Abstract
Among individuals presenting for monkeypox vaccination, transition from an opt-out protocol for sexually transmitted infection (STI) and HIV risk assessment and testing to an opt-in protocol was associated with a substantial increase in missed opportunities for HIV pre-exposure prophylaxis and STI testing at an ambulatory sexual health clinic.
Keywords: STI, monkeypox vaccination, PrEP
Since May 2022, nearly 30 000 monkeypox (mpox) cases have been diagnosed in the United States, with ∼400 diagnosed in Massachusetts, primarily among men who have sex with men (MSM) [1]. In response, public health agencies coordinated to vaccinate high-risk individuals against mpox. Sexual health clinics have served as a logical, low-barrier setting for individuals seeking mpox prevention, but may be ill-prepared for the challenge of responding to an emerging infectious disease while providing their usual services due to chronic underfunding that has been further exacerbated by the coronavirus disease 2019 (COVID-19) pandemic [2].
Sexually transmitted infections (STIs) have been rising steadily [3], and access to pre-exposure prophylaxis (PrEP) among Black and Hispanic MSM—one of the highest-risk groups for new HIV acquisition—has declined [4]. Although vital to the task of terminating mpox transmission, prioritizing vaccination in high-risk individuals risks diverting staff and financial resources away from competing public health threats and may inadvertently threaten efforts to end the HIV epidemic.
We evaluated the impact of mpox vaccine rollout on missed opportunities for HIV prevention care at an urban sexual health clinic.
METHODS
Boston Medical Center (BMC), affiliated with the Boston University Chobanian and Avedisian School of Medicine, is the largest safety-net hospital in New England [5]. BMC is located in one of the top 48 US counties for highest rates of new HIV diagnoses and is identified as a priority jurisdiction in the Ending the HIV Epidemic initiative [6]. Massachusetts Department of Public Health funding supports a Sexual Health Clinic at BMC, which, on July 11, 2022, was one of 4 Massachusetts sites that began mpox vaccinations.
At the initiation of mpox vaccine administration, individuals received STI/HIV risk assessment, testing, and PrEP as part of comprehensive care. Due to the need to scale up mpox vaccination access in the setting of a public health emergency and against the constraints of limited staffing, the strategy of comprehensive care subsequently shifted on August 1.
The Quality Manager for HIV Prevention Programs abstracted relevant demographic and clinical variables from the electronic medical record for all mpox-vaccinated patients and entered the data into a database to ensure that the quality of the clinic's standard STI and HIV prevention care was met. Demographic variables included race, ethnicity, date of birth, and gender identity. Clinical variables included vaccination date, bacterial STI testing ≤14 days, HIV status, PrEP status, and sexual risk factors.
This was a retrospective analysis to identify missed opportunities for STI and HIV prevention care as we switched to an opt-in protocol within a mass mpox vaccination setting.
Patients vaccinated from July 11, 2022, to August 17, 2022, were stratified into opt-out and opt-in time periods. During the opt-out period (July 11–29, 2022) each patient presenting to the clinic for mpox vaccination met with a public health nurse, underwent STI/HIV risk assessment and testing, and was evaluated with the clinic's standard protocol subject to patient opt-out. During the opt-in period (August 1–17, 2022), patients who presented for mpox vaccination at BMC's Sexual Health Clinic were not required to complete STI/HIV risk assessment and testing unless opting in. Data collection and distribution of subcutaneous vaccines in our clinic ended on August 17, 2022, the same day the Centers for Disease Control and Prevention first recommend an intradermal injection to extend the use of existing doses until the nationwide vaccine supply increases [7].
All individuals ≥18 years old vaccinated for mpox during our study period were evaluated. For race/ethnicity analyses, we excluded unknowns and combined race and ethnicity groups as follows: White/Non-Hispanic, Black/Non-Hispanic, Hispanic, and Asian. We used descriptive statistics to characterize the patients in the opt-out and opt-in periods.
To evaluate the impact of the opt-out vs opt-in periods of STI/HIV risk assessment protocol on receipt of comprehensive sexual health care, we used logistic regression to determine individual associations between covariates and time period. We adjusted our multivariable model for age, gender identity, and race/ethnicity regardless of significance. All other variables were considered for the multivariable model when unadjusted P values were <.2, to avoid excluding individually insignificant variables that would be significant in the multivariable model [8]. In cases where 2 variables were highly correlated, only the variable with greater clinical impact was included. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc, Cary, NC, USA).
Patient Consent
This study was approved by the Boston University Medical Campus Institutional Review Board and was granted exempt status (H-43183). This study did not include factors necessitating patient consent.
RESULTS
Among the entire cohort, 762 patients were vaccinated, including 232 in the opt-out period and 530 in the opt-in period (Table 1). Six hundred ninety-four (91.1%) of the patients identified as male, and 394 (51.7%) identified as White and non-Hispanic. Three hundred seventy-eight (49.6%) patients had unknown PrEP/HIV status, 247 (32.4%) patients were PrEP users, and 22 (2.9%) were persons with HIV (PWH).
Table 1.
Adjusted Models for STI Testing and PrEP Enrollments in Opt-Out vs Opt-In Protocols
| Variable | Opt-Out Protocol (n = 232), No. (%) | Opt-In Protocol (n = 530), No. (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Age, mean (SD), y | 36.4 (10.1) | 36.0 (12.1) | 1.00 (0.99–1.02) | 1.01 (0.99–1.02) |
| Gender identity | ||||
| Male | 225 (97.0) | 469 (88.5) | Ref. | Ref. |
| Female | 6 (2.6) | 51 (9.6) | 0.25 (0.10–0.58) | 0.32 (0.13–0.79) |
| Transgender Female | 1 (0.4) | 6 (1.1) | 0.35 (0.04–2.90) | 0.37 (0.04–3.40) |
| Transgender male | … | 3 (0.6) | … | … |
| Unknown | … | 1 (0.2) | … | … |
| Race/ethnicity | ||||
| White (non-Hispanic) | 122 (52.6) | 272 (51.3) | Ref. | Ref. |
| Black (non-Hispanic) | 27 (11.6) | 65 (12.3) | 0.93 (0.56–1.52) | 0.95 (0.56–1.64) |
| Hispanic | 38 (16.4) | 89 (16.7) | 0.95 (0.62–1.47) | 0.76 (0.47–1.22) |
| Asian | 22 (9.5) | 38 (7.2) | 1.29 (0.73–2.28) | 1.19 (0.65–2.19) |
| Unknown | 23 (9.9) | 66 (12.4) | 0.78 (0.46–1.31) | 0.96 (0.55–1.65) |
| Sexual risk | ||||
| MSM | 208 (89.7) | 145 (27.4) | 3.12 (1.08–9.28) | … |
| Hetero | 5 (2.2) | 11 (2.1) | Ref. | … |
| Unknown | 19 (8.2) | 374 (70.6) | 0.11 (0.04–0.35) | – |
| PrEP status | ||||
| PrEP user | 153 (65.9) | 94 (17.7) | 1.41 (0.82–2.41) | … |
| PrEP enrollment | 21 (9.1) | 25 (4.8) | 0.73 (0.34–1.54) | … |
| PrEP naive | 37 (15.9) | 32 (6.0) | Ref. | … |
| HIV+ a | 12 (5.2) | 10 (1.9) | 1.04 (0.40–2.72) | … |
| Unknown [2] | 9 (3.8) | 369 (69.6) | 0.02 (0.02–0.05) | … |
| STI tested (≤14 d) | ||||
| Yes | 117 (50.4) | 113 (21.1) | 3.75 (2.70–5.23) | 3.78 (2.67–5.36) |
| No | 115 (49.6) | 417 (78.9) | Ref. | Ref. |
Abbreviations: MSM, men who have sex with men; OR, odds ratio; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Includes 1 (0.1%) new HIV diagnosis during the study period.
Individuals in the group with an unknown PrEP/HIV status increased from 9 (3.8%) in the opt-out period to 369 (69.9%) in the opt-in period. This significant increase in the opt-in period was due to patients opting out of the STI/HIV risk assessment protocol. Therefore, patients were not screened for HIV status and PrEP use—making these data unavailable.
The unadjusted odds of being tested for STIs was significantly higher in the opt-out period compared with the opt-in period (OR, 3.75; 95% CI, 2.70–5.23). Sexual risk and PrEP status were excluded from the adjusted model due to being highly correlated with the STI tested variable. This is likely due to the large number of unknowns in both excluded variables for those who were not STI tested. Variables retained in the final multivariable model were age, gender identity, race/ethnicity, and STI tested. STI tested was statistically significant in the adjusted model, with those vaccinated in the opt-out period having 3.78 times the odds of being STI tested compared with the opt-in period (aOR, 3.78; 95% CI, 2.67–5.36).
Of the 230 total patients tested for HIV, syphilis, gonorrhea, and chlamydia, 1 (0.4%) patient was newly diagnosed with HIV and 24 (10.4%) tested positive for a bacterial STI (including 12 chlamydia cases, 10 gonorrhea cases, and 3 syphilis cases).
DISCUSSION
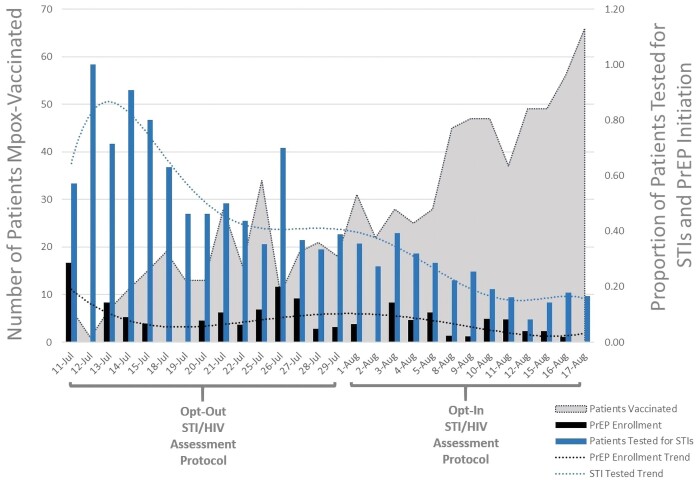
During the rollout of mpox vaccination, we observed a significant decline in the odds of PrEP enrollment and STI testing in a high-risk population following the switch from an opt-out to an opt-in protocol (Figure 1). This change in protocol was precipitated by a shift in vaccination strategy that prioritized high-volume vaccination to mitigate the national mpox outbreak over STI and HIV prevention services. This reduction in vital services was occurring during a time when an increase in STIs was consistently reported, particularly in MSM populations also at risk for mpox [9].
Figure 1.
PrEP enrollment and STI testing among mpox-vaccinated patients. Abbreviations: PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Sexual health clinics are uniquely positioned to respond to both the mpox outbreak and increasing STI rates among structurally vulnerable populations, but are constrained by chronic disinvestment. As these populations remain at high risk for STI and HIV acquisition, restructuring clinic activities to deprioritize STI care among patients seeking mpox vaccination will contribute to critical missed opportunities to prevent STI and HIV transmission.
Rapid and high-volume deployment of mpox vaccines to the highest-risk populations, combined with behavior change among at-risk individuals, has undoubtedly contributed to the current decline in cases nationally [10]. It is unlikely, however, that domestic mpox transmission will be eradicated in the near future [11], emphasizing the importance of integrating vaccination into existing routine services that are trusted and utilized by the most impacted populations. For MSM with sexual risk factors, vaccination represents a point in time during which personal and collective health benefits—through earlier disease detection and treatment and STI and HIV prevention—might be achieved with a comprehensive sexual health approach such as is encompassed by an opt-out protocol.
One limitation in assessing the true uptake of comprehensive sexual care during presentation for mpox vaccination is that unknown-status patients may opt out of screening because of current engagement in sexual health care with outside providers, thereby leading to possible underrepresentation of individuals who received services during the study. Another limitation, the inability to retrieve external medical records, impeded accurate assessment of the proportion of PrEP users or PWH. Finally, reliance on patient self-identification for vaccine eligibility may have resulted in a greater proportion of patients with low sexual risk. This change in sexual risk among our patient population would go undetected during the opt-in period, potentially contributing to an inappropriately inflated perception of missed STI testing and PrEP enrollment opportunities in an ineligible population.
CONCLUSIONS
Our findings illustrate missed opportunities in PrEP enrollment and STI prevention with utilization of an opt-in protocol for patients presenting to a sexual health clinic for mpox vaccination. Although prioritizing rapid and high-volume mpox vaccination has demonstrated efficacy in reducing mpox transmission rates, designing, staffing, and funding alternative strategies for vaccine deployment within sexual health clinics may intensify public health impact by simultaneously addressing critical HIV and STI health care issues of the at-risk population.
Acknowledgments
Financial support. This work was supported by a grant from the Massachusetts Department of Public Health, Bureau of Infectious Disease and Laboratory Sciences, Office of HIV/AIDS (INTF4944MM3181926007; Stewart, Bartkus, Ruiz-Mercado, Sperring, Johnson, Pierre).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the funders.
Author contributions. J.S. was responsible for the conception and design of the work with input and editing from M.B., H.S., G.R.M., S.J., and C.P. J.S. and M.B. were responsible for data collection. H.S. and M.B. were responsible for data analyses and interpretation of results. J.S. and C.P. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Contributor Information
Jessica Stewart, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA.
Mary Bartkus, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA.
Heather Sperring, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA.
Glorimar Ruiz-Mercado, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA.
Samantha Johnson, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA.
Cassandra Pierre, Section of Infectious Diseases, Boston University Chobanian and Avedisian School of Medicine & Boston Medical Center, Boston, Massachusetts, USA; Section of Infectious Diseases, Boston Medical Center, Boston, Massachusetts, USA.
References
- 1. Centers for Disease Control and Prevention . Monkeypox in the U.S. Published October 3, 2022. Available at:https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html. Accessed October 9, 2022.
- 2.National Coalition of STD Directors . Starved public health system in distress, STD first responders spring into action against COVID-19. Available at:https://www.prnewswire.com/news-releases/starved-public-health-system-in-distress-std-first-responders-spring-into-action-against-covid-19-301027621.html. Accessed September 30, 2022.
- 3. Tanne JH. Covid-19: sexually transmitted diseases surged in US during pandemic. BMJ 2022; 377:o1275. [DOI] [PubMed] [Google Scholar]
- 4. Morgan E, Caba AE, Eaton LA, Watson RJ. PrEP access affected by COVID-19 is associated with increased odds of HIV seroconversion. J Acquir Immune Defic Syndr 2022; 91:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boston Medical Center. About BMC. Available at:https://www.bmc.org/about-bmc. Accessed October 9, 2022.
- 6.Centers for Disease Control and Prevention, Ending the HIV Epidemic. Jurisdictions. Published June 13, 2022. Available at:https://www.cdc.gov/endhiv/jurisdictions.html. Accessed October 4, 2022.
- 7.Stolberg SG, Otterman S, Mandavilli A. Monkeypox vaccine plan prods cities and states to adopt new dosing regimen. New York Times. Published August 18, 2022. Updated November 28, 2022.
- 8. Malhotra RK. Errors in the use of multivariable logistic regression analysis: an empirical analysis. Indian J Community Med 2020; 45:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National overview. Published April 11, 2022. Available at:https://www.cdc.gov/std/statistics/2020/overview.htm. Accessed October 9, 2022.
- 10.NMAC Communications. The latest news on MPV. Published September 26, 2022. Available at:https://monkeypoxtruth.org/the-latest-news-on-mpv-3. Accessed October 9, 2022.
- 11. Centers for Disease Control and Prevention . Monkeypox technical reports. Published September 29, 2022. Available at:https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/report-3.html. Accessed October 9, 2022.