Abstract
The complete nucleotide sequence of pETB, a 38.2-kb Staphylococcus aureus plasmid encoding the exfoliative toxin B (ETB), was determined. A total of 50 open reading frames were identified on the plasmid genome and, among these, 32 showed sequence similarity to known proteins. pETB contains three copies of IS257, which divide the pETB genome into three regions: (i) a cadmium resistance operon-containing region, (ii) a lantibiotic production gene-containing region, and (iii) the remaining part where genes for plasmid replication and/or maintenance are dispersed. In the third region, genes of various kinds of functions are present among the replication- and maintenance-related genes. They include two virulence-related genes, the etb gene and a gene encoding a novel ADP-ribosyltransferase closely related to EDIN, which belongs to the C3 family of ADP-ribosyltransferases modifying Rho GTPases. They also include genes for a cell wall-anchoring surface protein and a phage resistance protein. Based on the determined sequence of pETB, the genome structures of etb-bearing plasmids (ETB plasmids) from various clinical isolates were analyzed by the PCR scanning method. The data indicate that, although the ETB plasmids are highly heterogeneous in genome size, the fundamental genome organization is well conserved. The size variation of the plasmid is mainly attributed to defined regions which may be hot spots for gene shuffling.
Exfoliative toxin (ET) is an exotoxin produced by staphylococcal species, causing blisters in human and animal skin (29). ET-producing Staphylococcus aureus is involved in staphylococcal scalded-skin syndrome (SSSS), or Ritter disease, and bullous impetigo in neonates (18, 29, 32). Serologically, ETs causing diseases in human have been divided into two major serotypes: ETA and ETB (5, 28). Both types cause intraepidermal cleavage in the granular layer, without epidermal necrolysis or inflammatory response of skin (5, 16, 28). The mechanism whereby ETs produce exfoliation of the skin has been unknown for a long time, but several lines of evidence have suggested that they are acting as a protease: (i) amino acid sequences of ETA and ETB show similarity with the S. aureus V8 serine protease (14), and the catalytic site of V8 protease is conserved in ETA (6); (ii) partially purified ETs preincubated with serine protease inhibitors exhibits delayed skin exfoliation (14); (iii) substitution of the serine residue with glycine in the putative catalytic site of ETA completely abolishes the exfoliative activity of the toxin (37, 38); and (iv) crystal structures of ETA (12, 56) and ETB (55) have recently been determined, and both types were shown to structurally belong to the chymotrypsin family of serine proteases. More recently, it has been shown that ETA digests desmoglein I, which is one of the major desmosome proteins present in the epidermal layer of human skin (4).
The genes for ETs are detected only in some S. aureus clinical strains. This limited distribution of the ET genes suggested that certain S. aureus strains acquired the genes by horizontal gene transfer. Recently, we actually demonstrated that the gene for ETA (eta) is carried on the genome of a temperate phage integrated into the S. aureus chromosome (62). On the other hand, the gene for ETB (etb) is located on large plasmids (21). Bacteriocin production and cadmium resistance have been also reported to be associated with etb-bearing plasmids (25, 57, 58). Despite the fact that etb-bearing plasmids play important roles in S. aureus skin infection, they have been poorly characterized at the molecular level so far. In this study, we determined the complete nucleotide sequence of an etb-bearing plasmid, pETB, and found that the plasmid encodes not only ETB but also a novel virulence factor, EDIN-C, which catalyzes the ADP-ribosylation of Rho GTPase, a member of the Ras small GTPase superfamily of eukaryotic cells. A PCR scanning analysis based on the determined pETB sequence revealed that the genome organizations of ETB plasmids from various clinical sources are well conserved, although the genome sizes are significantly diverged.
MATERIALS AND METHODS
Materials and chemicals.
EDIN-A was purified to homogeneity from S. aureus E-1 as described previously (45). Anti-EDIN-A serum was prepared as described previously (46). TSKgel HA-1000 and TSKgel G3000 SWXL were obtained from Tosoh, Tokyo, Japan. [α-32P]NAD was from DuPont NEN. Primers used in this study are listed in Table 1. The S. aureus-Escherichia coli shuttle vector pCL8 was kindly provided by Chia Y. Lee. Other materials and chemicals were from commercial sources.
TABLE 1.
Primers used in this study
| Character | Primer | Oligonucleotide sequence (5′-3′) | Location within ETB plasmid (bp) | Size of amplified products (bp) |
|---|---|---|---|---|
| etb | ET-3 | ATACACACATTACGGATAAT | 4847–4866 | 629 |
| ET-4 | CAAAGTGTCTCCAAAAGTAT | 5456–5475 | ||
| edin-C | ednC-1 | TATTAAGCATTCATTCAA | 2016–2033 | 629 |
| ednC-2 | AGTGTAGTCTGTTCCTCT | 1405–1422 | ||
| His6-edin-C | edn-C-Bam | GGATCCGATGATGTTAAAAATTTTACC | 1954–1974 | 731 |
| edn-C-Hind | AAGCTTGTCGATAGCACTTGCTT | 1256–1272 | ||
| pCL8-edin-C | edn-C-pCL8-Eco | GAATTCACCCCAGAACCTGATTG | 2393–2410 | 1,165 |
| ednC-pCL8-Bam | GGATCCGTCGATAGCACTTGC | 1256–1270 | ||
| ORF17 | ETB-17-1 | GCTATAAAGGGCGGCGTTGG | 10116–10135 | 577 |
| ETB-17-2 | GCTTAACCATATTGCCGA | 9559–9576 | ||
| ORF23 | ETB-23-1 | GAACCTGTTTATGGAGAA | 15221–15229 | 387 |
| ETB-23-2 | AACTTTACCTTGACTTGT | 15581–15598 | ||
| ORF24 | ORF24-3 | CAGAATTAAACAGCTACGG | 17795–17813 | 640 |
| ORF24-4 | GATATGTCATCCACATATCTCG | 17174–17195 | ||
| ORF34 | ORF34-1 | CGGTTGTTGCTGCTGCTG | 21232–21249 | 532 |
| ORF34-2 | ATAGCCATAATCCAACGA | 21746–21763 | ||
| ORF35 | ORF35-1 | CTTGTGATGTGATCTGTG | 21874–21891 | 205 |
| ORF35-2 | CTTTGCAACAGAACCCTC | 22061–22078 | ||
| ORF42 | ORF42-1 | CGTATAATGTGGTGTGGGG | 25546–25564 | 475 |
| ORF42-2 | GGAATAGGGCCGTTATTAG | 26002–26020 | ||
| ORF44 | ETB-44-1 | GAACAATGCAGACAATCG | 30327–30344 | 603 |
| ETB-44-2 | CGAACATAGACCTAAATTG | 30911–30929 | ||
| ORF47 | ETB-47-3 | CACGTAATGATACACTTTTGGG | 34944–34965 | 604 |
| ETB-47-4 | CCAACTAATGCAGGATAA | 35531–35548 | ||
| His6-rhoA | RhoA-BamHI | GGATCCGCTGCCATCCGGAAGAA | ||
| RhoA-END | TTACAAGACAAGGCACCCAGA | |||
| Rho-3 | CTGCCACATAGGCCTCAAACACCGTAGGGA | |||
| Rho-4 | GGTGTTTGAGGCCTATGTGGCAGATATTGA |
Bacterial strains.
S. aureus TY4 was isolated from skin lesions of patients diagnosed as SSSS in Hiroshima Citizen's Hospital. Other S. aureus strains used in this study were from our laboratory collection of clinical isolates producing ETB. E. coli DH5α (Difco Laboratories) was used as the host strain in preparing the shotgun library of pETB plasmid DNA. E. coli and S. aureus were grown aerobically in Luria-Bertani broth and heart infusion broth (HI; Difco) at 37°C, respectively. When necessary, ampicillin (at 100 μg ml−1) was added.
Manipulation of DNA.
Routine DNA manipulations were performed by standard procedures (42). Transformation of S. aureus by electroporation was performed as described previously (47). pETB DNA was extracted from TY4 by the method of Kado and Liu (26) and purified by using a Qiagen Midi kit. The plasmid DNA was further purified by CsCl equilibration centrifugation, followed by isopropanol precipitation. Southern blotting of DNA and hybridization were performed as described previously (46).
Sequence determination.
The nucleotide sequence of pETB was determined by shotgun approach as described previously (62). Collected sequences were assembled by using the SEQUENCHER DNA sequencing software (v.3.0; Gene Codes). The sequences of both strands were determined at least once for the entire region.
Computer analysis.
Searches for open reading frames (ORFs), ribosome-binding sites, and restriction sites were performed by using SEQUENCHER and GENE WORKS (v.5.1; IntelliGenetics). Protein and nucleotide sequences were compared with those in the sequence databases by using the BLAST and FASTA programs implemented at the DDBJ.
PCR scanning analysis.
Plasmid DNAs were isolated from ETB-producing S. aureus clinical strains in our laboratory stock and were used as templates for PCR. The strategy of the PCR scanning analysis of etb-bearing plasmids was essentially as previously described (34). All primers were designed according to the nucleotide sequence of pETB (Table 1).
Purification of EDIN-C.
The EDIN-C gene was amplified by PCR by using pETB as a template and cloned into S. aureus-E. coli shuttle vector pCL8 to generate pTY56. PCR primers were designed to introduce EcoRI and BamHI restriction sites into the upstream and downstream regions of the EDIN-C gene, respectively (Table 1). S. aureus RN4220 was transformed with pTY56, and the transformant TY2057 was used for overproduction and purification of the recombinant EDIN-C. TY2057 exponentially growing in HI was inoculated into 3 liters of the same fresh medium and incubated with continuous agitation by a rotary shaker for 18 h at 37°C until the stationary phase. The supernatant was collected by centrifugation at 10,000 × g for 30 min at 4°C. Concentrated culture filtrate was prepared by 80% ammonium sulfate precipitation. Concentrated culture filtrate was dialyzed against 100 mM phosphate buffer (pH 6.8) and applied to a TSKgel HA-1000 column (7.5 mm [inner diameter] by 75 mm long; Tosoh) which was equilibrated with 100 mM phosphate buffer (pH 6.8). The column was washed with 100 mM phosphate buffer (pH 6.8) until most of the unbound proteins passed through. The bound proteins were eluted with a linear gradient of 100 to 500 mM phosphate buffer (pH 6.8) at a flow rate of 1.0 ml/min. The fractions positive for EDIN-C were collected and loaded onto a TSKgel G3000SWXL (7.5 mm [inner diameter] by 300 mm) equilibrated with 100 mM phosphate buffer containing 100 mM Na2SO4, (pH 7.0), and the proteins were eluted at a flow rate of 0.5 ml/min. All of the chromatographic procedures were performed at room temperature. The N-terminal amino acid sequence of the purified protein was determined by a PROCYTE protein sequencer (Shimazu, Tokyo, Japan).
Preparation of antiserum.
To obtain anti-EDIN-C serum, we first constructed a recombinant plasmid encoding the His6-tagged EDIN-C protein. A DNA fragment corresponding to the processed form of EDIN-C was amplified by PCR and placed downstream of the His6-tag sequence of pQE30 (Qiagen). The recombinant plasmid was introduced into E. coli XL-II blue, and the overproduced His6-tagged EDIN-C protein was purified to homogeneity by using Ni-nitrilotriacetic acid agarose column (Qiagen) according to the manufacturer's instruction. The purified protein was emulsified with either Freund complete or incomplete adjuvant (Difco Laboratories, Detroit, Mich.) at 50 μg of protein per ml. A rabbit (2 kg) was immunized at day 1 with the sample emulsified with the complete adjuvant and at day 14 with that with the incomplete adjuvant. At day 28, the rabbit was injected intravenously with 50 μg of the protein. Antiserum was obtained 5 weeks after the first immunization. The affinity-purified antibody specific to His6-tagged EDIN-C was prepared as follows. Purified His6-tagged EDIN-C was coupled to CNBr-activated Sepharose 4B beads according to the manufacturer's instructions. A column packed with EDIN-C-coupled Sepharose 4B (1-ml bed volume) was charged with ca. 1 to 4 ml of the rabbit anti-His6-tagged EDIN-C serum and extensively washed with 0.1 M NaHCO3 (pH 8.3) containing 0.5 M NaCl. The antibody was eluted with 0.2 M Gly-Cl–0.2 M NaCl (pH 2.3). The eluate was immediately neutralized by adding 1 M Tris solution and extensively dialyzed against phosphate-buffered saline (PBS).
Preparation of recombinant small GTPases.
E. coli BL21(DE3) bearing pFLAG-RhoA, pFLAG-Rac1, or pFLAG-Cdc42 was prepared as described earlier (49). The recombinant proteins expressed as N-terminally FLAG-tagged proteins were purified by using anti-FLAG M2 affinity gel (Sigma). To obtain the His6-tagged RhoA mutant containing Asn41-to-Ala41 substitution, the RhoA gene was first amplified by PCR by using pFLAG-RhoA as a template and cloned into the BamHI-SacI site of pQE30. The PCR-based site-specific mutagenesis introducing the Asn41-to-Ala41 substitution was achieved by the overlap extension method by using four primers as described previously (24) (Table 1). The resulting PCR product was digested with BamHI and SacI, and cloned into the BamHI-SacI site of pQE30. His6-tagged proteins were purified as described above.
ADP-ribosylation assay.
The ADP-ribosylation assay was performed in a buffer containing 100 mM HEPES-NaOH (pH 8.0)–10 mM dithiothreitol–0.1 mM MgCl2–10 μM [α-32P]NAD (1,000 to 3,000 cpm/pmol) as described previously (48). The total volume of each reaction mixture was 100 μl, which contained 10 ng of EDIN-A or EDIN-C. Radioactivities incorporated into the small GTPases were determined by phosphorimaging with a BAS2000 (Fuji, Tokyo, Japan).
Other procedures.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (immunoblotting) were performed as described previously (50). Protein concentrations were determined by the method of Bradford (8), with bovine serum albumin as a standard.
Nucleotide sequence accession number.
The nucleotide sequence data determined here is included in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AP003088.
RESULTS
DNA sequence and general overview of the ETB plasmid.
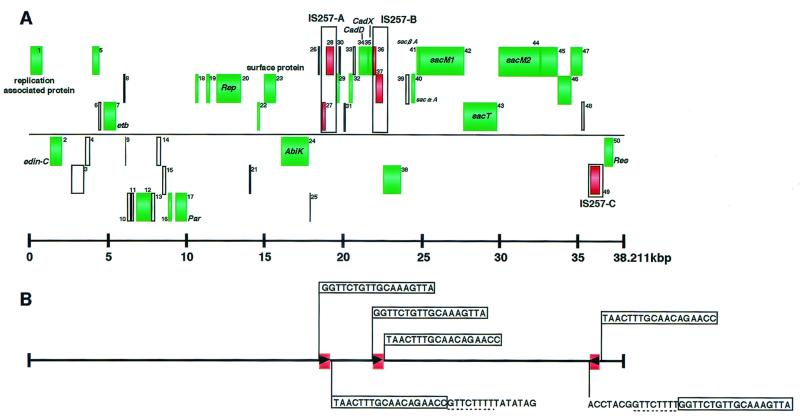
TY4 is a clinical strain of S. aureus isolated from an SSSS patient. A preliminary study by pulsed-field gel electrophoresis and Southern hybridization demonstrated that this strain possesses a large plasmid carrying the etb gene (designated pETB). The plasmid DNA was isolated from the strain and purified by CsCl gradient centrifugation. Subsequently, the complete nucleotide sequence of pETB was determined by a shotgun approach. Assembly of 716 underlying sequences (average length of 500 bp) resulted in a single contiguous sequence of 38,211 bp, with ca. 9.4 average redundancy. The average G+C content of pETB is 27.75%. To predict the protein coding regions, i.e., ORFs, we searched the entire nucleotide sequence for ORFs longer than 50 codons that started with ATG, GTG, or TTG. Each ORF was then examined for the presence of a ribosome-binding sequence and sequence similarity to known proteins. Intergenic regions longer than 100 bp were again searched for the presence of ORFs longer than 30 codons. In this way, we identified 50 potential protein-coding regions, including etb (tentatively named ORF1 to ORF50; etb corresponds to ORF7) (Fig. 1; Table 2). Among these, 32 ORFs showed sequence similarity to known proteins. These include a cadmium resistance operon, a set of genes for lantibiotic production, several genes for plasmid replication and maintenance, and a phage resistance gene. A gene encoding a novel ADP-ribosylating toxin closely related to EDIN was also identified.
FIG. 1.
Genome organiziation of pETB. (A) Identified ORFs are depicted as boxes. Boxes are at three different heights depending on the three frames on each strand. Green boxes indicate the ORFs that show sequence homology to known proteins. The positions of three copies of IS257 identified on pETB are also indicated by boxes, and ORFs on IS257 are indicated in red. (B) IS257 elements are represented as red boxes, and the directions of the transposase genes are indicated by arrowheads in the boxes. Note that the transposases on IS257-A and -B are disrupted by frameshift mutations. Sequences of the terminal inverted repeats of each IS element and the target site duplication-like direct repeats of 8 bp are shown.
TABLE 2.
Feature of ETB plasmid ORFsa
| No. | Location (bp) | Size (aa)a | Translation signalb | Homologue as determined
by BLAST and/or FASTA
|
||||
|---|---|---|---|---|---|---|---|---|
| Source | Description(s) | Identity (%) | Overlap (aa)c | Accession no. | ||||
| 1 | 12–803 | 268 | AGGTACCAATTTATG | S. aureus(pI9789) | Replication-associated protein | 256/256 | AAF63253 | |
| edin-C | 1336–2079 | 247 | AAGGAGTCTTTTATG | S. aureus E-1 | EDIN-A | 66 | 247/247 | AAA26616 |
| 3 | 2649–3479 | 276 | AAGGAGAATGAGGCATTG | |||||
| 4 | 3556–3870 | 104 | AAGGAGAGAAATAATG | |||||
| 5 | 4023–4439 | 138 | GAGGTGTATTAAAATG | S. aureus(pSK41) | ORF149 | 59 | 137/138 | AAC61933 |
| 6 | 4363–4515 | 50 | GGATATTGTAGAATGTG | |||||
| etb | 4681–5514 | 277 | AAGGAGGTTTTATATATG | S. aureus | ETB | 100 | 277/277 | AAA26628 |
| 8 | 5972–6082 | 36 | GAGCGTATGATTTATG | |||||
| 9 | 6085–6189 | 34 | GGAGTTTTTACTATG | |||||
| 10 | 6239–6478 | 79 | GGAGATGATAACTAATG | |||||
| 11 | 6581–6730 | 49 | AGGAGGCATTTATTATG | |||||
| 12 | 6857–7807 | 316 | AAGGAGTAGTTAAGATG | B. subtilis | ypuA | 32 | 317/290 | CAB14269 |
| 13 | 7871–8083 | 70 | GGAGGTAACCTAAATATG | |||||
| 14 | 8158–8502 | 114 | GGAACAATTG | |||||
| 15 | 8562–8774 | 70 | GAGGGTTTTACAAATG | |||||
| 16 | 8939–9181 | 80 | AGGAGAGATACTATG | L. lactis(pCL2000) | ORF1 | 45 | 44/75 | AAF27300 |
| 17 | 9350–10156 | 268 | GGAGGTGGAAGCAATG | L. lactis(pCL2000) | ParA | 46 | 256/262 | AAF27301 |
| 18 | 10643–10867 | 74 | GAGGTTTTTATTATG | S. aureus(pSK41) | Putative Rep-N terminal | 67 | 67/319 | AAC61944 |
| 19 | 11375–11629 | 84 | TAAGGAGGTTAATTAAAATTG | S. aureus(pSK41) | ORF86 | 31 | 75/86 | AAC61943 |
| 20 | 12002–13576 | 524 | AGGAGGTGCAGACAATG | E. faecalis(pAM-β1) | RepE | 31 | 504/496 | AAC38603 |
| 21 | 14121–14252 | 43 | AGGAGAATAATCATTACTTG | |||||
| 22 | 14809–14976 | 55 | - | S. aureus | Glycerol ester hydrolase | 38 | 49/682 | AAA26634 |
| 23 | 15059–15790 | 243 | GAGGTATTCTTAATAAATG | E. faecalis | Surface protein | 40 | 80/1873 | AAD09858 |
| 24 | 16150–17922 | 590 | AGGAGAAAGGCTATG | L. lactis | Abortive infection protein K | 49 | 600/599 | AAB53490 |
| 25 | 18068–18160 | 30 | AGGAGGTGCATTACTTTG | |||||
| 26 | 18642–18770 | 42 | GAGAATTTTG | |||||
| 27 | 18871–19095 | 74 | GAGGTGCAGAGGATG | S. aureus(pSK41) | Putative transposase | 70 | 68/218 | AAC61974 |
| 28 | 19017–19544 | 175 | - | S. aureus(pSK41) | Putative transposase | 87 | 174/224 | AAC61974 |
| 29 | 19709–19915 | 68 | - | S. aureus(pKH4) | Rep-C terminal | 94 | 63/334 | AAB47992 |
| 30 | 19920–20066 | 48 | TAAGGAGTTAAAAATATG | |||||
| 31 | 20194–20319 | 41 | TAAGCTTTTTATG | |||||
| 32 | 20615–20836 | 73 | GAGTAAGTCTTGTG | L. lactis(pBL1) | Hypothetical lactococcin972 immunity protein | 37 | 54/648 | AAF64055 |
| 33 | 20853–21023 | 56 | GAGGGGATTTTG | |||||
| CadD | 21222–21839 | 205 | GAGGTGTATTATG | S. aureus(pRW001) | CadD | 99 | 205/209 | AAB51227 |
| CadX | 21858–22082 | 74 | AGGGTGTGATTTTATATG | S. lugdunensis(pLUG10) | CadX | 93 | 70/115 | AAB18271 |
| 36 | 22122–22346 | 74 | GAGGTGCAGAGGATG | S. aureus(pSK41) | Putative transposase | 70 | 68/218 | AAC61974 |
| 37 | 22268–22795 | 175 | - | S. aureus(pSK41) | Putative transposase | 87 | 174/224 | AAC61974 |
| 38 | 22830–23972 | 380 | AAGGAGAAACTATG | C. jejuni | ATP/GTP binding protein Cj1550c | 25 | 370/588 | CAB73966 |
| 39 | 24101–24331 | 76 | TAAGCTGCTGTATATTATG | |||||
| SacαA | 24694–24882 | 62 | TAAAGCGTGGTGATTCTTATG | S. aureus C55 | SacαA | 100 | 62/62 | AAF47011 |
| SacβA | 24906–25109 | 67 | TAAGGTGGTATTTTTATG | S. aureus C55 | SacβA | 100 | 67/67 | AAD47012 |
| SacM1 | 25128–28025 | 965 | GGAGATAGTTCATAATG | S. aureus C55 | SacM1 | 99 | 965/965 | AAD47013 |
| SacT | 28027–30189 | 720 | GAGGTGTAATATG | S. aureus C55 | SacT | 99 | 720/720 | AAD47014 |
| SacM2 | 30186–32939 | 917 | AAGGAGTGTGGAGTTTG | L. lactis(pMRC01) | Lacticin 481 | 40 | 924/927 | AAC56013 |
| 45 | 32958–34055 | 365 | AGGAGCGTAAATATTTGATG | L. lactis(pMRC01) | ORF40 | 44 | 348/351 | AAC56039 |
| 46 | 34070–34939 | 289 | AAGGAGAATTCTGATG | B. subtilis | YdbJ-ATP binding protein | 28 | 209/308 | CAB12256 |
| 47 | 34923–35648 | 241 | GGAGGTTCTAAAATTG | B. subtilis | yojE | 29 | 139/263 | B69906 |
| 48 | 35677–35850 | 57 | GGAGGAATTTTAATG | |||||
| 49 | 36177–36851 | 224 | GAGGTGCAGAGGATG | S. aureus(pSK41) | Putative transposase | 88 | 224/224 | AAC61974 |
| 50 | 37066–37632 | 188 | GAGGTTATATTTGAATG | S. aureus(pSK41) | Putative Res | 73 | 185/185 | AAC61934 |
aa, amino acids.
Start codons are indicated in boldface. Putative ribosome binding sites complementary to the 3′ end of the 16s rRNA are underlined.
Overlap is indicated as the number of overlapping amino acids/total number of amino acids.
IS elements.
pETB contains three copies of IS257 (designated as IS257-A to -C), is 675 bp long, and has terminal inverted repeats of 18 bp. This IS element has been found on three S. aureus plasmids: pRW001 (13), pSK41 (7), and pSH6 (11). Among the three copies of IS257 on pETB, IS257-A and IS257-B contain authentic frameshift mutations in the putative transposase genes (nucleotide positions 19019 and 22270, respectively; Table 2). IS257 is known to create a target site duplication of 8 bp at the insertion site (7). By examining the boundary nucleotide sequences of the three IS257s, identical 8-bp sequnces (5′-GTTCTTTT-3′) were found at the right boundary of IS257-A and at the left boundary of IS257-C (Fig. 1B). This suggests that these two IS elements form a composite transposon (see below).
Cadmium resistance.
On a segment flanked by IS257-A (positions 18871 to 19544) and IS257-B (positions 22122 to 22795), a cadmium resistance operon and several small genes are present (Fig. 1; Table 2). ORF34 and ORF35 are virtually identical to a cadmium resistance gene, cadD, and its regulatory gene, cadX*, in S. aureus plasmid pRW001, respectively (99 and 93% amino acid sequence identity, respectively). PRW001 is also an etb-carrying plasmid that contains genes for bacteriocin BacR1 (13). The cadDX* operon of pRW001 is located on a segment flanked by two IS257 elements as well, and the nucleotide sequence of the ca. 4-kb pRW001 segment is 97% identical to that of the corresponding stretch of pETB. The functions of the other small genes on the segment are unknown, while ORF29 shows sequence similarities to the C-terminal parts of the Rep proteins of S. aureus plasmids pKH4 (accession no. AAB47992) and pST94 (23). This ORF is probably a remnant of a replication protein which was truncated by the insertion of IS257-A.
Two-component lantibiotic production system.
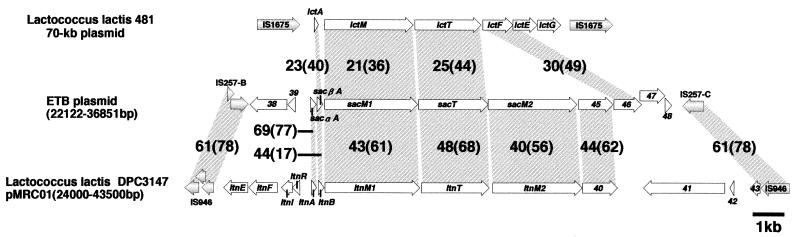
Genes for bacteriocin production are clustered in the 23- to 36-kb region flanked by IS257-B and IS257-C (positions 36177 to 36851) (Fig. 1). Eight genes (ORF40 to ORF48) form an operon-like structure, and most of them exhibit significant homology to the genes involved in the production and secretion of the posttranslationally modified lantibiotics of Lactococcus lactis DPC3147 (15) and/or L. lactis 481 (40) (Fig. 2). From S. aureus strain C55, a DNA fragment corresponding to part of this operon (ORF40 to ORF43) has been cloned and sequenced though the entire region of the C55 operon has not yet been analyzed (35). Thus, according to the nomenclature used for C55, ORF40 to ORF43 were designated sacαA, sacβA, sacM1, and sacT, respectively. Based on the homology to the two-component lacticin operon of strain DPC3147 (15), sacαA and sacβA were predicted to encode the precursors of the bacteriocin components, and sacM1 and sacT were predicted to be involved in the modification and transport of lantibiotics, respectively (15). Similarly, ORF44 (designated sacM2) is predicted to be involved in the modification of lantibiotics together with sacM1 since it encodes a protein showing 40% sequence identity to LtnM2. ORF45 also exhibits 44% sequence identity to an ORF of unknown function (ORF40) in the lacticin operon of DPC3147, while ORF46 is homologous to lctF, an ABC transporter gene involved in the immunity to the bacteriocin of L. lactis 481 (40) (Fig. 2; Table 2). The functions of ORF47 and ORF48 were not assigned by database search, but the absence of a transcriptional terminator for upstream gene(s) and potential promoter in front of these ORFs suggests that these are also a part of the lantibiotic production operon. Upstream of sacαA are two ORFs that are transcribed in the opposite direction of the sacαA-ORF48 operon. It is not known whether these ORFs are involved in the lantibiotic production, although ORF38 shows homology to a putative ATP/GTP-binding protein of Campylobacter jejuni (accession no. CAB73966).
FIG. 2.
Comparison of the lantibiotic production region on pETB with that on a 70-kb plasmid of L. lactis 481 and with that on pMRC01 of L. lactis DPC3147. All genes are drawn to scale. Homologous regions are indicated by gray shading. The numbers indicate the percentage of amino acid identity (similarity) between the depicted regions.
When strain TY4 was grown on a lawn of S. aureus FDA209P, the colony produced a zone of clearing after 24 h of incubation at 37°C. On the other hand, pETB-deleted TY4 failed to produce a zone of clearing. These data indicate that TY4 produces bacteriocin(s) and that pETB is involved in the bacteriocin production. Thus, the identified lantibiotic operon is likely to be active. Intriguingly, however, TY4 failed to produce a zone of clearing when pETB-deleted TY4 was used as an indicator strain. This implies that TY4 contains a chromosomal locus specifying the immunity to the lantibiotic, although the operon on pETB may also encode the immunity function. Therefore, the lantibiotic(s) may not be involved in the maintenance of the pETB in the population.
It is noteworthy that the lantibiotic production gene clusters are flanked by pairs of IS elements (or IS remnant) in both L. lactis DPC3147 and L. lactis 481. As mentioned above, this is also the case for the genes of pETB, suggesting that IS elements are deeply involved in the dissemination and diversification of the genetic determinants of lantibiotic production among both lactococcal strains and staphylococcal strains.
Genes for replication and stable maintenance of pETB.
All ORFs potentially involved in replication and partitioning of pETB are scattered in the region flanked by IS257-A and IS257-C, and this region occupies approximately half of the pETB genome (Fig. 1; Table 2). ORF20, designated rep, encodes a protein that exhibits significant similarity to the replication-initiation proteins of several plasmids of gram-positive origin, such as Enterococcus faecalis plasmid pAM-β1 (10), Streptococcus agalactiae plasmid pIP501 (9), and Streptococcus pyogenes plasmid pSM19035 (9). These are conjugative plasmids belonging to the class D theta replicating plasmid family (10). Class D plasmids are structurally similar to class A plasmids but require DNA polymerase I for replication (10). ORFs 16 to 18 are the homologues of the three contiguous ORFs in the replication-partition region of L. lactis plasmid pCL2000, i.e., ORF1, parA, and repA, respectively (27). The parA gene of pCL2000 has been demonstrated to be essential for stable plasmid maintenance (27). pCL2000 belongs to a newly classified group of theta replicating plasmids, the pLS32 family (53). Staphylococcal plasmids such as pSX267 (22) and pSK41 (7, 20) also belong to this group. ORF18 actually exhibits a high sequence similarity to the Rep protein of pSK41, and the following ORF19 exhibits a high sequence similarity to orf86, which is a neighboring gene of rep in pSK41. It should be noted, however, that ORF18 of pETB is apparently truncated and encodes only the N-terminal part of the replication protein. Besides the parA homologue, pETB contains an additional two genes probably involved in stable plasmid maintenance: ORF1 and ORF50. ORF1 is almost identical to orf245 of pSK1, which has been demonstrated to be required for the segregational stability of the pSK1 replicon (19). ORF50 encodes a protein belonging to the plasmid resolvase family, a subfamily of the recombinase superfamily, and is also likely to contribute to the segregational stability of pETB by facilitating efficient partitioning through conversion of plasmid multimers into monomers (51).
In the replication-maintenance region of pETB, various kinds of genes whose functions are apparently unrelated to replication or maintenance are present. They include two virulence-related genes: etb and ORF2. ORF2, located 1.2 kb upstream of etb, encodes a new ADP-ribosylating enzyme closely related to EDIN, as described in detail below. ORF23 showed weak homology to ESP, a cell surface protein of E. faecalis (44). ESP is a member of the protein family that covalently links to the cell wall with a consensus cell wall-anchoring motif LPXTGX existing in the C-terminal domain (36, 43). ORF23 also contains a motif sequence, LPNTGK, in the C-terminal region, and the motif is followed by a short stretch of hydrophobic amino acids, suggesting that ORF23 encodes a protein covalently linked to the cell wall. To our knowledge, this is the first plasmid-encoded cell wall-anchoring protein found in staphylococci. ORF24 exhibits high sequence similarities to phage resistance proteins of L. lactis, such as AbiK (17), and thus it probably functions as an abortive infection protein against certain phages of S. aureus.
EDIN-C, a new mono-ADP-ribosyltransferase.
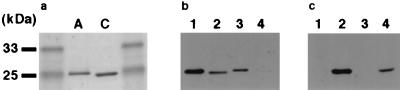
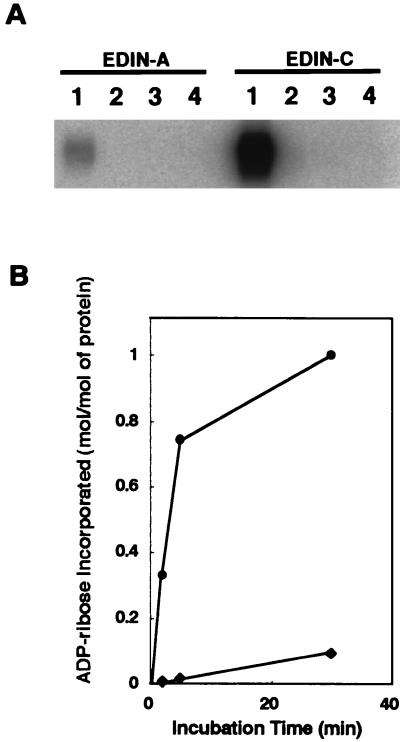
As mentioned above, ORF2 was found to display a high sequence similarity to EDIN (Table 2). EDIN was initially discovered as an inhibitor for morphologic differentiation of cultured epidermal keratinocytes and designated epidermal cell differentiation inhibitor (EDIN) (45). It was later shown to be a mono-ADP-ribosyltransferase that specifically modifies eukaryotic small GTP-binding proteins belonging to the Rho family (RhoA and RhoB) (48) and is now regarded as a member of the large family of bacterial Rho-specific mono-ADP-ribosyltransferases, the C3 family. Members of the family are the basic proteins with low molecular mass (∼25 kDa), but their amino acid sequences are considerably diverged (2). Recently, Wilde et al. (61) identified a novel mono-ADP-ribosyltransferase from S. aureus HM16 and designated it C3stau. However, C3stau showed 78% sequence identity to EDIN, while only 34% to C3 of Clostridium botulinum (Table 3), indicating that it is a close relative of EDIN. We therefore tentatively renamed the EDIN protein first identified in S. aureus E-1 as EDIN-A, C3stau as EDIN-B, and the third one discovered in this study as EDIN-C. EDIN-C shows 66% sequence identity to EDIN-A and 64% to EDIN-B (Table 3) and, in both cases, the homologies were observed throughout the molecules (Fig. 3). To confirm that EDIN-C is actually a mono-ADP-ribosyltransferase, the gene (ORF2) was cloned into S. aureus-E. coli shuttle vector pCL8 to generate pTY56, and the gene product was purified from the culture supernatant of TY2057, a derivative of S. aureus RN4220 which was transformed with pTY56. The molecular mass of the purified protein is 24 kDa (Fig. 4a), and the N-terminal sequence, DDVKN, exactly matched the sequence starting from the Asp residue at position 36 of ORF2. At the same time, we prepared the EDIN-C-specific antibody by using the recombinant His-tagged EDIN-C protein as described in Materials and Methods and then compared the serological properties of EDIN-A and EDIN-C. As shown in Fig. 4b and c, the anti-EDIN-A antibody weakly reacted with EDIN-C, but anti-EDIN-C did not recognize EDIN-A. The ADP-ribosylation assay revealed that EDIN-C, in the presence of [32P]NAD, preferentially radiolabeled RhoA but not Rac1 or Cdc42 as EDIN-A did. Furthermore, a RhoA mutant (RhoA A41), which contains a single amino acid substitution at the target residue (N41) of ADP-rybosylation by the C3 family including EDIN-A, was resistant to modification (Fig. 5A). The kinetics study with RhoA as a substrate revealed that ca. 1 mol of ADP-ribose was incorporated into 1 mol of RhoA by EDIN-C, whereas ca. 0.1 mol of ADP-ribose was incorporated by EDIN-A (Fig. 5B). These results indicate that EDIN-C catalyzes mono-ADP-ribosylation of RhoA more efficiently than EDIN-A and modifies the asparagine residue at position 41 in vitro.
TABLE 3.
Percent amino acid sequence identity among EDINs and C3
| EDIN or C3 | Size (aa)a | % Identity
(% homology) in:
|
||||
|---|---|---|---|---|---|---|
| EDIN-A | EDIN-B (C3stau) | EDIN-C | C. limosum C3 | C. botulinum C3 | ||
| EDIN-A | 247 | 70 (78) | 66 (78) | 30 (50) | 31 (49) | |
| EDIN-B (C3stau) | 247 | 70 (78) | 64 (76) | 31 (51) | 31 (49) | |
| EDIN-C | 247 | 66 (78) | 64 (76) | 30 (50) | 29 (50) | |
| C. limosum C3 | 250 | 30 (50) | 31 (51) | 30 (50) | 59 (74) | |
| C. botulinum C3 | 244 | 31 (49) | 31 (51) | 29 (50) | 59 (74) | |
aa, amino acids.
FIG. 3.
Alignment of EDIN and C3 transferases. Conserved identical amino acids among the members are boxed in gray. CLUSTAL W was used for the alignment. The accession numbers are M63917 (EDIN-A), AJ277173 (EDIN-B [C3stau]), AP003088 (EDIN-C), X87215 (C. limosum C3), and X59039 (C. botulinum C3).
FIG. 4.
Purification of recombinant EDIN-C from S. aureus TY2057. Purified EDIN-A and EDIN-C were resolved on an SDS–12% PAGE gel and stained with Coomassie brilliant blue (a) or transferred to nitrocellulose membrane (b and c). The membrane was then subjected to immunodetection by anti-EDIN-A antibody (b) or anti-EDIN-C affinity purified antibody (c). Lanes: A, EDIN-A (250 ng); B, EDIN-C (250 ng); 1, EDIN-A (25 ng); 2, EDIN-C (25 ng); 3, EDIN-A (5 ng); 4, EDIN-C (5 ng).
FIG. 5.
Protein substrate specificity of EDIN-C. (A) Comparison of protein substrate specificity of EDIN-C and EDIN-A. The recombinant small GTPases were incubated with EDIN-A or EDIN-C in the presence of [32P]NAD. The small GTPases were resolved by SDS-PAGE and subsequently analyzed by phosphorimaging by using a BAS 2000. Lanes: 1, RhoA; 2, Rac1; 3, Cdc42; and 4, RhoA N41A. (B) Kinetics of ADP-ribosylation of RhoA by EDIN-C. Recombinant RhoA was incubated with either EDIN-C (●) or EDIN-A (⧫). After the indicated incubation time, each sample was resolved by SDS-PAGE and analyzed by phosphorimaging. The amount of incorporated [32P]ADP-ribose was given in moles per moles of RhoA.
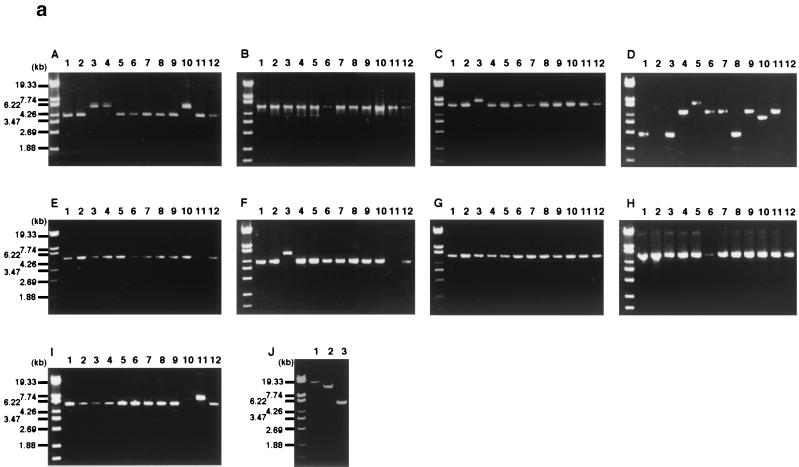
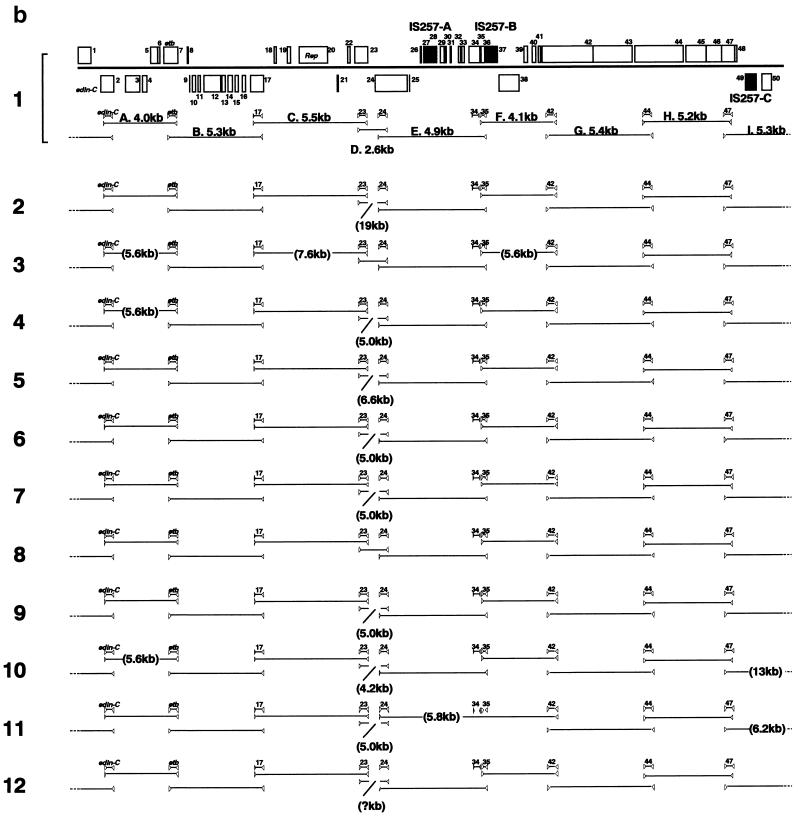
PCR scanning analysis of etb-carrying plasmid in clinical isolates. By analyzing the restriction profiles of etb-bearing plasmids from various S. aureus clinical isolates, it was shown that ETB plasmids are heterogeneous in size but exhibit somehow similar restriction profiles. This suggested that they are closely related to each other (59). To test this assumption, we examined the genome organization of etb-bearing plasmids isolated from 11 clinical isolates of various origins by the PCR scanning method (34). These plasmids are indeed remarkably diverse in the genome size, ranging from 38 to ∼55 kb (data not shown). First, 10 genes on pETB (edin-C, etb, and ORFs 17, 23, 24, 34, 35, 42, 44, and 47) were selected, and their presence in the 11 etb-carrying plasmids was examined by using 10 pairs of primers that were designed to specifically amplify each target gene. All pairs of primers yielded PCR products with expected sizes in all of the tested plasmids except for a plasmid from strain TY110 (Fig. 6a). This result clearly demonstrated that not only etb but also the other nine genes, which include the edin-C gene, are almost perfectly conserved in the ETB plasmids. In the ETB plasmid of TY110, only the two ORFs constituting the cadmium resistance operon, ORF34 and ORF35, are missing. Subsequently, the gene organizations of each plasmid genome were examined by using various combinations of the primers (Fig. 6a). As summarized in Fig. 6b, in all plasmids, the tested genes are located in the same order as that on pETB. This indicates that, although the ETB plasmids are significantly diverged in genome size, their genome organizations are surprisingly well conserved. In particular, the genome of the ETB plasmid from strain TY93 (no. 8 in Fig. 6) is virtually identical to that of pETB. In the other plasmids, some differences in fragment size were noted (one to three segments in each plasmid). However, the size variations were observed in limited regions: only two regions showed the size variation in more than 2 plasmids (regions A and D in Fig. 6). Although region A displayed only two patterns in size variation, region D is highly heterogeneous in size, and this region appears to mainly contribute to the genome size variation of etb-carrying plasmids.
FIG. 6.
PCR scanning analysis of ETB plasmids from 11 S. aureus clinical strains producing ETB. (a) The gene organizations of each ETB plasmid were examined by PCR by using various combinations of 20 primers that were targeted to the selected 10 genes on pETB. Panels A to I present the results of the PCR analysis of regions A to I indicated in panel b. By comparing the length of each amplified fragment with that from pETB, the regional heterogeneity was searched. Results with ETB plasmid from the following strains are shown in the indicated lanes: 1, TY4; 2, TY36; 3, TY49; 4, TY54; 5, TY56; 6, TY64; 7, TY69; 8, TY93; 9, TY97; 10, TY105; 11, TY110; and 12, TY119. In panel J, the regions where no PCR product was obtained in the first series of PCRs were examined by applying the modified protocols: prolonged extension times or lower annealing temperatures. Lanes: 1, region D of ETB plasmid from TY36 (20-min extension); 2, region I from TY105 (20-min extension); 3, region E from TY110 (annealing temperature lowered to 46°C). (b) Summary of the PCR scanning analysis of ETB plasmids from 11 S. aureus clinical strains. The gene arrangement of the pETB genome is presented at the top. Shaded boxes indicate the ORFs that show sequence homology to known proteins. Triangles indicate the positions of primers, and the target genes of each primer set are indicated in between. Solid lines between the triangles represent the regions amplified. When the length of the amplified fragment was different from that of pETB, the estimated size of the fragment is indicated in parentheses. The numbers at the left hand side correspond to the lane numbers in panel a. In the case of the ETB plasmid from TY110 (arrangement 12), the length of region D could not be determined since no PCR product was obtained even when the prolonged extension time was applied (up to 20 min).
DISCUSSION
Previous restriction enzyme (fingerprinting) analyses of etb-bearing plasmids isolated from different geographic locations and from strains with different phage patterns suggested that etb resides on a single family of large plasmids (59, 60). In the present study, we determined the complete nucleotide sequence of an etb-bearing plasmid, pETB. The PCR scanning analysis based on the determined sequence revealed that the genome structures of ETB plasmids are well conserved, verifying the above-mentioned idea. Although we did not examine pRW001, which was previously suggested to be the archetype of ETB plasmids, the reported size (37.5 kb) and restriction map of pRW001 (25) are similar to those deduced from the nucleotide sequence of pETB. It has also been reported that cadmium resistance and lantibiotic production are often associated with ETB plasmids (58, 59). Our data obtained by PCR scanning analysis clearly indicate that the regions containing the genes responsible for the two phenotypes are highly conserved among ETB plasmids (Fig. 6). The published nucleotide sequence of the cadmium resistance region of pRW001 (13) is almost identical to that of pETB. The 3.3-kb nucleotide sequence of a 32-kb ETB plasmid of strain C55 (35), which corresponds to the portion of the lantibiotic production region, is also virtually identical to that on pETB. Therefore, the nucleotide sequence of pETB determined in this study is regarded as the archetype of etb-bearing plasmids.
pETB contains three copies of IS257, and they divide the pETB genome into three regions which may represent the fundamental modules constituting the ETB plasmid genomes: (i) the cadmium resistance operon-containing region, (ii) the lantibiotic production gene-containing region, and (iii) the remaining part where replication- and/or maintenance-related genes are dispersed. A previous finding that cadmium resistance is sometimes not associated with ETB production (59) might be explained by IS257-mediated deletion of the region containing the cadmium resistance operon. The third large module containing replication- and maintenance-related genes appears to be a mosaic of several DNA segments of different origins and is assumed to have been generated by genetic shuffling through evolution. The origin of the replication machinery actually differs from those of plasmid maintenance genes; the rep gene of pETB is closely related to those of type D theta-replicating plasmids, while maintenance-related genes are similar to those on S. aureus multiresistance plasmids, such as pSK41 and pSK1, that belong to the pLS32 family (19). A remnant of a replication protein of the pLS32 family (ORF18) also remains in this module. A variety of genes identified on this module, the functions of which are apparently unrelated to each other, may be acquired during the process of genetic shuffling. The two segments of pETB that exhibit the highest size variability (regions A and D) are located in this module, and they may be the hot spots for genetic shuffling. Furthermore, this module itself may be able to move as a composite transposon because a possible target site duplication was identified at the boundaries of two IS elements flanking this module: IS257-A and IS257-C. The presence of another remnant replication protein (ORF29) at the right boundary of IS257-A implies that pETB was generated by integration of the module into a replicon carrying the cadmium resistance operon.
It is intriguing to know how the ETB determinant can be transmitted among staphylococcal strains. Since we could not find any trace of conjugal transfer (tra) gene cluster in the pETB sequence, pETB does not appear to be self-transmissible. The possibility of cotransfer with another conjugative plasmid remains elusive, but so far we could not detect any other plasmid in ETB-producing clinical isolates. Rogolsky et al. (41) demonstrated that pRW002, which carries the etb gene, was transferred to a plasmidless recipient strain by mixed-culture transduction. This suggests that ETB plasmid can be transmitted by some bacteriophage, but it remains unknown whether transduction is actually involved in the dissemination of pETB (or etb) in nature. Phylogenetic analysis of ETB-producing clinical strains may provide a clue to understanding the mechanism by which ETB plasmids spread among S. aureus strains.
The most medically important finding obtained in the present study is the identification of ORF2 encoding a novel ADP-ribosyltransferase closely related to EDIN, a member of the bacterial C3-like ADP-ribosyltransferase family, which specifically modifies and inactivates Rho GTPases (48). C3 exoenzymes of C. botulinum were first described as Rho-specific ADP-ribosyltransferase and, since then, proteins with similar activities have been identified from S. aureus, Clostridium limosum, and Bacillus cereus (2, 30, 39). EDIN was initially discovered as a factor inhibiting morphologic differentiation of epidermal keratinocytes (50) and later identified as an ADP-ribosyltransferase modifying Rho GTPases (48). ORF2 exhibits 66% sequence identity to EDIN, and the gene product specifically ADP-ribosylates RhoA but not Rac1 and Cdc42 (Table 3 and Fig. 5). C3stau, a Rho-specific ADP-ribosyltransferase recently identified from S. aureus HM16, also exhibits high sequence similarities to EDIN and ORF2 (70 and 64% identity, respectively) but remarkably less similarity to C3 enzymes from clostridia (Table 3). Thus, these three proteins are closely related to each other and form a distinct group among the C3 superfamily. We therefore propose that the original EDIN be renamed EDIN-A, that C3stau be renamed EDIN-B, and that the enzyme newly identified in this study (ORF2) be named EDIN-C. Recently, EDIN-B (C3stau) was shown to modify not only the RhoA, -B, and -C subtypes of the Rho family but also RhoE/Rnd3, whereas C3 confined its substrate specificity to RhoA, -B, and -C (61). It should be elucidated in the future whether EDIN-A and EDIN-C also modify the RhoE/Rnd3 subtype or not.
Rho GTPases are central regulators of actin cytoskeleton in eukaryotic cells and are involved in multiple cellular functions, including cell morphology, cell growth, cell cycle progression, motility, cell adhesion, phagocytosis, endocytosis, apoptosis, and smooth muscle contraction (31, 52, 54). Several bacterial Rho GTPase-inactivating proteins, such as large clostridial cytotoxins (3) and YopT of Yersinia enterocolitica (63), are known to be profoundly implicated in bacterial pathogenesis. It is also possible that EDINs play important roles in the pathogenesis of S. aureus skin infection, although no direct evidence for this has been reported thus far. In this regard, it is of interest that ETB-producing strains are more frequently isolated than ETA-producing strains from children with the generalized form of SSSS, while both types of strains are equally detected in the localized form of SSSS, bullous impetigo. This suggests that ETB-producing strains are more virulent (33). Furthermore, it was reported that ETB-producing strains sometimes cause the generalized form of SSSS in immunocompetent adults (33). Since the dermatologic effects of ETA and ETB are indistinguishable, there must be additional factor(s) involved in the exacerbation of the diseases caused by ETB-producing strains. In consequence, our finding here that etb and edin-C coexist on ETB plasmids is particularly important. Previously, Aepfelbacher et al. (1) demonstrated that EDIN-A inhibited endothelial wound repair and endothelial cell migration through inhibition of Rho GTPases. We have also shown that EDIN-A induces transient hyperplasia of the epidermis when injected into adult mouse skin, suggesting that EDIN-A affects epidermal cell differentiation and growth in vivo (48). It should be elucidated in the future whether EDIN-C is actually involved in the exacerbation of the diseases caused by ETB-producing strains.
ACKNOWLEDGMENTS
We are grateful to Mika Takahashi, Shuko Setsu, Kaori Satoh, and Yasuyuki Fudaba for technical assistance. We are also grateful to Yumiko Hayashi for editorial assistance and to Chia Y. Lee for S. aureus-E. coli shuttle plasmid. We thank the Research Center for Molecular Medicine, the Research Facility for Laboratory Animal Science, and the Radioisotope center in Kasumi Campus and the Research Facility, Hiroshima University Faculty of Dentistry, for allowing us to use their facilities.
REFERENCES
- 1.Aepfelbacher M, Essler M, Huber E, Sugai M, Weber P C. Bacterial toxins block endothelial wound repair. Arterioscler Thromb Vasc Biol. 1997;17:1623–1629. doi: 10.1161/01.atv.17.9.1623. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. doi: 10.1016/S0966-842X(97)01067-6. [DOI] [PubMed] [Google Scholar]
- 3.Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial toxins. Biol Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- 4.Amagai M, Matsuyoshi N, Wang Z H, Andl C, Stanley J R. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–1277. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- 5.Arbuthnott J P, Billcliffe B. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J Med Microbiol. 1976;9:191–201. doi: 10.1099/00222615-9-2-191. [DOI] [PubMed] [Google Scholar]
- 6.Bailey C J, Smith T P. The reactive serine residue of epidermolytic toxin A. Biochem J. 1990;269:535–537. doi: 10.1042/bj2690535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray R A. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brantl S D, Behnke D, Alonso J C. Molecular analysis of the replication region of the conjugative Streptococcus agalactiae plasmid pIP501 in Bacillus subtilis. Comparison with plasmids pAMβ1 and pSM19035. Nucleic Acids Res. 1990;18:4783–4790. doi: 10.1093/nar/18.16.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruand C, le Chatelier E, Ehrlich S D, Janniere L. A fourth class of theta-replicating plasmids: the pAMβ1 family from gram-positive bacteria. Proc Natl Acad Sci USA. 1993;90:11668–11672. doi: 10.1073/pnas.90.24.11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne M E, Gillespie M T, Skurray R A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from north American Staphylococcus aureusstrains. Antimicrob Agents Chemother. 1990;34:2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavarelli J, Prevost G, Bouquet W, Moulinier L, Chevrier B, Delagoutte B, Bilwas A, Mourey L, Rifai S, Piemont Y, Moras D. The structure of Staphylococcus aureusepidermolytic toxin A, an atypic serine protease, at 1.7 Å resolution. Structure. 1997;5:813–824. doi: 10.1016/s0969-2126(97)00235-9. [DOI] [PubMed] [Google Scholar]
- 13.Crupper S S, Worrell V, Stewart G C, Iandolo J J. Cloning and expression of cadD, a new cadmium resistance gene of Staphylococcus aureus. J Bacteriol. 1999;181:4071–4075. doi: 10.1128/jb.181.13.4071-4075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dancer S J, Noble W C. The epidermolytic toxins are serine proteases. FEBS Lett. 1990;268:129–132. doi: 10.1016/0014-5793(90)80990-z. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty B A, Hill C, Weidman J F, Richardson D R, Venter J C, Ross R P. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactisDPC3147. Mol Microbiol. 1998;29:1029–1038. doi: 10.1046/j.1365-2958.1998.00988.x. [DOI] [PubMed] [Google Scholar]
- 16.Elias P M, Fritsch P, Epstein J E H. Staphylococcal scalded skin syndrome. Clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207–219. doi: 10.1001/archderm.113.2.207. [DOI] [PubMed] [Google Scholar]
- 17.Emond E, Holler B J, Boucher I, Vandenbergh P A, Vedamuthu E R, Kondo J K, Moineau S. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl Environ Microbiol. 1997;63:1274–1283. doi: 10.1128/aem.63.4.1274-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell A M. Staphylococcal scalded-skin syndrome. Lancet. 1999;354:880–881. doi: 10.1016/s0140-6736(99)90120-4. [DOI] [PubMed] [Google Scholar]
- 19.Firth N, Apisiridej S, Berg T, O'rourke B A, Curnock S, Dyke K G H, Skurray R A. Replication of staphylococcal multiresistance plasmids. J Bacteriol. 2000;182:2170–2178. doi: 10.1128/jb.182.8.2170-2178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firth N, Ridgway K P, Byrne M E, Fink P D, Johnson L, Paulsen I T, Skurray R A. Analysis of a transfer region from the staphylococcal conjugative plasmid pSK41. Gene. 1993;136:13–25. doi: 10.1016/0378-1119(93)90442-6. [DOI] [PubMed] [Google Scholar]
- 21.Freer J, Arbuthnott J P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1983;19:55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- 22.Gering M, Gotz F, Bruckner R. Sequence and analysis of the replication region of the Staphylcoccus xylosusplasmid pSX267. Gene. 1996;182:117–122. doi: 10.1016/s0378-1119(96)00526-4. [DOI] [PubMed] [Google Scholar]
- 23.Heir E, Sundheim G, Holck A L. The Staphylococcus gacHgene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol Lett. 1998;163:49–56. doi: 10.1111/j.1574-6968.1998.tb13025.x. [DOI] [PubMed] [Google Scholar]
- 24.Ito W, Ishiguro H, Kurosawa Y. A general method for introducing a series of mutations into cloned DNA using the polymerase chain reaction. Gene. 1991;102:67–70. doi: 10.1016/0378-1119(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 25.Jackson M P, Iandolo J J. Cloning and expression of the exfoliative toxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166:574–580. doi: 10.1128/jb.166.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney K, Fitzgerald G F, Seegers J F M L. Identification and characterization of an active plasmid partition mechanism for the novel Lactococcus lactisplasmid pCI2000. J Bacteriol. 2000;182:30–37. doi: 10.1128/jb.182.1.30-37.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo I, Sakurai S, Sarai Y. Purification of exfoliatin produced by Staphylococcus aureusof bacteriophage group 2 and its physicochemical properties. Infect Immun. 1975;8:156–164. doi: 10.1128/iai.8.2.156-164.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladhani S, Joannou C L, Lochrie D P, Evans R W, Poston S M. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224–242. doi: 10.1128/cmr.12.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerm M, Schmidt G, Aktories K. Bacterial toxins targeting Rho GTPases. FEMS Microbiol Lett. 2000;188:1–6. doi: 10.1111/j.1574-6968.2000.tb09159.x. [DOI] [PubMed] [Google Scholar]
- 31.Matozaki T, Nakanishi H, Takai Y. Small G-protein networks: their crosstalk and signal cascades. Cell Signal. 2000;12:515–524. doi: 10.1016/s0898-6568(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 32.Melish M E, Glasgow L A. Staphylococcal scalded skin syndrome: the expanded clinical syndrome. J Pediatr. 1971;78:958–967. doi: 10.1016/s0022-3476(71)80425-0. [DOI] [PubMed] [Google Scholar]
- 33.Mulligan M E, Murray-Leisure K A, Ribner B S, Standiford H C, John H F, Korvick J A, Kauffman C A, Yu V L. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and treatment. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama K, Takashima K, Ishihara H, Shinomiya T, Kageyama M, Kanaya S, Ohnishi M, Murata T, Mori H, Hayashi T. The R-type pyocin of Pseudomonas aeruginosais related to P2 phage, and the F-type is related to lambda phage. Mol Microbiol. 2000;38:213–231. doi: 10.1046/j.1365-2958.2000.02135.x. [DOI] [PubMed] [Google Scholar]
- 35.Navaratna M A D B, Sahl H-G, Tagg J R. Identification of genes encoding two-component lantibiotic production in Staphylococcus aureus C55 and other phage group II S. aureusstrains and demonstration of an association with the exfoliative toxin B gene. Infect Immun. 1999;67:4268–4271. doi: 10.1128/iai.67.8.4268-4271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 37.Prevost G, Rifai S, Chaix L, Piemont Y. Functional evidence that Ser-195 residue of staphylococcal exfoliative toxin is essential for biological activity. Infect Immun. 1991;59:3337–3339. doi: 10.1128/iai.59.9.3337-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redpath M B, Foster T J, Bailey C J. The role of serine protease active site in the mode of action of epidermolytic toxin of S. aureus. FEMS Microbiol Lett. 1991;81:151–156. doi: 10.1016/0378-1097(91)90295-l. [DOI] [PubMed] [Google Scholar]
- 39.Richard J-F, Petit L, Gibert M, Marvaud J C, Bouchaud C, Popoff M R. Bacterial toxins modifying the actin cytoskeleton. Int Microbiol. 1999;2:185–194. [PubMed] [Google Scholar]
- 40.Rince A, Dufour A, Uguen P, le Pennec J-P, Haras D. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctGencode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol. 1997;63:4252–4260. doi: 10.1128/aem.63.11.4252-4260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogolsky M, Beall B W, Wiley B B. Transfer of the plasmid for exfoliative toxin B synthesis in mixed cultures on nitrocellulose membranes. Infect Immun. 1986;54:265–268. doi: 10.1128/iai.54.1.265-268.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 43.Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar V, Baghdayan A S, Huycke M M, Lindahl G, Gilmore M S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun. 1999;67:193–200. doi: 10.1128/iai.67.1.193-200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugai M, Enomoto T, Hashimoto K, Matsumoto K, Matuo Y, Ohgai H, Hong Y-M, Inoue S, Yoshikawa K, Suginaka H. A novel epidermal cell differentiation inhibitor (EDIN): purification and characterization from Staphylococcus aureus. Biochem Biophys Res Commun. 1990;173:92–98. doi: 10.1016/s0006-291x(05)81026-5. [DOI] [PubMed] [Google Scholar]
- 46.Sugai M, Fujiwara T, Akiyama T, Ohara M, Komatsuzawa H, Inoue S, Suginaka H. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitisEPK1. J Bacteriol. 1997;179:1193–1202. doi: 10.1128/jb.179.4.1193-1202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugai M, Fujiwara T, Komatsuzawa H, Suginaka H. Identification and molecular characterization of a gene homologous to epr (endopeptidase resistance gene) in Staphylococcus aureus. Gene. 1998;224:67–75. doi: 10.1016/s0378-1119(98)00508-3. [DOI] [PubMed] [Google Scholar]
- 48.Sugai M, Hashimoto K, Kikuchi A, Inoue S, Okumura H, Matsumoto K, Goto Y, Ohgai H, Moriishi K, Syuto B, et al. Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J Biol Chem. 1992;267:2600–2604. [PubMed] [Google Scholar]
- 49.Sugai M, Hatazaki K, Mogami A, Ohta H, Peres S, Herault F, Horiguchi Y, Masuda M, Ueno Y, Komatsuzawa H, Suginaka H, Oswald E. Cytotoxic necrotizing factor type 2 produced by pathogenic Escherichia colideamidates a Gln residue in the conserved G-3 domain of the Rho family and preferentially inhibits the GTPase activity of RhoA and Rac1. Infect Immun. 1999;67:6550–6557. doi: 10.1128/iai.67.12.6550-6557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugai M, Inoue S, Hino T, Kuwabara M, Hong Y-M, Miyake Y, Suginaka H. Purification of staphylococcal exfoliative toxin by high pressure liquid chromatography. Zentbl Bakteriol. 1990;273:5–11. doi: 10.1016/s0934-8840(11)80234-3. [DOI] [PubMed] [Google Scholar]
- 51.Swinfield T J, Janniere L, Ehrlich S D, Minton N P. Characterization of a region of the Enterococus faecalis plasmid pAMbeta1 which enhances the segregational stability of pAMβ1-derived cloning vectors in Bacillus subtilis. Plasmid. 1991;26:209–221. doi: 10.1016/0147-619x(91)90044-w. [DOI] [PubMed] [Google Scholar]
- 52.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka T, Ogura M. A novel Bacillus natto plasmid pLS32 capable of replication in Bacillus subtilis. FEBS Lett. 1998;422:243–246. doi: 10.1016/s0014-5793(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 54.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 55.Vath G M, Earhart C A, Monie D D, Iandolo J J, Schlievert P M, Ohlendorf D H. The crystal structure of exfoliative toxin B: a superantigen with enzymatic activity. Biochemistry. 1999;38:10239–10246. doi: 10.1021/bi990721e. [DOI] [PubMed] [Google Scholar]
- 56.Vath G M, Earhart C A, Rago J V, Kim M H, Bohach G A, Schlievert P M, Ohlendorf D H. The structure of the superantigen exfoliative toxin A suggests a novel regulation as a serine protease. Biochemistry. 1997;36:1559–1566. doi: 10.1021/bi962614f. [DOI] [PubMed] [Google Scholar]
- 57.Warren R, Rogolsky M, Wiley B B, Glasgow L A. Effect of ethidium bromide on elimination of exfoliative toxin and bacteriocin production in Staphylococcus aureus. J Bacteriol. 1974;118:980–985. doi: 10.1128/jb.118.3.980-985.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warren R, Rogolsky M, Wiley B B, Glasgow L A. Isolation of extrachromosomal deoxyribonucleic acid for exfoliative toxin production from phage group II Staphylococcus aureus. J Bacteriol. 1975;122:99–105. doi: 10.1128/jb.122.1.99-105.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warren R L. Exfoliative toxin plasmids of bacteriophage group 2 Staphylococcus aureus: sequence homology. Infect Immun. 1980;30:601–606. doi: 10.1128/iai.30.2.601-606.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warren R L. Restriction endonuclease map of phage group 2 Staphylococcus aureusexfoliative toxin plasmid. Infect Immun. 1981;33:7–10. doi: 10.1128/iai.33.1.7-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilde C, Chhatwal S, Schmalzing G, Aktories K, Just I. A novel C3-like ADP-ribosyltransferase from Staphylococcus aureusmodifying RhoE and Rnd3. J Biol Chem. 2001;276:9537–9542. doi: 10.1074/jbc.M011035200. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Hayashi T, Takami H, Nakasone K, Ohnishi M, Nakayama K, Yamada S, Komatsuzawa H, Sugai M. Phage conversion of exfoliative toxin A production in Staphylococcus aureus. Mol Microbiol. 2000;38:694–705. doi: 10.1046/j.1365-2958.2000.02169.x. [DOI] [PubMed] [Google Scholar]
- 63.Zumbihl R, Aepfelbacher M, Andor A, Jacobi C A, Ruckdeschel K, Rouot B, Heesemann J. The cytotoxin YopT of Yersinia enterocoliticainduces modification and cellular redistribution of the small GTP-binding protein RhoA. J Biol Chem. 1999;274:29289–29293. doi: 10.1074/jbc.274.41.29289. [DOI] [PubMed] [Google Scholar]