres Therapeutics' microbiota capsule SER-109 has passed clinical phase III. It may become the first FDA-approved microbiome therapy to treat recurrent Clostridium difficile infections, presenting the promises of microbiome-based drugs [17]. However, in NDD, most of the investigational products are in the early phases of clinical development, while few are in phase II. The gut-microbiome intervention technologies are now revolutionizing the development of therapeutic candidates targeting the gut-brain axis. For instance, 4D pharma has pioneered live biotherapeutic products, an emerging class of medicines based on single commensal bacterial strains. It's MicroRx platform enables us to understand how the individual bacteria affects the human body at the molecular level, allowing us to rationally pick live-biotherapeutic candidates with profiles appropriate to specific disease manifestations. It discovered two bacterial strains, MRx0005 and MRx0029, that have shown disease-modifying potential and targets numerous pathways involved in PD [18]. They decrease α-synuclein-induced neuroinflammation, protecting against oxidative stress, and boosting tight-junction protein expression, thereby improving gut barrier integrity.
Moreover, MRx0029 can generate a dopaminergic phenotype in undifferentiated neuronal cells, indicating its 'neuroregenerative' properties. Similarly, Enterin, a clinical-stage biopharmaceutical company, is also investigating the clinical efficacy of ENT-01 for treating non-motor symptoms, including constipation, dementia, and psychosis in PD (NCT04483479). Stellate therapeutics has synthesized clinical products STL-101 and STL-201 for therapeutic intervention in AD and PD, imparting neuroprotection [19]. In addition, Finch Therapeutics is also developing microbiome therapeutics such as FIN-211 for targeting gastrointestinal complications in ASD, employing its Human-First Discovery® platform. Instead, NuBiyota's microbial ecosystem therapeutic technology-based product MET-2 is under testing for anxiety and depression [20]. Furthermore, advanced biocomputation involving machine learning tools should be deployed by pharmaceutical companies to analyze complex microbiome data to develop promising microbiome-based drugs for therapeutic intervention in NDDs.
CONCLUSION AND FUTURE PROSPECTS
Although the MGB axis has gained more understanding in the past, important questions about the etiology, pathophysiology, and treatment are incomplete in neurodegenerative health and disease. Examining these as-yet-unknown factors will help develop effective pre-and probiotic treatments, but many unanswered questions remain. For instance, what role do post-biotics (such as SCFAs) play in the activity of prebiotics and probiotics and their interdependency? What are the quantitative and qualitative differences in the effects of these therapies on the structure and function of the gut microbiota? Importantly, how can dietary supplementation with probiotics and prebiotics affect signaling in the enteric nervous system and gut-brain axis? How do host variables like diet, genetic predisposition, prescribed medication, and age influence the therapeutic efficacy of dietary supplements that target the gut-brain axis? Moreover, their optimal doses and long-term safety concerns in the target populations are important.
Besides, many rodent discoveries have been successfully translated into human findings. Extrapolation from such findings and early clinical data requires extreme care. Additional human research employing prebiotic, probiotic, and synbiotic treatments is needed to target therapeutic regulation of the MGB axis. Furthermore, well-controlled large-scale longitudinal clinical trials are urgently needed to strengthen the present state of multi-omics-based targeted therapies for the MGB axis. Ultimately, using these best practices in relevant model systems with enough data on root-cause analyses and robustness might be a practical method to improve our pharmacological understanding. It will aid us in finding viable microbiome treatments and transform the future of our healthcare sector.
ACKNOWLEDGEMENTS
MIH thanks the Council of Scientific and Industrial Research for financial support [Project No. 27(0368)/20/EMR-II].
AUTHORS’ CONTRIBUTIONS
DK and MIH contributed to the conceptualization and study design. DK contributed to methodology, resources were provided by MIH. GMA and DK contributed to writing the original draft. GMA and MIH contributed to reviewing and editing of this manuscript, MIH supervised this research, MIH contributed to project administration, and GMA and MIH contributed to funding acquisition.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work is supported by the Indian Council of Medical Research (ISRM/12(22)/2020).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., Bendlin B.B., Rey F.E. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7(1):13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divyashri G., Krishna G. Muralidhara; Prapulla, S.G. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: in vitro and in vivo evidence. J. Med. Microbiol. 2015;64(12):1527–1540. doi: 10.1099/jmm.0.000184. [DOI] [PubMed] [Google Scholar]
- 4.Athari Nik Azm S., Djazayeri A., Safa M., Azami K., Ahmadvand B., Sabbaghziarani F., Sharifzadeh M., Vafa M. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1-42) injected rats. Appl. Physiol. Nutr. Metab. 2018;43(7):718–726. doi: 10.1139/apnm-2017-0648. [DOI] [PubMed] [Google Scholar]
- 5.Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D., Frisoni G., Neher J.J., Fåk F., Jucker M., Lasser T., Bolmont T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017;7(1):41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson T.R. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keshavarzian A., Engen P., Bonvegna S., Cilia R. Prog Brain Res. 2020. The gut microbiome in Parkinson’s disease: A culprit or a bystander? p. 252, 357-450. [DOI] [PubMed] [Google Scholar]
- 8.Wasser C.I., Mercieca E.C., Kong G., Hannan A.J., McKeown S.J., Glikmann-Johnston Y., Stout J.C. Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun. 2020;2(2):fcaa110. doi: 10.1093/braincomms/fcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blacher E., Bashiardes S., Shapiro H., Rothschild D., Mor U., Dori-Bachash M., Kleimeyer C., Moresi C., Harnik Y., Zur M., Zaba-ri M., Brik R.B., Kviatcovsky D., Zmora N., Cohen Y., Bar N., Levi I., Amar N., Mehlman T., Brandis A., Biton I., Kuperman Y., Tsoory M., Alfahel L., Harmelin A., Schwartz M., Israelson A., Arike L., Johansson M.E.V., Hansson G.C., Gotkine M., Segal E., Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 10.Duscha A. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067–1080. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018;9(1):21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112(Pt B):399-412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Ostapiuk A., Urbanska E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022;28(1):19–35. doi: 10.1111/cns.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao J., Wang T., Xu Y., Gu X., Li D., Niu K., Wang T., Zhao J., Zhou R., Wang H.L. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Transl. Psychiatry. 2020;10(1):25. doi: 10.1038/s41398-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme M., van de Wouw M., Bastiaanssen T.F.S., Olavarría-Ramírez L., Lyons K., Fouhy F., Golubeva A.V., Moloney G.M., Minuto C., Sandhu K.V., Scott K.A., Clarke G., Stanton C., Dinan T.G., Schellekens H., Cryan J.F. Mid-life microbiota crises: middle age is associated with pervasive neuroimmune alterations that are reversed by targeting the gut microbiome. Mol. Psychiatry. 2020;25(10):2567–2583. doi: 10.1038/s41380-019-0425-1. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira F.S., Biasibetti-Brendler H., Pierozan P., Schmitz F., Bertó C.G., Prezzi C.A., Manfredini V., Wyse A.T.S. Kynurenic acid restores Nrf2 levels and prevents quinolinic acid-induced toxicity in rat striatal slices. Mol. Neurobiol. 2018;55(11):8538–8549. doi: 10.1007/s12035-018-1003-2. [DOI] [PubMed] [Google Scholar]
- 17.Garber K. First microbiome-based drug clears phase III, in clinical trial turnaround. Nat. Rev. Drug Discov. 2020;19(10):655–656. doi: 10.1038/d41573-020-00163-4. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed S., Busetti A., Fotiadou P., Vincy Jose N., Reid S., Georgieva M., Brown S., Dunbar H., Beurket-Ascencio G., Delday M.I., Ettorre A., Mulder I.E. In vitro characterization of gut microbiota-derived bacterial strains with neuroprotective properties. Front. Cell. Neurosci. 2019;13:402. doi: 10.3389/fncel.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard P., Kozlowski L., Guillorit H., Garnier P., McKnight N.C., Danchin A., Manière X. Queuine, a bacterial-derived hypermodi-fied nucleobase, shows protection in in vitro models of neurodegeneration. PLoS One. 2021;16(8):e0253216. doi: 10.1371/journal.pone.0253216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinna M.A., Milev R. The safety, efficacy, and tolerability of microbial ecosystem therapeutic-2 in people with major depression and/or generalized anxiety disorder: protocol for a phase 1, open-label study. JMIR Res. Protoc. 2020;9(6):e17223–e17223. doi: 10.2196/17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R., Hamidi G.A., Salami M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016;8(256) doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamtaji O.R., Heidari-Soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E., Tajabadi-Ebrahimi M., Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019;38(6):2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Ton A.M.M., Campagnaro B.P., Alves G.A., Aires R., Côco L.Z., Arpini C.M., Guerra E.O.T., Campos-Toimil M., Meyrelles S.S., Pereira T.M.C., Vasquez E.C. Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid. Med. Cell. Longev. 2020;13(2638703) doi: 10.1155/2020/2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barichella M., Pacchetti C., Bolliri C., Cassani E., Iorio L., Pusani C., Pinelli G., Privitera G., Cesari I., Faierman S.A., Caccialanza R., Pezzoli G., Cereda E. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87(12):1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 25.Borzabadi S., Oryan S., Eidi A., Aghadavod E., Daneshvar Kakhaki R., Tamtaji O.R., Taghizadeh M., Asemi Z. The effects of probi-otic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: A randomized, double-blind, placebocontrolled trial. Arch. Iran Med. 2018;21(7):289–295. [PubMed] [Google Scholar]
- 26.Tamtaji O.R., Taghizadeh M., Daneshvar Kakhaki R., Kouchaki E., Bahmani F., Borzabadi S., Oryan S., Mafi A., Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38(3):1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]