Abstract
Objective
To assess the effect and safety of gum-chewing on the prevention of postoperative ileus after gynecological cancer surgery.
Methods
We conducted a systematic review of randomized controlled trials (RCTs) published between 2000 and 2022 in English and Chinese, using the EBSCO, Web of Science, Scopus, Cochrane Central Register of Controlled Trials (Cochrane database), PubMed, Medline (via Ovid), Chinese National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database, and Wan Fang databases. A total of 837 studies were screened using Endnote software, and those that met the inclusion criteria were selected for analysis. The main outcome of interest was the incidence of postoperative ileus, and secondary outcomes included time to first flatus, time to first bowel movement, and length of hospital stay.
Results
Two authors extracted data and performed quality assessment independently. The review included six RCTs with a total of 669 patients. Compared with routine care, gum-chewing could significantly reduce the incidence of postoperative ileus (RR 0.46, 95% CI: 0.30, 0.72, P=0.0006), shorten the time to first flatus (WMD -9.58, 95% CI: -15.04, -4.12, P=0.0006), first bowel movement (WMD -11.31, 95% CI: -21.05, -1.56, P=0.02), and the length of hospital stay (WMD -1.53, 95% CI: -2.08, -0.98, P<0.00001).
Conclusions
Gum-chewing is associated with early recovery of gastrointestinal function after gynecological cancer surgery and may be an effective and harmless intervention to prevent postoperative ileus.
Systemaic review registration
https://www.crd.york.ac.uk/prospero/#searchadvanced, identifier CRD42022384346.
Keywords: gastrointestinal function, gum-chewing, gynecological cancer surgery, meta-analysis, review
1. Introduction
Cancer is the leading cause of death worldwide and has become a major public health concern (1). Gynecological cancers are malignant tumors that affect the female reproductive system and account for more than 16% of all cancers among women (2). Globally, the top three most common gynecological cancers are cervical, ovarian, and uterine cancers. In the United States alone, approximately 109,000 new cases of these cancers were diagnosed in 2019, and there are an estimated 33,100 deaths from these diseases each year (3).
Surgery to treat cancer can help alleviate symptoms and extend a patient’s life, it may also result in a slower recovery of bowel function and cause postoperative complications such as nausea, vomiting, abdominal pain, abdominal distention, and even intestinal obstruction (4). Postoperative ileus is a temporary disruption of normal gastrointestinal function that is characterized by the inability to pass gas or stool due to non-mechanical causes (5). Despite the lack of a consistent definition, the incidence of postoperative ileus after abdominal surgery ranges from 5% to 25%, with an average incidence of 10% to 15% (6–8). The incidence of postoperative ileus in individuals receiving complete staging surgery for gynecologic cancers may be as high as 50% (9, 10). Postoperative ileus is reported to increase hospital costs by more than $5000 per patient and extend hospital stays by up to 5 days in the United States (11, 12). Therefore, postoperative ileus has a significant socio-economic impact due to the prolonged hospital stay and high overall hospital costs (13, 14).
Controversial theories exist about the cause of postoperative ileus. The development of postoperative ileus has been linked to sympathetic hyperactivity, pain fiber activation, and elevated catecholamine concentrations in the blood (15, 16). Another theory for the cause of postoperative ileus is aberrant electrolyte levels (17). Moreover, bowel manipulation-related pacemaker malfunction may possibly be another factor in postoperative ileus (18). Enhanced Recovery After Surgery (ERAS) is known to accelerate the motility of the gastrointestinal tract, and several interventions have been recommended to prevent postoperative ileus, including early mobilization, gum chewing, and minimally invasive surgery (19–21).
Previous research has shown that gum chewing is a form of sham feeding that can be used to restore gastrointestinal function without complications (22). It is believed that gum chewing may activate the cephalic-vagal axis, which can lead to the secretion of more gastrointestinal hormones (23). This, in turn, may help to prevent postoperative ileus by reducing inflammation of the intestinal wall (24). Gum chewing is a beneficial intervention that should be encouraged as a regular practice following gynecological surgery, based on several systematic reviews with meta-analyses (4, 25). One obvious limitation of these studies was their significant heterogeneity, and the majority of the participants in the trials were patients with benign gynecological diseases. Unlike benign gynecological surgery, gynecological cancer surgery often involves a larger abdominal incision and carries a higher risk of postoperative ileus (26). However, these findings are inconclusive, as there are some studies that suggest gum chewing may not promote gastrointestinal recovery after laparoscopic surgery (27–31), and there is no robust evidence to support the idea that gum chewing can improve the gastrointestinal function recovery for patients undergoing gynecological cancer surgery.
The aim of this study is to review randomized controlled trials that assess the effectiveness and safety of gum chewing for preventing postoperative ileus after gynecological cancer surgery. In addition to the incidence of postoperative ileus, which is the primary outcome of interest, the secondary outcomes include time to first flatus, time to first bowel movement, time to first defecation, and length of hospital stay.
2. Materials and methods
2.1. Search strategy
A comprehensive literature search for all RCTs was carried out between January 2000 and December 2022 in English and Chinese in EBSCO, Web of Science, Scopus, Cochrane Central Register of Controlled Trials (Cochrane database), PubMed, Medline (via Ovid), Chinese National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database, and Wan Fang. We combined the terms (“gynecological surgery” OR “gynecologic surgery” OR “gynecologic surgical procedures” OR “gynecologic surgical procedure” OR “gynecological surgical procedure” OR “gynecological surgical procedures” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”) AND (“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”) and snowball searches of all included studies were carried out to ensure full inclusion. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (32) was followed in conducting this systematic review and meta-analysis. Our protocol has been registered in the International prospective register of systematic reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/#joinuppage), the registration number is CRD42022384346.
2.2. Study selection
A total of 837 studies were reviewed by Endnote software, with two review authors completing preliminary exclusions based on title and abstract review and then through full-text review respectively. We selected the inclusion criteria according to the PICOS, patients (P) were subjects who received gynecological cancer surgery, interventions (I) were routine care with gum-chewing, comparison (C) was only routine care, outcomes (O) should include no less than one of the indicators below: incidence of postoperative ileus, time to first flatus, time to first bowel movement, or length of hospital stay, and study design (S) was limited to randomized controlled trials (RCTs). Papers were excluded if the full text was not available, published in a foreign language (except English and Chinese), and studies were conducted in children. Discussions or consultations with a third party (RJH) were used to settle disagreements between the two review writers.
2.3. Data extraction
A data extraction form was designed for all of the included articles, two authors (YYN and XH) independently assessed and extracted the following information: Author/year, country, sample size, type of surgery, methods of gum chewing, the definition of postoperative ileus, complication, and main outcomes. We resolved the differences between the two reviewers through consensus discussions with a third person on the review team (RJH) when necessary. When research data were not presented in the article, we contacted the authors of the original study to ask for the data. The data presented as “median and range” values were computed to mean and standard deviation (SD) data with the equations described by Wan et al. (33) and Luo et al. (34) to ensure that all of the relevant data could be included in the final quantitative synthesis.
2.4. Quality assessment of the included studies
In order to evaluate methodological quality and risk of bias in RCTs, our study adopted the Cochrane Collaboration’s “risk of bias” instrument (35). The following areas were evaluated in this analysis: representativeness of study population, random sequence generation, blinding of outcome assessors and participants, allocation concealment, selective reporting of outcomes, incomplete outcome data, and other potential sources of bias. Based on predetermined criteria, each of these domain was categorized as having a high, unclear, or low risk of bias.
2.5. Data synthesis and analysis
Review Manager (RevMan) version 5.3 software was used to format the extracted data for meta-analysis. Effect estimates were presented as weighted mean differences (WMDs) for continuous outcomes and risk ratios (RRs) for dichotomous data with 95% confidence intervals (95% CIs). For continuous data and dichotomous variables, the inverse variance estimate method and the Mantel-Haenzsel method, respectively, were used. The Higgins’ I2 statistic was used to determine the degree of statistical heterogeneity (I2 ≤ 25%, 25%<I2 ≤ 75%, and I2 >75 represent the low, moderate, and high heterogeneity, respectively) (36). Given the variability in the definition of postoperative ileus and the different surgical approaches (laparotomy versus laparoscopy), it was decided that a random effects model would be most appropriate for this meta-analysis (37). Subgroup analysis was presented if heterogeneity was observed among the studies. We used STATA software (Version 14.0) (Stata Corp., College Station, Texas) to perform sensitivity analysis and potential publication bias. Qualitative analysis was conducted if the heterogeneity was large.
3. Results
3.1. Characteristics of included studies
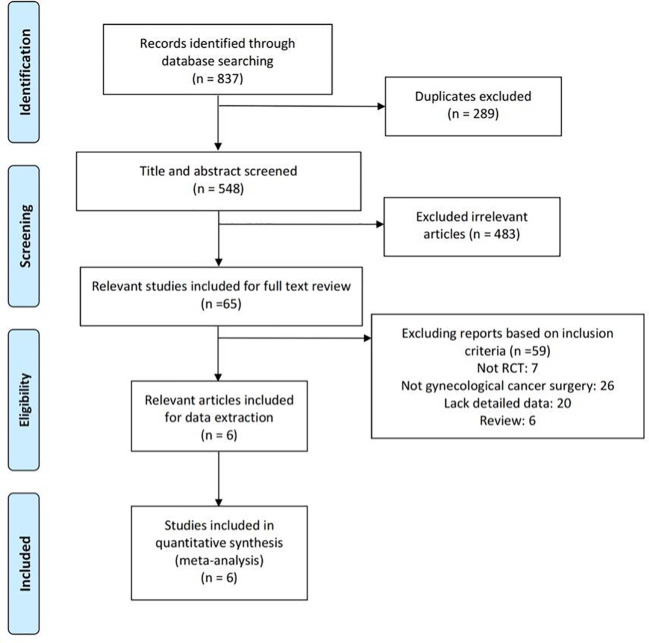
The literature search and selection are illustrated in the PRISMA flow diagram ( Figure 1 ). The search approach initially turned up 837 pertinent publications in total ( Table 1 ). Of these, 289 articles were excluded due to duplicates, and 483 trials were excluded after being screened based on their titles and abstracts. After two review authors re-viewed the full text, an additional 59 reports were excluded because they did not meet the inclusion criteria. The quantitative synthesis was completed with the inclusion of six research (3, 38–42).
Figure 1.
The flowchart of search result.
Table 1.
Search terms and results.
| Database | Search strategy | Results |
|---|---|---|
| Web of science | (TS=(“gynecological surgery” OR “gynecologic surgery” OR “gynecologic surgical procedures” OR “gynecologic surgical procedure” OR “gynecological surgical procedure” OR “gynecological surgical procedures” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”) ) AND (TS=(“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”)) | 61 |
| Scopus | ALL(“gynecological surgery” OR “gynecologic surgery” OR “gynecologic surgical procedures” OR “gynecologic surgical procedure” OR “gynecological surgical procedure” OR “gynecological surgical procedures” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”) AND ABS(“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”) AND PUBYEAR AFT 2000 | 108 |
| Cochrane Central Register of Controlled Trials (Cochrane database) | ALL Text ((“gynecological surgery” OR “gynecologic surgery” OR “gynecologic surgical procedures” OR “gynecologic surgical procedure” OR “gynecological surgical procedure” OR “gynecological surgical procedures” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”) AND (“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”)) | 53 |
| EBSCO | TX (“gynecological surgery” OR “gynecologic surgery” OR “gynecologic surgical procedures” OR “gynecologic surgical procedure” OR “gynecological surgical procedure” OR “gynecological surgical procedures” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”) AND SU (“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”) | 68 |
| Pubmed | (“Chewing Gum”[MeSH Terms] OR (“gum chewing”[All Fields] OR “gum”[All Fields] OR “gums”[All Fields] OR “sham feeding”[All Fields])) AND (“Gynecologic Surgical Procedures”[MeSH Terms] OR “hysterectomy”[MeSH Terms] OR “salpingo oophorectomy”[MeSH Terms] OR (“gynecological surgery”[All Fields] OR “gynecologic surgery”[All Fields] OR “gynecologic surgical procedure”[All Fields] OR “gynecological surgical procedure”[All Fields] OR “gynecological surgical procedures”[All Fields] OR “complete staging surgery”[All Fields] OR “comprehensive staging surgery”[All Fields] OR “modified hysterectomy”[All Fields] OR “radical hysterectomy”[All Fields] OR “laparotomy”[All Fields] OR “adnexal surgery”[All Fields] OR “uterine surgery”[All Fields])) | 64 |
| Medline (via Ovid) | (gynecological surgery or gynecologic surgery or gynecologic surgical procedures or gynecologic surgical procedure or gynecological surgical procedure or gynecological surgical procedures or complete staging surgery or comprehensive staging surgery or modified hysterectomy or salpingo-oophorectomy or radical hysterectomy or hysterectomy or laparotomy or adnexal surgery or uterine surgery).af. AND (gum chewing or chewing gum or gum or gums or sham feeding).af. AND limit 3 to yr=”2000-Current” | 44 |
| CNKI | FT = (“gum chewing” OR “gum” OR “sham feeding”)) AND FT = (“gynecological surgery” OR “complete staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”)) | 299 |
| Wan Fang | (TS: (“gum chewing” OR “gum” OR “sham feeding”)) AND (TS: (“gynecological surgery” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”)) | 49 |
| China Science and Technology Journal Database | (M=(“gum chewing” OR “chewing gum” OR “gum” OR “gums” OR “sham feeding”)) AND (M = (“gynecological surgery” OR “complete staging surgery” OR “comprehensive staging surgery” OR “modified hysterectomy” OR “salpingo-oophorectomy” OR “radical hysterectomy” OR “hysterectomy” OR “laparotomy” OR “adnexal surgery” OR “uterine surgery”)) | 91 |
SU, subject; TS, topic; ABS, abstract; SU, research area; FT, full text; TX, text; M, title or keyword, CNKI, Chinese National Knowledge Infrastructure.
Table 2 presents the fundamental traits of the six included trials, and Table 3 summarizes the results. In brief, a total of 669 patients with gynecological cancer had surgery, and patients were randomly assigned to either routine postoperative care groups (n = 336) or gum-chewing intervention groups (n = 333). The six trials were conducted in three different countries. No complications were reported by any of the participants.
Table 2.
Characteristics of gum chewing intervention in the six included randomized controlled studies.
| Author/year, | Country | Sample size | Type of surgery | Interventions of gum chewing | Definition of postoperative ileus | Main outcome | Complication |
|---|---|---|---|---|---|---|---|
| Ertas et al, 2013 (40) | Turkey | 149 | Laparotomy | Chewing sugar-free gum 3 times a day for 30 minutes starting from the first postoperative morning | “Mild”: if ileus symptoms spontaneously resolved in a few days with observation and basic support, “moderate” if vomiting was persisted and a nasogastric tube re-insertion was necessary, and “severe” if symptoms persisted for more than two days or resisted previous treatment | ①②③④ | None |
| Huang G K et al, 2016 (42) | China | 104 | Laparotomy | Chewing gum 3 times a day for 30 minutes starting from the first postoperative morning | Self-report | ①②③④ | None |
| Li W et al, 2016 (41) | China | 156 | Laparotomy | Chewing sugar-free gum 3 times a day for 30 minutes starting from the first postoperative morning | Abdominal distension, absence of bowel movement/flatus/defecation over 24 h, two or more episodes of nausea or vomiting, and the amount of vomiting is greater than 100 ml | ①②③④ | None |
| Chen L 2017 (38) | China | 78 | Laparotomy | Chewing gum 3 times a day for 30 minutes starting from the first postoperative morning | Self-report | ①②③④ | None |
| Wu L M 2020 (39) |
China | 100 | Laparotomy or laparoscopy | Chewing sugar-free gum 3 times a day for 30 minutes starting from the first postoperative morning | Self-report | ①③④ | None |
| Nanthiphatthanachai et al, 2020 ( 3) |
Thailand | 82 | Laparotomy or laparoscopy | Chewing sugar-free gum for 30 minutes starting on the first postoperative morning then every 8 hours and continue to the first passage of flatus | “Mild”: if ileus symptoms resolved spontaneously within a few days with only observation and basic support, “moderate” if vomiting persisted and reinsertion of the nasogastric tube was required, and “severe” if symptoms persisted for greater than two days or resisted treatment | ①②③④ | None |
①postoperative ileus, ②time to first flatus, ③time to first bowel movement, ④length of hospital stay.
Table 3.
Summary of outcomes across all included studies.
| Study | Postoperative ileus incidence (N) | Time to first flatus (h) | Time to first bowel movement (h) | Length of hospital stay (d) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||||||
| N (%) | Total | N (%) | Total | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Ertas 2013 (40) | 12 (16.22) | 74 | 37 (49.33) | 75 | 34.0±11.5 | 43.6±14.0 | 41.5±15.7 | 50.1±15.9 | 5.9±1.1 | 7.0±1.4 | ||||
| Huang G K 2016 (42) | 0 (0) | 52 | 1 (1.92) | 52 | 25.46±3.08 | 28.50±3.24 | 37.62±3.77 | 39.81±3.63 | 11.29±0.69 | 13.54±0.73 | ||||
| Li W 2016 (41) | 2 (2.56) | 78 | 5 (6.41) | 78 | 41.2±10.3 | 56.3±11.5 | 20.8±9.9 | 28.5±10.6 | 6.5±0.7 | 7.7±1.2 | ||||
| Chen L 2017 (38) | 2 (5.13) | 39 | 4 (10.26) | 39 | 23.6±3.7 | 35.4±4.2 | 37.6±5.2 | 58.4±5.8 | 2.9±1.2 | 4.6±1.5 | ||||
| Wu L M 2020 (39) | 1 (2.00) | 50 | 7 (14.00) | 50 | – | – | 45.0±4.6 | 72.2±4.8 | 3.2±0.8 | 4.2±1.6 | ||||
| Nanthiphatthana-chai 2020 (3) | 14 (35.00) | 40 | 20 (47.62) | 42 | 28.7±19.6 | 37.1±16.9 | 4.8±2.3 | 6.1±4.6 | 3.38±1.81 | 7.86±11.1 | ||||
3.2. Methodological quality
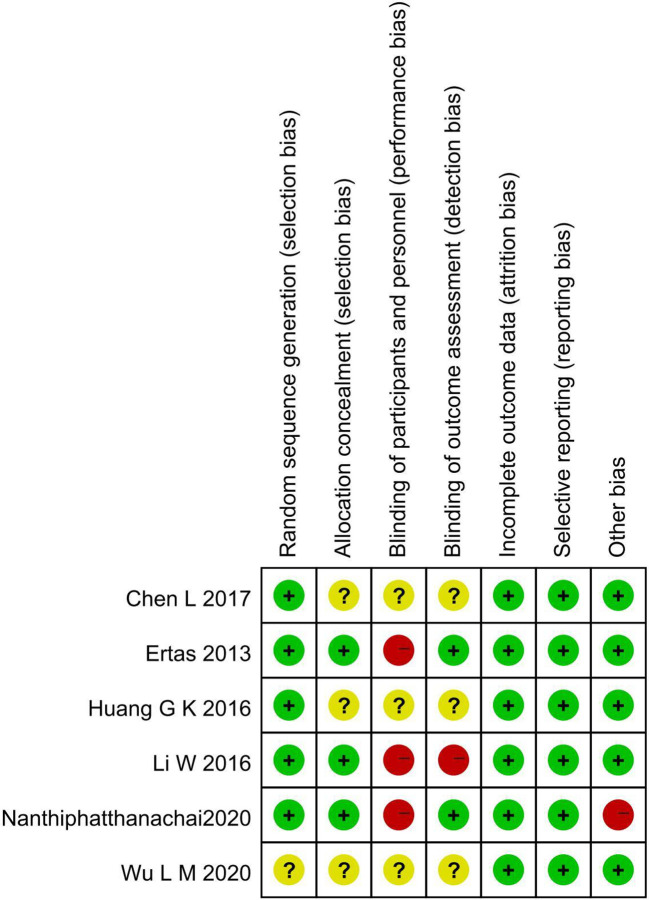
Cross-tabulation in Figure 2 shows the risk of bias for the included articles as determined by the Cochrane Risk of Bias Tool. While Chen L (38), Wu L M (39), and Huang G K 2016 (42) did not indicate the specific method of allocation concealment, three other included trials provided the right methods of random sequence generation and allocation concealment. Two studies chose to blind the outcome assessors (3, 40), and all trials did not blind subjects or not mentioned. There were no instances of selective or incomplete result reporting. The baseline was imbalanced in Nanthiphatthanachai 2020 (3), and no other obvious risk of potential bias was found in the included articles.
Figure 2.
Methodological quality and risk of included trials.
3.3. Primary outcome
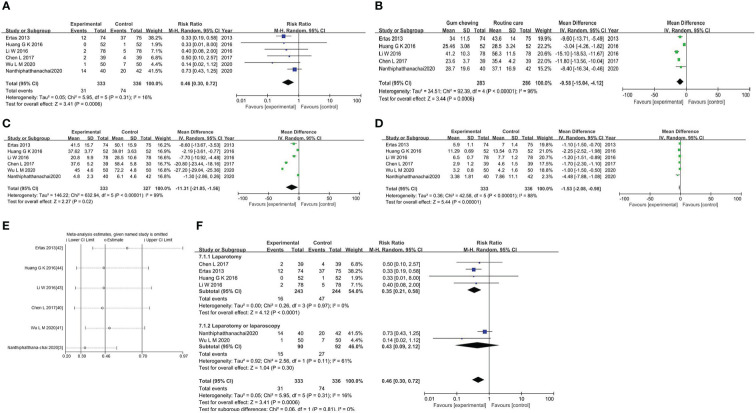
The meta-analysis of the incidence of postoperative ileus was conducted on all trials involving 669 individuals. The results showed that the gum-chewing group had a significantly lower incidence of postoperative ileus, with an RR of 0.46 (95% CI: 0.30, 0.72, P=0.0006), and moderate level of heterogeneity (I2 =16%) ( Figure 3A ).
Figure 3.
(A) Forest plot for the incidence of postoperative ileus. (B) Forest plot for the time to first flatus. (C) Forest plot for the time to first bowel movement. (D) Forest plot for the length of hospitalization. (E) Sensitivity analysis in studies assessing the impact of gum chewing. (F) Forest plot for the subgroup analysis.
3.4. Secondary outcomes
The time to first flatus in the gum-chewing group was significantly shorter than that in the routine care group. The WMD and I2 values were -9.58 (95% CI: -15.04, -4.12, P=0.0006) and I2 =96% ( Figure 3B ). Time to the first bowel movement in the gum-chewing group was significantly shorter than that in the routine care group. The WMD and I2 values were -11.31 (95% CI: -21.05, -1.56, P=0.02) and I2 = 99% ( Figure 3C ). The length of hospitalization was significantly shorter in the gum-chewing group compared to the routine care group. The WMD and I2 values were -1.53 (95% CI: -2.08, -0.98, P<0.00001) and I2 = 88% ( Figure 3D ).
Since the heterogeneity of secondary outcomes was large, we performed a qualitative analysis ( Table 4 ). Only Nanthiphatthanachai 2020 (3) was conducted with the implementation of ERAS, the main cancer types of patients in Huang G K 2016 (42) were different from others. The measurement method was described in two studies (3, 40), and only Ertas 2013 (40) mentioned the same surgical team performing all operations while others did not. Criteria for hospital discharge were only reported in Ertas 2013 (40) and Nanthiphatthanachai 2020 (3).
Table 4.
Qualitative analysis of the secondary outcomes in the included studies.
| Author, year | Implementation of ERAS | Main cancer types of patients | Description of measurement method | Same surgical team | Criteria for hospital discharge |
|---|---|---|---|---|---|
| Ertas 2013 (40) | No | Ovarian cancer, endometrial cancer, and cervical cancer | Yes | Yes | Yes |
| Huang G K 2016 (42) | No | Ovarian cancer, endometrial cancer, and carcinoma of the fallopian tube | No | No | No |
| Li W 2016 (41) | No | Ovarian cancer, endometrial cancer, and cervical cancer | No | No | No |
| Chen L 2017 (38) | No | Ovarian cancer, endometrial cancer, and cervical cancer | No | No | No |
| Wu L M 2020 (39) | No | Ovarian cancer, endometrial cancer, and cervical cancer | No | No | No |
| Nanthiphatthana-chai 2020 (3) | Yes | Ovarian cancer, endometrial cancer, and cervical cancer | Yes | No | Yes |
ERAS, Enhanced recovery after surgery.
3.5. Sensitivity analysis and subgroup analysis
We performed a sensitivity analysis by sequentially removing each study with STATA software, and the results indicated a consistent result among all studies ( Figure 3E ). The subgroup analysis based on the type of surgery showed that gum chewing in patients who underwent laparotomy had a lower incidence of postoperative ileus by an RR of 0.35 (95% CI: 0.21, 0.58, P<0.0001), and the heterogeneity was low with an I2 value of 0%. No statistically significant difference was observed among patients who underwent laparotomy or laparoscopy in two groups by an RR of 0.43 (95% CI: 0.09, 2.12, P=0.30), and heterogeneity among the studies was medium (I2 = 61%) ( Figure 3F ).
3.6. Publication bias analysis
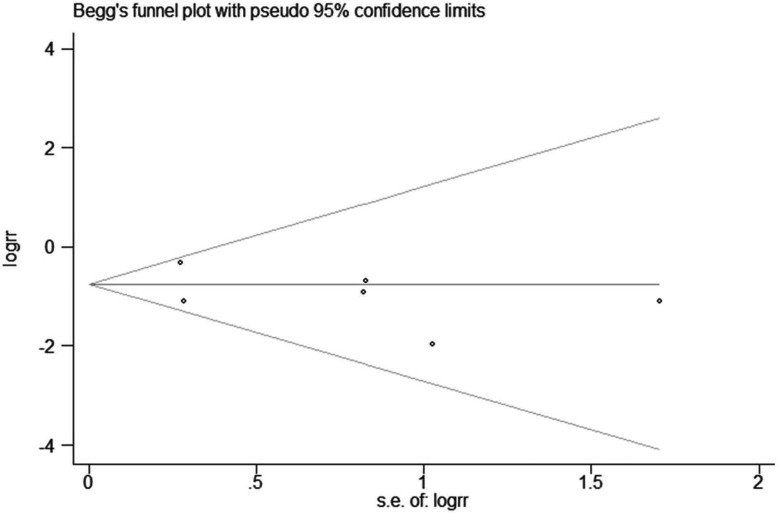
Funnel plots are commonly used to detect publication bias (36). We performed funnel plot analysis of the incidence of postoperative ileus ( Figure 4 ). The potential publication bias was assessed with the Egger’s and Begg’s tests, and no significant potential publication bias was observed (P >0.05).
Figure 4.
Begg’s funnel plot for the incidence of postoperative ileus.
4. Discussion
4.1. Quality of the evidence
Based on evidence from six RCTs involving 669 participants, we found that gum chewing after gynecological cancer surgery played an important role in reducing the incidence of postoperative ileus, shortening the time to first flatus, first bowel movement, and length of hospitalization. No complications were reported in the included trials. The method of allocation concealment was not revealed in three trials (38, 39, 42), which may lead to a risk of selection bias. Because the nature of the study does not permit complete blindness in both participants and outcome assessors, the risk of performance bias cannot be completely avoided. Most of the studies included in this analysis are single-blinded for outcome assessors (3, 40), which may introduce the possibility of a placebo effect. In addition, there was an imbalance in the underlying diseases between the two study groups in Nanthiphatthanachai 2020 (3).
4.2. Implications
In recent years, there has been increasing evidence that gum-chewing following abdominal surgery contributes to improved postoperative intestinal function in patients (43, 44). Dutch researchers found that 27% of participants in the gum-chewing group developed postoperative ileus after surgery, compared to 48% of participants in the control group, and they also found that gum-chewing was associated with a reduction of inflammatory markers (45). In a meta-analysis of gynecological surgery, Xu and his co-authors revealed that gum-chewing was an effective way to improve gastrointestinal function and reduce complications (4). These findings all concluded that gum-chewing was a novel, harmless strategy that should be advocated as a part of enhanced recovery after surgery (ERAS) protocol in patients who had abdominal surgery. Compared with our review, however, only two of the ten included trials where participants underwent gynecological cancer surgery in Xu’s study (4), and significant heterogeneity could not be avoided. The effect of gum-chewing in abdominal surgery was also inconclusive between studies. A multicenter randomized controlled trial (RCT) of 2000 patients undergoing abdominal surgery showed no differences in time to first flatus and time to defecation in both groups, and gum-chewing did not reduce hospital stays and postoperative complications (46). These findings were similar to previous studies (47, 48). These conflicting results suggest that caution should be taken when evaluating the effectiveness of gum-chewing after abdominal surgery, and further evidence should be gathered in future studies.
For gynecologic/oncology surgery, the enhanced recovery after surgery (ERAS) guidelines were developed in 2016 for postoperative care and has been proved to have many advantages, such as preventing postoperative ileus and accelerating bowel function (49). However, these mandatory requirements such as gum-chewing are not routinely implemented due to low recommendation grade and limited clinical benefits. One trial (3) of our review was conducted in relation to the implementation of ERAS, which may be a confounder, thus ERAS should be considered as an independent factor in future research. Other confounding factors that may affect outcomes include outcome indicators, type of surgery, and the definition of postoperative ileus. The first passage of flatus after surgery, a commonly reported outcome, is a signal that patients are recovering from the surgery and are no longer at risk of ileus (25). However, the measurement of this indicator is too subjective and lacks accuracy, since the data are derived from patients’ self-reports (50). This may explain the uncertain results and high heterogeneity found in previous meta-analyses. Postoperative ileus occurs after almost all types of surgery. However, bowel motility in laparoscopic surgery is not like in laparotomy surgery (51, 52). Since muscle function progressively decreases with increasing surgical manipulation of the bowel, and in laparoscopic surgery the bowel is only minimally manipulated (53). Of the six trials included in this study, two trials involved both laparoscopic and laparotomy surgery (3, 39), while the other four only involved laparotomy surgery (38, 40–42). The subgroup analysis based on the type of surgery showed that gum chewing in patients who underwent laparotomy had a lower incidence of postoperative ileus. No statistically significant difference was observed between the laparotomy and laparoscopy groups, indicating the type of surgery is a confounding factor.Additionally, the varying definition of postoperative ileus may also contribute to the heterogeneity. We found similar definitions reported in two studies (3, 40), which may reduce the potential for reporting bias. Similar gum-chewing interventions in the studies also contributed to the low heterogeneity. The main cancer types of patients and criteria for hospital discharge may also influence the heterogeneity. The main cancer types were ovarian cancer, endometrial cancer, and cervical cancer in five studies, in Huang G K 2016 (42), however, the main types of cancer were ovarian cancer, endometrial cancer, and carcinoma of the fallopian tube, which may cause potential confounding. Criteria for hospital discharge were reported in Nanthiphatthanachai 2020 (3) and Ertas 2013 (40), while others did not, which may influence the length of hospitalization and the result of heterogeneity.
4.3. Strengths and limitations
To our knowledge, this systematic review is one of the few to examine the effect of gum-chewing on the incidence of postoperative ileus after gynecological cancer surgery. In previous meta-analyses, gum-chewing was a major confounder, but in our review, the frequency and duration of gum-chewing were consistent across studies, reducing the risk of implementation bias. We chose postoperative ileus as the primary outcome of interest because it is more objective than time to first flatus. In addition, all of the trials included in our review were randomized controlled trials, which provide more reliable evidence of the association between gum-chewing and the incidence of postoperative ileus.
There are several limitations of this review that should be considered. Firstly, the number of trials included is relatively small (six RCTs), and more robust RCTs are needed to reach a conclusive result. Secondly, gum-chewing interventions were implemented in three different countries, which may lead to differences in the routine care received by control group participants. Finally, it seemed challenging to blind both outcome assessors and participants, which may have introduced detection and performance bias. Considering the potential influence of ethnicity or sociodemographic factors between different countries, the extrapolation of our results needs to be done with caution. We believe the influence could be minimal because gum chewing is a harmless practice and widely accepted in countries around the world.
5. Conclusions
In conclusion, this systematic review and meta-analysis of six RCTs suggests that gum-chewing is associated with early recovery of gastrointestinal function after gynecological cancer surgery and may be an effective and safe intervention for preventing postoperative ileus. Given the limited number of studies included in our analysis, multi-center studies with larger sample sizes and well-designed RCTs are warranted to assess the efficacy of gum-chewing on gastrointestinal function after gynecological cancer surgery.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Y-NY was involved in the conception design, literature review, and contributed to the first draft. HX was involved in the literature review, J-HR was involved in the consultation and participated in the data collection and analysis. N-JJ was involved in data analysis and interpretation, LD revised the manuscript and approved the final version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3. Nanthiphatthanachai A, Insin P. Effect of chewing gum on gastrointestinal function recovery after surgery of gynecological cancer patients at rajavithi hospital: a randomized controlled trial. Asian Pacific J Cancer Prevention: APJCP (2020) 21(3):761–70. doi: 10.31557/APJCP.2020.21.3.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu C, Peng J, Liu S, Qi DY. Effect of chewing gum on gastrointestinal function after gynecological surgery: A systematic literature review and meta-analysis. J Obstetrics Gynaecol Res (2018) 44(5):936–43. doi: 10.1111/jog.13602 [DOI] [PubMed] [Google Scholar]
- 5. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg (2001) 182(5):S3–S10. doi: 10.1016/S0002-9610(01)00781-4 [DOI] [PubMed] [Google Scholar]
- 6. LaRosa JA, Saywell JrRM, Zollinger TW, Oser TL, Erner BK, McClain E. The incidence of adynamic ileus in post-cesarean patients. patient-controlled analgesia versus intramuscular analgesia. J Reprod Med (1993) 38(4):293–300. [PubMed] [Google Scholar]
- 7. Andersson T, Bjerså K, Falk K, Olsén MF. Effects of chewing gum against postoperative ileus after pancreaticoduodenectomy–a randomized controlled trial. BMC Res Notes (2015) 8(1):1–5. doi: 10.1186/s13104-015-0996-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolff BG, Viscusi ER, Delaney CP, Du W, Techner L. Patterns of gastrointestinal recovery after bowel resection and total abdominal hysterectomy: pooled results from the placebo arms of alvimopan phase III north American clinical trials. J Am Coll Surgeons (2007) 205(1):43–51. doi: 10.1016/j.jamcollsurg.2007.02.026 [DOI] [PubMed] [Google Scholar]
- 9. Fujita K, Nagano T, Suzuki A, Sakakibara A, Takahashi S, Hirano T, et al. Incidence of postoperative ileus after paraaortic lymph node dissection in patients with malignant gynecologic tumors. Int J Clin Oncol (2005) 10(3):187–90. doi: 10.1007/s10147-005-0494-9 [DOI] [PubMed] [Google Scholar]
- 10. Tabata T, Kihira T, Shiozaki T, Tanida K, Kondo E, Nagao K, et al. Efficacy of a sodium hyaluronate-carboxycellulose membrane (seprafilm) for reducing the risk of early postoperative small bowel obstruction in patients with gynecologic malignancies. Int J Gynecologic Cancer (2010) 20(1):188–93. doi: 10.1111/IGC.0b013e3181c7fe84 [DOI] [PubMed] [Google Scholar]
- 11. Fitzgerald JEF, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg (2009) 33(12):2557–66. doi: 10.1007/s00268-009-0104-5 [DOI] [PubMed] [Google Scholar]
- 12. Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. J Am Coll Surgeons (2010) 210(2):228–31. doi: 10.1016/j.jamcollsurg.2009.09.028 [DOI] [PubMed] [Google Scholar]
- 13. Whitehead WE, Bradley CS, Brown MB, Brubaker L, Gutman RE, Varner RE, et al. Gastrointestinal complications following abdominal sacrocolpopexy for advanced pelvic organ prolapse. Am J obstetrics gynecology (2007) 197(1):78. e1–78. doi: 10.1016/j.ajog.2007.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen AM, Antosh DD, Grimes CL, Crisp CC, Smith AL, Friedman S, et al. Management of ileus and small-bowel obstruction following benign gynecologic surgery. Int J Gynecology Obstetrics (2013) 121(1):56–9. doi: 10.1016/j.ijgo.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 15. Tazegül Pekin A, Kerimoğlu OS, Doğan NU, Yilmaz SA, Kebapcilar AG, Gençoglu Bakbak BB, et al. Gum chewing reduces the time to first defaecation after pelvic surgery: A randomised controlled study. J Obstetrics Gynaecol (2015) 35(5):494–8. doi: 10.3109/01443615.2014.970146 [DOI] [PubMed] [Google Scholar]
- 16. Jepsen S, Klaerke A, Nielsen PH, Simonsen O. Negative effect of metoclopramide in postoperative adynamic ileus. a prospective, randomized, double blind study. J Br Surg (1986) 73(4):290–1. doi: 10.1002/bjs.1800730414 [DOI] [PubMed] [Google Scholar]
- 17. Basse L, Jakobsen DH, Billesbølle P, Billesbølle P, Werner M, Kehlet H, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg (2000) 232(1):51–7. doi: 10.1097/00000658-200007000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peeters T, Matthijs G, Depoortere I, Cachet T, Hoogmartens J, Vantrappen G, et al. Erythromycin is a motilin receptor agonist. Am J Physiology-Gastrointestinal Liver Physiol (1989) 257(3):G470–4. doi: 10.1152/ajpgi.1989.257.3.G470 [DOI] [PubMed] [Google Scholar]
- 19. Purkayastha S, Tilney HS, Darzi AW, Tekkis PP. Meta-analysis of randomized studies evaluating chewing gum to enhance postoperative recovery following colectomy. Arch Surg (2008) 143(8):788–93. doi: 10.1001/archsurg.143.8.788 [DOI] [PubMed] [Google Scholar]
- 20. Gero D, Gié O, Hübner M, Demartines N, Hahnloser D. Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbeck's Arch Surg (2017) 402(1):149–58. doi: 10.1007/s00423-016-1485-1 [DOI] [PubMed] [Google Scholar]
- 21. Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J visceral Surg (2016) 153(6):439–46. doi: 10.1016/j.jviscsurg.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 22. Noble EJ, Harris R, Hosie KB, Thomas S, Lewis SJ. Gum chewing reduces postoperative ileus? a systematic review and meta-analysis. Int J Surgery (2009) 7(2):100–5. doi: 10.1016/j.ijsu.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 23. Lunding JA, Nordström LM, Haukelid A, Gilja OH, Berstad A, Hausken T. Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neurogastroenterol Motility (2008) 20(6):618–24. doi: 10.1111/j.1365-2982.2007.01076.x [DOI] [PubMed] [Google Scholar]
- 24. Su'a BU, Hill AG. Perioperative use of chewing gum affects the inflammatory response and reduces postoperative ileus following major colorectal surgery. BMJ Evidence-Based Med (2015) 20(5):185–6. doi: 10.1136/ebmed-2015-110190 [DOI] [PubMed] [Google Scholar]
- 25. Park SH, Choi MS. Meta-analysis of the effect of gum chewing after gynecologic surgery. J Obstetric Gynecologic Neonatal Nurs (2018) 47(3):362–70. doi: 10.1016/j.jogn.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 26. Fagotti A, Fanfani F, Ercoli A, Giordano MA, Sallustio G, Scambia G. Postoperative ileus after para-aortic lymphadenectomy: a prospective study. Gynecologic Oncol (2007) 104(1):46–51. doi: 10.1016/j.ygyno.2006.06.040 [DOI] [PubMed] [Google Scholar]
- 27. Zaghiyan K, Felder S, Ovsepyan G, Murrell Z, Sokol T, Moore B, et al. A prospective randomized controlled trial of sugared chewing gum on gastrointestinal recovery after major colorectal surgery in patients managed with early enteral feeding. Dis colon rectum (2013) 56(3):328–35. doi: 10.1097/DCR.0b013e31827e4971 [DOI] [PubMed] [Google Scholar]
- 28. Lim P, Morris OJ, Nolan G, Moore S, Draganic B, Smith SR, et al. Sham feeding with chewing gum after elective colorectal resectional surgery: a randomized clinical trial. Ann Surg (2013) 257(6):1016–24. doi: 10.1097/SLA.0b013e318286504a [DOI] [PubMed] [Google Scholar]
- 29. Forrester DAT, Doyle-Munoz J, McTigue T, McTigue T, D'Andrea S, Natale-Ryan A, et al. The efficacy of gum chewing in reducing postoperative ileus: a multisite randomized controlled trial. J Wound Ostomy Continence Nurs (2014) 41(3):227–32. doi: 10.1097/WON.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 30. Matros E, Rocha F, Zinner M, Wang J, Ashley S, Breen E, et al. Does gum chewing ameliorate postoperative ileus? results of a prospective, randomized, placebo-controlled trial. J Am Coll Surgeons (2006) 202(5):773–8. doi: 10.1016/j.jamcollsurg.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 31. Quah HM, Samad A, Neathey AJ, Hay DJ, Maw A. Does gum chewing reduce postoperative ileus following open colectomy for left-sided colon and rectal cancer?–a prospective randomized controlled trial. Colorectal Dis (2006) 8(1):64–70. doi: 10.1111/j.1463-1318.2005.00884.x [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff JAD, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res methodol (2014) 14(1):1–13. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27(6):1785–805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Jama. (2001) 285(15):1987–91. doi: 10.1001/jama.285.15.1987 [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med (2001) 20(6):825–40. doi: 10.1002/sim.650 [DOI] [PubMed] [Google Scholar]
- 38. Chen L, He HJ. Effect of chewing gum on recovery of gastrointestinal function after abdominal surgery in gynecologic malignant tumors. Health Guide (2017) 227:137. (4). doi: 10.3969/j.issn.1006-6845.2017.04.215 [DOI] [Google Scholar]
- 39. Wu LM. The effect of chewing gum on gastrointestinal function recovery in patients with gynecologic malignant tumor after complete staging surgery. World Latest Med Inf (2020) 20(14):74–5. doi: 10.3969/j.issn.1671-3141.2020.14.040 [DOI] [Google Scholar]
- 40. Ertas IE, Gungorduk K, Ozdemir A, Solmaz U, Dogan A, Yildirim Y, et al. Influence of gum chewing on postoperative bowel activity after complete staging surgery for gynecological malignancies: a randomized controlled trial. Gynecologic Oncol (2013) 131(1):118–22. doi: 10.1016/j.ygyno.2013.07.098 [DOI] [PubMed] [Google Scholar]
- 41. Li W, Liu N, Zhang ST, Yu Z, Li B, Ou YL, et al. Influence of gum chewing on the recovery of gastrointestinal function in patients undergoing abdominal complete staging surgery for gynecological malignancies. Chin J Cancer Prev Treat (2016) 23(3):198–202. doi: 10.16073/j.cnki.cjcpt.2016.03.013 [DOI] [Google Scholar]
- 42. Huang GK, Lu QF, She YP, Jiang NN. The influence of chewing gum on the intestinal function recovery after complete staging surgery in patient with gynecological malignant tumor. Hebei Med (2016) 22(8):1257–60. doi: 10.3969/j.issn.1006-6233.2016.08.010 [DOI] [Google Scholar]
- 43. Li S, Liu Y, Peng Q, Xie L, Wang J, Qin X. Chewing gum reduces postoperative ileus following abdominal surgery: a meta-analysis of 17 randomized controlled trials. J Gastroenterol Hepatol (2013) 28(7):1122–32. doi: 10.1111/jgh.12206 [DOI] [PubMed] [Google Scholar]
- 44. Turkay Ü, Yavuz A, Hortu İ, Terzi H, Kale A. The impact of chewing gum on postoperative bowel activity and postoperative pain after total laparoscopic hysterectomy. J Obstetrics Gynaecol (2020) 40(5):705–9. doi: 10.1080/01443615.2019.1652891 [DOI] [PubMed] [Google Scholar]
- 45. Van den Heijkant TC, Costes LMM, van der Lee DGC, Aerts B, Osinga-de Jong M, Rutten HR, et al. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. J Br Surg (2015) 102(3):202–11. doi: 10.1002/bjs.9691 [DOI] [PubMed] [Google Scholar]
- 46. De Leede EM, Van Leersum NJ, Kroon HM, Van Weel V, Van Der Sijp JRM, Bonsing BA, et al. Multicentre randomized clinical trial of the effect of chewing gum after abdominal surgery. J Br Surg (2018) 105(7):820–8. doi: 10.1002/bjs.10828 [DOI] [PubMed] [Google Scholar]
- 47. Ge B, Zhao H, Lin R, Wang J, Chen Q, Liu L, et al. Influence of gum-chewing on postoperative bowel activity after laparoscopic surgery for gastric cancer: A randomized controlled trial. Medicine (2017) 96(13):e6501. doi: 10.1097/MD.0000000000006501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urological Assoc J (2011) 5(5):342–8. doi: 10.5489/cuaj.11002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced recovery after surgery (ERAS®) society recommendations-part II. Gynecologic Oncol (2016) 140(2):323–32. doi: 10.1016/j.ygyno.2015.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roslan F, Kushairi A, Cappuyns L, Daliya P, Adiamah A. The impact of sham feeding with chewing gum on postoperative ileus following colorectal surgery: a meta-analysis of randomised controlled trials. J Gastrointestinal Surg (2020) 24(11):2643–53. doi: 10.1007/s11605-019-04507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwenk W, Böhm B, Haase O, Junghans T, Müller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbeck's Arch Surg (1998) 383(1):49–55. doi: 10.1007/s004230050091 [DOI] [PubMed] [Google Scholar]
- 52. Leung KL, Lai PBS, Ho RLK, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg (2000) 231(4):506–11. doi: 10.1097/00000658-200004000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Husslein H, Franz M, Gutschi M, Worda C, Polterauer S, Leipold H. Postoperative gum chewing after gynecologic laparoscopic surgery: a randomized controlled trial. Obstetrics Gynecology (2013) 122(1):85–90. doi: 10.1097/AOG.0b013e3182983e92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.