Abstract
Organoids derived from stem cells or organ-specific progenitors are self-organizable, self-renewable, and multicellular three-dimensional (3D) structures that can mimic the function and structure of the derived tissue. Due to such characteristics, organoids are attracting attention as an excellent ex vivo model for drug screening at the stage of drug development. In addition, since the applicability of organoids as therapeutics for tissue regeneration has been embossed, the development of various organoids-based regenerative medicine has been rapidly progressing, reaching the clinical trial stage. In this review, we give a general overview of organoids and describe current status and prospects of organoid-based regenerative medicine, focusing on organoid-based regenerative therapeutics currently under development including clinical trials.
Keywords: Organoid, Precision medicine, Regenerative medicine, Stem cell, Therapeutics
INTRODUCTION
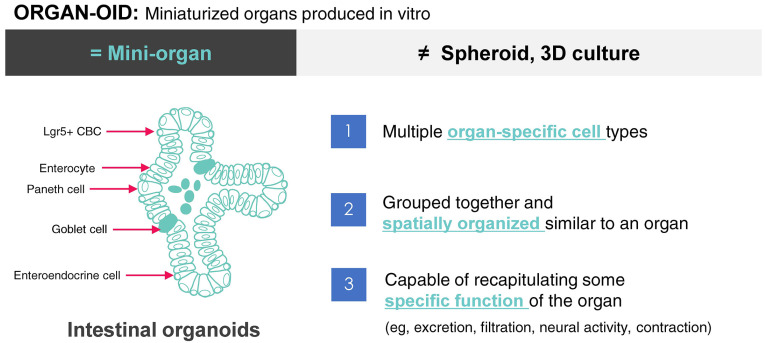
The term “organoid” derived from the word ‘organ’. The suffix ‘-oid’ means organisms resembling an organ. In the past, an organoid meant a three-dimensional (3D) cell aggregate that did not include the concept of mimicking an actual organ. However, advances in developmental and stem cell biology in the last few decades have helped us identify molecular and biological mechanisms underlying the generation and differentiation of organ-specific stem cells. Subsequently, tissue engineering technologies have enabled the creation of tissue or organ-like structures through self-organization of stem cells. As such, organoids are now defined as organ analogues produced in the described manner. Organoids must satisfy the following three criteria (Fig. 1): ① they are composed of cells from the target organ; ② they have the specific structure of the target organ; ③ they can reproduce specific functions of the target organ (1).
Fig. 1.
Definition of organoid. An organoid is in vitro miniaturized and simplified three-dimensional (3D) structure having multiple organ-specific cell types constituting the organ. For example, intestinal organoids are composed of enterocytes, Paneth ells, goblet cells, enteroendocrine cells and stem cells (Lgr5+ crypt base columnar cells), which constitute the actual intestinal epithelium. In addition, organoid have organ-like structures (e.g., crypt and villus structures in intestinal organoids) and mimic their specific functions (e.g., secretory and absorptive functions in intestinal organoids).
This definition of an organoid was first introduced by Yoshiki Sasai (2) and Hans Clevers (3). Yoshiki Sasai reproduced the brain development process using pluripotent stem cells and produced the cerebral cortex and optic cup tissue (2). Meanwhile, Hans Clevers has developed a method to establish intestinal organoids from adult intestinal tissue-derived stem cells (3). These two discoveries are considered initial studies of organoids. Based on these studies, subsequent studies by different groups have successfully produced various organoids derived from adult stem cells and pluripotent stem cells.
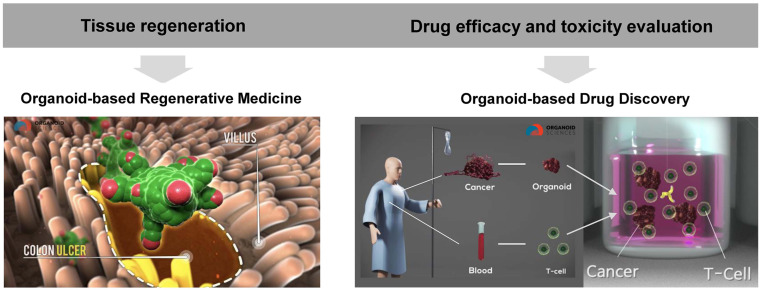
Organoids can be used as disease models similar to actual organs. They can also be used for tissue regeneration in regenerative medicine (Fig. 2). For the former, organoid-based disease models comprise human organ models that can be produced using human tissues. This can overcome limitations of human–animal differences observed in previous animal model studies. Organoid-based disease models also have a 3D structure, which allows researchers to obtain results that can more closely resemble those associated with the living body as compared with results based on conventional monolayer culture or simply organized cells.
Fig. 2.
Application of organoids. Organoids can be used as regenerative medicine for damaged tissues or for in vitro disease modeling to test the efficacy/toxicity of new drugs. For example, colon organoids can be transplanted into colon ulcer to reconstruct the colon epithelium (Left), and the advanced in vitro model system of cancer organoids with immune cells can be used to test the efficacy of drugs such as cancer immunotherapy (Right).
In addition, as only organs of interest are simplified/miniaturized, organoids have better experimental accessibility than organism-based studies. They are also more useful for identifying molecular mechanisms and applying the latest research techniques. Organoids can be used to study development and regeneration mechanisms through normal organ modeling. They can also be used for pathogenesis research and drug development through disease modeling. In particular, cancer organoids can be used for various purposes in most oncology studies, including identifying tumor formation mechanisms, enabling precision medicine, and developing anti-cancer drugs. Furthermore, in organoid-based regenerative medicine, organoids as fundamental therapeutic agents can be directly transplanted into damaged tissues for repair. This review focuses on current status and prospects of organoid-based regenerative medicine.
CONCEPT OF ORGANOID-BASED REGENERATIVE MEDICINE
The first study that introduced the concept of regenerative medicine using organoid was a paper published in Nature Medicine in 2012 by Hans Clevers (from the Netherlands) and Watanabe Momorou (from Japan) (3). That study reported therapeutic effects of intestinal organoid transplantation using an animal model of inflammatory bowel disease (3). In the study, intestinal stem cells were injected into colonic mucosal damaged by colitis-inducing dextran sulfate sodium (DSS). After a certain period, injected intestinal stem cells were stably transplanted into the intestinal epithelium. Upon evaluating various intestinal epithelial cell markers in injected intestinal stem cells, cells were found to have differentiated into intestinal cells such as goblet cells, endocrine cells, and epithelial cells, meaning successful regeneration of damaged tissues through injected cells directly.
Results of that study demonstrated that in vitro cultured intestinal organoids could be transplanted into damaged tissues to directly induce tissue regeneration through engraftment and differentiation. Their study was the first to highlight the potential of organoids for regenerative medicine. Organoid-based regenerative medicine has the following advantages over existing regenerative therapies. First, the securement of cells is easier with organoid-based regenerative medicine than with other regenerative therapies. Most tissues required for organoids can be easily collected through minimal invasive procedure such as biopsy. The required amount of tissue does not post any burden to the donor as it can be obtained through simple procedures. Second, organoids are capable of long-term mass propagation because their culture environment closely resembles human tissues. Therefore, they can be cultured in large quantities for a prolonged period despite using adult tissues, which could facilitate their use as a therapeutic agent. Third, organoids can differentiate into specific cells constituting target tissues. Tissue-specific adult stem cells in organoids have outstanding ability to differentiate into relevant organs or tissues. This can induce direct tissue regeneration upon transplantation, maximizing effects of regenerative treatment. Fourth, organoids are generated using adult stem cells having a lower risk of tumor formation. Therefore, they are safe after transplantation. Moreover, as they are directly transplanted into the lesion site, they have a low risk of movement and distribution to other organs. The use of autologous cells for organoids can also minimize the risk of immune rejection. Finally, transplantation procedures for organoids are safe and patient friendly because most indications for organoid as therapeutics require minimally invasive local transplantation using endoscopes and ultrasound.
TISSUE SPECIFIC ORGANOID-BASED REGENERATIVE MEDICINE
Various studies have demonstrated that organoid-based regenerative medicine can be applied to different diseases, such as intestinal organoids for inflammatory bowel disease, salivary organoids, liver organoids, biliary tract organoids, and lacrimal gland organoids (Table 1).
Table 1.
Status of organoid regeneration medicine development
| Organoid type | Indication | Development stage | Institutions |
|---|---|---|---|
| Intestinal organoid | Ulcerative enteritis (3), Refractory crohn’s disease (4) | Phase I: clinical | Japan, TMDU/ |
| Korea, ORGANOIDSCIENCES Ltd. | |||
| Radiation proctitis (11) | Clinical | Japan, TMDU/ | |
| Korea, ORGANOIDSCIENCES Ltd. | |||
| Short bowel syndrome | Proof of concept | Japan, Keio University | |
| Salivary gland organoid | Radiation-induced xerostomia (5), Sjogren’s syndrome | Phase I: | Netherland, University of Groningen/ |
| Clinical/non-clinical | Korea, ORGANOIDSCIENCES Ltd. | ||
| Liver organoid | Inherited metabolic disease (6, 7) | Non-clinical | Netherlands, Hubrecht institute/ |
| Netherlands, Utrecht University | |||
| Lacrimal gland organoid | Dry eyes (8, 9) | Proof of concept | Netherlands, Hubrecht institute/ |
| Korea, ORGANOIDSCIENCES Ltd. | |||
| Biliary tract organoid | Biliary tract damage disease (10) | Proof of concept | UK, Wellcome-MRC Cambridge Stem Cell Institute |
| Thyroid organoid | Hypothyroidism (12) | Proof of concept | Netherlands, University of Groningen |
| Hair follicle organoid | Hair loss (13) | Proof of concept | US, Harvard University |
| Pancreas organoid | Diabetes (14) | Organoid development | China, University of Chinese Academy of Sciences |
| Gastric organoid | Refractory gastric ulcer (15) | Organoid development | Netherlands, Hubrecht institute |
| Uterine organoid | Uterine adhesion (16) | Organoid development | UK, University of Cambridge |
Intestinal organoid
As described earlier, Mamoru Watanabe reported the first study on intestinal organoid therapeutics. They were the first to start clinical development of organoid-based regenerative medicine. In 2020, they completed the establishment of manufacturing and quality control for clinical applications and received investigational new drug (IND) approval for clinical trials in Japan. A clinical trial targeting patients with ulcerative colitis was started in that year. Additionally, Organoidsciences Ltd., the first company to develop organoid-based regenerative therapeutic agents in Korea, reported therapeutic effects of intestinal organoids on radiation proctitis in a non-clinical study (4).
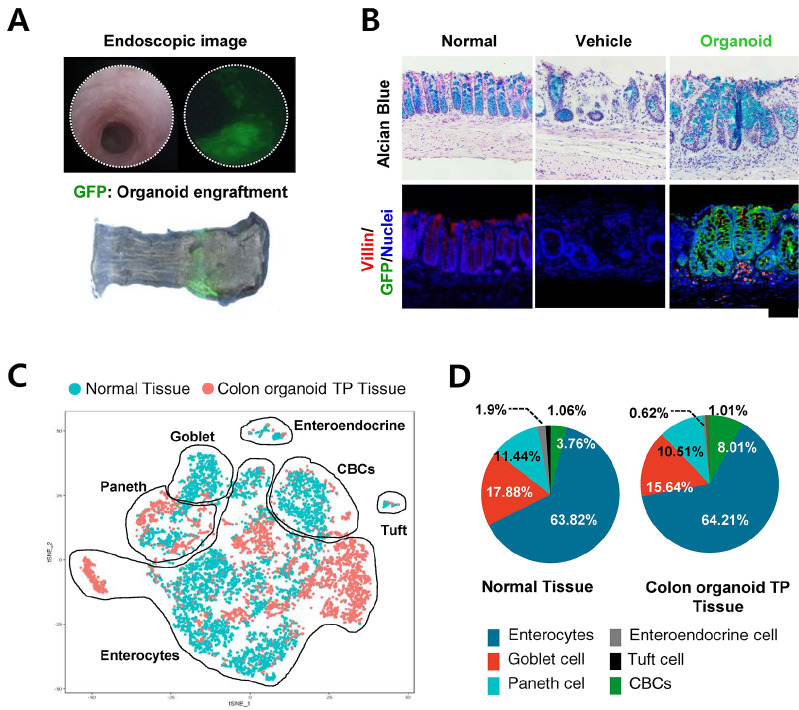
Radiation proctitis, which develops after pelvic radiation therapy, currently has no fundamental treatment. They showed that organoid transplantation could be used as the fundamental treatment of radiation proctitis through successful intestinal tissue regeneration in an animal study (Fig. 3). Based on these findings, Organoidsciences Ltd. has been approved to perform clinical studies using organoid-based regenerative therapeutic agents for intestinal Behçet’s disease and radiation proctitis.
Fig. 3.
Repair of mucosal epithelium by colon organoid transplantation in radiation proctitis. (A) Fluorescence endoscopic tracing and (B) histological analysis of mouse colon organoid (Green) engrafted tissue at 4 weeks after transplantation. The mucosal epithelium damaged by radiation was structurally restored by engraftment of colon organoid transplantation. (C) Unsupervised clustering of scRNA-seq data from normal and colon organoid transplanted. (D) Proportion of cells (Enterocyte, Goblet cells, Paneth cells, Enteroendocrine cells, Tuft cells and CBCs: Crypt-base columnar cells) in normal and colon organoid TP tissue. TP; transplantation (Jee et al. Biomaterials. 2021).
Salivary gland organoid
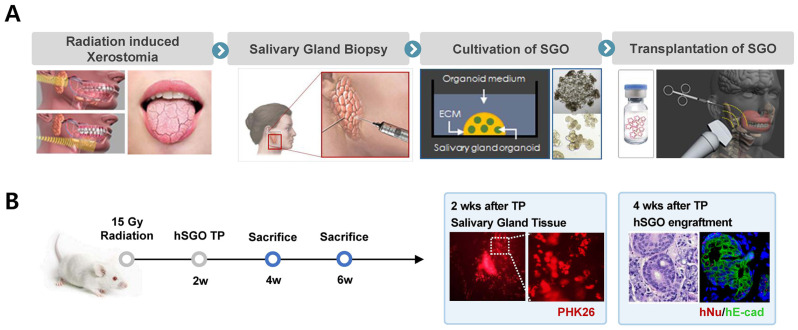
Studies on the treatment of xerostomia using salivary gland organoids are also actively being conducted. Xerostomia is caused by various factors such as radiation therapy for head and neck disease and Sjogren’s syndrome. This disease causes various symptoms such as oral infections and tooth damage induced by decreased salivation. However, owing to a lack of fundamental treatment, regenerative treatment is expected to be the only treatment option for xerostomia. Robert P. Coppes et al. have established salivary gland organoids from salivary gland tissues. After these organoids were transplanted into a mouse model of radiation-induced salivary gland dysfunction, salivation was improved. Currently, a clinical trial is ongoing after an IND approval in 2021 (5). Furthermore, Organoidsciences Ltd. in Korea is developing a salivary gland organoid-based regenerative medicine to target radiation treatment- and Sjogren’s syndrome-related xerostomia (Fig. 4).
Fig. 4.
Salivary gland organoid as a therapeutic strategy for the xerostomia. (A) The therapeutic strategy of hSGO for radiation-induced xerostomia. (B) Engraftment of hSGO at 2, 4 weeks after transplantation in the radiation induced mouse xerostomia model. hSGO; human salivary gland organoid, TP; transplantation, hNu; human nucleoli, hE-cad; human E-cadherin.
Liver organoid
Liver organoids are being actively studied for treating inherited metabolic diseases. Establishment of liver organoids were first reported by Hans Clevers (6). That study demonstrated that the transplantation of normal liver organoids into liver tissues of an animal model with tyrosinemia generated normal hepatocytes in the tissue and improved the survival period (6). Additionally, Bart Spee et al. have established disease organoids from liver tissues of COMMD1-deficient dogs with inherited copper metabolic insufficiency (7). Normal liver organoids were generated by COMMD1 gene transfection into the established disease organoids. After proliferation, they were transplanted into the liver through the portal vein. After transplantation, COMMD1 gene was expressed for a prolonged period and disease signs caused by copper accumulation in the body were improved, indicating the potential of using organoids as ex vivo gene therapeutic agents (7).
Lacrimal gland organoid
Hans Clevers and our group (8, 9) have recently optimized culture conditions for lacrimal gland organoids to secrete tear components and shown that lacrimal tissue stem cells can be cultured in large quantities for an extended period. Hand Clevers et al. have confirmed the intra-tissue engraftment of lacrimal gland organoids after injection into lacrimal gland tissues of wild type mice (8). Similarly, we implanted normal lacrimal gland organoids into a mouse model of lacrimal gland inflammation and observed regeneration of damaged tissues (9). Based on these results, it is expected that lacrimal gland organoids can be applied to treat ophthalmologic diseases such as dry eye and Sjorgen’s syndrome.
Biliary tract organoid
Recently, Ludovic Vallier et al. have demonstrated the regenerative effect by engrafting biliary tract cells derived organoids after transplanting them into a biliary tract injured animal model and human biliary tracts (10). Additionally, Toshiro Sato et al. have suggested a development strategy for intestinal organoid based-regenerative medicine for short bowel syndrome and demonstrated its therapeutic efficacy in a mouse short bowel disease model (11). Various culture conditions for organoids such as thyroid (12), hair follicle (13), pancreas (14), stomach (15), and endometrium (16) organoids have also been optimized. Regenerative medicine based on these organoids targeting a wider range of diseases is expected to be developed in the future.
CONCLUSION
Organoids that can be transplanted into damaged tissues to induce regeneration are currently being actively studied due to their fundamental treatment effects for various disease. To commercialize and use these organoids for the treatment of patients with intractable diseases, the following problems must be resolved. First, to use organoids as pharmaceuticals, the diversity of organoids according to the composition and size of various cells that form the 3D structure should be precisely identified and issues associated with quality assurance and mass production must be overcome. Second, to transplant organoids into damaged tissues and induce engraftment, administration techniques with clinically applicable scaffolds are required. They must be optimized according to the type of organoid, target tissue, and disease status. Third, suitable indications and patient medical case that can properly show the effectiveness of organoid regenerative medicine must be selected. They should be reflected in clinical trials and product approval. Although many limitations must be overcome for organoid-based regenerative therapy to be successful, these efforts might provide a bridgehead for the development of artificial organs in the future.
ACKNOWLEDGEMENTS
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program-3D-TissueChip Based Drug Discovery Platform Program) (2000 9773, Commercialization of 3D Multifunction Tissue Mimetics Based Drug Evaluation Platform) and the Technology Innovation Program (or Industrial Strategic Technology Development Program, 20018578, Production standardization and development of analysis to verify the quality and characterization of organoid based regeneration medicine) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea) and the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (HH21C0010).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 2.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 4.Jee J, Park JH, Im JH, et al. Functional recovery by colon organoid transplantation in a mouse model of radiation proctitis. Biomaterials. 2021;275:120925. doi: 10.1016/j.biomaterials.2021.120925. [DOI] [PubMed] [Google Scholar]
- 5.Pringle S, Maimets M, van der Zwaag M, et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells. 2016;34:640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 6.Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruitwagen HS, Oosterhoff LA, van Wolferen ME, et al. Long-term survival of transplanted autologous canine liver organoids in a COMMD1-deficient dog model of metabolic liver disease. Cells. 2020;9:410. doi: 10.3390/cells9020410.c674d01025924804a5b649c947eca488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannier-Helaouet M, Post Y, Korving J, et al. Exploring the human lacrimal gland using organoids and single-cell sequencing. Cell Stem Cell. 2021;28:1221–1232. doi: 10.1016/j.stem.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Jeong SY, Choi WH, Jeon SG, et al. Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Res Ther. 2021;12:247. doi: 10.1186/s13287-021-02133-y.73f38065616d455fa29b00649108398e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampaziotis F, Muraro D, Tysoe OC, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto S, Kobayashi E, Fujii M, et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature. 2021;592:99–104. doi: 10.1038/s41586-021-03247-2. [DOI] [PubMed] [Google Scholar]
- 12.Ogundipe VML, Groen AH, Hosper N, et al. Generation and differentiation of adult tissue-derived human thyroid organoids. Stem Cell Reports. 2021;16:913–925. doi: 10.1016/j.stemcr.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Rabbani CC, Gao H, et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582:399–404. doi: 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Wang J, Bai L, et al. Long-term expansion of pancreatic islet organoids from resident procr+ progenitors. Cell. 2020;180:1198–1211. doi: 10.1016/j.cell.2020.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turco MY, Gardner L, Hughes J, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]