Abstract
Interleukin-4 (IL-4) has been shown to be crucial in parasite expulsion in several gastrointestinal nematode infection models. Data from both epidemiological studies with humans and experimental infections in animals imply a critical role for the type II helper response, dominated by IL-4, in host protection. Here we utilized inbred mice on two distinct backgrounds to document the involvement of IL-4 in the clearance of a primary infection of Brugia from the murine host. Our data from infections of IL-4 receptor−/− and Stat6−/− mice further indicate that IL-4 exerts its effects by activating the Stat6 molecule in host target cells, a finding which links clearance requirements of a gastrointestinal tract-dwelling nematode with those of a tissue-dwelling nematode. Additionally, we show that the requirements for IL-4 receptor binding and Stat6 activation extend to accelerated clearance of a secondary infection as well. The data shown here, including analysis of cell populations at the site of infection and infection of immunoglobulin E (IgE)−/− mice, lead us to suggest that deficiencies in eosinophil recruitment and isotype switching to IgE production may be at least partially responsible for slower parasite clearance in the absence of IL-4.
In rodent models of helminth infections, a Th2-like response is generally associated with parasite clearance, while a Th1-dominated response leads to a state of chronic infection (11). This protective role for Th2 host responses has been documented most extensively in the gastrointestinal nematode models, in which an absolute requirement for the prototypical Th2 cytokine interleukin-4 (IL-4), the IL-4 receptor (IL-4R), or Stat6 signaling has been shown for the clearance of Heligmosomoides polygyrus, Trichuris muris, Trichinella spiralis, and Nippostrongylus brasiliensis from the murine host (4, 7, 8, 38–40). In contrast, gamma interferon mediates exacerbation of infection, at least in N. brasiliensis infections (36).
The human-infecting parasite Brugia malayi and its feline-infecting counterpart Brugia pahangi are tissue-dwelling nematodes against which a Th2-dominated response also seems to be rapidly induced and beneficial in a rodent host. The murine model has been extensively utilized in studies of Brugia infection. Immunocompetent mice are able to clear an infection with Brugia before patency, thereby providing a mammalian model of filarial infection in which host immune responses are successful in clearance of the parasite. Antigen-presenting cells responding to a Brugia infection in wild-type (WT) mice induce Th2 differentiation of naive T cells (17). Analysis of draining lymph node cells as well as splenocytes following intraperitoneal (i.p.), subcutaneous, or hind-footpad injections with live Brugia L3 infective-stage larvae (hereafter referred to as L3) shows an induction of Th2-like cytokines and an impairment in concanavalin A-driven Th1 cell proliferation and IL-2 and gamma interferon production (15, 27, 28). If the L3 are irradiated prior to subcutaneous injection, a strong Th2 response is still elicited (3). Osborne and Devaney have demonstrated that increased levels of mRNA encoding IL-4 can be detected in draining lymph nodes as early as 24 h postinfection with B. pahangi L3, from T-cell receptor αβ+ CD4− CD8− cells (27). A critical requirement for IL-4 in this system has been described (2), although studies with IL-4-deficient mice have shown variable results, seemingly dependent upon the background strains used (11, 15).
Although the necessity for IL-4 signaling has been well established in the clearance of several gastrointestinal nematodes, the precise role(s) of IL-4 has been more difficult to determine. Targets of IL-4 signaling in general include B cells, T cells, macrophages, stromal cells, hematopoietic precursors, intestinal epithelial cells, fibroblasts, and muscle cells (18, 26, 29, 30). Effects of IL-4 include inhibition of superoxide anion production by macrophages (34), upregulation of isotype switching to immunoglobulin G1 (IgG1) and IgE and major histocompatibility complex class II and CD23 expression on B cells (9, 33, 41), enhancement of eosinophil extravasation and recruitment (7, 21, 22, 32), and induction of Th2 cell differentiation in T cells (23). Finkelman et al. (10) and Urban et al. (37–39) have shown a dependence on both T cells and mast cells for the function of Stat6 signaling in clearance of Trichinella spiralis, while the Stat6 requirement in a model of N. brasiliensis infection is independent of B cells, T cells, or mast cells. It has been hypothesized that the target cell within the latter system is the intestinal epithelial cell (10, 37–39). Despite the target cells involved, the clearance mechanisms of these models involve expulsion of the live parasite from the gastrointestinal tract of the host.
A tissue-dwelling nematode such as Brugia differs in this respect from its gastrointestinal relatives in that elimination of the parasite load can be accomplished only by killing the worm while it is still inside the host. It is therefore very probable that the final effector mechanisms resulting in parasite clearance will be very different in the two nematode groups. Nonetheless, it is tempting to speculate that successful clearance of both gastrointestinal tract- and tissue-dwelling nematodes from the murine host shares the characteristic of inducing the IL-4R α chain–Stat6 signaling pathway within a host target cell(s).
In this study we utilized inbred mice on two distinct backgrounds to make a stronger case for the necessity of IL-4 in clearance of a primary infection of Brugia from the murine host. Further, through infection of IL-4R α chain−/− (hereafter IL-4R−/−) and Stat6−/− mice, we show that IL-4-induced Stat6 signaling within a host target cell(s) does in fact play a role in the killing of a tissue-dwelling parasite. We have begun to further elucidate the mechanisms by which IL-4 may aid in clearance of Brugia from the murine host through cellular analysis of intact and IL-4-deficient animals at the site of infection, in addition to infections of mutant mice with deficiencies downstream of Stat6 signaling.
MATERIALS AND METHODS
Mice.
BALB/c-Pkrdcscid/Pkrdcscid, BALB/c Stat6−/−, BALB/cByJ+/+, C57BL/6+/+, C57BL/6-Il4tm1Nnt (IL-4−/−), and BALB/c-Il4tm2Nnt (IL-4−/−) animals were obtained from the Jackson Laboratory (Bar Harbor, Maine). BALB/c-Il4ratm1Sz (IL-4R−/−) mice were initially obtained from the Jackson Laboratory and were subsequently bred and housed under specific-pathogen-free conditions in microisolator cages in the American Association for the Accreditation of Laboratory Animal Care-accredited vivarium of the University of Connecticut Health Center. BALB/c IgE−/− mice were originally obtained as a gift from M. Oettgen (Harvard University School of Medicine) and subsequently bred at our facility. All mice used were males between 6 and 12 weeks of age. Confirmation of the genotype of the IL-4R−/− animals was carried out by random PCR analysis of tail DNA using primers specific for the neomycin resistance gene as well as specific segments of the intact IL-4R gene as described previously (25). Leakiness of the SCID phenotype was ruled out through serum Ouchterloney tests. The IgE deficiency of IgE−/− animals was periodically confirmed using an IgE-specific enzyme-linked immunosorbent assay (ELISA) of serum.
Parasite.
B. malayi was harvested at the insectarium of Thomas Klei (Louisiana State University, Baton Rouge) from infected Aedes aegypti mosquitoes and shipped overnight in RPMI medium containing antibiotics and fluconazole. B. pahangi was harvested from infected mosquitoes at the University of Georgia and shipped in a similar manner.
Experimental infection.
Mice were inoculated with 50 to 80 B. pahangi or B. malayi L3 i.p. using a 25 5/8-gauge needle for a primary infection. For challenge infections, 50 L3 of the same species were injected i.p. into mice previously sensitized with 30 to 50 L3 4 to 6 weeks earlier.
Worm recovery.
Mice were sacrificed at various time points postinfection and subjected to a cardiac bleed for retrieval of serum. Peritoneal lavages were performed using RPMI medium supplemented with 5 U of heparin per ml. At time points of 4 weeks and later, lavage was extracted from the peritoneal cavity using a soft plastic pipette to prevent shearing of the adult worms. Following lavage, intestines were removed and soaked in phosphate-buffered saline (PBS). Testes were cut, and carcasses were placed in PBS for further soaking. Carcasses were then rinsed several times with PBS. Viable worms were counted from peritoneal lavage, intestinal washes, and carcass soaks under a dissecting microscope.
Preparation of inflammatory nodules.
Inflammatory nodules recovered from peritoneal lavages were transferred to petri dishes containing RPMI with 5 U of heparin per ml as a washing step. Nodules were then transferred to a 4% glutaraldehyde fixative and stored at 4°C prior to embedding.
IgE ELISA.
Total serum IgE-specific sandwich ELISA was performed following standard BD Pharmingen (San Diego, Calif.) protocol. Purified anti-mouse IgE R35-72 (Pharmingen catalog no. 02111D) was used as a capture antibody, and alkaline phosphatase-conjugated anti-mouse IgE (Pharmingen catalog no. 02133E) was used as a detecting antibody. Total concentrations in serum were determined by comparison to a purified mouse IgE standard (Pharmingen catalog no. 03121D).
Statistical analysis.
Statistical significance was analyzed by parametric as well as nonparametric methods. The Student t test (Stt) was used in the parametric analysis, and the Mann-Whitney test (MWt) was used in nonparametric determinations. P values of less than 0.05 were considered statistically significant.
Fluorescence-activated cell sorting.
Peritoneal lavage cells were collected and passed through nylon mesh to remove debris. Conjugated monoclonal antibodies (CD19-phycoerythrin, CD3-fluorescein isothiocyanate, Ly6G-fluorescein isothiocyanate, CD43-biotin, CD11b-biotin, and CD138-phycoerythrin) were obtained from Pharmingen and used at a dilution of 1:100 unless otherwise stated. Streptavidin-CyChrome (Pharmingen catalog no. 554062) was used at a dilution of 1:400 as a secondary antibody with biotin-conjugated antibodies. Stained cells were fixed with paraformaldehyde, and data were acquired through the CellQuest software using the FACSCalibur (Becton Dickinson). Data were subsequently analyzed using WinMDI software. Cell sorts were performed using the FACSVANTAGE SE.
Cytospins.
Cells (104) were taken from peritoneal lavages prior to fluorescence-activated cell sorter staining or following sorting and resuspended in 1% bovine serum albumin in PBS. Using a Cytospin 2, these cells were transferred to slides. Staining of slides was carried out with the Platinum Line Quick-Dip three-part staining system (Mercedes Medical, Inc.).
RESULTS
IL-4 is required for clearance of Brugia from C57BL/6 mice.
Our previous studies showed that BALB/c mice deficient in IL-4 were significantly more permissive to infection with B. malayi than WT mice at 6, 10, and 12 weeks postinfection. In addition, microfilariae (Mf) were found in the IL-4−/− mice at the 10- and 12-week time points (2). These results were in conflict with those obtained by Lawrence et al. (15) using IL-4−/− mice on the segregating (C57BL/6J × 129) background, suggesting that a requirement for IL-4 in host protection may be a phenomenon depending on the background strain of the murine host. In order to determine if the requirement for IL-4 for resistance to infection was unique to the BALB/c strain or a more universal requirement, we examined C57BL/6 mice. Although both BALB/c and C57BL/6 mice have been shown to be “resistant” to patent infection, our laboratory has consistently found that clearance of an i.p. injection of L3 from a C57BL/6 mouse occurs significantly more rapidly than clearance from a BALB/c mouse (unpublished data).
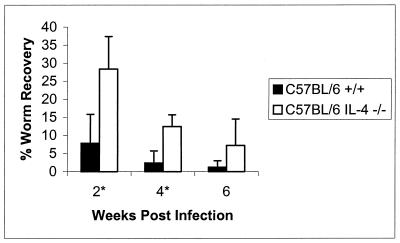
Figure 1 compares the clearance kinetics of C57BL/6 WT and IL-4-deficient mice. As early as 2 weeks following an i.p. injection of L3, a significantly higher percentage of live worms were recoverable from the C57BL/6 IL-4-deficient cohort. In this experiment (which is representative of four independent experiments), WT animals supported survival of 8% ± 8% of injected worms at 2 weeks, while their IL-4-deficient counterparts harbored 28% ± 9% (Stt, P = 0.005; MWt, P = 0.021). This difference in live worm recoveries remained significant until 4 weeks postinfection, at which point 2% ± 3% of worms remained in the WT animals, while the IL-4-deficient cohort still maintained 12% ± 3% (Stt, P = 0.001; MWt, P = 0.008). By 6 weeks postinfection both WT and IL-4-deficient mice supported less than 10% of injected worms. Unlike the case for BALB/c IL-4−/− mice, we have not detected Mf in B6 IL-4−/− animals. Similar results were obtained upon infection with B. pahangi (data not shown).
FIG. 1.
C57BL/6 mice are delayed in their ability to clear a primary infection of Brugia. Groups of 5 to 10 C57BL/6+/+ or IL-4−/− mice were injected i.p. with 50 L3 of B. malayi and necropsied at indicated time points postinfection. The data represent the percentage of the initial inoculation recovered as live worms. ∗, statistical significance (P < 0.05 by both parametric and nonparametric analyses) between percentages of worm recoveries from the WT and IL-4−/− groups. Error bars indicate standard deviations.
IL-4-induced parasite clearance requires signaling through IL-4R and activation of Stat6 within host cells.
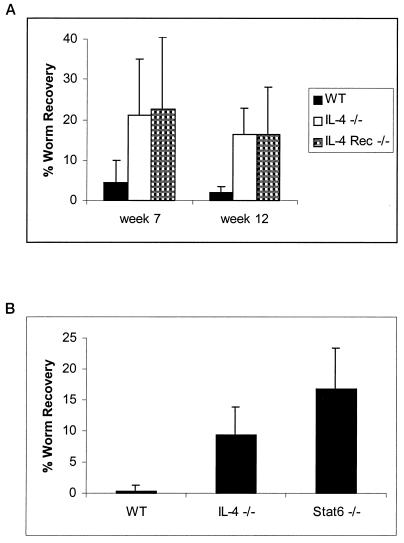
To differentiate between an activity of IL-4 directly against the worm and a function requiring signaling through an intermediary host cell, we utilized BALB/c IL-4R−/− mice. As shown in Fig. 2A, at 7 weeks postinfection 23% ± 18% of injected worms were recovered from IL-4R−/− mice, while cohorts of intact mice at this time point harbored only 4% ± 6% of injected worms. At 12 weeks postinfection IL-4R−/− mice continued to harbor significantly more worms than their WT counterparts, with IL-4R−/− mice supporting 17% ± 12% of injected worms, while fewer than 3% of worms could be recovered from the WT cohort (Stt, P = 0.02; MWt, P = 0.013). In addition, live worm recoveries from IL-4−/− cohorts in the same experiment were nearly identical to those from the IL-4R−/− animals. This experiment is representative of eight independent experiments analyzing various time points from 4 to 12 weeks postinfection.
FIG. 2.
IL-4 exerts its effects by signaling a host target cell(s) through IL-4R and subsequent Stat6 activation. (A) Groups of 5 to 10 BALB/c+/+ or BALB/c IL-4R−/− mice were injected i.p. with 50 B. malayi L3 and necropsied at 7 or 12 weeks following infection. The data represent the average percentage of the original inoculation recovered as live worms. P values at weeks 7 and 12 between WT and IL-4R−/− are 0.06 and 0.02, respectively, by Stt and 0.098 and 0.013, respectively, by MWt analysis. Error bars indicate standard deviations. (B) Groups of five BALB/c+/+, IL-4−/−, or Stat6−/− mice were injected i.p. with 50 B. malayi L3 and necropsied at 6 weeks postinfection. P values between WT and Stat6−/− mice are 0.0006 by Stt and 0.007 by MWt.
To ascertain whether signaling of IL-4 through IL-4R involved activation of the Stat6 pathway, we utilized BALB/c mice in which the Stat6 gene had been disrupted. Figure 2B shows the results of one representative experiment of three, which highlights significantly higher recoveries from Stat6−/− mice than from WT mice. At 6 weeks postinfection fewer than 1% of injected worms were recovered from WT mice, while IL-4−/− and Stat6−/− mice still harbored 9% ± 5% and 17% ± 7%, respectively, of the initial inoculum.
Effects of IL-4 defect compared to severe combined immunodeficiency.
Immunodeficient SCID mice, which lack T and B cells, have been utilized as an example of a completely susceptible host for Brugia infection (24). At 10 to 12 weeks postinfection with L3, SCID mice become patent for Mf. Although IL-4R−/− mice on the BALB/c background maintained significant worm burdens at late time points and became patent for Mf, live worm recoveries from these animals were consistently lower than those from completely immunodeficient SCID mice (data not shown).
A requirement for IL-4 signaling extends to a challenge infection.
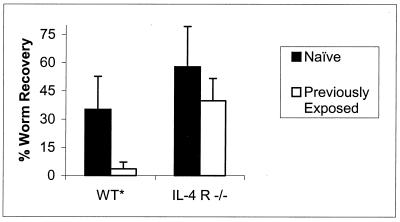
Immunocompetent BALB/c mice previously exposed to live Brugia L3 exhibit accelerated clearance of a second infection with L3 given 4 to 6 weeks later. Worms remaining from the primary infection are easily distinguishable from those remaining from the challenge infection due to their much larger size. In an effort to establish a role for IL-4 signaling in this rapid secondary clearance, BALB/c IL-4R−/− animals were subjected to the same challenge protocol. As shown in Fig. 3, worm recoveries from naive WT mice averaged 35%, while previously exposed WT mice harbored 4% of the challenge injection (Stt, P = 0.004; MWt, P = 0.008). In contrast, naive IL-4R−/− mice harbored 58% of injected worms, while previously exposed IL-4R−/− mice supported 40% of the challenge injection at this same time point (Stt, P = 0.129; MWt, P = 0.1).
FIG. 3.
IL-4 signaling is required for accelerated clearance of a challenge infection with Brugia. Five previously exposed or naive BALB/c+/+ or IL-4R−/− mice were challenged with 50 B. malayi L3 i.p. and necropsied at 14 days postchallenge. The data represent the average percentage of the challenge inoculation recovered as live worms. ∗, statistical significance (P < 0.5) between naive and previously exposed cohorts by both parametric and nonparametric analyses. Error bars indicate standard deviations.
Cellular analysis of the site of infection.
In search of mechanisms underlying the requirement for IL-4 signaling in our system, we analyzed the cellular compartment responding to the worms at the site of infection at several time points throughout infection. Peritoneal exudate cells (PEC) recovered from a peritoneal lavage were analyzed cytologically as well as by flow cytometry.
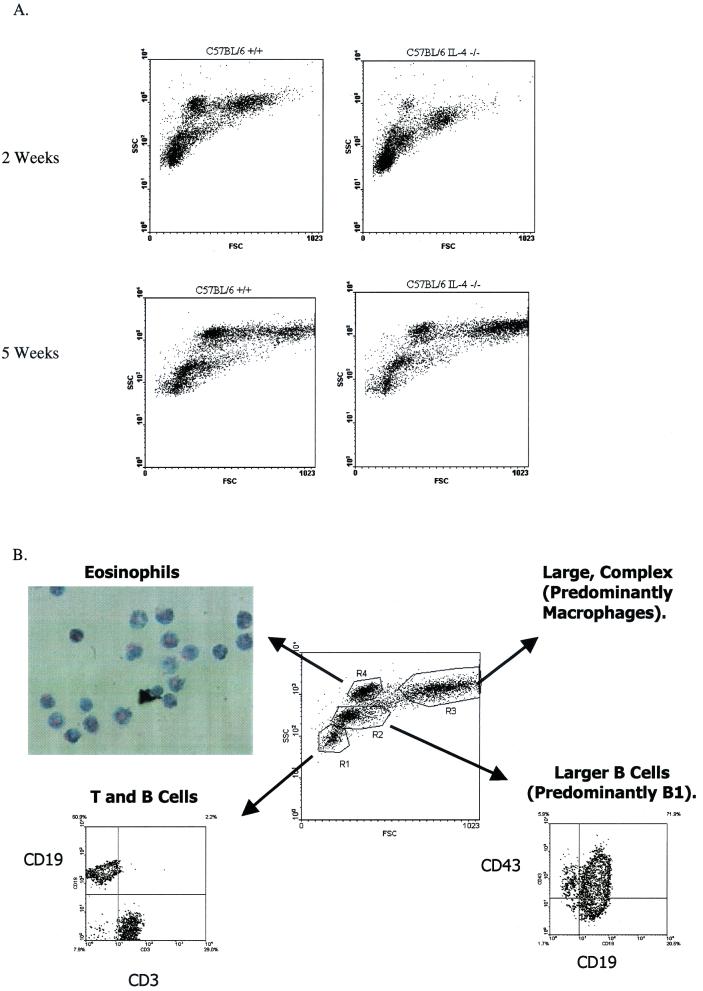
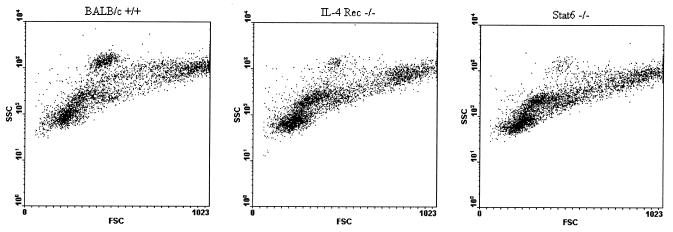
Figure 4A shows representative forward- and side-scatter plots generated from PEC isolated from C57BL/6 WT and IL-4−/− mice at 2 and 5 weeks postinfection. In these plots the y axis is a measurement of the relative complexity, or granularity, of the cells (side scatter) and the x axis represents the relative sizes of the cells (forward scatter). Thus, the cells in the lowest, leftmost regions are the smallest, least granular cells, while cells found at the rightmost, higher region of the plot represent the largest, most complex populations. Staining with fluorochrome-conjugated monoclonal antibodies has allowed us to identify the discreet populations which appear on this plot, as illustrated in Fig. 4B. Thus, the region arbitrarily designated R1 consists predominantly of T and B cells (CD3+ and CD19+ staining, respectively), while R2 appears to be predominantly larger B1 cells with some B2 cells (CD19+ CD43+ and CD19+ CD43−, respectively). The region designated R3 presumably represents a predominantly macrophage population, as suggested by the high degree of nonspecific staining and autofluorescence seen in this population and a strong reaction with CD11b (Mac-1) antibody (data not shown), as well as the large size and complexity implied by its location on the scatter plot (Fig. 4B). Positive staining for CD138 has also been observed in this region, indicating the presence of plasma cells (data not shown). The R4 region has been difficult to identify by cell surface staining techniques, although intermediate Ly6G (Gr-1) staining is consistently measured. These cells appear to be eosinophils, based on the observation that upon sorting of this region a population enriched for eosinophils is recovered (Fig. 4B). Further support for this identification comes from findings that this population is significantly reduced in IL-5−/− mice, as well as in WT mice treated with a monoclonal antibody against CCR3 (unpublished data).
FIG. 4.
Infected C57BL/6 IL-4−/− mice display an increased percentage of small lymphocytes and an impairment in their ability to recruit eosinophils to the site of infection. (A) PEC were recovered from C57BL/6+/+ or IL-4−/− mice at 2 or 5 weeks postinfection and analyzed by flow cytometry using the FACSCalibur. Results are shown as a forward-scatter (FSC)–side-scatter (SSC) plot. (B) Discrete cell populations of forward-scatter–side-scatter plots were gated and analyzed for expression of specific fluorochrome-conjugated antibodies. The R4 gate was sorted using the FACSVANTAGE SE and reanalyzed with the FACSCalibur to ensure purity of the sorted population. Sorted cells were then transferred to slides with a Cytospin 2 and stained.
The cellular compositions of the peritoneal cavities of WT and IL-4−/− mice at 2 and 5 weeks postinfection are calculated in Table 1 from a representative experiment involving five mice in each group. Amounts of different cell types are expressed as percentages of total cells, to account for any variability in lavage efficiency or mouse size. However, as Table 2 illustrates, a comparison of total cell numbers yields the same results. At 2 weeks postinfection, a significant difference was observed between the percentages of worms recovered from WT and IL-4−/− mice, with WT mice harboring 8% ± 8% of the injected worms, while IL-4−/− mice supported 28% ± 9%. Comparison of the cellular compositions of these animals at this time point indicates that the two cohorts differed significantly in the numbers of small lymphocytes (comprising 45% ± 6% of the IL-4−/− PEC and 25% ± 0.6% of the WT PEC [Stt, P = 0.00009]) and eosinophils (comprising 4% ± 1% of the IL-4−/− PEC and 14% ± 2% of the WT PEC [Stt, P = 0.0001]). By 5 weeks postinfection the IL-4−/− cohort had begun to decrease its worm burden, as 12% ± 4% of the injected worms were recovered, while the WT mice harbored 1% ± 2%. Analysis of the cellular compartments at this time point, as shown in Table 1, indicates no significant differences in the compositions of PEC between the two groups. As seen in Table 2, a comparison of total cell numbers yields similar results, with a fivefold decrease in the absolute number of eosinophils seen in IL-4−/− mice in comparison to their WT counterparts at 2 weeks postinfection and no significant discrepancies in any cell population at 5 weeks postinfection.
TABLE 1.
Percentages of different cell types in PEC after infection of WT and IL-4−/− C57BL/6 mice with B. malayia
| Time postinfection (wk) | Mice | % Worm recovery (mean ± SD) | % of total PEC (mean ± SD)
|
|||
|---|---|---|---|---|---|---|
| R1 (small lymphocytes) | R2 (large lymphocytes) | R3 (large, granular cells) | R4 (eosinophils) | |||
| 2 | C57BL/6+/+ | 8 ± 8 | 25 ± 0.6 | 20 ± 2 | 27 ± 9 | 14 ± 2 |
| C57BL/6 IL-4−/− | 28 ± 9 | 45 ± 6 | 25 ± 5 | 17 ± 4 | 4 ± 1 | |
| Pb | 0.005 | 0.00009 | 0.072 | 0.083 | 0.0001 | |
| 5 | C57BL/6+/+ | 1 ± 2 | 11 ± 1 | 29 ± 9 | 27 ± 6 | 10 ± 7 |
| C57BL/6 IL-4−/− | 12 ± 4 | 20 ± 8 | 24 ± 6 | 22 ± 12 | 8 ± 2 | |
| P | 0.0007 | 0.060 | 0.275 | 0.489 | 0.456 | |
Groups of 5 to 10 C57BL/6+/+ or IL-4−/− mice were injected i.p. with 50 L3 of B. malayi and necropsied at 2 or 5 weeks postinfection. PEC were collected and analyzed by flow cytometry on the basis of size and granularity.
Between WT and IL-4−/− mice, determined by Stt.
TABLE 2.
Total cell numbers in PEC after infection of WT and IL-4R−/− mice with B. malayia
| Time postinfection (wk) | Mice | Total cell number, 106 (mean ± SD)
|
|||
|---|---|---|---|---|---|
| R1 (small lymphocytes) | R2 (large lymphocytes) | R3 (large, granular cells) | R4 (eosinophils) | ||
| 2 | C57BL/6+/+ | 2.2 ± 1.4 | 1.7 ± 0.8 | 3.3 ± 2.4 | 1.2 ± 0.8 |
| C57BL/6 IL-4−/− | 3.9 ± 2 | 1.8 ± 0.7 | 1.7 ± 0.9 | 0.24 ± 0.1 | |
| 5 | C57BL/6+/+ | 2.1 ± 0.8 | 4.7 ± 1.4 | 5.6 ± 2.5 | 1.9 ± 1.9 |
| C57BL/6 IL-4−/− | 3.3 ± 0.6 | 4.3 ± 1.9 | 7.5 ± 7.8 | 1.4 ± 1.3 | |
See Table 1, footnote a.
Analysis of PEC from IL-4R−/− and Stat6−/− mice in comparison to WT mice highlights a similar increase in small lymphocytes and decrease in eosinophils seen in the mutant cohorts, as shown in Fig. 5 and Table 3. At 2 weeks postinfection, an average of 11% ± 16% of injected worms were recovered from five BALB/c WT mice, while five each of IL-4R−/− and Stat6−/− mice harbored averages of 75% ± 19% and 43% ± 23%, respectively. At this time point 38% ± 13% of PEC from IL-4R−/− mice compared to only 23% ± 3% of PEC from WT mice are small lymphocytes (Stt, P = 0.04). Similar to the case for IL-4R−/− mice, small lymphocytes represent 37% ± 3% of PEC in Stat6−/− mice (Stt, P = 0.00004 between Stat6−/− and WT mice). The eosinophil deficiency is seen in both the IL-4R−/− and Stat6−/− mice as well, as eosinophils comprise 1% ± 0.8% of PEC from IL-4R−/− mice, 1% ± 0.9% of PEC from Stat6−/− mice, and 11% ± 2% of PEC from WT mice (Stt, P = 0.00003 between IL-4R−/− and WT mice and P = 0.00003 between Stat6−/− and WT mice). Unlike the comparison between the C57BL/6 WT and IL-4−/− cohorts, the percentage of total PEC made up of large, complex cells was also significantly deficient in IL-4R−/− and Stat6−/− mice.
FIG. 5.
Infected BALB/c IL-4R−/− and Stat6−/− mice display an increased percentage of small lymphocytes and an impairment in their ability to recruit eosinophils to the site of infection. PEC were recovered from BALB/c+/+, IL-4R−/−, or Stat6−/− mice at 2 weeks postinfection. Cells were analyzed by flow cytometry as in Fig. 4 and are shown as forward-scatter (FSC)–side-scatter (SSC) plots.
TABLE 3.
Percentages of different cell types in PEC after infection of WT, IL-4R−/−, and Stat6−/− BALB/c mice with B. malayia
| Mice | % Worm recovery (mean ± SD) | % of total PEC (mean ± SD)
|
|||
|---|---|---|---|---|---|
| R1 (small lymphocytes) | R2 (large lymphocytes) | R3 (large, granular cells) | R4 (eosinophils) | ||
| BALB/c+/+ | 11 ± 16 | 23 ± 3 | 19 ± 4 | 25 ± 6 | 11 ± 2 |
| BALB/c IL-4R−/− | 75 ± 19 | 38 ± 13 | 22 ± 3 | 18 ± 2 | 1 ± 0.8 |
| BALB/c Stat6−/− | 43 ± 23 | 37 ± 3 | 28 ± 1 | 15 ± 2 | 1 ± 0.9 |
| P value between WT and IL-4R−/− mice (Stt) | 0.0004 | 0.04 | 0.194 | 0.036 | 0.00003 |
| P value between WT and Stat6−/− mice (Stt) | 0.035 | 0.00004 | 0.0005 | 0.009 | 0.00003 |
Groups of 5 to 10 WT, IL-4R−/−, or Stat6−/− BALB/c mice were injected i.p. with 50 L3 of B. malayi and necropsied at 2 weeks postinfection. PEC were collected and analyzed by flow cytometry on the basis of size and granularity.
Examination of cytospins obtained from IL-4−/−, IL-4R−/−, Stat6−/−, and WT PEC confirms the flow cytometric results (data not shown).
Analysis of PEC during challenge infection.
Table 4 lists the breakdown of the PEC compartment from WT or IL-4R−/− mice 2 weeks following a challenge infection. Again, an increase in small lymphocytes is observed in IL-4R−/− (21% ± 3%) compared to WT (8% ± 3%) animals, as is a deficit in the percentage of eosinophils found within the peritoneal cavity upon challenge infection of IL-4R−/− mice (13% ± 6%) compared to WT controls (28% ± 5%). In addition, a significantly higher percentage of the PEC are comprised of large, granular cells and a significantly lower percentage of large lymphocytes are found within the WT cohort compared with the mutant animals.
TABLE 4.
Percentages of different cell types in PEC after challenge infection of previously exposed WT and IL-4R−/− BALB/c mice
| Mice | % Worm recovery (mean ± SD) | % of total PEC (mean ± SD)
|
|||
|---|---|---|---|---|---|
| R1 (small lymphocytes) | R2 (large lymphocytes) | R3 (large, granular cells) | R4 (eosinophils) | ||
| BALB/c+/+ | 2 ± 4 | 8 ± 3 | 12 ± 3 | 36 ± 5 | 28 ± 5 |
| BALB/c IL-4R−/− | 64 ± 15 | 21 ± 3 | 29 ± 2 | 19 ± 4 | 13 ± 6 |
| P value between WT and IL-4R−/− mice (Stt) | 0.000 | 0.000 | 0.000 | 0.001 | 0.007 |
Groups of 5 to 10 previously exposed WT or IL-4R−/− BALB/c mice received a challenge injection of 50 B. malayi L3 and were necropsied 2 weeks later. PEC were collected and analyzed by flow cytometry on the basis of size and granularity.
Importance of eosinophils in worm clearance.
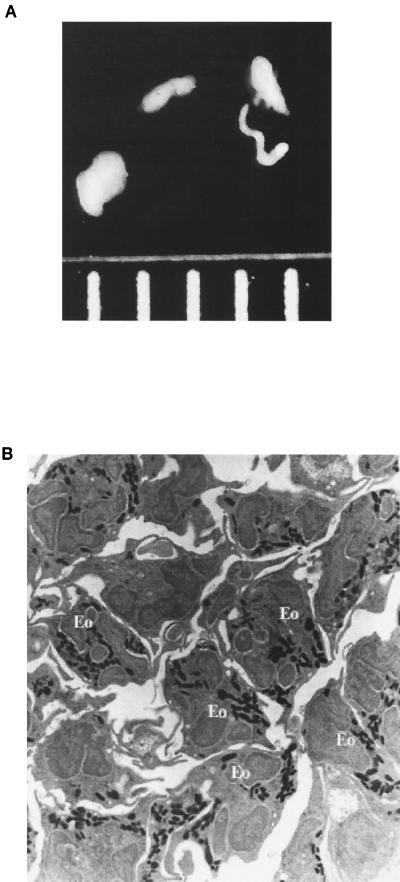
The inability of IL-4−/−, IL-4R−/−, and Stat6−/− mice to recruit eosinophils to the infection site in a timely manner is not surprising, as a role for IL-4 in eosinophil recruitment has been well documented (7, 21, 22, 32). A role for eosinophils in clearance of filarial infections has not been formally documented. However, evidence exists for their inclusion in host cell nodules encompassing worms within a murine host (12, 13, 20, 35). These “coated worms” (Fig. 6A) are found within the peritoneal cavity during phases of maximum worm clearance and presumably represent a final step before worm death, as illustrated by the necrotic appearance of portions of the encased worms when observed by electron microscopy. Analysis of the cellular composition of these nodules by light microscopy and transmission electron microscopy indicates that the predominant host cell types are eosinophils and macrophages. Figure 6B illustrates the high level of eosinophil involvement in host cell nodules. Further analysis of this nodule revealed eosinophil involvement ranging from 10 to 80% in every field examined. This dramatic variation in eosinophil numbers makes a precise quantitation of eosinophils nearly impossible.
FIG. 6.
Worms recovered from peritoneal cavities encased in host cell nodules contain a high percentage of eosinophils. (A) L4 stage worms encased in host cells recovered from a WT mouse at 2 weeks postinfection with B. malayi. Measuring marks represent 1-mm increments. (B) Transmission electron micrograph of a section of a host cell nodule recovered from a WT mouse at 2 weeks postinfection with B. malayi. Magnification, ×900.
Isotype switching to IgE as a possible target of IL-4.
Another IL-4-mediated effect which has been attributed to Stat6 activation is the induction of isotype switching to IgE production upon costimulation of B cells through CD40 (5, 6, 42). IL-4−/− mice have been shown to produce significantly less parasite-specific IgE than WT mice in response to B. malayi (2). Similarly, we find little to no IgE in sera from IL-4R−/− and Stat6−/− mice following a primary infection with Brugia (Table 5).
TABLE 5.
IgE levels in BALB/c++, BALB/c IL-4R−/−, and BALB/c Stat6−/− mice infected with B. malayi
| Mice | Total IgE levela (ng/ml) at wk postinfection:
|
||
|---|---|---|---|
| 2 | 4 | 5 | |
| BALB/c++ | 1,460 ± 2,212 | 792 ± 384 | 873 ± 650 |
| BALB/c IL-4R−/− | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BALB/c Stat6−/− | NMb | NM | 37 ± 83 |
Total IgE levels (means ± standard deviations) in sera of infected mice were measured by sandwich ELISA.
NM, not measured.
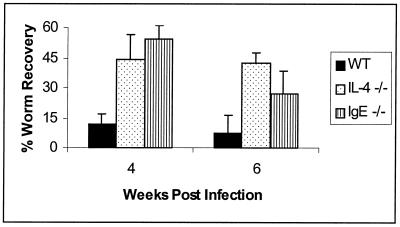
In order to ascertain whether the decrease in IgE production in these animals may be responsible for the impairment in worm clearance abilities, we infected BALB/c IgE−/− mice. A comparison of worm clearance kinetics among WT, IL-4−/−, and IgE−/− mice is shown in Fig. 7. By 4 weeks postinfection, IL-4−/− and IgE−/− mice harbored 44% ± 13% and 54% ± 7% of injected worms, respectively. In contrast, the WT animals had decreased their worm burdens to 12% ± 5% at this time point. This increase in worm recoveries from IgE−/− compared to WT mice is maintained at least until week 6 postinfection, at which point 8% ± 9% of injected worms were recovered in the WT mice, while 27% ± 12% were found in the IgE−/− group. It is important to note, however, that worm recoveries from IgE−/− mice at 6 weeks postinfection were significantly lower than those seen at 4 weeks postinfection (Stt, P = 0.003; MWt, P = 0.014). Additionally, the recoveries at 6 weeks were significantly lower in IgE−/− animals than in IL-4−/− mice (Stt, P = 0.034; MWt, P = 0.019).
FIG. 7.
IgE is involved in clearance of a primary infection with B. malayi. Ten BALB/c+/+, 10 IL-4−/−, and 10 IgE−/− mice were injected with 35 B. malayi L3. Five mice from each of these cohorts were necropsied at each time point following injection. The data represent the average percentage of the initial inoculation recovered as live worms. Error bars indicate standard deviations.
Using our challenge infection protocol, we analyzed the levels of circulating serum IgE in BALB/c+/+ and IL-4R−/− mice. In agreement with other published reports, previously exposed WT mice displayed an increased level of circulating IgE at 2 weeks after a challenge infection. As measured by sandwich ELISA, serum IgE levels in WT mice were 5,058 ± 3,672 ng/ml. In contrast, no serum IgE could be detected in any of the IL-4R−/− mice previously exposed to L3. Thus, the presence of circulating IgE appears to correlate with the ability of the murine host to clear a challenge injection of L3 in an accelerated manner.
DISCUSSION
We chose to utilize BALB/c and C57BL/6 mice to address the question of a role for IL-4 in murine immunity to Brugia for two reasons: (i) the two strains differ in major histocompatibility complex haplotype and follow slightly different kinetics of worm clearance, and (ii) both strains are “resistant” to infection, as they are eventually able to reduce parasite numbers to zero or near zero, and Mf are not found, even at very late time points. The data shown here therefore indicate that IL-4 does in fact play a role in otherwise resistant strains, irrespective of haplotype or worm clearance kinetics. The lack of IL-4 signaling significantly delays the worm clearance kinetics of mice on the C57BL/6 as well as the BALB/c background. Due to the slower killing kinetics seen in BALB/c mice infected with Brugia, the further delay caused by a deficiency in IL-4 leads to patency for Mf. This effect of IL-4 deficiency was seen following infection with B. pahangi as well as B. malayi.
We hypothesized that IL-4 was acting through a host target cell(s) rather than through a direct cytotoxic effect on the worm itself. This hypothesis was based on the observations that although SCID mice lack T cells as a source of IL-4, other IL-4-producing cells such as NK cells, mast cells, and eosinophils are present, and yet these animals are unable to eliminate a substantial number of worms (24). Additionally, in vitro studies with worms recovered from WT or IL-4−/− hosts showed identical survival times in culture at all time points analyzed, suggesting that the mere presence of IL-4 within the host does not predispose these worms to earlier destruction (data not shown). Two receptors have been described for IL-4 (type I and type II) (14, 16, 31). The type I receptor is composed of the IL-4R alpha chain complexed to the common gamma chain, while the type II receptor is made up of the IL-4R alpha chain and the IL-13R alpha chain. IL-13 has been shown to bind to the type II form of the receptor (44). In order to more convincingly differentiate between a direct or indirect effect of IL-4, we infected mice in which the gene encoding the alpha chain of IL-4R had been disrupted, thus disabling both type I and type II receptors. These IL-4R−/− mice exhibit an impairment in worm clearance capabilities comparable to that of the IL-4−/− animals, indicating that IL-4 is indeed exerting its effects by signaling through an intermediary host cell. In addition, these data suggest that IL-13 is not able to compensate for the absence of IL-4 in a primary-infection model.
This dependence upon IL-4R signaling is reminiscent of several gastrointestinal nematode models, including T. muris, H. polygyrus, T. spiralis, and N. brasiliensis. In these models the crucial IL-4 signaling has been shown to work through a Stat6-dependent pathway, to induce mastocytosis or act on the intestinal epithelial cell to induce expulsion of the parasites. Our data indicate a similar requirement for Stat6 activation downstream of IL-4R in clearance of B. malayi and B. pahangi from the murine host, a novel concept in that in this system the action of IL-4 must result in destruction of the parasite while it is still within the host, rather than induction of a mechanism for expulsion of the live worms.
Having established a role for IL-4R–Stat6 signaling in a primary infection, we questioned whether there existed a similar requirement for accelerated worm clearance in a challenge infection model. As shown in Fig. 3, IL-4R–Stat6 signaling is necessary for accelerated clearance of a challenge infection as well, at least at the time points analyzed. It should be noted that in the challenge infection protocol, adult worms remaining from the primary infection are seldom if ever found within WT animals but are nearly always present in the IL-4R−/− and Stat6−/− animals. The argument may be made that the inability of the IL-4R−/− and Stat6−/− mice to clear the challenge L3 may therefore be attributable to the existence of proliferative suppressive capabilities of the PEC induced by the adult worms remaining from the primary injection. The concept of lymphocytic proliferative suppression has been described for murine B. malayi infections. In studies where adult B. malayi organisms were implanted into the peritoneal cavities of mice, adherent peritoneal cells removed from these animals suppressed lymphocytic proliferation ex vivo, while appearing to leave cytokine production unhampered (1). In later studies a similar suppressive ability in response to L3 was described as well (19). Work by MacDonald et al. highlights an indirect requirement for IL-4 in the induction of this suppressive PEC population, arguing against the ability of adult worms to elicit such a response in the IL-4R−/− animals (19).
Although these data have identified an essential role for Stat6 activation in both a primary and a challenge infection of B. malayi, the crucial effector mechanism(s) downstream of this Stat6 signaling remains elusive. Analysis of the cellular components responding to the site of infection by both cytological and flow cytometric analyses highlights a significant defect in the ability of IL-4-, IL-4R-, and Stat6-deficient mice to recruit eosinophils at time points when a significant difference in live worm recoveries is observed. The importance of IL-4 signaling in eosinophil recruitment is not a new concept but has been well established in models such as the cellular response to schistosomal egg antigens in rodents and allergy in humans (7, 21, 22, 32). Human studies have shown that IL-4R is expressed constitutively on the surface of eosinophils (7). While a directly cytotoxic role for eosinophils in the host response to Brugia has not yet been established, eosinophil involvement in host cell nodules encasing the worms is shown here (Fig. 6B) and has been extensively described in the literature (12, 13, 20, 35). It is therefore likely that one factor contributing to the impairment of the worm clearance capabilities of a mouse deficient in IL-4 signaling will be the inability to recruit eosinophils to the site of worm invasion.
An increase in the percentage of small lymphocytes was observed in all groups of mutant animals, and a significant decrease in percentages of large, granular cells responding to infection was observed in IL-4R−/− and Stat6−/− animals compared with WT controls. The significance of these cellular abnormalities has yet to be determined. The high level of involvement of macrophages in inflammatory nodules in conjunction with the many known effects of IL-4 signaling on these cells suggests that this population may also contribute to the involvement of IL-4 signaling in worm clearance.
In addition to these deficiencies in cellular recruitment, it is tempting to speculate that another important player may be the induction of IgE production from B cells. B cells are induced to undergo class switching to the IgE isotype following signaling through IL-4R and CD40. Analyses of antibody isotypes responding to a primary infection with Brugia illustrate a severe defect in IgE production from IL-4−/−, IL-4R−/−, and Stat6−/− mice (2) (Table 5). A further increase in IgE levels has been observed in the challenge infection in WT mice (43). IL-4R−/− and Stat6−/− mice continue to exhibit an inability to mount a substantial IgE response in the challenge infection protocol as well; thus, the absence of IgE appears to correlate with a deficiency in worm clearance. Further support for the importance of IgE in parasite clearance is provided by the decreased ability of IgE−/− mice to clear a primary infection in comparison to WT mice. In contrast to our findings, Watanabe et al. have found no difference in worm clearance abilities between control mice and mice treated from birth with an antiepsilon monoclonal antibody (43). The discrepancies between our results and those of Watanabe et al. may reflect an incomplete depletion of IgE in their protocol of neonatal antibody neutralization. Our system utilized mice deficient in IgE due to a targeted gene disruption. This strategy is also subject to misleading results due to the ability of organisms to compensate for a missing gene when forced to develop in its absence. In general, compensation would result in a normal phenotype. Since our data suggest that the absence of IgE alters the phenotype compared to WT controls, it is unlikely that compensation is playing a major role.
Although the data generated here suggest roles for both eosinophil recruitment and IgE production in the clearance of tissue-dwelling filarial worms, we have no data to support any commonalities between these mechanisms except for a mutual dependence upon IL-4R signaling for their implementation. Unlike their human counterparts, murine eosinophils do not express the high-affinity IgE receptor. In light of this, it may be interesting to look at the contribution, if any, of the low-affinity IgE receptor in parasite killing.
IL-4 signaling and subsequent activation of Stat6 have been shown to be a key component of a successful host response to gastrointestinal nematodes. Despite the vast differences in mechanisms of parasite clearance, the data shown here indicate that a similar signaling pattern is utilized for the elimination of Brugia, a tissue-dwelling nematode, from the rodent host. Identification of the crucial IL-4-mediated mechanism(s) has yet to be achieved; however, it is likely that at least two of these mechanisms may be the inability of the murine host, in the absence of IL-4, to produce sufficient levels of IgE and to recruit eosinophils to the site of infection.
ACKNOWLEDGMENTS
This work was made possible by grants AI 39705 and AI 42362 to T.V.R.
We thank Lynn Puddington and Steve Wikel for critical review of the manuscript, and we thank Sharon Coleman and Thomas Klei for some of the infectious larvae used in this study.
REFERENCES
- 1.Allen J E, Lawrence R A, Maizels R M. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. doi: 10.1093/intimm/8.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Babu S, Ganley L M, Klei T R, Shultz L D, Rajan T V. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect Immun. 2000;68:3034–3035. doi: 10.1128/iai.68.5.3034-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancroft A J, Grencis R K, Else K J, Devaney E. Cytokine production in BALB/c mice immunized with radiation attenuated third stage larvae of the filarial nematode, Brugia pahangi. J Immunol. 1993;150:1395–1402. [PubMed] [Google Scholar]
- 4.Bancroft A J, McKenzie A N, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 5.Berton M T, Linehan L A. IL-4 activates a latent DNA-binding factor that binds a shared IFN-gamma and IL-4 response element present in the germ-line gamma 1 Ig promoter. J Immunol. 1995;154:4513–4525. [PubMed] [Google Scholar]
- 6.Delphin S, Stavnezer J. Characterization of an interleukin 4 (IL-4) responsive region in the immunoglobulin heavy chain germline epsilon promoter: regulation by NF-IL-4, a C/EBP family member and NF-kappa B/p50. J Exp Med. 1995;181:181–192. doi: 10.1084/jem.181.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Else K J, Finkelman F D, Maliszewski C R, Grencis R K. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlin W G, Severinson E, Strom L, Heath A W, Coffman R L, Ferrick D A, Howard M C. CD40 signaling induces interleukin-4-independent IgE switching in vivo. Eur J Immunol. 1998;28:525–531. doi: 10.1002/eji.1830261216. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman F D, Katona I M, Urban J F, Jr, Holmes J, Ohara J, Tung A S, Sample J V, Paul W E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 10.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 11.Grencis R K. Th2-mediated host protective immunity to intestinal nematode infections. Philos Trans R Soc London B. 1997;352:1377–1384. doi: 10.1098/rstb.1997.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffers G W, Klei T R, Enright F M. Activation of jird (Meriones unguiculatus) macrophages by the filarial parasite Brugia pahangi. Infect Immun. 1984;43:43–48. doi: 10.1128/iai.43.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffers G W, Klei T R, Enright F M, Henk W G. The granulomatous inflammatory response in jirds, Meriones unguiculatus, to Brugia pahangi: an ultrastructural and histochemical comparison of the reaction in the lymphatics and peritoneal cavity. J Parasitol. 1987;73:1220–1233. [PubMed] [Google Scholar]
- 14.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence R A, Allen J E, Gregory W F, Kopf M, Maizels R M. Infection of IL-4-deficient mice with the parasitic nematode Brugia malayi demonstrates that host resistance is not dependent on a T helper 2-dominated immune response. J Immunol. 1995;154:5995–6001. [PubMed] [Google Scholar]
- 16.Lin J X, Migone T S, Tsang M, Friedmann M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 17.Loke P, MacDonald A S, Allen J E. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4(+) T cells. Eur J Immunol. 2000;30:1127–1135. doi: 10.1002/(SICI)1521-4141(200004)30:4<1127::AID-IMMU1127>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Lowenthal J W, Castle B E, Christiansen J, Schreurs J, Rennick D, Arai N, Hoy P, Takebe Y, Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988;140:456–464. [PubMed] [Google Scholar]
- 19.MacDonald A S, Maizels R M, Lawrence R A, Dransfield I, Allen J E. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J Immunol. 1998;160:4124–4132. [PubMed] [Google Scholar]
- 20.Mackenzie C D, Oxenham S L, Gatrill A, Andrew S, Grennan D, Denham D A. Mononuclear and multinuclear macrophages in filarial infections. Immunol Lett. 1985;11:239–246. doi: 10.1016/0165-2478(85)90174-9. [DOI] [PubMed] [Google Scholar]
- 21.Moser R, Fehr J, Bruijnzeel P L. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992;149:1432–1438. [PubMed] [Google Scholar]
- 22.Moser R, Groscurth P, Carballido J M, Bruijnzeel P L, Blaser K, Heusser C H, Fehr J. Interleukin-4 induces tissue eosinophilia in mice: correlation with its in vitro capacity to stimulate the endothelial cell-dependent selective transmigration of human eosinophils. J Lab Clin Med. 1993;122:567–575. [PubMed] [Google Scholar]
- 23.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Nelson F K, Greiner D L, Shultz L D, Rajan T V. The immunodeficient scid mouse as a model for human lymphatic filariasis. J Exp Med. 1991;173:659–663. doi: 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noben-Trauth N, Shultz L D, Brombacher F, Urban J F, Jr, Gu H, Paul W E. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohara J, Paul W E. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- 27.Osborne J, Devaney E. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4-CD8-alphabeta T cell population. Int Immunol. 1998;10:1583–1590. doi: 10.1093/intimm/10.10.1583. [DOI] [PubMed] [Google Scholar]
- 28.Osborne J, Hunter S J, Devaney E. Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi. Infect Immun. 1996;64:3461–3466. doi: 10.1128/iai.64.9.3461-3466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan P Y, Rothman P. IL-4 receptor mutations. Curr Opin Immunol. 1999;11:615–620. doi: 10.1016/s0952-7915(99)00026-6. [DOI] [PubMed] [Google Scholar]
- 30.Paul W E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 31.Russell S M, Keegan A D, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann M C, Miyajima A, Puri R K, Paul W E, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 32.Ruth J H, Warmington K S, Shang X, Lincoln P, Evanoff H, Kunkel S L, Chensue S W. Interleukin 4 and 13 participation in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation: multiparameter analysis of cellular recruitment, chemokine expression and cytokine networks. Cytokine. 2000;12:432–444. doi: 10.1006/cyto.1999.0595. [DOI] [PubMed] [Google Scholar]
- 33.Sato T A, Widmer M B, Finkelman F D, Madani H, Jacobs C A, Grabstein K H, Maliszewski C R. Recombinant soluble murine IL-4 receptor can inhibit or enhance IgE responses in vivo. J Immunol. 1993;150:2717–2723. [PubMed] [Google Scholar]
- 34.Schreiber S, Heinig T, Panzer U, Reinking R, Bouchard A, Stahl P D, Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995;108:21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 35.Thompson J P, Bentley A G, Crandall R B, Crandall C A. The histology and ultrastructure of the Meyers-Kouwenaar body in ferrets infected with Brugia malayi. Am J Trop Med Hyg. 1984;33:1141–1146. doi: 10.4269/ajtmh.1984.33.1141. [DOI] [PubMed] [Google Scholar]
- 36.Urban J F, Jr, Madden K B, Cheever A W, Trotta P P, Katona I M, Finkelman F D. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus brasiliensis. J Immunol. 1993;151:7086–7094. [PubMed] [Google Scholar]
- 37.Urban J F, Jr, Maliszewski C R, Madden K B, Katona I M, Finkelman F D. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol. 1995;154:4675–4684. [PubMed] [Google Scholar]
- 38.Urban J F, Jr, Noben-Trauth N, Donaldson D D, Madden K B, Morris S C, Collins M, Finkelman F D. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 39.Urban J F, Jr, Schopf L, Morris S C, Orekhova T, Madden K B, Betts C J, Gamble H R, Byrd C, Donaldson D, Else K, Finkelman F D. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 40.Urban J J, Katona I M, Paul W E, Finkelman F D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vercelli D, De Monte L, Monticelli S, Di Bartolo C, Agresti A. To E or not to E? Can an IL-4-induced B cell choose between IgE and IgG4? Int Arch Allergy Immunol. 1998;116:1–4. doi: 10.1159/000023918. [DOI] [PubMed] [Google Scholar]
- 42.Warren W D, Roberts K L, Linehan L A, Berton M T. Regulation of the germline immunoglobulin Cgamma1 promoter by CD40 ligand and IL-4: dual role for tandem NF-kappaB binding sites. Mol Immunol. 1999;36:31–44. doi: 10.1016/s0161-5890(98)00114-x. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe N, Hayashi Y, Kobayashi A, Ohtomo H. Brugia malayi infection in mice with selective suppression of IgE production. Int Arch Allergy Immunol. 1996;109:192–196. doi: 10.1159/000237219. [DOI] [PubMed] [Google Scholar]
- 44.Zurawski G, de Vries J E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]