Abstract
Background
Limited outcome data exist regarding partial-oral antibiotic therapy, defined as oral antibiotics as part of a patient's treatment, for bone and joint infections (BJIs) in people who inject drugs (PWID).
Methods
We conducted a retrospective study of all PWID reporting drug use within 3 months and BJIs requiring ≥6 weeks of antibiotics in an urban safety-net hospital between February 1, 2019, and February 1, 2021. Treatment outcomes were assessed by chart review. Rates of failure, defined as death, symptoms, or signs concerning for worsening or recurrent infections, were assessed 90 and 180 days after completion of antibiotics. Univariate logistic regression was used to explore the association between covariates and failure.
Results
Of 705 patients with BJI, 88 (13%) were PWID. Eighty-six patients were included in the final cohort. Forty-four (51%) were homeless, 50 (58%) had spine infection, 68 (79%) had surgery, and 32 of 68 (47%) had postoperatively retained hardware. Twelve (14%) of 86 patients received exclusively intravenous (IV) antibiotics, and 74 (86%) received partial-oral antibiotics. Twelve (14%) of 86 patients had patient-directed discharge. In those who received partial-oral antibiotics, the failure rate was 20% at 90 days and 21% at 180 days after completion of intended treatment. Discharge to a medical respite and follow-up with infectious diseases (ID) or surgery were negatively associated with odds of failure.
Conclusions
Partial-oral treatment of BJI in PWID was a common practice and often successful when paired with medical respite and follow-up with ID or surgery.
Keywords: people who inject drugs, bone and joint infection, homeless, partial-oral antibiotics, patient-directed discharge
People who inject drugs (PWID) constitute a significant and increasing proportion of patients with bone and joint infections (BJIs) [1, 2]. Treatment of BJI for PWID can pose challenges. Traditionally, 6–12 weeks of intravenous (IV) antibiotics has been recommended. Some care providers are reluctant to discharge PWID with indwelling intravenous catheters, fearing loss to follow-up, line tampering, and secondary bacteremia. Consequently, a common practice is to keep PWID with BJI in the hospital for the duration of the treatment course. However, this approach is resource-intensive and can diminish patient autonomy. Therefore, PWID frequently have patient-directed discharges (PDDs) and are often readmitted for the same or related problems [3], delaying management and increasing health care costs [1, 4–6].
Alternatives to IV antibiotic treatment are urgently needed for PWID with BJI [7–9] to improve patient-centered care and reduce the cost of inpatient hospitalization, but the safety and efficacy of alternate approaches have not been well studied. A randomized clinical trial of early transition to oral vs prolonged IV antibiotics (OVIVA) for BJI demonstrated noninferiority of early transition to oral antibiotics [10], but the study excluded PWID.
A prior study of PWID with BJI treated with prolonged IV antibiotics at our institution demonstrated a 50% cure rate, even with bundled interventions of infectious diseases (ID) consultation, addiction medicine consultation, case management, medications for opioid use disorder (MOUD), and postdischarge care in a medical respite [11, 12]. The cure rate was lower than the 60%–90% cure rates reported by BJI studies that excluded PWID [10, 13]. Inspired by the OVIVA trial, we define partial-oral antibiotic treatment as using oral antibiotics as part of a patient's treatment. The aim of this study was to evaluate the frequency and outcomes of partial-oral antibiotic treatment in PWID with BJI.
METHODS
Setting and Study Population
We conducted a retrospective cohort study at a university-affiliated urban teaching hospital, Harborview Medical Center (HMC), located in Seattle, Washington. It is a public safety-net hospital for King County and a level-1 trauma and burn center for Washington, Wyoming, Alaska, Montana, and Idaho. Patients aged ≥18 with recent injection drug use (self-reported use within 3 months) and clinically diagnosed BJI by the treating teams (ID, orthopedic surgery, or spinal surgery) at HMC between February 1, 2019, and February 1, 2021, were analyzed. The date of February 1, 2019, was chosen to be the date of print publication of the OVIVA trial. Self-reported injection drug use was ascertained through documentation in clinical notes from the ID, addiction medicine, and/or admitting team. If injection drug use was not noted by these teams, it was assumed not to be present for the patient. The patients were identified through an outpatient parental antibiotic therapy (OPAT) database maintained by the ID service. On occasions where patients were discharged with long-term antibiotics without enrollment in the OPAT database, they were identified through search of the electronic medical records (EMRs) (detailed methods in Supplementary Table 1). Exclusion criteria included (1) any infection that required <6 weeks of antibiotic therapy, as defined in the OVIVA trial; (2) any infection for which the treating ID service concluded that there were no suitable oral antibiotics based on drug susceptibility, drug allergies, contraindications, adverse effects, intolerance, or drug interactions; (3) any infection that was treated with chronic suppression without curative intent; (4) any infection that would not respond to antibacterial treatment (caused by fungal or parasitic agents) or would need prolonged treatment >6–12 weeks (caused by mycobacteria, Actinomyces spp,. Nocardia spp., Brucella spp., or Coxiella burnetii); (5) receipt of partial-oral antibiotics as well as dalbavancin, because the long half-life of dalbavancin could have covered the entire treatment duration. Only the first admissions were included for patients with multiple admissions for BJI during the study period. Notably, we included those with concurrent Staphylococcus aureus bacteremia or bacterial infective endocarditis who were excluded from the OVIVA trial. Furthermore, we excluded septic arthritis, which was treated for 4 weeks in our institution.
Definitions and Ascertainment of Treatment Duration and Outcomes
Treatment duration and outcomes were ascertained through retrospective chart review. Day 1 of effective antibiotic therapy was decided by the ID consulting team. This was the day of definitive source control through surgery or drainage or the first day of sterile blood culture on susceptible antibiotics when there was no intervention, whichever came later. The treatment duration was at the discretion of the ID team. Dalbavancin treatment duration was calculated such that 7 days were counted toward IV antibiotics if the patient received 1 dose and that 42 days were counted if the patient received 2 doses, based on a clinical trial [14] and pharmacokinetic studies [15]. Treatment outcomes were determined between treatment initiation and 90 or 180 days after completion of intended antibiotic treatment. Because intermittent outpatient follow-up was common, outcomes were assessed based on chart review of clinical documentation by any medical provider who evaluated patients after hospital discharge, including those from ID, surgical specialties, addiction medicine, general medicine, family medicine, or emergency medicine. If there was no documentation of symptoms or signs consistent with worsening or recurrent infections, it was assumed that they were not present.
We used a dichotomized primary outcome, which was whether someone had failure or cure. Failure included inpatient death and/or treatment failure. Treatment failure was defined as any clinical symptoms or signs concerning for worsening or recurrent infection within 90 or 180 days of completion of intended treatment, which included but were not limited to local erythema, induration, swelling, pain, pus drainage, and poor healing of the infection site, with or without systemic infectious symptoms. Cure was defined as the absence of treatment failure for those with clinical evaluation within 90 or 180 days of completion of intended treatment. Detailed methods of ascertaining outcomes are described in Supplementary Table 2. Loss to follow-up was defined as when a patient did not have an evaluation by any HMC provider after hospital discharge, nor was there information through shared EMRs. Transfer of care was defined as when a patient chose to follow up with providers outside of HMC after discharge without available information through shared EMRs. PDD was defined as premature discharge of a patient despite contrary recommendation by the treating team. Depending on the follow-up of those who had PDD, outcomes of these patients could be failure, cure, or loss to follow-up. To account for the fact that oral antibiotics were often given as a harm reduction approach in the setting of PDD, we further divided the treatment groups into 3 groups: (1) all-IV treatment; (2) planned partial-oral therapy, in which the ID team recommended antibiotic transition; and (3) reactive partial-oral therapy, in which oral antibiotic treatment was prescribed in response to PDD. Some patients with PDD left before an antibiotic prescription was given. If they did not pick up the prescription or follow up, they would have received incomplete IV treatment and no oral antibiotics. Secondary outcomes were length of stay (LOS), percentages of PDD, and any postdischarge follow-up with ID or surgery before completion of intended antibiotic treatment.
Statistical Analyses
The prevalence of recent injection drug use was calculated among all individuals with BJI. Among PWID with BJI, we calculated estimates for the following outcomes: (1) the percentages of patients in the all-IV and partial-oral antibiotic groups and the duration spent receiving IV and oral antibiotics; (2) baseline demographic, clinical characteristics, and outcomes of the all-IV and partial-oral groups; and (3) factors associated with failure in the full PWID BJI cohort and the subcohort of patients who received planned partial-oral antibiotic therapy.
Results were reported as a percentage for categorical variables; as mean, standard deviation, median, and interquartile range (IQR) for continuous variables; and as an odds ratio (OR) for logistic regression. Because of the small sample size in the all-IV treatment group, statistical comparisons between the all-IV and partial-oral groups were not performed. Univariate logistic regression was used to explore the association between baseline and clinical variables and failure. The variables were selected based on an earlier study [11] and clinical plausibility to be associated with outcomes. Logistic regression was performed using the full cohort as well as the subset that received planned partial oral therapy. Multivariate analyses were not performed due to the small number of failures. Missing data were <3% for all variables, except for baseline hepatitis C (HCV) antibody status (8%). The data points were removed from the denominator in the case of missing data. Given that the primary outcome data were missing for patients who were lost to follow-up or transferred care, we performed sensitivity analyses to calculate failure rates, which assumed that patients who were lost to follow-up or transferred care all failed treatment.
The significance level for P values was set at .05. Stata, version 16 (StataCorp, College Station, TX, USA), was used for statistical analyses. The study was approved by the University of Washington's Institutional Review Board. The manuscript was prepared according to the STROBE guideline [16].
RESULTS
Prevalence of PWID in Patients With BJI and Description of the PWID Cohort
During the study period, 705 patients with BJI were identified. Among these, 88 (13%) had documented self-reported injection drug use within the prior 3 months. Eighty-six patients were included in the final study cohort (Figure 1). As shown in Table 1, the study population was predominantly White and male, with a mean age in the mid-forties. Over half of the patients were homeless at the time of admission. Spine infection accounted for almost 60% of cases. Twenty (40%) patients with spine infection had concurrent epidural abscess, and 15 (75%) underwent surgery. Eighty percent of all patients with BJI underwent surgical debridement, and 46% had postoperatively retained hardware. Twelve (14%) of the 86 patients received all-IV antibiotics; 66 (77%) received planned partial-oral antibiotics therapy; and 8 (9%) received reactive antibiotic therapy (Table 1; Supplementary Table 3). Notably, 18 (90%) patients with spinal infection and concurrent epidural abscess received partial-oral antibiotics. Twenty-one (24%) of the 86 patients received concurrent rifampin, including 20 patients in the partial-oral treatment group. The oral antibiotic regimen for the 74 patients in the partial-oral treatment group included fluoroquinolone (34 levofloxacin, 3 moxifloxacin) for 37 (50%) patients, trimethoprim/sulfamethoxazole for 29 (39%), doxycycline for 12 (16%), and beta-lactam for 6 (8%) (Supplementary Table 4). Six (7%) of 86 patients were lost to follow-up, and only 56 (65%) followed up with ID or surgery after discharge (Table 2). Loss to follow-up and transfer of care only occurred in the partial-oral group. None of the 10 patients in the all-IV group who survived to be discharged were discharged with a central venous catheter to unsupervised settings.
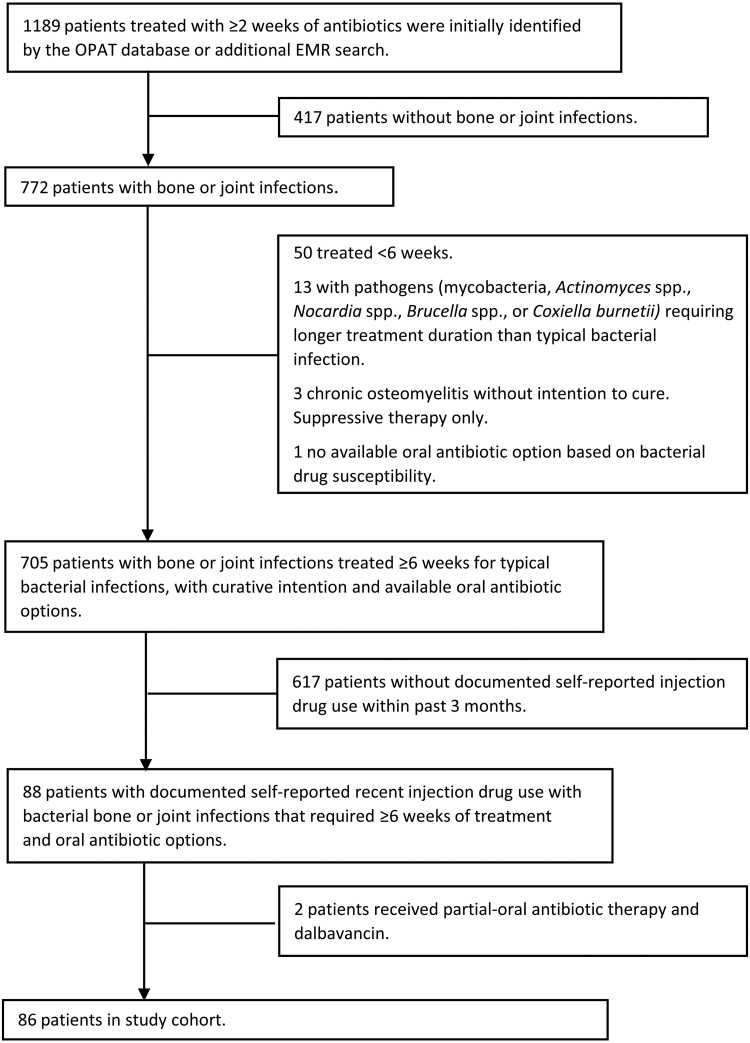
Figure 1.
Study inclusion flowchart to identify PWID with BJI requiring ≥6 weeks of antibiotic treatment in Harborview Medical Center (Seattle, WA, USA) between February 1, 2019, and February 1, 2021. Abbreviations: BJI, bone and joint infection; EMR, electronic medical record; OPAT, outpatient parental antibiotic therapy; PWID, people who inject drugs.
Table 1.
Baseline Demographic and Clinical Characteristics of PWID With BJI Between All-IV and Partial-Oral Treatment Groups
| All IV (n = 12) | Partial Oral (n = 74) | Overall (n = 86) | |
|---|---|---|---|
| Age, median (IQR), y | 50 (14) | 44 (21) | 44 (20) |
| Male sex at birth, No. (%) | 8 (67) | 45 (61) | 53 (62) |
| Race/ethnicitya | |||
| White, No. (%) | 8 (67) | 63 (86) | 71 (85) |
| Black, No. (%) | 3 (25) | 2 (3) | 5 (6) |
| Native American, No. (%) | 0 | 5 (7) | 5 (6) |
| Asian, No. (%) | 0 | 1 (1) | 1 (1) |
| Multiple, No. (%) | 1 (8) | 3 (4) | 4 (5) |
| HIV infection at baseline, No. (%) | 1 (8) | 4 (5) | 5 (6) |
| Positive HCV antibody at baseline, No. (%) | 10 (83) | 54 (73) | 64 (74) |
| Injection-related infection in past year, No. (%) | 9 (75) | 32 (43) | 41 (48) |
| Homeless upon admission, No. (%) | 6 (50) | 38 (51) | 44 (51) |
| Substance(s) | |||
| Opioid only, No. (%) | 3 (25) | 30 (41) | 33 (38) |
| Opioid and stimulant, No. (%) | 8 (67) | 35 (47) | 43 (50) |
| Stimulant only, No. (%) | 1 (8) | 9 (12) | 10 (12) |
| Addiction service consultation, No. (%) | 9 (75) | 62 (84) | 71 (83) |
| MOUD during hospitalization, No. (%) | 10 (91) | 53 (83) | 63 (84) |
| Site(s) of bone or joint infection | |||
| Spine, No. (%) | 8 (67) | 42 (57) | 50 (58) |
| Foot, No. (%) | 0 | 8 (11) | 8 (9) |
| Other lower extremity, No. (%) | 3 (25) | 9 (12) | 12 (14) |
| Hand, No. (%) | 1 (8) | 1 (1) | 2 (2) |
| Other upper extremity, No. (%) | 1 (8) | 6 (8) | 7 (8) |
| Other sites, No. (%) | 1 (8) | 11 (15) | 12 (14) |
| Multiple sites, No. (%) | 2 (17) | 5 (7) | 7 (8) |
| Bacterial culture from infected site(s) | |||
| MRSA, No. (%) | 7 (58) | 25 (34) | 32 (37) |
| MSSA, No. (%) | 2 (17) | 33 (45) | 35 (41) |
| CoNS, No. (%) | 0 | 4 (5) | 4 (5) |
| GAS, No. (%) | 0 | 6 (8) | 6 (7) |
| Other Streptococcus, Enterococcus, Granuicatella, No. (%) | 1 (8) | 8 (11) | 9 (11) |
| Arcanobacterium haemolyticum, No. (%) | 0 | 3 (4) | 3 (3) |
| Gram negatives, No. (%) | 0 | 3 (4) | 3 (3) |
| Mixed infection, No. (%) | 0 | 12 (16) | 12 (14) |
| No culture result, No. (%) | 2 (17) | 5 (7) | 7 (8) |
| Bacteremia, No. (%) | 6 (50) | 22 (30) | 28 (33) |
| Staphylococcus aureus bacteremia without endocarditis, No. (%) | 3 (25) | 14 (19) | 17 (20) |
| Staphylococcus aureus endocarditis, No. (%) | 3 (25) | 6 (8) | 9 (10) |
| Surgery, No. (%) | 9 (75) | 59 (80) | 68 (79) |
| Retained hardware, No. (%) | 3 (33) | 29 (49) | 32 (47) |
| Dalbavancin use,b No. (%) | 5 (42) | 0 | 5 (6) |
Abbreviations: BJI, bone and joint infection; CoNS, coagulase-negative Staphylococcus; GAS, group A Streptococcus; HCV, hepatitis C virus; IQR, interquartile range; IV, intravenous; MOUD, medication for opioid use disorder; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; PWID, people who inject drugs.
Seven (8%) out of 86 patients identified as Hispanic.
Patients in the all-IV group received dalbavancin before discharge as the last part of IV antibiotics while hospitalized. No patient was discharged with indwelling venous catheter to unsupervised settings.
Table 2.
Clinical Outcomes of PWID With BJI Between All-IV and Partial-Oral Treatment Groups
| All IV (n = 12) | Partial Oral (n = 74) | |
|---|---|---|
| Days of definitive IV antibiotics,a mean (SD) | 35.1 (12.5) | 16.0 (15.3) |
| Days of definitive oral antibiotics, mean (SD) | 0 | 45.4 (22.3) |
| Days of total definitive antibiotics, mean (SD) | 35.1 (12.5) | 60.7 (22.9) |
| Days of hospital stay, mean (SD) | 35.4 (49.9) | 17.0 (13.) |
| Disposition after hospital dischargeb | ||
| Housed,c No. (%) | 4 (40) | 34 (46) |
| Medical respite, No. (%) | 2 (20) | 20 (27) |
| Postacute facilities, No. (%) | 1 (10) | 11 (15) |
| Hospitalized entire duration of antibiotic treatment, No. (%) | 2 (20) | 0 |
| Mobile home, No. (%) | 0 | 2 (3) |
| Tent, shelter, or street, No. (%) | 1 (10) | 2 (3) |
| Unknown, No. (%) | 0 | 5 (7) |
| Patient-directed discharge, No. (%) | 1 (9) | 11 (14) |
| Follow-up with infectious diseases or surgery, No. (%) | 7 (78) | 49 (66) |
| 90-d failure rate, No. (%) | 6 (50) | 13 (20) |
| 90-d failure rate by sensitivity analysis,d No. (%) | 6 (50) | 21 (28) |
| 180-d failure rate, No. (%) | 7 (58) | 14 (21) |
| 180-d failure rate by sensitivity analysis,d No. (%) | 7 (58) | 22 (30) |
Primary outcome was dichotomized. Failure included death before definitive treatment plan and treatment failure, and cure included confirmed and presumed cure.
Abbreviations: BJI, bone and joint infection; IV, intravenous; MSSA, methicillin-susceptible Staphylococcus aureus; NA, not applicable; PWID, people who inject drugs.
Duration of dalbavancin: 7 days were counted toward IV antibiotics if the patient only received 1 dose of it; 42 days if the patient received 2 doses.
In the all-IV group, the percentage was calculated for those who survived to be discharged. Two deaths occurred in the all-IV group. One patient with concurrent spinal osteomyelitis and MSSA aortic valve endocarditis died after the valvular surgery, and the other died of respiratory failure from high cervical osteomyelitis–associated myelopathy.
Patient’s own home or home of friends or family, support housing, or motel.
Sensitivity analyses for failure rates assumed all patients who were lost to follow-up or transferred care failed treatment.
Treatment Outcomes
Percentages of failure at 90 days and 180 days were similar in the all-IV and partial-oral groups. Among 74 patients in the partial-oral group, the failure rates were around 20% at 90 and 180 days. These failure rates were around 30% in sensitivity analyses (Table 2). As shown in Supplementary Table 3, for the group that received planned partial-oral antibiotics, the failure rates were 18% at 90 days and 20% at 180 days. In sensitivity analysis, the failure rates were 26% at 90 days and 27% at 180 days. Of note, patients with spinal infection, including those with concurrent epidural abscesses, had similar failure rates at 90 and 180 days.
Patient-Directed Discharges
Twelve (14%) of 86 patients had PDDs, and 8 (75%) of these were readmitted. Among these patients, 7 (88%) were readmitted within 30 days. Nine (75%) of 12 patients with PDD were homeless upon hospitalization. Only 7 (58%) of the 12 patients were included in the reactive partial-oral treatment group, because 4 of them were readmitted within 3 days of PDD without signs of worsening infection and subsequently completed all-IV or planned partial-oral antibiotic treatment. Of 12 patients with PDD, 4 (33%) experienced treatment failure; 1 (8%) died after surgery, and 2 (17%) were lost to follow-up. Reasons for PDD were documented in 10 of the 12 (83%) patients: 3 were related to drug use and were all within 1 week of hospitalization, 4 reported the need to take care of personal affairs or belongings, 1 reported concern for hospitalization-related cost, 1 reported visitor restriction, and 1 was frustrated with prolonged hospitalization.
Variables Associated With Failure
Homelessness upon hospitalization, follow-up with ID or surgery, and discharge to medical respite were associated with lower OR of failure (Supplementary Table 5). The low OR associated with homelessness appeared to be mediated by discharge to medical respite (Supplementary Table 6).
DISCUSSION
Our study described real-world practice experience in caring for PWID with BJI for whom ≥6 weeks of antibiotic therapy was recommended. Cure rates of 70%–80%, depending on the assumptions used for failure, were achieved within 6 months of completion of the intended antibiotics in the planned partial-oral antibiotic group (the largest and intended group). A recent study included BJI as part of complicated staphylococcal bacteremia–related outcomes in PWID [17]. It showed noninferiority of partial-oral antibiotic therapy compared with all-IV therapy. With a more clearly defined BJI study population, our study enhances the partial-oral antibiotic treatment outcome data in PWID with BJI. The cure rate was lower than has been reported in BJI trials that did not include PWID (>90%) [10, 13], but is an encouraging benchmark for program implementation and further improvement of care delivery to PWID with BJI. We showed improvement in cure rates compared with an earlier study from our institution (90-day failure rate 50%) [11]. This improvement underscores the importance of multidisciplinary efforts—the interval increase of our addiction medicine consult team's capacity and the ongoing partnership between the ID, medical respite [12], and care coordination teams. The improvement likely reflects the maturation of the care model infrastructure.
The second highlight from our data is the significant number of PWID with spinal BJI and the demonstration of comparable cure rates compared with other BJI, including those with retained hardware. As our institution is a regional referral center for spinal surgery, 50 (58%) of our patients had spinal BJI, a group under-represented in previous non-PWID BJI treatment trials [10, 19]. Interestingly, the outcomes of patients with concurrent epidural abscess were similar. Over 90% of these patients underwent surgery. Our observation provided reassuring evidence that partial-oral antibiotic therapy can be considered in spinal BJI with concurrent epidural abscess, provided surgical source control was achieved.
The last highlight is the outcome observed with the use of oral trimethoprim/sulfamethoxazole. The OVIVA trial did not include this drug as an oral antibiotic option. Twenty-nine patients received it in our study, including 25 patients who were given monotherapy. The treatment outcomes were similar to the rest of the patients. Our real-world practice experience with a highly bioavailable antibiotic that is commonly used for Staphylococcus aureus can be very valuable in caring for PWID with BJI.
Twelve (14%) of the patients in our study had PDD. We identified contributing factors to PDD, similar to published observations [3, 20, 21]. As PDD is associated with worse outcomes [22, 23], a standardized proactive approach can prevent PDD [20], which should include substance withdrawal treatment and pain control; compassionate, nonjudgmental communication; and proactive management of physical symptoms and concerns related to personal belongings and other obligations. Fortunately, only 8 (11%) of 74 patients in the partial-oral group received oral antibiotics in the setting of PDD, and only 2 of them were truly loss to follow-up. This further reflects the importance of inpatient addiction care and care coordination. Proactive low-barrier linkage to outpatient ID care and post-PDD care coordination [24] can be used to further mitigate potential poor outcomes associated with PDD. Only 66% of the 86 patients (70% of the 66 patients in the planned partial-oral group) followed up with surgery or ID after discharge. Standardized checklists [24, 25] and published care models [24–28] can strengthen postdischarge follow-up and retention in care.
The major limitations of our study were its observational nature and small sample size in the all-IV group. Statistical comparisons between the all-IV and partial-oral groups were not possible, and the point estimates of cure rates in the all-IV group are sensitive to slight change in the number of failures. Misclassifications of PWID status and outcomes were possible. We used self-reported injection drug use in the past 3 months, which may be under-reported by patients or incompletely documented in the EMR. Outcomes were ascertained through EMR review, so patients could have encountered treatment failures elsewhere that were not captured by shared EMRs. We were also not able to ascertain compliance with oral antibiotic treatment.
Additionally, our study was subject to confounding by indication [29], in both the selection of all-IV vs partial-oral treatment and reactive partial-oral treatment. First, differences between the all-IV and partial-oral groups likely reflected that patients with more severe infections received all-IV treatment. The all-IV group included more patients with bacteremia or Staphylococcus aureus endocarditis, and the only 2 deaths were in this group. In addition, despite few Black patients, they were over-represented in the all-IV group. Higher severity from delayed presentation related to racial disparities in access to care needs to be considered [30]. Second, patients with PDD were prescribed reactive partial-oral antibiotics therapy as a harm reduction strategy. They had higher failure rates, which may be related more closely to the underlying reasons for PDD or poor follow-up than the inefficacy of antibiotic treatment.
The last limitation includes multiple comparisons in the univariate logistic analyses for associations with failure. This analysis was exploratory and hypothesis-generating; thus we did not perform any correction for multiple comparisons.
Despite the limitations, our study provides a useful analytical framework and identifies potential variables for future research. Our results can be generalizable if multidisciplinary care, including medical respite, is achievable. We also provide grounds to justify partial-oral antibiotics and implementation details to care for PWID with BJI.
CONCLUSIONS
Our practice experience demonstrated that successful treatment of PWID with BJI was attainable with planned partial-oral antibiotics within the context of support services and postdischarge care coordination. We recommend proactive patient-centered care to prevent PDD. Care coordination in discharge planning, including offering medical respite care for patients experiencing homelessness, and postdischarge follow-up with ID or surgery may be instrumental in contributing to higher cure rates.
Supplementary Material
Acknowledgments
The authors thank Ms. Ashley Katzer for her tireless efforts creating and maintaining the OPAT database. This work also benefited from the support of the University of Washington Division of Allergy & Infectious Diseases (AID) Research Collaboratory.
Financial support. This work was supported by National Institutes of Health T32 grant number 5T32AI007044 (W.T.Y.).
Author contributions. W.T.Y., S.D., A.M.B., J.C.D., and S.N.G. developed the research questions and analytical plans. A.M.B., H.N.K., and K.F.L. generated lists of patients. W.T.Y. performed chart review. W.T.Y., K.F.L., and H.N.K. performed data extraction. W.T.Y. performed data analyses and wrote the first draft of the manuscript. All authors critically reviewed and approved the final manuscript.
Patient consent. The study was approved and granted a waiver of consent by the University of Washington's Institutional Review Board before any research activities were performed.
Contributor Information
Wei-Teng Yang, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Julia C Dombrowski, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; HIV/STD Program, Public Health-Seattle & King County, Seattle, Washington, USA.
Sara N Glick, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; HIV/STD Program, Public Health-Seattle & King County, Seattle, Washington, USA.
H Nina Kim, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Alison M Beieler, Harborview Medical Center, Seattle, Washington, USA.
Kristine F Lan, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Shireesha Dhanireddy, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Oh DHW, Wurcel AG, Tybor DJ, Burke D, Menendez ME, Salzler MJ. Increased mortality and reoperation rates after treatment for septic arthritis of the knee in people who inject drugs: nationwide inpatient sample 2000–2013. Clin Orthop Relat Res 2018; 476:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross JJ, Ard KL, Carlile N. Septic arthritis and the opioid epidemic: 1465 cases of culture-positive native joint septic arthritis from 1990–2018. Open Forum Infect Dis 2020; 7(3):ofaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment': an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med 2014; 105:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libertin CR, Camsari UM, Hellinger WC, Schneekloth TD, Rummans TA. The cost of a recalcitrant intravenous drug user with serial cases of endocarditis: need for guidelines to improve the continuum of care. IDCases 2017; 8:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toppo AJ, Oh DHW, Tybor DJ, et al. Hospital stays and medical expenses increase nationwide among patients with septic arthritis of the shoulder who inject drugs. Orthopedics 2020; 43:e270–7. [DOI] [PubMed] [Google Scholar]
- 6. Toppo AJ, Rogerson A, Oh DHW, Tybor DJ, Wurcel AG, Salzler MJ. Injection drug use in patients with spinal epidural abscess: nationwide data 2000 to 2013. Spine (Phila Pa 1976) 2020; 45:843–50. [DOI] [PubMed] [Google Scholar]
- 7. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. [DOI] [PubMed] [Google Scholar]
- 8. Springer SA, Barocas JA, Wurcel A, et al. Federal and state action needed to end the infectious complications of illicit drug use in the United States: IDSA and HIVMA's Advocacy agenda. J Infect Dis 2020; 222:S230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurley H, Sikka M, Jenkins T, Cari EV, Thornton A. Outpatient antimicrobial treatment for people who inject drugs. Infect Dis Clin North Am 2020; 34:525–38. [DOI] [PubMed] [Google Scholar]
- 10. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beieler AM, Klein JW, Bhatraju E, Iles-Shih M, Enzian L, Dhanireddy S. Evaluation of bundled interventions for patients with opioid use disorder experiencing homelessness receiving extended antibiotics for severe infection. Open Forum Infect Dis 2021; 8(6):ofab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med 2016; 11:531–5. [DOI] [PubMed] [Google Scholar]
- 13. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019; 6(1):ofy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 2015; 59:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–9. [DOI] [PubMed] [Google Scholar]
- 17. Wildenthal JA, Atkinson A, Lewis S, et al. Outcomes of partial oral antibiotic treatment for complicated S. aureus bacteremia in people who inject drugs. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gelman SS, Stenehjem E, Foster RA, Tinker N, Grisel N, Webb BJ. A novel program to provide drug recovery assistance and outpatient parenteral antibiotic therapy in people who inject drugs. Open Forum Infect Dis 2022; 9(2):ofab629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N Engl J Med 2021; 384:1991–2001. [DOI] [PubMed] [Google Scholar]
- 20. Holmes EG, Cooley BS, Fleisch SB, Rosenstein DL. Against medical advice discharge: a narrative review and recommendations for a systematic approach. Am J Med 2021; 134:721–6. [DOI] [PubMed] [Google Scholar]
- 21. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus 2020; 41:519–25. [DOI] [PubMed] [Google Scholar]
- 22. Eaton EF, Westfall AO, McClesky B, et al. In-Hospital illicit drug use and patient-directed discharge: barriers to care for patients with injection-related infections. Open Forum Infect Dis 2020; 7(3):ofaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Appa A, Adamo M, Le S, et al. Patient-directed discharges among persons who use drugs hospitalized with invasive Staphylococcus aureus infections: opportunities for improvement. Am J Med 2022; 135:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis S, Liang SY, Schwarz ES, et al. Patients with serious injection drug use-related infections who experience patient-directed discharges on oral antibiotics have high rates of antibiotic adherence but require multidisciplinary outpatient support for retention in care. Open Forum Infect Dis 2022; 9(2):ofab633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serota DP, Tookes HE, Hervera B, et al. Harm reduction for the treatment of patients with severe injection-related infections: description of the Jackson SIRI team. Ann Med 2021; 53:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dombrowski JC, Ramchandani M, Dhanireddy S, Harrington RD, Moore A, Golden MR. The Max Clinic: medical care designed to engage the hardest-to-reach persons living with HIV in Seattle and King County, Washington. AIDS Patient Care STDS 2018; 32:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sikka MK, Gore S, Vega T, Strnad L, Gregg J, Englander H. “OPTIONS-DC”, a feasible discharge planning conference to expand infection treatment options for people with substance use disorder. BMC Infect Dis 2021; 21:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooksey GE, Epps JL, Moye RA, Patel N, Shorman MA, Veve MP. Impact of a plan of care protocol on patient outcomes in people who inject drugs with infective endocarditis. J Infect Dis 2020; 222:S506–12. [DOI] [PubMed] [Google Scholar]
- 29. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016; 316:1818–9. [DOI] [PubMed] [Google Scholar]
- 30. Matsuzaka S, Knapp M. Anti-racism and substance use treatment: addiction does not discriminate, but do we? J Ethn Subst Abuse 2020; 19:567–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.