Abstract
Sexual (and gender)-dimorphism in tolerance to hypobaric hypoxia increasingly matters for a differential surveillance of human activities at high altitude (HA). At low altitudes, the prevalence of anxiety and depression in women has already been found to double when compared with men; it could be expected to even increase on exposure to HA. In purposefully caring for the health of women at HA, the present work explores the potential involvement of the tryptophan (Trp)–melatonin axis in mood changes on exposure to hypobaric hypoxia. The present work highlights some already known anxiogenic effects of HA exposure. Hypoxia and insomnia reduce serotonin (5-HT) availability; the latter defect being expressed as failure of brown adipose tissue (BAT) activation and mood disorders. Rapid eye movement (REM) sleep organization and synapsis restoration that are additionally affected by hypoxia impair memory consolidation. Affective complaints may thus surge, evolving into anxiety and depression. Sex-related differences in neural network organization and hormonal changes during the menstrual cycle, and certainly also during the life cycle, underscore the possibility of 5-HT–related mood alterations, particularly in women on HA exposure. The mean brain rate of 5-HT synthesis at sea level is already 1.5-fold higher in males than in females. sexual dimorphism also evidences the overexpression effects of SERT, a 5-HT transporter protein. Gonadal and thyroid hormones, as influenced by HA exposure, further modulate 5-HT availability and its effects in women. Besides caring for adequate oxygenation and maintenance of one’s body core temperature, special precautions concerning women sojourning at HA should include close observations of hormonal cycles and, perhaps, also trials with targeted antidepressants.
Keywords: women’s mood, high altitude, serotonin, tryptophan, REM sleep, thermogenesis
1 Introduction
One’s mood tends to change on high altitude (HA) exposure (Wang et al., 2014; Kanekar et al., 2015; Das et al., 2018; Kious et al., 2018; Reno et al., 2018). HA exposure may even provoke suicide (Young, 2013; Bocchetta and Traccis, 2017), with various factors possibly involved (Reno et al., 2018). In fact, atmospheric pressure and completed suicide rates evidence a highly significant inverse correlation (Frutos et al., 2018). Mood changes, the quality of life, and cognitive performance are, however, greatly improved by adequate oxygenation in hypoxic patients (Krop et al., 1973). Women appear to be more susceptible to acute mountain sickness than do men (Richalet et al., 2012; Richalet et al., 2020; Canouï-Poitrine et al., 2014). On exposure to HA, peripheral edema also occurs more frequently in women than in men (Canouï-Poitrine et al., 2014; Richalet et al., 2020). Rotating night shift work, mostly implicit to working at HA, notably increases the risk of ischemic stroke, again particularly in women (Brown et al., 2009). Moreover, night shift work that represents a potential carcinogenetic condition has also been especially proven in women (Gehlert and Clanton, 2020).
Female rats were shown to be more prone to mental alterations on exposure to HA than their male counterparts (Kanekar et al., 2015). Reproductive issues have been shown to change on exposure to HA (Gonzales and Carrillo, 1993; Escudero et al., 1996; Gonzales et al., 2002); puberty, menstrual cycle, pregnancy, and menopause are all known to modulate the onset, recurrence, and exacerbation of affective disorders (Altemus et al., 2014). Oral contraception, menopause, and hormonal treatment do not appear to influence HA effects (Richalet et al., 2020). Sleep changes, however, occur during the menstrual cycle (Baker and Lee, 2018). Being a woman features indeed among the independent predictors of significant anxiety at HA (Boos et al., 2018). Anxiety levels and even somatic alterations at HA correlate with pre-expedition propensity (Boos et al., 2018). However, sexual (and gender)-dimorphism, referring to mood changes on exposure to HA, remains to be more closely investigated; specifically, the serotonin (5-HT) system that constitutes a part of the tryptophan (Trp)–melatonin axis and is known for many sex-related differences (Pitychoutis et al., 2012). 5-HT is involved in sleep regulation and mood expression, and additionally influences appetite, gastrointestinal motility, sexual behavior, pain, and certainly also thermoregulation.
HA exposure alters the circadian rhythm of melatonin that is often related to energy metabolism (Behn and De Gregorio, 2020). This finding prompted us to explore the Trp–melatonin axis, as a field full of neurochemically active and anxiogenic derivatives, strongly linked to sex hormones, inflammation, and energy metabolism (Cipolla-Neto et al., 2022). Hypoxia-induced decreases in energy metabolism affect thermoregulation, the latter being further impaired by the failure of 5-HT–dependent brown adipose tissue (BAT) activation. HA-related insomnia additionally interferes with thermoregulation and probably also with sleep-related neural circuitry restoration. Memory consolidation reduced by inadequate REM sleep predisposes one to anxiety and depression. Affective disorders impair the safety and productivity at work (Matamala Pizarro and Aguayo Fuenzalida, 2021). The main topics discussed here are potential HA effects on women’s moods related to sexual dimorphism in 5-HT availability, energy metabolism, thermoregulation, and sleep-related neurocircuitry repair. Sexual dimorphism of the Trp–melatonin axis may provide some clues, in this respect, for a more thorough understanding of the mental health risks at HA.
2 Special topics
2.1 sexual dimorphism, 5-HT, and mood disorders
Sex (biological constructs) and gender (social constructs) have to be considered in all adaptive physiological responses (Mauvais-Jarvis et al., 2020; Riveros-Rivera et al., 2022). “Sex” refers to biological characteristics and “gender” to social factors associated with being male or female (Habib and Messing, 2012; Laberge et al., 2020). sexual dimorphism, at least partially, includes the brain structure and function (Choleris et al., 2018; McEwen and Milner, 2017). Estrogen and progesterone receptors have been detected in the cortex, limbic system, hypothalamus, and pituitary gland (Risco, 2010). Early developmental and final permanent effects of the reproductive hormones determine a sex-related brain dimorphism, the latter basically consisting of organizational differences of the neural networks (Littlejohn et al., 2020), their sex-specific genetic architecture presumably resulting from a combination of sex-specific and sex-independent loci (Weiss et al., 2005). Neuropsychiatric disorders, such as post-traumatic stress, autism, and schizophrenia, and general social behavior and sensory processing appear often linked to alterations of the serotonergic and dopaminergic neurotransmitter systems (Lyon et al., 2020). Particularly, 5-HT synthesis represents a key for neuroendocrine disorders (Rahman et al., 2011). 5-HT synthesis occurs in the brain neurons and gut enterochromaffin cells. Peripheral 5-HT contributes to constitute the gut–brain axis. The mean rate of 5-HT synthesis in the brain is 1.5-fold higher in males than in females (Nishizawa et al., 1997). The platelet 5-HT content, on the other hand, is higher in women than in men (Gonzales, 1980; Weiss et al., 2005; Chakraborti et al., 2020). On chronic exposure to hypobaric hypoxia, brain neurotransmitters tend to associate with those in the peripheral blood, possibly because of the blood–brain barrier permeabilization (Zhong et al., 2021). Trp depletion is associated with depression (Cowen, 2008). Abnormal metabolism of 5-HT has also been related to depression (Lu et al., 2017). The lower levels of platelet 5-HT are associated with suicide risk (Giurgiuca et al., 2016). Low GH and low IGF-1 levels have also been associated with major depressive disorder (MDD) and anxiety (Santi et al., 2018; Karachaliou et al., 2021; Wainberg et al., 2021). Increasing brain 5-HT activity in humans elevates plasma levels of prolactin, growth hormone (GH), and adrenocorticotropic hormone (Cowen et al., 1990).
Most of body 5-HT is derived from dietary Trp (Figure 1). After its hydroxylation and subsequent decarboxylation, Trp leads to 5-HT, a highly conserved monoamine neurotransmitter involved in behavior and regulation of metabolism (Yabut et al., 2019). Approximately 1% of Trp is converted into 5-HT and other downstream metabolites (Mondanelli and Volpi, 2021). Trp hydroxylases (TPHs), which finally yield 5-hydroxyindoleacetic acid (5-HIAA), have two isoforms: TPH2 in the neurons and TPH1 in the peripheral organs (Schoenichen et al., 2019). Estradiol increases TPH2 activity, thereby enhancing 5-HT synthesis and also 5-HT transporter (SERT) expression (Hernández-Hernández et al., 2019). 5-HT reuptake in the presynaptic neuron through SERT, a membrane 5-HT transporter, terminates neurotransmission (Nagayasu, 2022). The enhancement of SERT function predominantly affecting female mice (Haase et al., 2021) enhances 5-HT clearance, thereby directly reducing 5-HT receptor responsiveness. Female mice overexpressing the 5-HT transporter (SERT + mice) develop pulmonary arterial hypertension, whereas male SERT + mice remain unaffected (White et al., 2011). SERT is also the target of most antidepressants (Bouali et al., 2003). Arylalkylamine-N-acetyltransferase (AANAT) N-acetylates converts 5-HT to N-acetylserotonin, which is subsequently converted by hydroxyindole-O-methyltransferase, thus leading finally to melatonin (N-acetyl-5-methoxytryptamine). Melatonin, increasing during the luteal phase of the menstrual cycle (Cipolla-Neto et al., 2022), is supposed to elicit female aggressive behavior by releasing androgens from adrenal cells (Rendon et al., 2015).
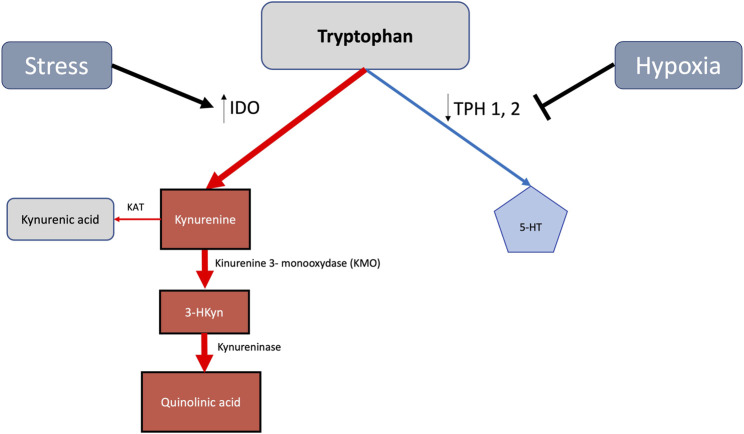
FIGURE 1.
Stress and hypoxia mediated increase of the tryptophan derived metabolic kynurenine 128 pathway in detriment of 5-HT generation. Indoleamine 2,3-dioxygenase (IDO), being upregulated 129 by stress, enhances toxic kynurenine signaling. By reducing tryptophan hidroxylase (TPH) activities, 130 hypoxia concomitantly diminishes 5-HT availability.
At sea level, anxiety and depression occur twice as often in women than in men (Weissman et al., 1996; 1994; Gater et al., 1998). Depression contributing to drug abuse (Brook et al., 2002) occurs more frequently in females who also appear to be more vulnerable toward addiction (Becker and Chartoff, 2019). Moreover, women are twice more likely than men to develop post-traumatic stress disorder (PTSD), a condition evolving with increasing sympathetic reactivity and decreasing parasympathetic activity, with chronic inflammation also appearing to be involved (Fonkoue et al., 2020). Pro-inflammatory cytokines seem to participate in causing depression. Patients treated with interferon alpha (IFN-α) show reduced levels of Trp, augmented levels of kynurenine (Kyn), and elevated Kyn/Trp ratio activity together with an increase in depressive symptoms (Correia and Vale, 2022). An elevated Trp to kynurenine ratio, conversely, reflects endogenous adaptation to stress (Poeggeler et al., 2022).
Trp hydroxylases (TPHs) require molecular oxygen as a substrate, with their activities potentially being curtailed by hypoxia (Davis, 1975). Hypoxia and stress reduce TPH activity, thereby diminishing 5-HT generation (Young, 2013). The Trp–kynurenine pathway, on the contrary, concomitantly becomes enhanced (Figure 1) and also increases Trp degradation into kynurenine and quinolinic acid (Mohapatra et al., 2021). The latter anxiogenic agents (Figure 1) potentially trigger panic disorder, phobias, and post-traumatic stress (Kim and Jeon, 2018). Indoleamine-2,3-dioxygenase (IDO), a heme enzyme, catalyzes the rate-limiting step in the kynurenine pathway of Trp metabolism (Figure 1). Hypoxia increases IDO generation in dendritic cells (Song et al., 2018). Activating the Trp–kynurenine pathway, IDO may lead to abnormal 5-HT levels in the brain and trigger depressive disorders (Lu et al., 2017). 5-HT blood levels appear to be reduced 1.64 times in post-menopausal women at low altitudes and 1.25 times at HA (Gonzales and Carrillo, 1993). Whole blood 5-HT increases in men native to high altitude but does not change in women (Gonzales, 2014). 5-HT concentrations in the hippocampus, cerebral cortex, cerebrospinal fluid, and plasma decrease on exposure to HA in rats and in the plasma of men (Kious et al., 2018). Severe hypobaric hypoxia reduces rat brain 5-HT levels by approximately 30% (Prioux-Guyonneau et al., 1982). Together with a decrease in 5-HT content in the forebrain and lungs, hypoxia leads to an accumulation of 5-hydroxy-Trp (Izikki et al., 2007). Hypoxia also increases resistance to 5-HT reuptake inhibitors (Kanekar et al., 2018) and blocks the 5-HT1A receptor (Dutta et al., 2022). HA-associated cognitive impairment correlates with concomitant decrements of 5-HT plasma levels (Zhong et al., 2021). Under hypoxic conditions, Trp is also increasingly converted to tryptamine (Mohapatra et al., 2021). Tryptamine resulting from melatonin degradation also causes hallucinations (Malaca et al., 2020). Hypoxia-related enhancement of IDO is at least partially reverted by estrogen (Songtachalert et al., 2018). Hypoxia-induced TPH inhibition is also partially reverted by estrogen (Rahman and Thomas, 2013). An increase in oxygen availability by the continuous positive airway pressure (CPAP), on the other hand, improves sleep patterns in humans and restores plasma 5-HT concentration (Madaeva et al., 2021).
2.2 5-HT, REM sleep, and thermoregulation
Sleep-related complaints also appear to be more frequent in women than in men (Krishnan and Collop, 2006; Jehan et al., 2016), eventually becoming even more common with aging (Goel et al., 2005). Hypoxia increases sleep disturbances particularly in women which apparently increases even more when combined with bed rest applied as a quasi-weightlessness condition to simulate spacecraft and/or planetary environments (Morrison et al., 2017; Van Cutsem et al., 2022). Nocturnal oxygen saturation inversely relates to serum melatonin concentration, at least in males (Calderón-Jofré et al., 2021), suggesting that melatonin may also be involved in altitude-associated sleep impairments. Sleep issues are strongly linked to psychiatric disorders (Benca, 1992; Wetter et al., 2008). The network organization of REM sleep seems to be more vulnerable in women than in men (Palagini et al., 2013; Baglioni et al., 2016; Hein et al., 2020). Short REM sleep latency (the interval between sleep onset and the first REM sleep period), an increase in REM sleep duration, and the density of REMs during this state are considered important biological markers of depression (Torterolo et al., 2015). Patients with REM sleep behavior disorder (RBD) tend to develop neurodegenerative diseases (Schenck and Mahowald, 2002; Boeve et al., 2003). Memory processing and learning are thought to occur by selective pruning of synapses (Li et al., 2017). Synapse efficacy, reduced by daily wear, is restored during sleep, thus allowing contextual restraints of the brain to be coordinated and reinforced. Memory consolidation is also implicit for emotion handling (Wagner et al., 2001), the latter, however, being more recently questioned (Lehmann et al., 2016). An adequate REM sleep diminishes the possibility of picture memories becoming excessively intrusive (Werner et al., 2021).
Besides a potential role in memory consolidation (Boyce et al., 2016), REM sleep, although rather passive, appears to be involved in thermoregulation (Kavanau, 1997; Komagata et al., 2019; Cerri and Amici, 2021). Total sleep deprivation and selective REM sleep deprivation induce hypothermia, which even lead to death in rats (Rechtschaffen and Bergmann, 2002). Besides hypothermia, syndromes induced by sustained REM sleep deprivation include a notorious increase in food intake and weight loss (Rechtschaffen and Bergmann, 2002). Interestingly, acute exposure to HA often leads to a very similar pattern. Appropriately sustaining thermogenesis should be particularly important at HA, an environment that lends to sleep deficiency and also potential exposure to cold. Women have been reported to shiver less in cold stress than do men (Iyoho et al., 2017). Cold-induced thermogenesis, on the other hand, has been reported to be higher in women than in men, a condition independently associated with estradiol (Herz et al., 2021). Chronic stress, as supposed to prevail on exposure to chronic hypoxia, disrupts the normal pattern of daily body temperature by decreasing the presence of 5-HT in the medial preoptic area (mPOA). 5-HT agonist injected into the mPOA, on the contrary, restores normal body temperature cycling (Natarajan et al., 2015). The mPOA represents a sex dimorphic structure (Wei et al., 2018) that also coordinates emotions and behavior (Zhang et al., 2021). By tightly interacting with the melanin-concentrating hormone (MCH) in the median raphe nucleus, 5-HT is involved in REM sleep and mood control (Pascovich et al., 2021). Mediated by reactive oxygen species (ROS) generated in the hypothalamus through an NADPH oxidase–dependent pathway, 5-HT acts as an anorexigenic agent (Fang et al., 2013), in contrast to the orexigenic MCH (Izawa et al., 2019), where the latter reduces energy expenditure (Zheng et al., 2005) and decreases body temperature (Zheng et al., 2016; Ishihara et al., 2021). The greatest firing rate of MCHergic neurons occurs during REM sleep, with the optogenetic stimulation of the latter neurons inducing sleep (Torterolo et al., 2015). Neurons producing MCH lead REM sleep to promote memory loss. The fine-tuned REM sleep expression and regulation, moreover, require a strong interplay between 5-HT systems and hypocretin/orexin (Seifinejad et al., 2020). The orexin/orexin receptor system appears to constitute an anxiogenic organization, also being more dominant in women than in men (Freeman and Aston-Jones, 2020). Orexin levels in the cerebrospinal fluid have been found to be elevated in patients with anxiety disorder (Bi et al., 2022). 5-HT inhibits orexin neurons (Muraki et al., 2004; Saito et al., 2018), but its availability is diminished at HA.
REM sleep, thought to support CNS maturation and optimization (Chen et al., 2022), tends to decrease on exposure to cold (Komagata et al., 2019). 5-HT neurons lead BAT to enhance heat generation by increasing the sympathetic drive (Yabut et al., 2019). By activating adrenergic receptors in BAT, norepinephrine stimulates triglycerides stored in white adipose tissue (WAT) to hydrolyze into free fatty acids (FFAs). The latter activates BAT-specific uncoupling protein 1 (UCP1) in the inner mitochondrial membrane. UCP1 activation generates heat by uncoupling oxidative phosphorylation from ATP synthesis. The mitochondrial inner membrane uncoupling protein 2 (UCP2), an anion carrier, senses changes in oxygen levels. The upregulation of UCP2 is essential for metabolic adaptation in hypoxic environments (Wu et al., 2021; Rigaud et al., 2022). UCP2, moreover, plays an essential role in the development of cognitive ability and resistance to anxiety (Wang et al., 2014). The blockade of UCP2, on the other hand, produces cognitive impairment and anxiety in young mice. Serotoninergic fluoxetine in rats overfed during the suckling period improves their mitochondrial function and oxidative balance and, moreover, activates signaling pathways that increase mitochondrial biogenesis and metabolism (Zhong et al., 2021). Heat generated by BAT may contribute to supporting the core temperature during REM sleep. BAT appears to be more developed in females than in males (Keuper and Jastroch, 2021) but requires 5-HT to be fully activated. In a cold and hypoxic environment such as in HA, that is additionally devoid of 5-HT, a lack of energy availability may lead to a resource allocation tradeoff favoring the maintenance of body temperature at the expense of synapse repairs (Figure 2). The failure of sleep-related synapse reinforcement implies an accumulation of rather degraded circuitry and possibly underlying mood disorders (Kavanau, 2000).
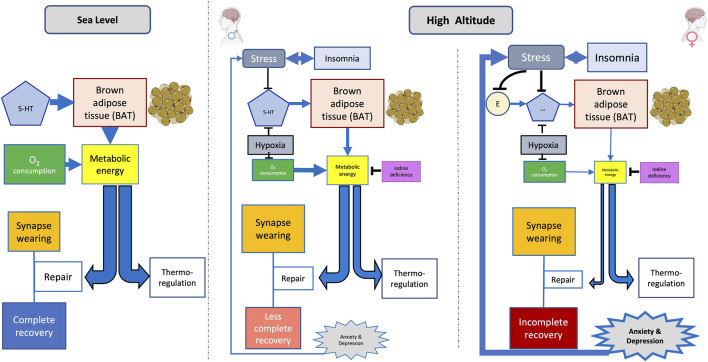
FIGURE 2.
Hypothetical sex-related mental disorder on exposure to high altitude (HA). Stress and hypoxia decrease the availability of 5-HT at HA; brain synthesis of the latter already being lower in women than in men. Moreover, estrogen (E)-mediated support of 5-HT is blocked by stress. The lack of 5-HT diminishes brown adipose tissue (BAT)–dependent heat generation. Metabolic energy already being reduced by hypoxia, and possibly also by goiter, which may consequently generate a tradeoff in order to prioritize the energy requirements of thermoregulation, possibly at the expense of synapse repair. Memory consolidation thereby being interfered could allow mood changes to surge; the latter, in turn, additionally enhancing stress and insomnia. A vicious circle may thus be installed, potentially triggering the generation of mental disorders. Line thickness indicates the magnitude of effects.
2.3 5-HT and monoamino oxidases
Bound to the outer mitochondrial membrane, monoamino oxidases (MAOs) are ROS generators. Catechol-O-methyl transferase and MAOs appear to be less affected by HA than are tyrosine and TPHs, but their activities always tend to decrease (Vaccari et al., 1978). MAO activity in the cerebral cortex on exposure to HA is occasionally been found to be somewhat lower in females than in males (Vaccari et al., 1978). Corresponding with a possibly higher dopaminergic activity at HA, serum prolactin levels appear to be diminished both in women and men living in the Peruvian highlands (Gonzales et al., 1992; Gonzales and Carrillo, 1993; Solís et al., 2011). The plasma dopamine-beta-hydroxylase (E.C. 1.14.17.1) activity increases in rats acutely exposed to HA; this increase is significantly more notorious in females than in males (Koudelová and Mourek, 1990). Dopamine and noradrenaline turnovers accordingly appear to be higher in the female carotid body and brainstem noradrenergic cell groups than in the corresponding male structures in rats reared at an altitude of 3,000 m. Orchidectomy increases dopamine and noradrenaline turnover in the carotid body and brainstem noradrenergic cell groups and ovariectomy decreases it (Pequignot et al., 1997). Peripheral plasma DOPA, dopamine, 5-HT, 5-HIAA, and glutamate levels are associated with brain neurotransmitter levels after chronic HA exposure in rats. Chronic HA exposure decreases dopamine but increases DOPA levels in the human plasma. 5-HT, on the other hand, decreases and 5-HIAA levels increases in humans on chronic HA exposure (Zhong et al., 2021).
2.4 5-HT, thyroid, and gonadal hormones
The Trp–5-HT axis also links with thyroid function, with both pathways influencing mood (Bauer et al., 2002) and thermoregulation. Thyroid replacement therapy reverses the reduced responsiveness to 5-HT in hypothyroid patients and represents an effective adjunct for treatment of affective disorders (Bauer et al., 2002). Selective 5-HT reuptake inhibitors, on the other hand, seem to decrease thyroid function (Caye et al., 2020). Iodine deficiency in mountainous regions and corresponding hypothyroidism must be considered in this respect (Figure 2). Goiter being more prevalent in iodine-deficient areas, particularly affects females (Pretell et al., 1969; Malboosbaf et al., 2013).
HA exposure tends to suppress the gonadal axis (von Wolff et al., 2018). Estrogens upregulate 5-HT2A receptors in the brain (Cyr et al., 1998). Their effect on mood may be also mediated through influences on 5-HT2A and 5-HT2C receptor expressions (Birzniece et al., 2002). Estrogen receptor 2 (ESR2) mediates estrogen effects on TH1 and TH2 expressions (Hiroi and Handa, 2013). Estradiol acting on estrogen receptor β (ERβ) inhibits 5-HT reuptake by downregulating the gene expression of SERT in the neurons and astrocytes. Estradiol may thus act as an antidepressant by increasing 5-HT availability (Gu et al., 2022). Estrogen treatment alone or in combination with testosterone decreases SERT in several cortical, limbic, and striatal regions of surgically ovariectomized women (Jovanovic et al., 2015). Low estrogenic activity, on the other hand, may result in low 5-HT availability. Cognitive performance improves in the luteal phase when compared with the follicular phase of the menstrual cycle (Grant et al., 2021). Premenstrual syndrome (PMS) mostly expresses as an increase in irritability and fatigue during the late luteal or premenstrual phase and finally disappears when menses initiates (Pearlstein et al., 2005). Selective 5-HT reuptake inhibitors (SSRIs), applied along the luteal phase, are effective in treating premenstrual syndrome (Yonkers and Simoni, 2018; Carlini and Deligiannidis, 2021). 5-HT2A receptors regulate responses to stress (Czesak et al., 2012). On the other hand, elevated expressions of the 5-HT1A receptor gene and reduced raphe 5-HT levels appear to be involved in the generation of major depressive disorder (MDD) and other mood changes. 5-HT1A receptors in the dorsal raphe nucleus appear to be upregulated during the depressive state (Li et al., 2020).
Remarkably, estrogen levels during pregnancy appear to be higher in Andean residents than in European residents at HA (Charles et al., 2014). In ovulating women, the luteal phase increase of serum estradiol occurred earlier at sea level than at HA (Escudero et al., 1996). By modulating the expression of the 5-HT receptor—5-HT2BR—estrogens decrease cardiomyocyte death that occurs in response to hypoxia/re-oxygenation injury. Glucocorticoids, on the other hand, prevent 5-HT–mediated estrogen cardioprotection, exacerbating the size of the infarct areas in myocardial infarction (Dhaibar et al., 2021). 5-HT protection by estrogens, being cancelled by glucocorticoids, evidence the deleterious effects that stress may have on women’s well-being, particularly under hypoxic conditions.
3 Discussion and conclusion
A surveillance of human activities at HA increasingly requires sexual (and gender)-dimorphism to be considered in tolerance to hypobaric hypoxia. HA exposure decreases slow-wave sleep (SWS), REM sleep, sleep efficiency, and the total sleep time (Weil, 2004). Hypoxia affects REM sleep (Riveros-Rivera et al., 2022), the organization level of the latter having been shown to be more vulnerable in women than in men (Palagini et al., 2013; Baglioni et al., 2016; Hein et al., 2020). REM sleep disruption may affect memory consolidation, which includes the emotional state—mood alterations, anger, and higher fatigue scores thus arise (Stavrou et al., 2018). Besides affecting mood stability, HA exposure decreases attention, visual and working memory, concentration, executive functions, inhibitory control, and the speed of mental processing (Aquino Lemos et al., 2012; Bloch et al., 2015). Involving the Trp–melatonin axis, hypoxia also reduces 5-HT synthesis (Young, 2013). The failure of BAT activation due to lack of 5-HT (Yabut et al., 2019) additionally favors the development of hypothermia.
Figure 2 summarizes the postulated sexual dimorphism of 5-HT availability at HA and the related pathogenesis of mood disorders. Hypoxia affects metabolic energy generation. The shortage of metabolic energy at HA generates tradeoffs between different energy destinations, which include thermoregulation and sleep-related neural circuitry repair. Hypoxia-induced insomnia, which includes REM sleep deprivation, concomitantly with the lack of 5-HT, may lead to energy being preferentially diverted into thermoregulation in women, perhaps also at the expense of adequate synapse restoration. Defective neurocircuitry repair may affect the consolidation of memory such as the emotional state, with mood disorders thus emerging. Stress and insomnia are, thereby additionally enhanced, in turn reinforcing already prevailing mood disorders. A vicious circle may thus evolve, possibly affecting mental health. Hormonal changes during the menstrual cycle, and certainly also during the life cycle, as well as enhanced stress susceptibility, underscore the possibility of 5-HT–related mood alterations in women exposed to HA. Besides caring for adequate oxygen supply, maintenance of adequate body temperature, stress mitigation, and close observation of menstrual cycles, trials with selective 5-HT reuptake inhibitors may also be envisaged for health, well-being, and safety of women sojourning at HA.
Acknowledgments
The authors thank the many colleagues and students for their helpful discussions and comments.
Author contributions
DA-Z and CB devised the study and drafted the manuscript. GG and MB-C reviewed the literature on women natives at HA, participated in the writing and critically reviewed the manuscript. NDG and NL edited the manuscript and corrected the reference style. PAC and CIV reviewd the literature referring to women at HA at occupational settings. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, editors, and reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Altemus M., Sarvaiya N., Neill Epperson C. (2014). Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35, 320–330. 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino Lemos V., Antunes H. K. M., Santos R. V. T., Lira F. S., Tufik S., Mello M. T. (2012). High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 49, 1298–1306. 10.1111/j.1469-8986.2012.01411.x [DOI] [PubMed] [Google Scholar]
- Baglioni C., Nanovska S., Regen W., Spiegelhalder K., Feige B., Nissen C., et al. (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 142, 969–990. 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F. C., Lee K. A. (2018). Menstrual cycle effects on sleep. Sleep. Med. Clin. 13, 283–294. 10.1016/j.jsmc.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Bauer M., Heinz A., Whybrow P. (2002). Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatry 7, 140–156. 10.1038/sj.mp.4000963 [DOI] [PubMed] [Google Scholar]
- Becker J., Chartoff E. (2019). Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. 10.1038/s41386-018-0125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behn C., De Gregorio N. (2020). Melatonin relations with energy metabolism as possibly involved in fatal mountain road traffic accidents. Int. J. Mol. Sci. 21, 2184. 10.3390/ijms21062184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca R. M., Obermeyer W. H., Thisted R. A., Gillin J. C. (1992). Sleep and psychiatric disorders. A meta-analysis. Arch. Gen. Psychiatry 49, 651–668. 10.1001/archpsyc.1992.01820080059010 [DOI] [PubMed] [Google Scholar]
- Bi X., Guo M., Cao J., Hao Y. (2022). The study of face processing in social anxiety disorder based on face-specific N170 component. J. Healthc. Eng. 2022, 6003973. 10.1155/2022/6003973 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Birzniece V., Johansson I., Wang M., Bäckström T., Olsson T. (2002). Ovarian hormone effects on 5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptor mRNA expression in the ventral hippocampus and frontal cortex of female rats. Neurosci. Lett. 319, 157–161. 10.1016/s0304-3940(01)02570-8 [DOI] [PubMed] [Google Scholar]
- Bloch K. E., Buenzli J. C., Latshang T. D., Ulrich S. (2015). Sleep at high altitude: Guesses and facts. J. Appl. Physiol. 119, 1466–1480. 10.1152/japplphysiol.00448.2015 [DOI] [PubMed] [Google Scholar]
- Bocchetta A., Traccis F. (2017). The Sardinian puzzle: Concentration of major psychoses and suicide in the same sub-regions across one century. Clin. Pract. Epidemiol. Ment. Heal. 13, 246–254. 10.2174/1745017901713010246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve B. F., Silber M. H., Parisi J. E., Dickson D. W., Ferman T. J., Benarroch E. E., et al. (2003). Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 61, 40–45. 10.1212/01.WNL.0000073619.94467.B0 [DOI] [PubMed] [Google Scholar]
- Boos C. J., Bass M., O’Hara J. P., Vincent E., Mellor A., Sevier L., et al. (2018). The relationship between anxiety and acute mountain sickness. PLoS One 13, e0197147. 10.1371/journal.pone.0197147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouali S., Evrard A., Chastanet M., Lesch K.-P., Hamon M., Adrien J. (2003). Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur. J. Neurosci. 18, 2203–2212. 10.1046/j.1460-9568.2003.02960.x [DOI] [PubMed] [Google Scholar]
- Boyce R., Glasgow S. D., Williams S., Adamantidis A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 80, 812–816. 10.1126/science.aad5252 [DOI] [PubMed] [Google Scholar]
- Brook D., Brook J., Zhang C., Cohen P., Whiteman M. (2002). Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch. Gen. Psychiatry 59, 1039–1044. 10.1001/archpsyc.59.11.1039 [DOI] [PubMed] [Google Scholar]
- Brown D. L., Feskanich D., Sánchez B. N., Rexrode K. M., Schernhammer E. S., Lisabeth L. D. (2009). Rotating night shift work and the risk of ischemic stroke. Am. J. Epidemiol. 169, 1370–1377. 10.1093/aje/kwp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Jofré R., Moraga D., Moraga F. A. (2021). The effect of chronic intermittent hypobaric hypoxia on sleep quality and melatonin serum levels in Chilean miners. Front. Physiol. 12, 809360. 10.3389/fphys.2021.809360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canouï-Poitrine F., Veerabudun K., Larmignat P., Letournel M., Bastuji-Garin S., Richalet J.-P. (2014). Risk prediction score for severe high altitude illness: A cohort study. PLoS One 9, e100642. 10.1371/journal.pone.0100642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini S. V., Deligiannidis K. M. (2021). Evidence-based treatment of premenstrual dysphoric disorder: A concise review. J. Clin. Psychiatry 81, 19ac13071–5. 10.4088/JCP.19ac13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caye A., Pilz L., Maia A., Hidalgo M., Furukawa T., Kieling C. (2020). The impact of selective serotonin reuptake inhibitors on the thyroid function among patients with major depressive disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 33, 139–145. 10.1016/j.euroneuro.2020.01.011 [DOI] [PubMed] [Google Scholar]
- Cerri M., Amici R. (2021). Thermoregulation and sleep: Functional interaction and central nervous control. Compr. Physiol. 11, 1591–1604. 10.1002/cphy.c140012 [DOI] [PubMed] [Google Scholar]
- Chakraborti B., Verma D., Guhathakurta S., Jaiswal P., Singh A. S., Sinha S., et al. (2020). Gender-specific effect of 5-HT and 5-HIAA on threshold level of behavioral symptoms and sex-bias in prevalence of autism spectrum disorder. Front. Neurosci. 13, 1375. 10.3389/fnins.2019.01375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. M., Julian C. G., Vargas E., Moore L. G. (2014). Higher estrogen levels during pregnancy in Andean than European residents of high altitude suggest differences in aromatase activity. J. Clin. Endocrinol. Metab. 99, 2908–2916. 10.1210/jc.2013-4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Gao J., Chen Y., Xie J., Xie Y., Spruyt K., et al. (2022). Rapid eye movement sleep during early life: A comprehensive narrative review. Int. J. Env. Res. Public Heal. 19, 13101. 10.3390/ijerph192013101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E., Galea L. A. M., Sohrabji F., Frick K. M. (2018). Sex differences in the brain: Implications for behavioral and biomedical research. Neurosci. Biobehav. Rev. 85, 126–145. 10.1016/j.neubiorev.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla-Neto J., Amaral F. G., Soares J. M., Jr, Gallo C. C., Furtado A., Cavaco J. E., et al. (2022). The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology 112, 115–129. 10.1159/000516148 [DOI] [PubMed] [Google Scholar]
- Correia A. S., Vale N. (2022). Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci. 23, 8493. 10.3390/ijms23158493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen P. J., Anderson I. M., Gartside S. E. (1990). Endocrinological responses to 5-HT. Ann. N. Y. Acad. Sci. 600, 250–257. 10.1111/j.1749-6632.1990.tb16887.x [DOI] [PubMed] [Google Scholar]
- Cowen P. J. (2008). Serotonin and depression: Pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci. 29, 433–436. 10.1016/J.TIPS.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Cyr M., Bossé R., Di Paolo T. (1998). Gonadal hormones modulate 5-hydroxytryptamine2A receptors: Emphasis on the rat frontal cortex. Neuroscience 83, 829–836. 10.1016/s0306-4522(97)00445-4 [DOI] [PubMed] [Google Scholar]
- Czesak M., Le François B., Millar A. M., Deria M., Daigle M., Visvader J. E., et al. (2012). Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J. Biol. Chem. 287, 6615–6627. 10.1074/jbc.M111.293027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Dhar P., Sharma V. K., Barhwal K., Hota S. K., Norboo T., et al. (2018). High altitude with monotonous environment has significant impact on mood and cognitive performance of acclimatized lowlanders: Possible role of altered serum BDNF and plasma homocysteine level. J. Affect Disord. 237, 94–103. 10.1016/j.jad.2018.04.106 [DOI] [PubMed] [Google Scholar]
- Davis J. (1975). Adaptation of brain monoamine synthesis to hypoxia in the rat. J. Appl. Physiol. 39, 215–220. 10.1152/jappl.1975.39.2.215 [DOI] [PubMed] [Google Scholar]
- Dhaibar H. A., Carroll N. G., Amatya S., Kamberov L., Khanna P., Orr A. W., et al. (2021). Glucocorticoid inhibition of estrogen regulation of the serotonin receptor 2B in cardiomyocytes exacerbates cell death in hypoxia/reoxygenation injury. J. Am. Heart Assoc. 10, e015868. 10.1161/JAHA.120.015868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Sarkar P., Shrivastava S., Chattopadhyay A. (2022). Effect of hypoxia on the function of the human Serotonin1A receptor. ACS Chem. Neurosci. 13, 1456–1466. 10.1021/acschemneuro.2c00181 [DOI] [PubMed] [Google Scholar]
- Escudero F., Gonzales G. F., Góñez C. (1996). Hormone profile during the menstrual cycle at high altitude. Int. J. Gynaecol. Obs. 55, 49–58. 10.1016/0020-7292(96)02697-5 [DOI] [PubMed] [Google Scholar]
- Fang X.-L., Shu G., Yu J.-J., Wang L.-N., Yang J., Zeng Q.-J., et al. (2013). The anorexigenic effect of serotonin is mediated by the generation of NADPH oxidase-dependent ROS. PLoS One 8, e53142. 10.1371/journal.pone.0053142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkoue I. T., Michopoulos V., Park J. (2020). Sex differences in post-traumatic stress disorder risk: Autonomic control and inflammation. Clin. Auton. Res. 30, 409–421. 10.1007/s10286-020-00729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. R., Aston-Jones G. (2020). Activation of medial hypothalamic orexin neurons during a Go/No-Go task. Brain Res. 1731, 145928. 10.1016/j.brainres.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos A. M., Sloan C. D., Merrill R. M. (2018). Modeling the effects of atmospheric pressure on suicide rates in the USA using geographically weighted regression. PLoS One 13, e0206992. 10.1371/journal.pone.0206992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater R., Tansella M., Korten A., Tiemens B. G., Mavreas V. G., Olatawura M. O. (1998). Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: Report from the world health organization collaborative study on psychological problems in general health care. Arch. Gen. Psychiatry 55, 405–413. 10.1001/archpsyc.55.5.405 [DOI] [PubMed] [Google Scholar]
- Gehlert S., Clanton M., On Behalf Of The Shift Work And Breast Cancer Strategic Advisory Group (2020). Shift work and breast cancer. Int. J. Environ. Res. Public Health 17, 9544–9548. 10.3390/ijerph17249544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgiuca A., Schipor S., Caragheorgheopol A., Crasan A., Postolache E., Tudose C., et al. (2016). Platelet serotonin as biomarker for assessing suicidal behaviour in patients with bipolar I disorder. Acta Endocrinol. 12, 275–281. 10.4183/AEB.2016.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N., Kim H., Lao R. P. (2005). Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol. Int. 22, 905–915. 10.1080/07420520500263235 [DOI] [PubMed] [Google Scholar]
- Gonzales G. F. (1980). Blood levels of 5-hydroxytryptamine in human beings under several physiological situations. Life Sci. 27, 647–650. 10.1016/0024-3205(80)90004-1 [DOI] [PubMed] [Google Scholar]
- Gonzales G. F., Carrillo C. (1993). Blood serotonin levels in postmenopausal women: Effects of age and serum oestradiol levels. Maturitas 17, 23–29. 10.1016/0378-5122(93)90120-7 [DOI] [PubMed] [Google Scholar]
- Gonzales G. F., Góñez C., Villena A. (2002). Adrenopause or decline of serum adrenal androgens with age in women living at sea level or at high altitude. J. Endocrinol. 173, 95–101. 10.1677/joe.0.1730095 [DOI] [PubMed] [Google Scholar]
- Gonzales G. F., Kaneku L., Goñez C. (1992). Physiology and pathology of reproduction at high altitude. Acta Andin. 1992, 3–16. [Google Scholar]
- Gonzales G. F. (2014). Metabolismo en las grandes alturas. Acta Andin. 9, 31–32. 10.20453/.v9i1-2.1823 [DOI] [Google Scholar]
- Grant I., Giussani D., Aiken C. (2021). Fetal growth and spontaneous preterm birth in high-altitude pregnancy: A systematic review, meta-analysis, and meta-regression. Int. J. Gynaecol. Obs. 10.1002/ijqo.13779 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhang N., Zhu S., Lu S., Jiang H., Zhou H. (2022). Estradiol reduced 5-HT reuptake by downregulating the gene expression of Plasma Membrane Monoamine Transporter (PMAT, Slc29a4) through estrogen receptor β and the MAPK/ERK signaling pathway. Eur. J. Pharmacol. 924, 174939. 10.1016/j.ejphar.2022.174939 [DOI] [PubMed] [Google Scholar]
- Haase J., Jones A. K. C., Mc Veigh C. J., Brown E., Clarke G., Ahnert-Hilger G. (2021). Sex and brain region-specific regulation of serotonin transporter activity in synaptosomes in guanine nucleotide-binding protein G(q) alpha knockout mice. J. Neurochem. 159, 156–171. 10.1111/jnc.15482 [DOI] [PubMed] [Google Scholar]
- Habib R., Messing K. (2012). Gender, women’s work and ergonomics. Ergonomics 55, 129–132. 10.1080/00140139.2011.646322 [DOI] [PubMed] [Google Scholar]
- Hein M., Lanquart J.-P., Loas G., Hubain P., Linkowski P. (2020). Alterations of neural network organization during REM sleep in women: Implication for sex differences in vulnerability to mood disorders. Biol. Sex. Differ. 11, 22. 10.1186/s13293-020-00297-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández O. T., Martínez-Mota L., Herrera-Pérez J. J., Jiménez-Rubio G. (2019). Role of estradiol in the expression of genes involved in serotonin neurotransmission: Implications for female depression. Curr. Neuropharmacol. 17, 459–471. 10.2174/1570159X16666180628165107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz C. T., Kulterer O. C., Prager M., Marculescu R., Langer F. B., Prager G., et al. (2021). Sex differences in Brown adipose tissue activity and cold-induced thermogenesis. Mol. Cell. Endocrinol. 534, 111365. 10.1016/j.mce.2021.111365 [DOI] [PubMed] [Google Scholar]
- Hiroi R., Handa R. J. (2013). Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 127, 487–495. 10.1111/jnc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A., Park I., Suzuki Y., Yajima K., Cui H., Yanagisawa M., et al. (2021). Metabolic responses to polychromatic LED and OLED light at night. Sci. Rep. 11, 12402. 10.1038/s41598-021-91828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoho A. E., Ng L. J., Macfadden L. (2017). Modeling of gender differences in thermoregulation. Mil. Med. 182, 295–303. 10.7205/MILMED-D-16-00213 [DOI] [PubMed] [Google Scholar]
- Izawa S., Chowdhury S., Miyazaki T., Mukai Y., Ono D., Inoue R., et al. (2019). REM sleep-active MCH neurons are involved in forgetting hippocampusdependent memories. Sci. (80) 365, 1308–1313. 10.1126/science.aax9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikki M., Hanoun N., Marcos E., Savale L., Barlier-Mur A. M., Saurini F., et al. (2007). Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: Effects on hypoxic pulmonary hypertension in mice. Am. J. Physiol. - Lung Cell. Mol. Physiol. 293, 1045–1052. 10.1152/ajplung.00082.2007 [DOI] [PubMed] [Google Scholar]
- Jehan S., Auguste E., Hussain M., Pandi-Perumal S. R., Brzezinski A., Gupta R., et al. (2016). Obstructive sleep apnea: Women's perspective. J. Sleep. Med. Disord. 3, 1064. [PMC free article] [PubMed] [Google Scholar]
- Jovanovic H., Kocoska-Maras L., Rådestad A. F., Halldin C., Borg J., Hirschberg A. L., et al. (2015). Effects of estrogen and testosterone treatment on serotonin transporter binding in the brain of surgically postmenopausal women – A PET study. NeuroImage 106, 47–54. 10.1016/j.neuroimage.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Kanekar S., Bogdanova O. V., Olson P. R., Sung Y.-H., D’Anci K. E., Renshaw P. F. (2015). Hypobaric hypoxia induces depression-like behavior in female sprague-dawley rats, but not in males. High. Alt. Med. Biol. 16, 52–60. 10.1089/ham.2014.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar S., Sheth C. S., Ombach H. J., Olson P. R., Bogdanova O. V., Petersen M., et al. (2018). Hypobaric hypoxia exposure in rats differentially alters antidepressant efficacy of the selective serotonin reuptake inhibitors fluoxetine, paroxetine, escitalopram and sertraline. Pharmacol. Biochem. Behav. 170, 25–35. 10.1016/j.pbb.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Karachaliou F.-H., Karavanaki K., Simatou A., Tsintzou E., Skarakis N. S., Kanaka-Gatenbein C. (2021). Association of growth hormone deficiency (GHD) with anxiety and depression: Experimental data and evidence from GHD children and adolescents. Hormones 20, 679–689. 10.1007/s42000-021-00306-1 [DOI] [PubMed] [Google Scholar]
- Kavanau J. (1997). Origin and evolution of sleep: Roles of vision and endothermy. Brain Res. Bull. 42, 245–264. 10.1016/s0361-9230(96)00331-0 [DOI] [PubMed] [Google Scholar]
- Kavanau J. (2000). Sleep, memory maintenance, and mental disorders. J. Neuropsychiatry Clin. Neurosci. 12, 199–208. 10.1176/jnp.12.2.199 [DOI] [PubMed] [Google Scholar]
- Keuper M., Jastroch M. (2021). The good and the BAT of metabolic sex differences in thermogenic human adipose tissue. Mol. Cell Endocrinol. 533, 111337. 10.1016/j.mce.2021.111337 [DOI] [PubMed] [Google Scholar]
- Kim Y.-K., Jeon S. W. (2018). Neuroinflammation and the immune-kynurenine pathway in anxiety disorders. Curr. Neuropharmacol. 16, 574–582. 10.2174/1570159x15666170913110426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kious B. M., Kondo D. G., Renshaw P. F. (2018). Living high and feeling low: Altitude, suicide, and depression. Harv. Rev. Psychiatry 26, 43–56. 10.1097/HRP.0000000000000158 [DOI] [PubMed] [Google Scholar]
- Komagata N., Latifi B., Rusterholz T., Bassetti C. L. A., Adamantidis A., Schmidt M. H. (2019). Dynamic REM sleep modulation by ambient temperature and the critical role of the melanin-concentrating hormone system. Curr. Biol. 29, 1976–1987. e4. 10.1016/J.CUB.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Koudelová J., Mourek J. (1990). Influence of age, sex and hypoxia on plasma dopamine-beta-hydroxylase activity in the rat. Physiol. Bohemoslov. 39, 409–416. [PubMed] [Google Scholar]
- Krishnan V., Collop N. A. (2006). Gender differences in sleep disorders. Curr. Opin. Pulm. Med. 12, 383–389. 10.1097/01.mcp.0000245705.69440.6a [DOI] [PubMed] [Google Scholar]
- Krop H. D., Block A. J., Cohen E. (1973). Neuropsychologic effects of continuous oxygen therapy in chronic obstructive pulmonary disease. Chest 64, 317–322. 10.1378/chest.64.3.317 [DOI] [PubMed] [Google Scholar]
- Laberge M., Blanchette-Luong V., Blanchard A., Sultan-Taïeb H., Riel J., Lederer V., et al. (2020). Impacts of considering sex and gender during intervention studies in occupational health: Researchers’ perspectives. Appl. Ergon. 82, 102960. 10.1016/j.apergo.2019.102960 [DOI] [PubMed] [Google Scholar]
- Lehmann M., Schreiner T., Seifritz E., Rasch B. (2016). Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Scitific Rep. 2016, 39229. 10.1038/srep39229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ma L., Yang G., Gan W.-B. (2017). REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 20, 427–437. 10.1038/nn.4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sun X., Sun J., Zu Y., Zhao S., Sun X., et al. (2020). Depressive-like state sensitizes 5-HT1A and 5-HT1B auto-receptors in the dorsal raphe nucleus sub-system. Behav. Brain Res. 389, 112618. 10.1016/j.bbr.2020.112618 [DOI] [PubMed] [Google Scholar]
- Littlejohn E. L., Fedorchak S., Boychuk C. R. (2020). Sex-steroid-dependent plasticity of brain-stem autonomic circuits. Am. J. Physiol. Integr. Comp. Physiol. 319, R60. 10.1152/ajpregu.00357.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wang Y., Liu C., Wang Y. (2017). Depressive disorder and gastrointestinal dysfunction after myocardial infarct are associated with abnormal tryptophan-5-hydroxytryptamine metabolism in rats. PLoS One 12, e0172339. 10.1371/JOURNAL.PONE.0172339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon K. A., Rood B. D., Wu L., Senft R. A., Goodrich L. V., Dymecki S. M. (2020). Sex-specific role for dopamine receptor D2 in dorsal raphe serotonergic neuron modulation of defensive acoustic startle and dominance behavior. eNeuro 7, ENEURO.0202. 10.1523/ENEURO.0202-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaeva I. M., Berdina O. N., Kurashova N. A., Semenova N. V., Ukhinov E. B., Belskikh A. V., et al. (2021). Sleep apnea and serum serotonin level pre- and post-PAP therapy: A preliminary study. Neurol. Ther. 10, 1095–1102. 10.1007/s40120-021-00290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaca S., Lo Faro A. F., Tamborra A., Pichini S., Busardò F. P., Huestis M. A. (2020). Toxicology and analysis of psychoactive tryptamines. Int. J. Mol. Sci. 21, 9279–9338. 10.3390/ijms21239279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malboosbaf R., Hosseinpanah F., Mojarrad M., Jambarsang S., Azizi F. (2013). Relationship between goiter and gender: A systematic review and meta-analysis. Endocrine 43, 539–547. 10.1007/s12020-012-9831-8 [DOI] [PubMed] [Google Scholar]
- Matamala Pizarro J., Aguayo Fuenzalida F. (2021). Mental health in mine workers: A literature review. Ind. Health 59, 343–370. 10.2486/indhealth.2020-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Bairey Merz N., Barnes P. J., Brinton R. D., Carrero J. J., DeMeo D. L., et al. (2020). Sex and gender: Modifiers of health, disease, and medicine. Lancet 396, 565–582. 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Milner T. A. (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 95, 24–39. 10.1002/jnr.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. R., Sadik A., Sharma S., Poschet G., Gegner H. M., Lanz T. V., et al. (2021). Hypoxia routes tryptophan homeostasis towards increased tryptamine production. Front. Immunol. 12, 590532–590613. 10.3389/fimmu.2021.590532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondanelli G., Volpi C. (2021). The double life of serotonin metabolites: In the mood for joining neuronal and immune systems. Curr. Opin. Immunol. 70, 1–6. 10.1016/J.COI.2020.11.008 [DOI] [PubMed] [Google Scholar]
- Morrison S. A., Mirnik D., Korsic S., Eiken O., Mekjavic I. B., Dolenc-Groselj L. (2017). Bed rest and hypoxic exposure affect sleep architecture and breathing stability. Front. Physiol. 8, 410. 10.3389/fphys.2017.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki Y., Yamanaka A., Tsujino N., Kilduff T. S., Goto K., Sakurai T. (2004). Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J. Neurosci. 24, 7159–7166. 10.1523/JNEUROSCI.1027-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayasu K. (2022). Serotonin transporter: Recent progress of in silico ligand prediction methods and structural biology towards structure-guided in silico design of therapeutic agents. J. Pharmacol. Sci. 148, 295–299. 10.1016/J.JPHS.2022.01.004 [DOI] [PubMed] [Google Scholar]
- Natarajan R., Northrop N. A., Yamamoto B. K. (2015). Protracted effects of chronic stress on serotonin-dependent thermoregulation. Stress 18, 668–676. 10.3109/10253890.2015.1087502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa S., Benkelfat C., Young S. N., Leyton M., Mzengeza S., De Montigny C., et al. (1997). Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. 94, 5308–5313. 10.1073/PNAS.94.10.5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D. (2013). REM sleep dysregulation in depression: State of the art. Sleep. Med. Rev. 17, 377–390. 10.1016/j.smrv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Pascovich C., Niño S., Mondino A., Lopez-Hill X., Urbanavicius J., Monti J., et al. (2021). Microinjection of melanin-concentrating hormone (MCH) into the median raphe nucleus promotes REM sleep in rats. Sleep. Sci. 14, 229–235. 10.5935/1984-0063.20200075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein T., Yonkers K., Fayyad R., Gillespie J. (2005). Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J. Affect Disord. 85, 275–282. 10.1016/j.jad.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Pequignot J. M., Spielvogel H., Caceres E., Rodriguez A., Semporé B., Pequignot J., et al. (1997). Influence of gender and endogenous sex steroids on catecholaminergic structures involved in physiological adaptation to hypoxia. Pflügers Arch. Eur. J. Physiol. 433, 580–586. 10.1007/s004240050317 [DOI] [PubMed] [Google Scholar]
- Pitychoutis P. M., Dalla C., Sideris A. C., Tsonis P. A., Papadopoulou-Daifoti Z. (2012). 5-HT(1A), 5-HT(2A) and 5-HT(2C) receptor mRNA modulation by antidepressant treatment in the chronic mild stress model of depression: Sex differences exposed. Neuroscience 210, 152–167. 10.1016/j.neuroscience.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Poeggeler B., Singh S. K., Pappolla M. A. (2022). Tryptophan in nutrition and health. Int. J. Mol. Sci. 23, 5455–5512. 10.3390/ijms23105455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretell E. A., Moncloa F., Salinas R., Kawano A., Guerra-Garcia R., Gutierrez L., et al. (1969). Prophylaxis and treatment of endemic goiter in Peru with iodized oil. J. Clin. Endocrinol. Metab. 29, 1586–1595. 10.1210/JCEM-29-12-1586 [DOI] [PubMed] [Google Scholar]
- Prioux-Guyonneau M., Mocaër-Cretet E., Redjimi-Hafsi F., Jacquot C. (1982). Changes in brain 5-hydroxytryptamine metabolism induced by hypobaric hypoxia. Gen. Pharmacol. 13, 251–254. 10.1016/0306-3623(82)90097-0 [DOI] [PubMed] [Google Scholar]
- Rahman M. S., Khan I. A., Thomas P. (2011). Tryptophan hydroxylase: A target for neuroendocrine disruption. J. Toxicol. Environ. Health B Crit. Rev. 14, 473. 10.1080/10937404.2011.578563 [DOI] [PubMed] [Google Scholar]
- Rahman M., Thomas P. (2013). Interactive effects of hypoxia with estradiol-17β on tryptophan hydroxylase activity and serotonin levels in the Atlantic croaker hypothalamus. Gen. Comp. Endocrinol. 192, 71–76. 10.1016/j.ygcen.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A., Bergmann B. (2002). Sleep deprivation in the rat: An update of the 1989 paper. Sleep 25, 18–24. 10.1093/sleep/25.1.18 [DOI] [PubMed] [Google Scholar]
- Rendon N. M., Rudolph L. M., Sengelaub D. R., Demas G. E. (2015). The agonistic adrenal: Melatonin elicits female aggression via regulation of adrenal androgens. Proc. R. Soc. B Biol. Sci. 282, 20152080. 10.1098/rspb.2015.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno E., Brown T. L., Betz M. E., Allen M. H., Hoffecker L., Reitinger J., et al. (2018). Suicide and high altitude: An integrative review. High. Alt. Med. Biol. 19, 99–108. 10.1089/ham.2016.0131 [DOI] [PubMed] [Google Scholar]
- Richalet J.-P., Larmignat P., Poitrine E., Letournel M., Canouï-Poitrine F. (2012). Physiological risk factors for severe high-altitude illness: A prospective cohort study. Am. J. Respir. Crit. Care Med. 185, 192–198. 10.1164/rccm.201108-1396OC [DOI] [PubMed] [Google Scholar]
- Richalet J.-P., Lhuissier F., Jean D. (2020). Ventilatory response to hypoxia and tolerance to high altitude in women: Influence of menstrual cycle, oral contraception, and menopause. High. Alt. Med. Biol. 21, 12–19. 10.1089/ham.2019.0063 [DOI] [PubMed] [Google Scholar]
- Rigaud V. O. C., Zarka C., Kurian J., Harlamova D., Elia A., Kasatkin N., et al. (2022). UCP2 modulates cardiomyocyte cell cycle activity, acetyl-CoA, and histone acetylation in response to moderate hypoxia. JCI Insight 7, e155475. 10.1172/JCI.INSIGHT.155475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco L. (2010). Menopausia: Efectos de cambios hormonales en ánimo y cognición. Medwave 10, 4407. 10.5867/medwave.2010.03.4407 [DOI] [Google Scholar]
- Riveros-Rivera A., Penzel T., Gunga H. C., Opatz O., Paul F., Klug L., et al. (2022). Hypoxia differentially affects healthy men and women during a daytime nap with a dose-response relationship: A randomized, cross-over pilot study. Front. Physiol. 13, 899636. 10.3389/fphys.2022.899636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. C., Tsujino N., Abe M., Yamazaki M., Sakimura K., Sakurai T. (2018). Serotonergic input to orexin neurons plays a role in maintaining wakefulness and REM sleep architecture. Front. Neurosci. 12, 892. 10.3389/fnins.2018.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A., Bot M., Aleman A., Penninx B. W. J. H., Aleman I. T., AlemAn A. (2018). Circulating insulin-like growth factor I modulates mood and is a biomarker of vulnerability to stress: From mouse to man. Transl. Psychiatry 8, 142. 10.1038/s41398-018-0196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck C. H., Mahowald M. W. (2002). REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 Years after its formal identification in SLEEP. Sleep 25, 120–138. 10.1093/sleep/25.2.120 [DOI] [PubMed] [Google Scholar]
- Schoenichen C., Bode C., Duerschmied D. (2019). Role of platelet serotonin in innate immune cell recruitment. Front. Biosci. (Landmark Ed. 24, 514–526. 10.2741/4732 [DOI] [PubMed] [Google Scholar]
- Seifinejad A., Li S., Possovre M. L., Vassalli A., Tafti M. (2020). Hypocretinergic interactions with the serotonergic system regulate REM sleep and cataplexy. Nat. Commun. 11, 6034. 10.1038/s41467-020-19862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís J., Guerra-García R., Acosta S., Hurtado J. (2011). Secreción de prolactina en hombres nativos de las grandes alturas. An. Fac. Med. 72, 45–50. [Google Scholar]
- Song X., Zhang Y., Zhang I., Song W., Shi L. (2018). Hypoxia enhances indoleamine 2, 3-dioxygenase production in dendritic cells. Oncotarget 9, 11572–11580. 10.18632/oncotarget.24098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songtachalert T., Roomruangwong C., Carvalho A., Bourin M., Maes M. (2018). Anxiety disorders: Sex differences in serotonin and tryptophan metabolism. Curr. Top. Med. Chem. 18, 1704–1715. 10.2174/1568026618666181115093136 [DOI] [PubMed] [Google Scholar]
- Stavrou N. A. M., Debevec T., Eiken O., Mekjavic I. B. (2018). Hypoxia exacerbates negative emotional state during inactivity: The effect of 21 days hypoxic bed rest and confinement. Front. Physiol. 9, 26–11. 10.3389/fphys.2018.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P., Scorza C., Lagos P., Urbanavicius J., Benedetto L., Pascovich C., et al. (2015). Melanin-concentrating hormone (MCH): Role in REM sleep and depression. Front. Neurosci. 9, 475. 10.3389/fnins.2015.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari A., Brotman S., Cimino J., Timiras P. S. (1978). Adaptive changes induced by high altitude in the development of brain monoamine enzymes. Neurochem. Res. 3, 295–311. 10.1007/BF00965576 [DOI] [PubMed] [Google Scholar]
- Van Cutsem J., Pattyn N., Mairesse O., Delwiche B., Fernandez Tellez H., van Puyvelde M., et al. (2022). Adult female sleep during hypoxic bed rest. Front. Neurosci. 16, 852741. 10.3389/fnins.2022.852741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wolff M., Nakas C., Tobler M., Merz T., Hilty M., Veldhuis J., et al. (2018). Adrenal, thyroid and gonadal axes are affected at high altitude. Endocr. Connect. 7, 1081–1089. 10.1530/EC-18-0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U., Gais S., Born J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 8, 112–119. 10.1101/lm.36801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M., Kloiber S., Diniz B., McIntyre R. S., Felsky D., Tripathy S. J. (2021). Clinical laboratory tests and five-year incidence of major depressive disorder: A prospective cohort study of 433, 890 participants from the UK biobank. Psychiatry 111, 380. 10.1038/s41398-021-01505-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhai X., Chen P., Yang M., Zhao J., Dong J., et al. (2014). Hippocampal UCP2 is essential for cognition and resistance to anxiety but not required for the benefits of exercise. Neuroscience 277, 36–44. 10.1016/J.NEUROSCIENCE.2014.06.060 [DOI] [PubMed] [Google Scholar]
- Wei Y. C., Wang S. R., Jiao Z. L., Zhang W., Lin J. K., Li X. Y., et al. (2018). Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat. Commun. 9, 279. 10.1038/s41467-017-02648-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J. V. (2004). Sleep at high altitude. High. Alt. Med. Biol. 5, 180–189. 10.1089/1527029041352162 [DOI] [PubMed] [Google Scholar]
- Weiss L. A., Abney M., Cook E. H., Ober C. (2005). Sex-specific genetic architecture of whole blood serotonin levels. Am. J. Hum. Genet. 76, 33–41. 10.1086/426697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M., Bland R., Canino C., Faravelli C., Greenwald S., Hwu H., et al. (1996). Cross-national epidemiology of major depression and bipolar disorder. J. Am. Med. Assoc. 276, 293–299. 10.1001/jama.1996.03540040037030 [DOI] [PubMed] [Google Scholar]
- Weissman M., Bland R., Canino G., Greenwald S., Hwu H., Lee C., et al. (1994). The cross national epidemiology of obsessive compulsive disorder: The cross national collaborative group. J. Clin. Psychiatry 55, 5–10. [PubMed] [Google Scholar]
- Werner G. G., Schabus M., Blechert J., Wilhelm F. H. (2021). Differential effects of REM sleep on emotional processing: Initial evidence for increased short-term emotional responses and reduced long-term intrusive memories. Behav. Sleep. Med. 19, 83–98. 10.1080/15402002.2020.1713134 [DOI] [PubMed] [Google Scholar]
- Wetter T., Zils E., Fulda S. (2008). Sleep and psychiatric disorders, In Smith, H., Comella C. (Ed), Sleep medicine. Cambridge University Press, Cambridge, 170–185. 10.1017/CBO9780511545085.012 [DOI] [Google Scholar]
- White K., Loughlin L., Maqbool Z., Nilsen M., McClure J., Dempsie Y., et al. (2011). Serotonin transporter, sex, and hypoxia: Microarray analysis in the pulmonary arteries of mice identifies genes with relevance to human PAH. Physiol. Genomics 43, 417–437. 10.1152/physiolgenomics.00249.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. F., Zhang K. L., Wang Z. M., Yang Y., Li S. H., Wang J. Q., Ma J., et al. (2021). Benefit of a single simulated hypobaric hypoxia in healthy mice performance and analysis of mitochondria-related gene changess. Sci. Rep. 11 (1), 4494. 10.1038/s41598-020-80425-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabut J. M., Crane J. D., Green A. E., Keating D. J., Khan W. I., Steinberg G. R. (2019). Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 40, 1092–1107. 10.1210/ER.2018-00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers K. A., Simoni M. K. (2018). Premenstrual disorders. Am. J. Obstet. Gynecol. 218, 68–74. 10.1016/j.ajog.2017.05.045 [DOI] [PubMed] [Google Scholar]
- Young S. (2013). Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: Possible role of hypoxia causing decreased serotonin synthesis. J. Psychiatry Neurosci. 38, 423–426. 10.1503/jpn.130002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. W., Shen L., Tao C., Jung A. H., Peng B., Li Z., et al. (2021). Medial preoptic area antagonistically mediates stress-induced anxiety and parental behavior. Nat. Neurosci. 24, 516–528. 10.1038/s41593-020-00784-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Kim Y. J., Moran T. H., Li H., Bi S. (2016). Central transthyretin acts to decrease food intake and body weight. Sci. Rep. 6, 24238. 10.1038/srep24238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Patterson L. M., Morrison C., Banfield B. W., Randall J. A., Browning K. N., et al. (2005). Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience 135, 611–625. 10.1016/j.neuroscience.2005.06.055 [DOI] [PubMed] [Google Scholar]
- Zhong Z., Zhou S., Xiang B., Wu Y., Xie J., Li P. (2021). Association of peripheral plasma neurotransmitters with cognitive performance in chronic high-altitude exposure. Neuroscience 463, 97–107. 10.1016/J.NEUROSCIENCE.2021.01.031 [DOI] [PubMed] [Google Scholar]