Abstract
The obligate intracellular pathogen Chlamydia (Chlamydophila) pneumoniae is known to be associated with some chronic inflammatory diseases, such as atherosclerosis. Interaction between C. pneumoniae and immune cells is important in the development of such diseases. However, susceptibility of immune cells, particularly lymphocytes, to C. pneumoniae infection has not been reported, even though lymphocytes play a pivotal role in the development of the diseases caused by this bacterium. In this regard, we examined the susceptibility of lymphocytes to C. pneumoniae infection in vitro. The results demonstrated that human peripheral blood lymphocytes as well as mouse spleen lymphocytes could be infected with C. pneumoniae. Furthermore, purified T lymphocytes as well as established T-lymphocyte cell line cells showed an obvious susceptibility to C. pneumoniae infection, indicating that T cells could be one of the host cells for this bacterial infection. These findings reveal a new infection site for C. pneumoniae, i.e., lymphocytes.
Chlamydia (Chlamydophila) pneumoniae is an obligate intracellular bacterium which causes a variety of respiratory illnesses, including community-acquired pneumonia, bronchitis, pharyngitis, and sinusitis (25). Current studies also indicate the possible involvement of C. pneumoniae in chronic inflammatory as well as other diseases, such as asthma (10), arthritis (8), and atherosclerosis (23), besides respiratory illness. However, even though there are a large number of reports relating C. pneumoniae infection and certain chronic inflammatory diseases, the mechanisms for development of such diseases by Chlamydia infection is not clear. In the case of atherosclerosis, for instance, how Chlamydia organisms reach the intima, which is the main site of atherosclerosis, and how Chlamydia spp. are involved in the chronic inflammatory reaction characterized in atherosclerosis are not well understood. It is known that lymphocytes always play a central role in the development of chronic inflammatory diseases by their diverse functions. In this regard, a recent study by Kaul et al. (18) showed an interesting finding indicating that Chlamydia DNA could be recovered from CD3+ peripheral blood leukocytes obtained from the patients attending a cardiology clinic. This finding suggests the possibility that lymphocytes may be a host cell for C. pneumoniae.
Although C. pneumoniae is known to preferentially infect the epithelial tissue of the respiratory tract, this bacterium can also multiply in vitro in monocytes/macrophages, endothelial cells, and aortic smooth muscle cells (1, 3, 4, 9, 17, 26). However, there are no reports regarding experimental in vitro infection of lymphocytes with C. pneumoniae which demonstrate that lymphocytes can be a host cell. The study reported here demonstrates that lymphocytes, particularly T lymphocytes, can be infected with C. pneumoniae and support the growth of this bacterium in vitro. These findings may reveal a possible new Chlamydia infection pathway, i.e., lymphocytes.
MATERIALS AND METHODS
Bacterial strain, propagation, and purification.
C. pneumoniae (CM-1, ATCC VR-1360) was obtained from the American Type Culture Collection, Manassas, Va., and propagated in HEp-2 cell cultures as described (22). Chlamydial elementary bodies (EBs) were purified by density gradient centrifugation with Percoll (Sigma Chemical, St. Louis, Mo.) (13). Purified EBs were suspended in sucrose-phosphate-glutamic acid (0.2 M sucrose, 3.8 mM KH2PO4, 6.7 mM Na2HPO4, 5 mM l-glutamic acid) buffer (pH 7.4) and stored in small aliquots at −70°C until used. Inclusion forming units (IFUs) of the Chlamydia preparation were determined by counting Chlamydia inclusions in HEp-2 cells as described elsewhere (29).
Lymphocytes.
Human peripheral blood lymphocytes were isolated from buffy coats provided by the Florida Blood Services, St. Petersburg, Fla., by density gradient centrifugation with Histopaque-1077 (Sigma). The resulting peripheral blood mononuclear cells were washed three times with Hanks' balanced salt solution (HBSS) and suspended in RPMI 1640 medium containing 10% heat-inactivated human AB blood type serum (Sigma). The peripheral blood mononuclear cell suspensions were then dispensed in tissue culture flasks and incubated for 2 h at 37°C in 5% CO2 to adhere out the monocytes. After incubation, nonadherent cells were collected, washed with HBSS, and resuspended in RPMI 1640 medium containing 10% AB serum. Cytocentrifuged preparations of the resulting lymphocyte fractions stained with Giemsa showed greater than 95% lymphocytes by morphology.
Mouse spleen lymphocytes were prepared from the spleens of BALB/c female mice (Jackson Laboratory, Bar Harbor, Maine), 10 to 12 weeks of age, using a stomacher 80 lab blender (Tekmer, Cincinnati, Ohio), and the erythrocytes were lysed by ammonium chloride. Spleen cell suspensions in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS; HyClone, Logan, Utah) were cultured on tissue culture dishes to adhere out macrophages for 2 h at 37°C in 5% CO2. The nonadherent lymphocytes were collected, washed with HBSS two times, and resuspended in the medium. In some experiments, T lymphocytes were purified from the isolated spleen lymphocytes by Stem Sep System (Stem Cell Technologies, Vancouver, Canada) in accordance with the manufacture's manual. The T-cell purification was confirmed by flow cytometry with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD3 and -CD19 antibodies (BD Pharmingen, San Diego, Calif.). The T-cell-enriched fraction showed that CD3-positive and CD19-positive cells were more than 95% and less than 0.02%, respectively.
T-lymphocyte cell line.
The human T-lymphocyte cell line Molt 3 was kindly provided by R. Widen, Tampa General Hospital, Tampa, Fla. Cultures of the cell line (106 cells) were infected with either viable bacteria (107 IFUs) or UV-treated bacteria (107 organisms; prepared by placing cultures 1.0-cm distance under UV light for 15 min) or cultured without bacteria in 2.0 ml of RPMI 1640 medium containing 10% heat-inactivated FCS and antibiotics (gentamicin, 10 μg/ml; vancomycin, 10 μg/ml; and amphotericin B, 1 μg/ml) for 3 h at 37°C in 5% CO2 with gentle shaking. After two washes with HBSS by centrifugation, the cultures were resuspended in 10 ml of medium and then incubated for 72 h in the presence of cycloheximide (1 μg/ml; Sigma).
Infection of lymphocytes.
Lymphocyte suspensions in RPMI 1640 medium supplemented with either 10% AB serum for human lymphocytes or 10% FCS for mouse lymphocytes and 2 mM l-glutamine were dispensed in six-well tissue culture plates at 2 × 106 cells/3.0 ml/well. The lymphocyte cultures were then infected with 108 IFUs of EBs/well by gentle shaking with a shaker for 2 h at 37°C. After infection, the cells were washed with HBSS two times by centrifugation and resuspended in RPMI 1640 medium containing cycloheximide (0.5 μg/ml) and either 10% AB serum or FCS for human lymphocytes and mouse lymphocytes, respectively. The infected lymphocytes were then incubated at 37°C in 5% CO2 for up to 4 days.
Assessment of Chlamydia infection by microscopy.
After 1 to 4 days of incubation, the infected lymphocytes were centrifuged on a microscopic slide by a Cytospin (Shandon, Sewickley, Pa.). After fixing with ethanol, cells were stained with Chlamydia genus-specific FITC-conjugated monoclonal antibody (specific to Chlamydia lipopolysaccharide; Research Diagnostics, Flanders, N.J.). The presence of inclusion bodies in the sample was then determined with a fluorescence microscope. In some experiments, isolated mouse lymphocytes infected with C. pneumoniae were double stained with PE-conjugated anti-mouse CD3 antibody (BD Pharmingen) and FITC-conjugated anti-Chlamydia lipopolysaccharide (LPS) monoclonal antibody (Fitzgerald International Inc., Concord, Mass.). In brief, the cells on a microscopic slide were stained with anti-CD3 antibody and then treated with a Fix and Perm cell permeabilization kit (Caltag Laboratories, Burlingame, Calif.) with anti-Chlamydia antibody in accordance with the manufacture's protocol. The double-stained cells were then analyzed with a fluorescence microscope.
ELISA.
Chlamydia LPS antigen in lymphocyte cultures was detected by an enzyme-linked immunosorbent assay (ELISA) kit (IDEIA PCE Chlamydia; Dako Ltd., Ely, United Kingdom). C. pneumoniae-infected lymphocytes (106 cells) were suspended in 1.0 ml of triethanolamine buffer and heated at 95°C for 15 min to extract Chlamydia LPS. The specimen (200 μl) containing Chlamydia LPS was then determined by sandwich ELISA using monoclonal anti-Chlamydia and alkaline phosphatase-conjugated anti-Chlamydia antibodies supplied in the kit. As a standard for Chlamydia LPS, a series of diluted EBs were spiked into the cell cultures and then Chlamydia LPS was extracted from the cultures. The relative number of Chlamydia organisms was calculated from the standard curve. The lower detection limit was 5 × 104 EBs/assay.
PCR.
To detect C. pneumoniae-specific DNA, infected lymphocytes (106 cells) were collected at appropriate time points, and DNA was isolated using the QIAmp DNA minikit (Qiagen, Valencia, Calif.) in accordance with the manufacturer's manual. The PCR was performed with the Universal Amplification and Detection system (Intergen, Purchase, N.Y.) with primers specific for the C. pneumoniae major outer membrane protein gene (omp1) (14) in a Minicycler (MJ Research, Watertown, Mass.) in accordance with the manufacturer's manual. The first cycle, consisting of a 5-min degradation at 94°C, was followed by 50 cycles of 30 s at 94°C, 45 s at 50°C, and 1 min 30 s at 72°C, with a final extension for 10 min at 72°C. The PCR products were analyzed by a Fmax fluorometer (Molecular Devices, Sunnyvale, Calif.).
Electron microscopy.
For transmission electron microscopy (TEM), after incubation the lymphocytes were immersed in a fixative containing 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h at 4°C and then rinsed in 2.5% sucrose in 0.1 M phosphate buffer at pH 7.4. They were postfixed with 1% osmium tetroxide in 0.1 M phosphate (pH 7.4) at 4°C for 45 min. After a brief rinse in 2.5% sucrose, they were processed for alcohol dehydration and embedding in Epon 812 as described previously (19). Ultrathin sections of the cells were stained with lead citrate and uranium acetate before viewing on an electron microscope.
RESULTS
When either human or murine lymphocytes were infected with C. pneumoniae, centrifuged to remove free bacteria from the cells, and then incubated for up to 4 days, Chlamydia organisms obviously multiplied within the lymphocytes, as demonstrated by four methods: detection of inclusion bodies by staining with anti-Chlamydia monoclonal antibody conjugated with FITC in lymphocytes, stained with lymphocyte cell surface markers, demonstration of chlamydiae in lymphocytes by TEM, and detection of increased Chlamydia antigen by ELISA and of C. pneumoniae-specific DNA in the lymphocyte cultures by PCR at various times after infection.
Chlamydia inclusion body formation in lymphocytes.
We initially determined whether human peripheral blood lymphocytes isolated from a buffy coat are susceptible to in vitro C. pneumonia infection. Lymphocytes obtained from different donors were infected with Chlamydia EBs and incubated in the presence of cycloheximide, which is a common method for the enhancement of Chlamydia growth in cell cultures (7), and then incubated further. At various time points after infection, such as 0, 1, 2, 3, and 4 days, the cells were harvested on a glass slide, fixed, and stained with genus-specific anti-Chlamydia monoclonal antibody conjugated with FITC. Figure 1A and B show representative results that obvious Chlamydia inclusion bodies stained with anti-Chlamydia antibody in a lymphocyte were evident 3 days after infection. Wright staining of lymphocyte cultures showed that the lymphocyte cultures used were highly purified, as determined by morphology (more than 95% lymphocytes) (data not shown). However, it is notable that the infection of lymphocytes obtained from individual donor buffy coats with C. pneumoniae was positive for only 4 of 11 donors tested.
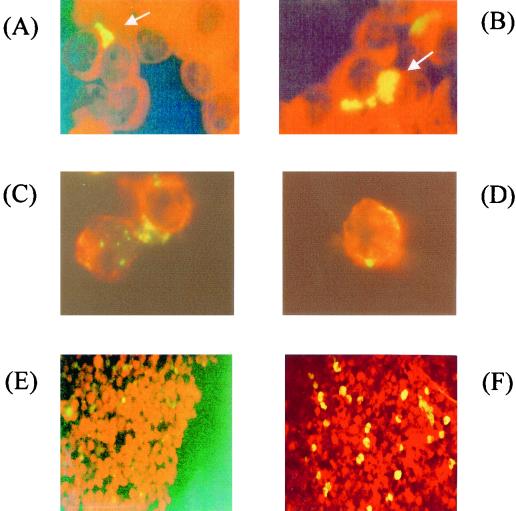
FIG. 1.
Fluorescence stain micrographs of C. pneumoniae-infected lymphocytes. (A and B) Human peripheral blood lymphocytes were infected with C. pneumoniae by gentle shaking and then incubated for 3 days at 37°C in 5% CO2. The infected cells were fixed on a glass slide by Cytospin and stained with FITC-conjugated anti-Chlamydia monoclonal antibody. Chlamydia inclusion bodies (arrow) were demonstrated in lymphocytes. Magnification, ×1,000. (C and D) Immunofluoresence micrographs of C. pneumoniae-infected mouse spleen lymphocytes. The mouse spleen lymphocytes were infected with C. pneumoniae by gentle shaking, incubated for 3 days, and then stained with PE-conjugated anti-mouse IgG CD3 antibody and FITC-conjugated anti-Chlamydia antibody after treatment with the permeabilization kit. Red color indicates surface molecules of lymphocytes. Green to yellow color indicates C. pneumoniae. Magnification, ×1,000. (E and F) Fluorescence micrographs of mouse T-cell-enriched lymphocyte cultures infected with C. pneumoniae. The purity of T cells in the cultures was more than 95% as determined by FACS analysis with anti-CD3 antibody. The T-cell cultures showed a few chlamydiae at 0 h (E). At 3 days after infection, there were many Chlamydia inclusion bodies (yellow spots) stained with FITC-conjugated anti-Chlamydia antibody in the culture (F). Magnification, ×200.
In order to confirm the finding of Chlamydia infection in lymphocytes, mouse spleen lymphocytes were used because of their genetic homogeneity in susceptibility to infection as well as their specific-pathogen-free status. Mouse spleen lymphocytes, which showed more than 95% lymphocytes by morphology, obtained from BALB/c mice were infected with C. pneumoniae in the same way as human peripheral lymphocytes. Three days after infection, the cultures showed many inclusion bodies stained with anti-Chlamydia antibody similar to that seen in the human lymphocytes (data not shown). The time zero cultures after infection with chlamydiae showed a few Chlamydia-positive (FITC positive) spots, but the number and intensity of these observed spots were limited compared with the day 3 cultures. The analysis of infected cells by double staining with PE-conjugated anti-CD3 and FITC-conjugated anti-Chlamydia antibody confirmed that CD3-positive T lymphocytes were infected and allowed the growth of C. pneumoniae in the cells (Fig. 1C and D).
TEM analysis of infected lymphocytes.
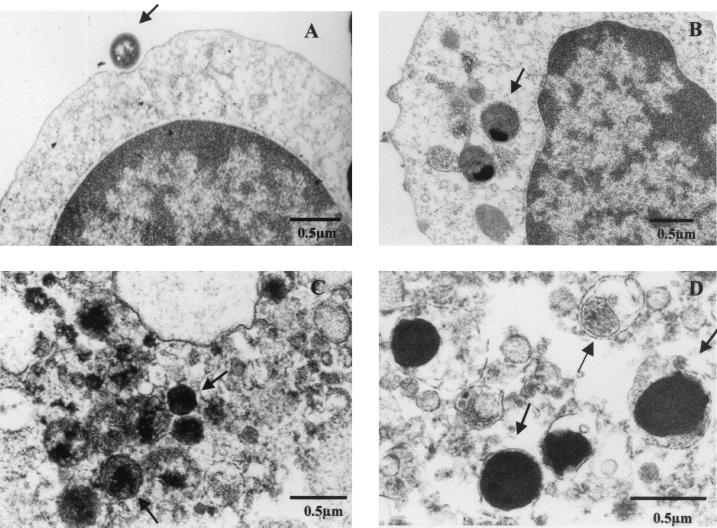
In order to analyze in detail the infection in a cell, C. pneumoniae-infected human as well as mouse lymphocytes were examined by TEM. Figure 2 shows a representative electron micrograph of chlamydiae attached to or in human peripheral blood lymphocytes. At an early infection point, such as just after infection, chlamydiae attached to the surface of a lymphocyte as well as chlamydiae within the cytoplasm of the lymphocyte were observed (Fig. 2A and B). At a later time after infection, such as 3 days after infection, there were many but variably sized Chlamydia particles (0.3 to 0.5 μm), including reticulate-like or intermediate bodies, which were not as big as seen in HEp-2 cells, in the lymphocyte cytoplasm (Fig. 2C and D). The Chlamydia particles in an inclusion body with membrane were also observed (data not shown). Similar findings were noted in the mouse spleen lymphocytes after infection with C. pneumoniae (data not shown).
FIG. 2.
Transmission electron micrographs of C. pneumoniae-infected human lymphocytes. The analysis of C. pneumoniae-infected human lymphocytes (A and B, time zero after infection; C and D, 3 days after infection) by electron microscopy showed the attachment of chlamydiae to the surface of a lymphocyte (A), internalization of chlamydiae in a lymphocyte (B), and many Chlamydia particles of various sizes in a cell (C and D). Arrows indicate Chlamydia particles.
Chlamydia antigen in infected lymphocytes.
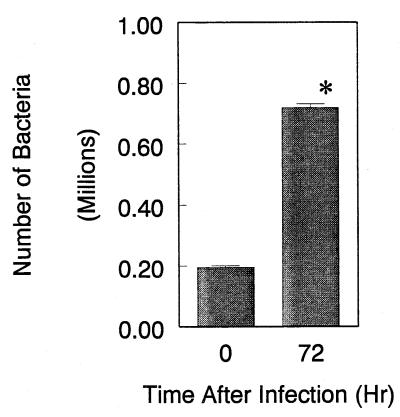
To measure the quantity of chlamydiae in infected lymphocytes, the amount of Chlamydia LPS in mouse lymphocyte cultures was assessed by ELISA, which has been established previously (20), at different incubation time points after infection. The ELISA specific for Chlamydia LPS could quantify Chlamydia organisms in lymphocyte lysates of between 5 × 104 and 5 × 107 EBs/assay using the standard lymphocyte cultures spiked with Chlamydia EBs. As shown in Fig. 3, at the beginning of infection relatively few chlamydiae were recovered from the lymphocyte cultures. However, the relative number of Chlamydia organisms, which was calculated from the LPS concentrations determined by ELISA, in the cultures was increased during the infection and almost reached a plateau at 3 to 4 days after infection due to increased dead lymphocytes (data not shown). As is obvious in Fig. 3, the relative number of Chlamydia organisms recovered from the cultures at 3 days after infection was significantly increased compared to the beginning of infection.
FIG. 3.
Relative number of Chlamydia organisms determined by ELISA in lymphocyte cultures. The amount of Chlamydia LPS antigen in mouse lymphocyte cultures infected with C. pneumoniae was measured by ELISA and converted to a relative number of Chlamydia organisms from the standard curve. The lymphocytes (106 cells) at time zero and 72 h after infection were collected, and Chlamydia LPS in lymphocytes was extracted (1.0 ml). The amount of LPS in extracts (200 μl) was measured by ELISA. The data represent means ± standard deviation (SD) for three experiments. ∗, P < 0.05 compared to time zero culture, analyzed by Student's t test.
Increased C. pneumoniae-specific DNA in infected lymphocytes.
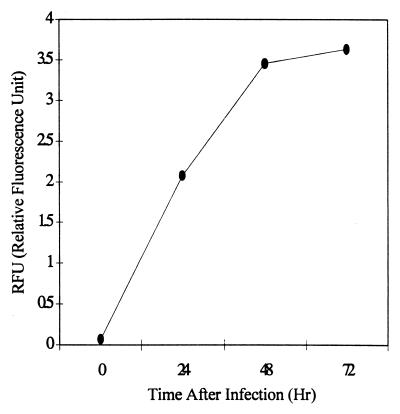
Since analysis by both microscopy with FITC-conjugated anti-Chlamydia antibody and the ELISA specific for Chlamydia LPS was genus specific, not C. pneumoniae specific, C. pneumoniae-specific DNA in the cultures was assessed by PCR with primers specific for C. pneumoniae omp1. The adjusted amount of extracted DNA (0.2 μg) obtained from lymphocyte cultures at different time points was subjected to PCR, and the PCR products were semiquantified. As shown in Fig. 4, C. pneumoniae-specific DNA in the lymphocyte cultures increased during infection similar to that seen in the ELISA for Chlamydia LPS.
FIG. 4.
C. pneumoniae DNA in lymphocyte cultures. The amount of C. pneumoniae DNA in mouse lymphocyte cultures was semiquantified by Amplifluor-PCR with primers specific for C. pneumoniae omp1. DNA (50 μl) was isolated from the infected lymphocytes (106 cells) at the indicated time points, and 2 μl of DNA extracts was subjected to PCR. The data presented are representative of three experiments.
Infection of purified T lymphocytes with C. pneumoniae.
In order to determine whether T lymphocytes are susceptible to Chlamydia infection, T-enriched lymphocyte cultures were prepared using a T-cell enrichment column with mouse spleen lymphocytes. The prepared T-cell fractions were more than 95% CD3 positive and had only trace numbers of CD19-positive cells, as determined by fluorescence-activated cell sorting (FACS) analysis. Growth of C. pneumoniae in the T-cell cultures was assessed by microscopy using FITC-conjugated anti-Chlamydia antibody to detect Chlamydia inclusion bodies (Fig. 1E and F). At the beginning of infection, only a chlamydiae were observed by fluorescence microscopy (Fig. 1E). In contrast, at 3 days after infection, the T-lymphocyte cultures showed an obvious increase in number and size of Chlamydia inclusion bodies in the cultures (Fig. 1F).
Infection of T-lymphocyte cell lines.
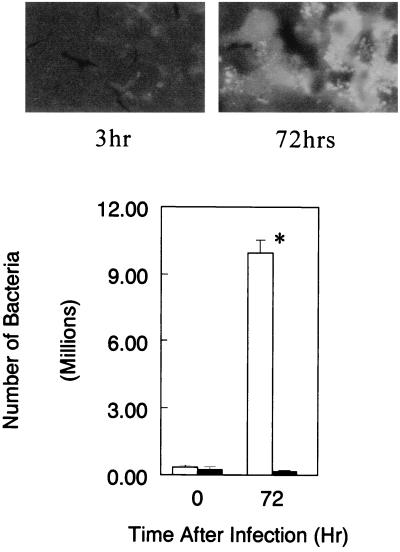
Since it is difficult to prepare a 100% pure culture of lymphocytes with a primary culture of lymphocytes, using an established T-lymphocyte cell line is another way to demonstrate possible infection of lymphocytes with C. pneumoniae. In this regard, Molt 3 T-lymphocyte cells were used for in vitro infection with C. pneumoniae. As shown in Fig. 5, the relative number of Chlamydia organisms recovered from cultures infected with viable bacteria was significantly increased during cultivation for 3 days. In contrast, UV-killed bacteria-treated cultures did not show any increase in bacteria during cultivation. The observations of Chlamydia inclusions in the cells determined by microscopy with FITC-conjugated anti-Chlamydia antibody were parallel with the findings of the bacterial antigen analysis (Fig. 5).
FIG. 5.
Fluorescence micrographs and relative number of Chlamydia organisms in T-lymphocyte cell line Molt 3 cultures infected with C. pneumoniae. See the legend to Fig. 3 for details. The cultures (106 cells) at time zero and 72 h after infection were collected and stained with FITC-conjugated anti-Chlamydia antibody, or Chlamydia LPS in cultures was extracted (1.0 ml). Fluorescence micrographs show the culture at time zero and 72 h after infection with viable C. pneumoniae. Relative number of Chlamydia organisms was calculated from the LPS concentrations determined by ELISA. The data shown as the relative bacterial number represent means ± SD for three experiments. Open bars, infected with viable bacteria; solid bars, infected with UV-killed bacteria. ∗, P < 0.05 compared to time zero culture, analyzed by Student's t test. Magnification, ×1,000.
DISCUSSION
Infection of host cells with C. pneumoniae may change a variety of host cell functions. For instance, when Chlamydia spp. infect monocytes, the infection inhibits host cell apoptosis due, at least partially, to interleukin-10 (IL-10) induction during the infection (6). Macrophage foam cells are inducible by Chlamydia infection by changing the uptake of lipids in infected macrophages (14, 15). A variety of proinflammatory cytokines are also induced from monocytes infected with C. pneumoniae (12, 17). These modulated host cell functions may play an important role in the development of the diseases associated with C. pneumoniae.
Lymphocytes are another major immune cell type besides macrophages in the development of chronic inflammatory diseases, including atherosclerosis. It is well documented that the lesions of atherosclerosis contain a focal accumulation of macrophages and T lymphocytes with the capacity to secret cytokines (2, 11, 24, 27, 28). This inflammatory response may result in plaque rupture and thrombosis, eventually causing stroke or myocardial infarction (30). Therefore, an immune response including regulation of inflammation by immune cells to C. pneumoniae may be critical for the development of atherosclerosis associated with C. pneumoniae. In this regard, if lymphocytes permit Chlamydia infection, involvement of infected lymphocytes in the development of atherosclerosis or other inflammatory diseases caused by C. pneumoniae infection may have a critical role in the pathogenesis of the diseases.
The demonstration of in vitro C. pneumoniae infection of lymphocytes in this study was achieved by several criteria, including morphology as well as demonstration of antigen and DNA levels. All of the criteria tested supported the conclusion that chlamydiae multiplied in lymphocyte cultures, which contained more than 95% lymphocytes, as determined by morphology. However, possible contamination with monocytes, which support the growth of chlamydiae, in the lymphocyte cultures was still a possibility, even though there was only a small number of monocytes. Therefore, the amount of Chlamydia antigen recovered from the lymphocyte cultures at the end of infection may be associated with some chlamydiae in monocytes, but this is unlikely because there were few monocytes in the cultures. On the other hand, the microscopic study clearly showed that lymphocytes defined by morphology as well as lymphocyte-specific surface molecules, such as CD3 for T lymphocytes, obviously had inclusion bodies, as determined with FITC-conjugated anti-Chlamydia monoclonal antibody.
The TEM analysis of infected lymphocytes supported the findings of fluorescence microscopy. In addition, it was demonstrated that purified T-lymphocyte cultures which showed a greater than 95% purity of CD3-positive cells allowed the growth of C. pneumoniae. Finally, demonstration of the growth of C. pneumoniae in the established T-lymphocyte cell line Molt 3 cultures supported the possible infection of lymphocytes with this bacterium. Thus, these findings indicate that C. pneumoniae can infect and multiply in lymphocytes, particularly T lymphocytes, in vitro.
Although we extensively examined propagation of the bacteria in lysates of lymphocytes using the HEp-2 cell culture system, which is considered the most conventional culture system for C. pneumoniae growth, only specimens obtained from the cells just after infection were positive. Similar results were obtained in the case of human peripheral blood monocytes and the human monocytic cell lines U937 and RAW. That is, the propagation of infected C. pneumoniae in the lysates of these cells was not successively detected (1, 4). Therefore, it can be conjectured that the adaptation of C. pneumoniae to host cells or immature EB formation in infected lymphocytes may have occurred.
It is notable that human peripheral lymphocytes did not always allow Chlamydia infection. The reason for the variation in the susceptibility to Chlamydia infection among lymphocytes obtained from different donors is not known. C. pneumoniae is one of the most common pathogens of humans, supported by the fact that the prevalence of antibodies to this bacterium increases with age and about 50% of the population has been infected with this organism by age 50 (9). Therefore, it seems likely that besides the genetic background, the immunological background of a donor may also affect the susceptibility of these lymphocytes to this organism. This speculation is at least partly supported by the results that mouse lymphocytes, which have the same genetic background and have not been infected with chlamydiae, showed a consistent susceptibility to in vitro Chlamydia infection.
The BALB/c mouse strain has been described as resistant for experimental C. pneumoniae infection (16), but the BALB/cAnN mouse strain shows susceptibility to Chlamydia infection (32). In addition, a recent study showed successful experimental C. pneumoniae infection in BALB/c mice (5). Thus, the susceptibility of the BALB/c mouse strain, which was used as a source for the lymphocyte preparation in this study, is not consistent with other reports because of the different experimental conditions between studies. Nevertheless, in vitro susceptibility of lymphocytes to Chlamydia infection may not be directly comparable with in vivo susceptibility due to the different complexities between the systems.
The frequency of inclusion body formation of each lymphocyte in the cultures was not high compared with HEp-2 cells, which are used for the propagation of Chlamydia spp., even though the infectivity ratio was similar between the two cultures (data not shown). The relatively low frequency of inclusion body formation in lymphocytes may be related to several reasons. One of the possible reasons is the use of lymphocyte mixtures, which consist of several subpopulations of lymphocytes, such as B and T cells. If only a particular lymphocyte subset is susceptible to Chlamydia infection, the frequency of Chlamydia infection of each lymphocyte in lymphocyte cultures may be limited. This possibility seems likely because purified T-lymphocyte cultures showed more frequent Chlamydia inclusion bodies after infection than nonpurified lymphocyte cultures (data not shown).
In vivo susceptibility of lymphocytes to chlamydiae during infection is not known. However, the finding of the Chlamydia DNA recovery from CD3+ peripheral blood leukocytes from patients (18) suggests that lymphocytes may serve as a host cell for chlamydiae in vivo. Furthermore, Moazed et al. reported that in experimental infection of mice with C. pneumoniae, it was demonstrated that the bacteria were spread via peripheral blood mononuclear cells, and the authors speculated that the responsive cell vehicle may be monocytes/macrophages (21). However, since the study did not fractionate the peripheral blood mononuclear cells to determine the cell vehicle for C. pneumoniae, it was not clear which peripheral blood mononuclear cells were responsible as the vehicle. Nevertheless, these reports support the possibility that lymphocytes may serve as a host cell in vivo.
The ultrastructural study of infected lymphocytes by TEM revealed that infected chlamydiae were located in the cytoplasm of the cell at the early phase of the infection. The overall shape of such Chlamydia particles observed in a lymphocyte was similar to the finding in HeLa cells (31), which showed a characteristic size and condensed nucleotide structure. In the late phase of infection, such as 3 days after infection, apparent growth of chlamydiae in a cell was observed. In contrast to the early phase of infection, many variable Chlamydia particle sizes, including reticulate-like bodies with a large periplasmic space, were seen in a cell. These ultrastructural findings of such variable sizes of Chlamydia particles in a cell were similar to our other observations in human monocytes (data not shown) as well as in a previous report utilizing human monocytes (1), except that there were relatively smaller numbers of Chlamydia particles in an inclusion. Thus, the overall morphology of Chlamydia particles in lymphocytes was nearly comparable to the previous report (1), with minor differences at the late phase of infection.
Positive Chlamydia infection of lymphocytes may impair lymphocyte functions, particularly cellular immunity, because T lymphocytes are a target for infection. However, no data are yet available as to how Chlamydia infection may impair or affect lymphocyte functions. Nevertheless, the evidence that C. pneumoniae can replicate in lymphocytes suggests that these cells may be an important host cell for dissemination of the organisms as well as possibly alter lymphocyte functions and certain immune mechanisms in an infected individual. Thus, the results presented in this study may provide a new direction for understanding C. pneumoniae infection and immunity.
ACKNOWLEDGMENTS
We thank Akira Matsumoto, Kawasaki Medical School, Kawasaki, Japan, for cooperation in training S.H. We also thank Raymond Widen, Tampa General Hospital, Tampa, Fla., for performance of the flow cytometric analysis.
REFERENCES
- 1.Airenne S, Surcel H M, Alakärppä H, Laitinen K, Paavonen J, Saikku P, Laurila A. Chlamydia pneumoniae infection in human monocytes. Infect Immun. 1999;67:1445–1449. doi: 10.1128/iai.67.3.1445-1449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emeson E E, Robertson A L. T lymphocytes in aortic and coronary intimas: their potential role in atherogenesis. Am J Pathol. 1988;135:369–376. [PMC free article] [PubMed] [Google Scholar]
- 3.Fryer R H, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Investig Med. 1994;45:168–174. [PubMed] [Google Scholar]
- 4.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng Y, Berencsi K, Gyulai Z, Valyi-Nagy T, Gonczol E, Trinchieri G. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect Immun. 2000;68:2245–2253. doi: 10.1128/iai.68.4.2245-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng Y, Shane R B, Berencsi K, Gonczol E, Zaki M H, Margolis D J, Trinchieri G, Rook A H. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol. 2000;164:5522–5529. doi: 10.4049/jimmunol.164.10.5522. [DOI] [PubMed] [Google Scholar]
- 7.Godzik K L, O'Brien E R, Wang S K, Kuo C C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gran J T, Hjetland R, Andreassen A H. Pneumonia, myocarditis and reactive arthritis due to Chlamydia pneumoniae. Scand J Rheumatol. 1993;22:43–44. doi: 10.3109/03009749309095111. [DOI] [PubMed] [Google Scholar]
- 9.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saikku P, Thom D H, Wang S-P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 10.Hahn D L. Chlamydia pneumoniae infection and asthma. Lancet. 1992;339:1173–1174. doi: 10.1016/0140-6736(92)90775-x. [DOI] [PubMed] [Google Scholar]
- 11.Hansson G K, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jantos C A, Roggendorf R, Wuppermann F N, Hagemann J H. Rapid detection of Chlamydia pneumoniae by PCR-enzyme immunoassay. J Clin Microbiol. 1998;36:1890–1894. doi: 10.1128/jcm.36.7.1890-1894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalayoglu M V, Byrne G I. Chlamydia pneumoniae component that induces macrophage foam cell formation is Chlamydial lipopolysaccharide. Infect Immun. 1998;66:5067–5072. doi: 10.1128/iai.66.11.5067-5072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalayoglu M V, Byrne G I. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–729. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 16.Kaukoranta-Tolvanen S S, Laurila A L, Saikku P, Leinonen M, Liesirova L, Laitinen K. Experimental infection of Chlamydia pneumoniae in mice. Microb Pathog. 1993;15:293–302. doi: 10.1006/mpat.1993.1079. [DOI] [PubMed] [Google Scholar]
- 17.Kaukoranta-Tolvanen S S, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 18.Kaul R, Uphoff J, Wideman J, Yadlapalli S, Wenman W M. Detection of Chlamydia pneumoniae DNA in CD3+ lymphocytes from healthy blood donors and patients with coronary artery disease. Circulation. 2000;102:2341–2346. doi: 10.1161/01.cir.102.19.2341. [DOI] [PubMed] [Google Scholar]
- 19.Klein T W, Yamamoto Y, Brown H K, Friedman H. Interferon-γ induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukoc Biol. 1991;49:98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- 20.Miyashita N, Matsumoto A. Establishment of a particle-counting methof for purified elementary bodies of Chlamydia and evaluation of the IDEIA Chlamydia kit and DNA probe by using the purified elementary bodies. J Clin Microbiol. 1992;30:2911–2916. doi: 10.1128/jcm.30.11.2911-2916.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moazed T C, Kuo C C, Grayston J T, Campbell L E. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998;177:1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 22.Molestina R E, Dean D, Miller R D, Ramirez J A, Summersgill J T. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect Immun. 1998;66:1370–1376. doi: 10.1128/iai.66.4.1370-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlstein J B. Bacterial infections and atherosclerosis. J Investig Med. 1998;46:396–402. [PubMed] [Google Scholar]
- 24.Munro J M, van der Walt J D, Munro C S, Chalmers J A, Cox E L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987;18:375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- 25.Pechere J-C. In: Intracellular bacterial infections. Pechere J-C, editor. West Sussex, England: Cambridge Medical Publications; 1996. [Google Scholar]
- 26.Quinn T C, Gaydos C A. In vitro infection and pathogenesis of Chlamydia pneumoniae in endovascular cells. Am Heart J. 1999;138:507–511. doi: 10.1016/s0002-8703(99)70287-5. [DOI] [PubMed] [Google Scholar]
- 27.Ramshaw A L, Parums D V. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17:543–552. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 28.Stemme S, Holm J, Hansson G K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 29.Summersgill J T, Shaney N N, Gaydos C A, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in Hep-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyemura K, Demer L L, Castle S C, Jullien D, Berliner J A, Gately M K, Warrier R R, Pham N, Fogelman A M, Modlin R L. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Investig. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf K, Fisher E, Hackstadt T. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect Immun. 2000;68:2379–2385. doi: 10.1128/iai.68.4.2379-2385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z P, Kuo C C, Grayston J T. A mouse model of Chlamydia pneumoniae strain TWAR pneumonitis. Infect Immun. 1993;61:2037–2040. doi: 10.1128/iai.61.5.2037-2040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]