Abstract.
Osteocytes are dendritic-shaped cells embedded in the bone matrix and are terminally differentiated from osteoblasts. Inaccessibility due to their location has hindered the understanding of the molecular functions of osteocytes. However, scientific advances in the past few decades have revealed that osteocytes play critical roles in bone and mineral metabolism through their paracrine and endocrine functions. Sclerostin produced by osteocytes regulates bone formation and resorption by inhibiting Wnt/β-catenin signaling in osteoblast-lineage cells. Receptor activator of nuclear factor κ B ligand (RANKL) derived from osteocytes is essential for osteoclastogenesis and osteoclast activation during postnatal life. Osteocytes also secrete fibroblast growth factor 23 (FGF23), an endocrine FGF that regulates phosphate metabolism mainly by increasing phosphate excretion and decreasing 1, 25-dihydroxyvitamin D production in the kidneys. The regulation of FGF23 production in osteocytes is complex and multifactorial, involving many local and systemic regulators. Antibodies against sclerostin, RANKL, and FGF23 have emerged as new strategies for the treatment of metabolic bone diseases. Improved undrstanding of the paracrine and endocrine functions of osteocytes will provide insight into future therapeutic options.
Keywords: osteocytes, sclerostin, bone mass, fibroblast growth factor 23, phosphate metabolism
Highlights
● Osteocytes embedded in bone matrix produce paracrine and endocrine factors.
● Osteocytes control bone mass through the secretion of sclerostin and RANKL.
● Osteocytes play a central role in phosphate metabolism by producing FGF23.
Introduction
Osteocytes are dendritic-shaped cells with long processes embedded deeply in the mineralized bone matrix and terminally differentiated from bone-forming osteoblasts (1, 2). They are the most abundant cells among cells of adult bone, but their location and inaccessibility have hindered molecular analyses of their function. However, in the past few decades, evidence has accumulated indicating that osteocytes profoundly impact bone and mineral metabolism via their paracrine and endocrine functions, as summarized in Fig. 1. Osteocytes seem to control bone mass through the production of sclerostin, a potent inhibitor of bone formation, and receptor activator of nuclear factor κ B ligand (RANKL), an essential molecule for osteoclast formation and activation (3,4,5). Furthermore, osteocyte secrete fibroblast growth factor 23 (FGF23), an endocrine FGF that plays a central role in phosphate homeostasis (6, 7). Neutralizing antibodies against sclerostin, RANKL, and FGF23 have been developed for therapeutic use (8,9,10). Thus, osteocytes are drawing attention as cellular targets for research and drug discovery.
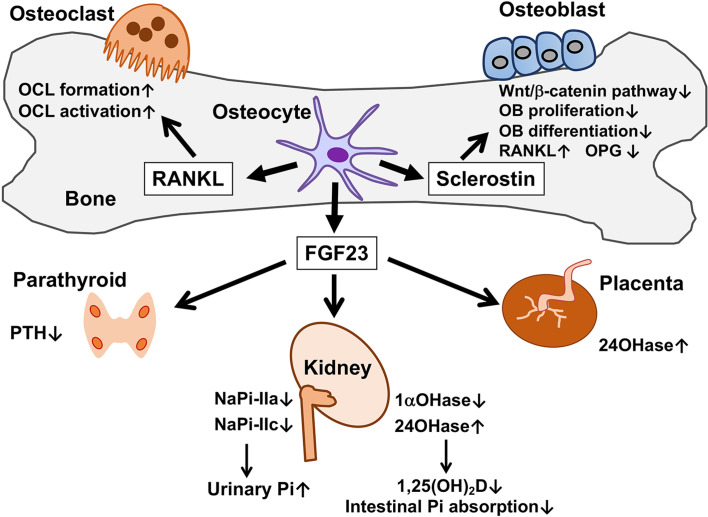
Fig. 1.
Paracrine and endocrine functions of osteocytes. Osteocytes produce paracrine factors, sclerostin and receptor activator of NF-κB ligand (RANKL). Sclerostin produced by osteocytes inhibits Wnt/β-catenin signaling in osteoblasts (OB) in a paracrine manner, leading to the suppression of their proliferation and differentiation, the up-regulation of RANKL and the down-regulation of osteoprotegerin (OPG). Although RANKL is expressed in osteoblasts as well as osteocytes, RANKL derived from osteocytes plays a major role in the formation and activation of osteoclasts (OCL) in postnatal life. FGF23 secreted by osteocytes exerts its effects on distant targets in an endocrine manner. In the kidneys, FGF23 suppresses the expression of NaPi-IIa and NaPi-IIc to increase Pi excretion. Moreover, FGF23 reduces the expression of 25-hydroxyvitamin D-1α-hydroxylase (1αOHase) and induces that of 25-hydroxyvitamin D-24-hydroxylase (24OHase). The resultant decrease in serum 1,25(OH)2D level leads to the reduction in the intestinal Pi absorption. Animal studies have suggested that FGF23 also suppresses the secretion of PTH in the parathyroid glands and induces the expression of 24OHase in the placenta.
This review aims to provide an updated overview of osteocytes’ paracrine and endocrine functions that regulate bone and mineral metabolism.
Osteoblast-to-Osteocyte Differentiation
Osteocytes have long lifespans and comprise 90–95% of all bone cells in the adult skeleton (1, 2). A subpopulation of osteoblasts on the bone surface becomes embedded in the matrix proteins they produce and terminally differentiate into dendritic-shaped osteocytes, while the remainder of osteoblasts become flattened bone lining cells or undergo apoptosis (2). Osteocytes reside in small cavities known as “lacunae” in the bone matrix, and are interconnected with each other and osteoblasts on the bone surface by their long processes extending through the tunnels called “canaliculi”. The lacuna-canaliculi system is filled with extracellular fluids. According to Buenzli et al., the average human skeleton contains ~ 42 billion osteocytes with 23 trillion connections, and the total surface area of the lacuna-canaliculi network is 215 m2. The complexity of the communication network of osteocytes is similar to that of neurons (11). The maturation of osteoblasts into osteocytes is associated with a decreased production of matrix proteins, marked changes in morphology, and the expression of genes characteristic of osteocytes, including those involved in bone homeostasis and mineral metabolism (1, 2).
Paracrine Regulation of Bone Formation by Osteocytes
Osteocytes embedded in the mineralized bone matrix sense mechanical forces and exert bone anabolic signals on other bone cells via their lacuna-canaliculi system in a paracrine manner. Genetic ablation of osteocytes in mice causes marked bone loss with the suppression of mechanically induced bone formation (12).
Osteocytes regulate bone formation mainly through the production of sclerostin, a secreted inhibitor of Wnt/β-catenin signaling. In bones, the activation of Wnt/β-catenin signaling promotes the commitment of mesenchymal progenitor cells into osteoblasts and accelerates the proliferation and differentiation of committed osteoblasts (13, 14). Wnt/β-catenin signaling also suppresses the relative expression of RANKL in osteoblast-lineage cells to reduce the formation and activation of osteoclasts, which are responsible for bone resorption (13, 14). Sclerostin inhibits Wnt/β-catenin signaling by binding to Wnt co-receptors low-density lipoprotein receptor-related protein 5 (LRP5), and LRP6 (15). The critical role of sclerostin in controlling the bone mass has been suggested by the discovery that inactivation or reduced expression of SOST, the gene for sclerostin, is responsible for rare bone-sclerosing genetic disorders such as sclerosteosis 1 (MIM #269500) and van Buchem disease (MIM #239100). Sclerosteosis 1 is an autosomal recessive disease characterized by sclerosing bones, progressive skeletal overgrowth, and syndactyly. It is frequent in the Afrikaner population in South Africa and is caused by the inactivation of variants in the SOST gene (16). Van Buchem disease is an autosomal recessive disease characterized by marked osteosclerosis in the skull, lower jaw, clavicles, ribs, and diaphysis of the long bones and short tubular bones, resulting in increased cortical bone density. In a large consanguineous Dutch family with van Buchem disease, a homozygous 52-kb deletion located downstream of the SOST gene has been identified as a responsible factor for the disease (17). This deletion results in suppressed gene expression. Sost-knockout mice also exhibit a high bone mass phenotype with increased bone formation and strength (18).
Sclerostin expression is regulated by mechanical forces. Animal experiments by Robling et al. demonstrated that Sost transcripts and sclerostin protein levels in osteocytes were dramatically reduced by mechanical loading, and this effect was both local and regional (3). Hence, osteocytes may coordinate local and regional bone formation by modulating sclerostin levels in response to mechanical forces. Recently, a mechano-sensor channel, Piezo1, was suggested to mediate mechanical force-induced suppression of Sost (19).
It is well established that intermittent administration of parathyroid hormone (PTH) exerts anabolic effects on the bone (20). Teriparatide, a recombinant human PTH[1–34], is the first approved anabolic agent that increases bone formation and is widely used to treat osteoporosis (21). PTH-induced bone formation is also mediated by downregulation of Sost in osteocytes (22, 23). A recent study suggested that PTH signaling leads to the phosphorylation of salt-inducible kinase 2 (SIK2), which causes nuclear translocation of histone deacetylase 4 (HDAC4) and HDAC5. In the nucleus, HDAC4/5 inhibits myocyte enhancer factor 2C (MEF2C)-mediated transactivation of SOST (24). The expression of SOST is also regulated by other factors, such as prostaglandin E2 (25), transforming growth factor (TGF) β (26), bone morphogenetic proteins (BMPs) (27), hypoxia (28), and gp130 signaling (29).
Because the sclerostin expression is virtually restricted to osteocytes, it has emerged as an attractive therapeutic target for treating skeletal diseases characterized by low bone mass. Anti-sclerostin-neutralizing antibodies stimulate bone formation and suppress bone resorption by inhibiting Wnt/β-catenin signaling in the bone. Clinical trials of the anti-sclerostin antibody romosozumab suggested that the risk of cardiovascular events might increase when compared with bisphosphonate alendronate (30); however, romosozumab was approved in January 2019 in Japan for the treatment of osteoporosis in patients at high fracture risk and was launched in March 2020, followed by approval in the United States, Canada, and Europe for treating the postmenopausal women at high fracture risk (31). A recent systematic review and meta-analysis have demonstrated the efficacy and safety of romosozumab in the treatment of postmenopausal osteoporosis (8).
Osteogenesis imperfecta (OI) is a heterogeneous genetic disorder characterized by low bone mass and increased bone fragility (32). The beneficial effects of anti-sclerostin antibodies on bone mass and strength have been demonstrated in some mouse models of OI, such as Brtl/+ mice harboring a heterozygous glycine to cysteine substitution in Col1a1 (33), Crtap–/– mice, a model of OI type VII (34), and oim/oim mice, a model for OI type III (35). In humans, in a randomized phase 2a trial, the anti-sclerostin antibody BPS804 stimulated bone formation, reduced bone resorption, and increased lumbar spine areal bone mineral density in adult patients with moderate OI (36). Antibody-mediated inhibition of sclerostin is a promising approach to tackle various conditions with low bone mass and bone fragility.
Paracrine Regulation of Bone Resorption by Osteocytes
Osteoclasts are multinucleated cells responsible for bone resorption and are formed from monocyte/macrophage-lineage cells. RANKL is a membrane-associated cytokine expressed in osteoblast-lineage cells, including osteocytes, that plays an essential role in osteoclastogenesis (37). RANKL binds to its cognate receptor, RANK, on the surface of osteoclast precursors to induce osteoclast differentiation. Mature osteoclasts also express RANK, which activates their bone-resorbing activity (37). Osteoprotegerin is a soluble decoy receptor for RANKL that inhibits osteoclast differentiation and activation by preventing RANKL-RANK binding (37). Denosumab is a humanized monoclonal antibody against RANKL (9) that is used to treat osteoporosis, rheumatoid arthritis, and bone disease associated with solid cancers and multiple myeloma. However, pediatric data on the use of denosumab is limited (38). The suppressive effects of denosumab on bone turnover rapidly disappear after its discontinuation, and rebound increases in bone turnover may cause severe hypercalcemia (38).
In 2011, two groups reported that osteocyte-specific deletion of RANKL in mice using dentin matrix protein 1 (Dmp1)-Cre transgene caused postnatal progressive osteopetrosis despite normal skeletal development and bone mass at birth (4, 5). Sost-Cre-mediated deletion of RANKL from osteocytes results in a similar high bone mass phenotype (39). Using a series of Cre-deleter mouse strains, Xiong et al. demonstrated that hypertrophic chondrocyte-derived RANKL regulates the resorption of mineralized cartilage and that osteocyte-derived RANKL is essential for bone remodeling and controls unloading-induced bone loss. In contrast, RANKL derived from osteoblasts and bone-lining cells contribute less to bone remodeling (5, 39). It has also been suggested that osteocyte-derived RANKL is upregulated by senescence and is involved in age-related cortical bone loss (40). These findings indicated that osteocyte-derived RANKL is required for postnatal bone homeostasis. However, how RANKL on the surface of osteocytes located in the bone matrix reaches osteoclast precursors to induce their differentiation and activation remains to be elucidated.
Endocrine Regulation of Phosphate Metabolism by Osteocytes
Osteocytes also function as endocrine cells. Fibroblast growth factor 23 (FGF23), a central regulator of phosphate metabolism, is produced mainly by osteocytes in the bone and exerts its effects on distant organs such as the kidneys in an endocrine manner (1, 2, 6, 7, 41).
In mammals, phosphate homeostasis is maintained by the influx and efflux of inorganic phosphate (Pi) in the intestines, kidneys, bones, and soft tissues, and endocrine factors such as 1,25-dihydroxyvitamin D (1,25(OH)2D), PTH, and FGF23 mediate interorgan communication to regulate the fluxes of Pi (42). 1,25(OH)2D, which is an active vitamin D metabolite, is predominantly produced in the kidneys and increases the intestinal absorption of Pi through the upregulation of the type IIb sodium/Pi (Na+/Pi) co-transporter NaPi-IIb (43). PTH secreted from parathyroid cells decreases renal Pi reabsorption by reducing the amounts of type IIa and IIc Na+/Pi co-transporters (NaPi-IIa and NaPi-IIc, respectively) on the brush border membrane of proximal tubules (44, 45).
The kidneys are the main target of FGF23, which increases Pi excretion by suppressing the expression of NaPi-IIa and NaPi-IIc. Furthermore, FGF23 decreases the level of 1,25(OH)2D by suppressing the expression of 25-hydroxyvitamin D 1α-hydroxylase and inducing the expression of 25-hydroxyvitamin D 24-hydroxylase (46). The decreased level of 1,25(OH)2D leads to the reduced Pi absorption in the intestines. Bioactive intact FGF23 is inactivated by cleavage between Arg179 and Ser180 by subtilisin-like proprotein convertase (47).
In contrast to canonical FGFs, which function as autocrine and/or paracrine factors, FGF23 acts as an endocrine factor in distant organs. It has been suggested that its low binding affinity to heparin/heparan sulfate enables FGF23 to enter the circulation while escaping capture by extracellular matrices (48). At physiological concentrations, FGF23 requires the single-pass transmembrane protein αKlotho as a cofactor to evoke its signal through FGF receptors (FGFRs) (49, 50). Hence, organs and tissues expressing both FGFR and αKlotho, such as the kidneys, parathyroid glands, and placenta, may be physiological targets for FGF23 (51,52,53).
The FGF23-mediated interaction between the bone and kidney plays a central role in Pi homeostasis, and the activity of this interaction appears to determine serum Pi levels. Our recent mouse studies have demonstrated that the production of FGF23 in osteocytes increases from youth to adulthood, which leads to growth-related enhancement of the FGF23-mediated bone-kidney axis and a decline in serum Pi levels (7).
The parathyroid glands express both FGFR and αKlotho, and animal studies have demonstrated that administration of recombinant FGF23 suppresses PTH gene expression and secretion via the mitogen-activated protein kinase (MAPK) signaling pathway (51). However, since FGF23-induced suppression of PTH secretion was still observed in mice with parathyroid-specific conditional deletion of αKlotho (54), FGF23 is likely to regulate PTH secretion in both α a Klotho-dependent and -independent manner.
The placenta can also be a target of FGF23. The placenta contains both maternal and fetal tissues, and FGFR1 and αKlotho are colocalized at the feto-maternal interface of the placenta in both mice and humans (52). Using pregnant mothers of hypophosphatemic Hyp mice, a murine model for X-linked hypophosphatemic rickets (XLH, MIM #307800), we have found that maternal FGF23 produced by osteocytes of pregnant mothers, not fetal FGF23, exerts its effects on the placenta and influences fetal vitamin D metabolism by increasing the placental expression of Cyp24a1 encoding 25-hydrpxyvitamin D 24-hydroxylase. In contrast, maternal FGF23 did not affect the placental expression of Na+/Pi co-transporters or fetal serum Pi levels (52). Thus, the role of FGF23 in mineral metabolism may differ between fetal and postnatal life. Our results in Hyp pregnancies have demonstrated that pathologically elevated maternal FGF23 regulate placental vitamin D metabolism; however, the physiological significance of this observation remains to be elucidated.
FGF23-Related Hyperphosphatemic and Hypophosphatemic Disorders
The bone-kidney axis mediated by FGF23/FGFR/αKlotho signaling is crucial for Pi homeostasis, its disruption or excess causes diseases with abnormal serum Pi levels. Inactivating variants of FGF23 and KLOTHO have been identified as causative elements for hyperphosphatemic familial tumoral calcinosis-2 (HFTC2, MIM #617993) and HFTC3 (MIM #617994), respectively, both of which are associated with hyperphosphatemia, normal to elevated serum 1,25(OH)2D levels, and ectopic calcification (55, 56). HFTC1 (MIM #211900) is caused by inactivation of variants in the GALNT3 gene encoding the enzyme UDP-N-acetyl-α-D-galacosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3), which mediates the O-glycosylation of FGF23 on Thr178 (57, 58). O-glycosylation has been suggested to prevent cleavage-mediated inactivation of FGF23 (58).
Excessive expression of FGF23 leads to hypophosphatemic diseases characterized by urinary Pi wasting, hypophosphatemia, inappropriately normal to low levels of serum 1,25(OH)2D, and impaired skeletal mineralization, such as rickets and osteomalacia. These disorders are collectively called FGF23-related hypophosphatemic rickets/osteomalacia, which includes tumor-induced osteomalacia (TIO) caused by the overproduction of FGF23 by tumors and genetic disorders such as XLH, autosomal dominant hypophosphatemic rickets (ADHR, MIM #193100), autosomal recessive hypophosphatemic rickets 1 (ARHR1, MIM #241520), ARHR2 (MIM #613312), and Raine syndrome (RNS, MIM #259775) (59, 60).
Among hereditary FGF23-related hypophosphatemic rickets/osteomalacia, ADHR is caused by variants in FGF23 at Arg176 or Arg179, which make the protein resistant to cleavage-mediated inactivation (61). However, the penetrance of ADHR is incomplete, and it has been suggested that iron deficiency triggers the accumulation of uncleaved bioactive FGF23 and leads to the onset of symptoms (62, 63).
XLH is the most common form of hereditary hypophosphatemic rickets and is caused by the inactivation of variants in the phosphate-regulating gene homologous to endopeptidase on the X chromosome (PHEX) (64, 65). PHEX is highly expressed in osteocytes, as is FGF23 (6, 7). Patients with XLH show increased levels of serum intact FGF23, which leads to renal Pi wasting, hypophosphatemia, and reduced levels of 1,25(OH)2D (64). Although the mechanisms underlying FGF23 overproduction in XLH remain elusive, studies using Phex-deficient Hyp mice have suggested the involvement of enhanced FGFR signaling (6, 66, 67). We demonstrated that the osteocytic expression of canonical FGF ligands (Fgf1 and Fgf2), FGF receptors (Fgfr1–3), and early growth response 1, a downstream target for FGFR activation, was markedly higher in Hyp mice than in wild-type mice (6). Furthermore, Xiao et al. reported that osteocyte-specific ablation of Fgfr1 in Hyp mice partially restored the elevation of serum FGF23 levels, and ameliorated hypophosphatemia and rickets (66). Enhanced FGFR signaling in Hyp osteocytes is interesting since previous studies, including ours, have implicated that FGFR might play a role in the transduction of signals evoked by increased extracellular Pi and/or Pi sensing (42, 68,69,70,71).
ARHR1 is caused by inactivating variants of the DMP1 gene, which is highly expressed in osteocytes in bone and odontoblasts in dentin (72, 73). DMP1 encodes a matrix protein belonging to the small integrin-binding ligand N-linked glycoprotein (SIBLINGs) family. Deletion of Dmp1 in mice resulted in increased FGF23 expression in osteocytes and impaired skeletal mineralization, similar to the clinical manifestations in patients with ARHR1. Overproduction of FGF23 in Dmp1-null mice has also been attributed to enhanced FGFR signaling in osteocytes (59).
ARHR2 is caused by inactivating variants of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), which encodes an ectoenzyme that produces pyrophosphate (PPi) (74, 75). As PPi is a potent inhibitor of mineralization, inactivating variants in the ENPP1 gene also cause disorders characterized by ectopic calcification, such as generalized arterial calcification of infancy 1 (CAGI1, MIM #208000) (76). The expression of ENPP1 is broadly detected, with higher expression in vascular smooth muscle cells, chondrocytes, osteoblasts, and osteocytes. It was reported that Enpp1-deficient mice showed increased expression of Fgf23 in the bone, and dosing these mice with ENPP1-Fc recombinant protein demonstrated a negative correlation between Enpp1 and Fgf23 transcription (77).
RNS is an autosomal recessive disorder caused by inactivating variants in the family with sequence similarity 20, member C (FAM20C) gene, and is characterized by osteosclerotic bone dysplasia and cranial malformation (78). The prognosis of RNS is usually poor, and patients who survive infancy may manifest elevated levels of serum FGF23, hypophosphatemia, and dental anomalies (79, 80). FAM20C encodes a secreted protein kinase (47, 81) that is highly expressed in osteocytes (82). FAM20C directly phosphorylates FGF23 at Ser180, which may prevent its O-glycosylation at Thr178 and facilitate cleavage-mediated inactivation (47). Hence, inactivation of FAM20C in RNS leads to reduced phosphorylation at Ser180, increased O-glycosylation at Thr178, and impaired FGF23 inactivation.
Regulators of FGF23
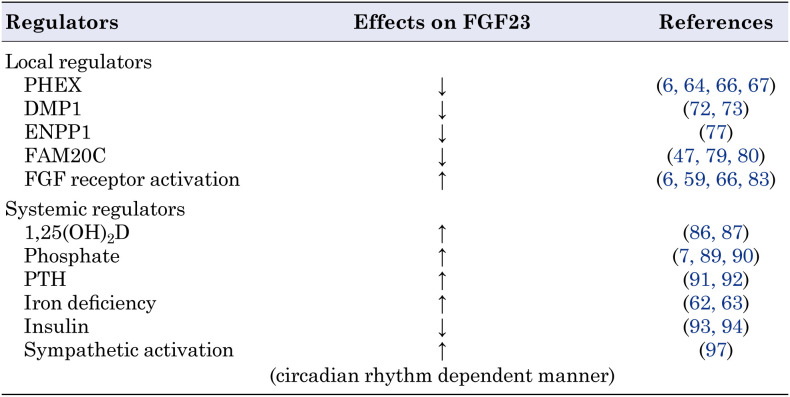
Since PHEX, DMP1, ENPP1, and FAM20C are expressed in osteocytes, and their inactivating variants cause overproduction of FGF23, these molecules are considered to function as local negative regulators of FGF23. However, as described above, analysis of Hyp mice and Dmp1-null mice has suggested that activation of FGFR signaling in osteocytes positively regulates FGF23 production (6, 59, 66). The regulation of FGF23 production by FGFR signaling is also supported by the fact that osteoglophonic dysplasia, a human disease caused by activating mutations in FGFR1, may be associated with FGF23-related hypophosphatemia (83). In addition to these local regulators, other systemic factors have been suggested to regulate FGF23 production in osteocytes. Systemic regulators of FGF23 include 1,25(OH)2D, Pi, PTH, iron, insulin, and circadian rhythms. Thus, the regulation of FGF23 is complex and contextual. Since excellent reviews on the regulators of FGF23 can be found elsewhere (84, 85), only some of them are described here. Table 1 summarizes local and systemic regulators of FGF23 expression.
Table 1. Local and systemic regulators of FGF23 discussed in this article.
FGF23 transcription is stimulated by 1,25(OH)2D in osteoblast lineage cells via the vitamin D receptor (86, 87). The importance of 1,25(OH)2D in regulatingFGF23 is supported by the observation that patients with vitamin D deficiency have low serum FGF23 levels (88).
Pi also stimulates FGF23 production, hence, dietary phosphorus loading increases serum FGF23 levels (89, 90). Treatment with high Pi levels increased the production of FGF23 by primary murine osteocytic cells, which appeared to occur at the protein level rather than at the mRNA level (7). Elevated levels of Pi upregulated the expression of Galnt3 in osteoblast lineage cells, leading to increased FGF23 production without increasing its mRNA expression (71).
PTH has also been shown to increase FGF23 production. Patients with Jansen-type metaphyseal chondrodysplasia (MIM #156400), a disease caused by activating variants of PTH1R encoding PTH receptor 1, may show elevated levels of serum FGF23 and hypophosphatemia (91). Furthermore, mice with constitutive activation of PTH1R in osteocytes exhibit increased expression of Fgf23 (92).
Iron deficiency stimulates transactivation of the Fgf23 promoter through the accumulation of hypoxia-inducible factor 1α (HIF1α) (63). In healthy human subjects, iron deficiency accelerates both the production and cleavage-mediated inactivation of FGF23 in a coupled manner, leading to normal levels of intact FGF23 in the serum and normophosphatemia. In contrast, in patients with ADHR, impaired cleavage leads to the elevation of bioactive FGF23 during iron deficiency, leading to hypophosphatemia (62).
Insulin signaling may also influence FGF23 production in osteocytes. Bar et al. reported a negative correlation between plasma FGF23 levels and increased plasma insulin levels following oral glucose load in women (93). The authors also demonstrated that insulin and insulin-like growth factor 1 (IGF-1) reduced the production of FGF23 in cultured osteoblastic cells (93). To further confirm that osteocytes respond to insulin to regulate FGF23 production, we generated mice with an osteocyte-specific deletion of phosphatase and tensin homolog deleted from chromosome 10 (PTEN), a molecule that antagonizes insulin/IGF-1-induced AKT activation. These mice exhibit decreased skeletal and serum levels of intact FGF23, reduced urinary Pi excretion, and elevated serum Pi levels (94). Our in vitro studies suggest the involvement of the AKT/mechanistic target of rapamycin complex 1 (mTORC1) in insulin/IGF-1-induced suppression of FGF23 (94).
The circadian clock regulates physiology and metabolism for the optimal adaptation of living organisms to environmental changes, including nutrient availability (95, 96). Our mouse studies demonstrated that skeletal expression of Fgf23 was higher in the dark phase. The duration of food intake determines the circadian profile of skeletal Fgf23 expression via sympathetic activation, which is regulated by a peripheral clock in the skeleton (97).
Conclusion
Osteocytes deeply embedded in the bone matrix play an important role in controlling of bone mass and Pi homeostasis by secreting paracrine and endocrine factors. Sclerostin produced by osteocytes inhibits Wnt/β-catenin signaling in osteoblast-lineage cells, stimulating bone formation and suppressing bone resorption. RANKL, derived from osteocytes, plays a significant role in osteoclastogenesis and osteoclast activation during postnatal life. FGF23, the central regulator of Pi metabolism, is secreted by osteocytes and exerts its effects on distant organs such as the kidneys in an endocrine fashion. Osteocytes also express PHEX, DMP1, ENPP1, and FAM20C, the genes responsible for FGF23-related hypophosphatemic rickets/osteomalacia, indicating that these cells function as command centers for Pi homeostasis. Since many local and systemic factors are involved, the regulation of FGF23 in osteocytes is complex. Further clarification of the paracrine and endocrine functions of osteocytes will contribute to the development of new strategies for the diagnosis and treatment of bone and mineral metabolism disorders.
Conflict of interests
Toshimi Michigami received lecture fees from Kyowa Kirin Co., Ltd.
References
- 1.Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26: 229–38. doi: 10.1002/jbmr.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado-Calle J, Bellido T. The osteocyte as a signaling cell. Physiol Rev 2022;102: 379–410. doi: 10.1152/physrev.00043.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008;283: 5866–75. doi: 10.1074/jbc.M705092200 [DOI] [PubMed] [Google Scholar]
- 4.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011;17: 1231–4. doi: 10.1038/nm.2452 [DOI] [PubMed] [Google Scholar]
- 5.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 2011;17: 1235–41. doi: 10.1038/nm.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyagawa K, Yamazaki M, Kawai M, Nishino J, Koshimizu T, Ohata Y, et al. Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice. PLoS One 2014;9: e93840. doi: 10.1371/journal.pone.0093840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michigami T, Tachikawa K, Yamazaki M, Nakanishi T, Kawai M, Ozono K. Growth-related skeletal changes and alterations in phosphate metabolism. Bone 2022;161: 116430. doi: 10.1016/j.bone.2022.116430 [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Dutta S, Khasbage S, Kumar T, Sachin J, Sharma J, et al. A systematic review and meta-analysis of efficacy and safety of Romosozumab in postmenopausal osteoporosis. Osteoporos Int 2022;33: 1–12. doi: 10.1007/s00198-021-06095-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol 2011;7: 631–8. doi: 10.1038/nrrheum.2011.130 [DOI] [PubMed] [Google Scholar]
- 10.Carpenter TO, Whyte MP, Imel EA, Boot AM, Högler W, Linglart A, et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N Engl J Med 2018;378: 1987–98. doi: 10.1056/NEJMoa1714641 [DOI] [PubMed] [Google Scholar]
- 11.Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone 2015;75: 144–50. doi: 10.1016/j.bone.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 2007;5: 464–75. doi: 10.1016/j.cmet.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Kubota T, Michigami T, Ozono K. Wnt signaling in bone metabolism. J Bone Miner Metab 2009;27: 265–71. doi: 10.1007/s00774-009-0064-8 [DOI] [PubMed] [Google Scholar]
- 14.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013;19: 179–92. doi: 10.1038/nm.3074 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 2005;280: 19883–7. doi: 10.1074/jbc.M413274200 [DOI] [PubMed] [Google Scholar]
- 16.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 2001;68: 577–89. doi: 10.1086/318811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 2002;39: 91–7. doi: 10.1136/jmg.39.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 2008;23: 860–9. doi: 10.1359/jbmr.080216 [DOI] [PubMed] [Google Scholar]
- 19.Sasaki F, Hayashi M, Mouri Y, Nakamura S, Adachi T, Nakashima T. Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem Biophys Res Commun 2020;521: 806–13. doi: 10.1016/j.bbrc.2019.10.174 [DOI] [PubMed] [Google Scholar]
- 20.Poole KE, Reeve J. Parathyroid hormone - a bone anabolic and catabolic agent. Curr Opin Pharmacol 2005;5: 612–7. doi: 10.1016/j.coph.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 21.Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int 2016;27: 2395–410. doi: 10.1007/s00198-016-3534-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 2007;22: 1957–67. doi: 10.1359/jbmr.070804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 2010;25: 178–89. doi: 10.1359/jbmr.090730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wein MN, Liang Y, Goransson O, Sundberg TB, Wang J, Williams EA, et al. SIKs control osteocyte responses to parathyroid hormone. Nat Commun 2016;7: 13176. doi: 10.1038/ncomms13176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One 2011;6: e17772. doi: 10.1371/journal.pone.0017772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone 2012;50: 663–9. doi: 10.1016/j.bone.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland MK, Geoghegan JC, Yu C, Winkler DG, Latham JA. Unique regulation of SOST, the sclerosteosis gene, by BMPs and steroid hormones in human osteoblasts. Bone 2004;35: 448–54. doi: 10.1016/j.bone.2004.04.019 [DOI] [PubMed] [Google Scholar]
- 28.Genetos DC, Toupadakis CA, Raheja LF, Wong A, Papanicolaou SE, Fyhrie DP, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem 2010;110: 457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RW, Brennan HJ, Vrahnas C, Poulton IJ, McGregor NE, Standal T, et al. The primary function of gp130 signaling in osteoblasts is to maintain bone formation and strength, rather than promote osteoclast formation. J Bone Miner Res 2014;29: 1492–505. doi: 10.1002/jbmr.2159 [DOI] [PubMed] [Google Scholar]
- 30.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017;377: 1417–27. doi: 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Matsumoto T. Sclerostin: from bench to bedside. J Bone Miner Metab 2021;39: 332–40. doi: 10.1007/s00774-020-01176-0 [DOI] [PubMed] [Google Scholar]
- 32.Mäkitie RE, Costantini A, Kämpe A, Alm JJ, Mäkitie O. New insights into monogenic causes of osteoporosis. Front Endocrinol (Lausanne) 2019;10: 70. doi: 10.3389/fendo.2019.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res 2013;28: 73–80. doi: 10.1002/jbmr.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, et al. Sclerostin antibody treatment improves the bone phenotype of Crtap(-/-) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res 2016;31: 1030–40. doi: 10.1002/jbmr.2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardinal M, Tys J, Roels T, Lafont S, Ominsky MS, Devogelaer JP, et al. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 2019;124: 137–47. doi: 10.1016/j.bone.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, et al. BPS804 Anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J Bone Miner Res 2017;32: 1496–504. doi: 10.1002/jbmr.3143 [DOI] [PubMed] [Google Scholar]
- 37.Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 2021;39: 19–26. doi: 10.1007/s00774-020-01162-6 [DOI] [PubMed] [Google Scholar]
- 38.Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep 2017;15: 283–92. doi: 10.1007/s11914-017-0380-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, et al. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 2015;10: e0138189. doi: 10.1371/journal.pone.0138189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HN, Xiong J, MacLeod RS, Iyer S, Fujiwara Y, Cawley KM, et al. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight 2020;5: e138815. doi: 10.1172/jci.insight.138815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 2009;5: 611–9. doi: 10.1038/nrendo.2009.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michigami T, Kawai M, Yamazaki M, Ozono K. Phosphate as a signaling molecule and its sensing mechanism. Physiol Rev 2018;98: 2317–48. doi: 10.1152/physrev.00022.2017 [DOI] [PubMed] [Google Scholar]
- 43.Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, et al. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am J Physiol Cell Physiol 2005;288: C429–34. doi: 10.1152/ajpcell.00331.2004 [DOI] [PubMed] [Google Scholar]
- 44.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 2006;69: 495–503. doi: 10.1038/sj.ki.5000148 [DOI] [PubMed] [Google Scholar]
- 45.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, et al. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 2007;292: F395–403. doi: 10.1152/ajprenal.00100.2006 [DOI] [PubMed] [Google Scholar]
- 46.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 2001;98: 6500–5. doi: 10.1073/pnas.101545198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA 2014;111: 5520–5. doi: 10.1073/pnas.1402218111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 2007;27: 3417–28. doi: 10.1128/MCB.02249-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444: 770–4. doi: 10.1038/nature05315 [DOI] [PubMed] [Google Scholar]
- 50.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006;281: 6120–3. doi: 10.1074/jbc.C500457200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007;117: 4003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohata Y, Yamazaki M, Kawai M, Tsugawa N, Tachikawa K, Koinuma T, et al. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res 2014;29: 1627–38. doi: 10.1002/jbmr.2186 [DOI] [PubMed] [Google Scholar]
- 53.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390: 45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 54.Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R, et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet 2013;9: e1003975. doi: 10.1371/journal.pgen.1003975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 2005;14: 385–90. doi: 10.1093/hmg/ddi034 [DOI] [PubMed] [Google Scholar]
- 56.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 2007;117: 2684–91. doi: 10.1172/JCI31330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 2004;36: 579–81. doi: 10.1038/ng1358 [DOI] [PubMed] [Google Scholar]
- 58.Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 2006;281: 18370–7. doi: 10.1074/jbc.M602469200 [DOI] [PubMed] [Google Scholar]
- 59.Fukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, et al. Pathogenesis and diagnostic criteria for rickets and osteomalacia--proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J Bone Miner Metab 2015;33: 467–73. doi: 10.1007/s00774-015-0698-7 [DOI] [PubMed] [Google Scholar]
- 60.Fukumoto S. FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol 2021;66: R57–65. doi: 10.1530/JME-20-0089 [DOI] [PubMed] [Google Scholar]
- 61.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 2000;26: 345–8. doi: 10.1038/81664 [DOI] [PubMed] [Google Scholar]
- 62.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 2011;96: 3541–9. doi: 10.1210/jc.2011-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA 2011;108: E1146–55. doi: 10.1073/pnas.1110905108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 2019;15: 435–55. doi: 10.1038/s41581-019-0152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, et al. The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 1995;11: 130–6. doi: 10.1038/ng1095-130 [DOI] [PubMed] [Google Scholar]
- 66.Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One 2014;9: e104154. doi: 10.1371/journal.pone.0104154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J 2011;25: 2551–62. doi: 10.1096/fj.10-177816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y, et al. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J Cell Biochem 2010;111: 1210–21. doi: 10.1002/jcb.22842 [DOI] [PubMed] [Google Scholar]
- 69.Kimata M, Michigami T, Tachikawa K, Okada T, Koshimizu T, Yamazaki M, et al. Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/Pi cotransporter Pit-1 and Raf/MEK/ERK pathway. Bone 2010;47: 938–47. doi: 10.1016/j.bone.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 70.Nishino J, Yamazaki M, Kawai M, Tachikawa K, Yamamoto K, Miyagawa K, et al. Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J Cell Biochem 2017;118: 1151–63. doi: 10.1002/jcb.25742 [DOI] [PubMed] [Google Scholar]
- 71.Takashi Y, Kosako H, Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK, et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci USA 2019;116: 11418–27. doi: 10.1073/pnas.1815166116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 2006;38: 1310–5. doi: 10.1038/ng1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 2006;38: 1248–50. doi: 10.1038/ng1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 2010;86: 267–72. doi: 10.1016/j.ajhg.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet 2010;86: 273–8. doi: 10.1016/j.ajhg.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 2003;34: 379–81. doi: 10.1038/ng1221 [DOI] [PubMed] [Google Scholar]
- 77.Maulding ND, Kavanagh D, Zimmerman K, Coppola G, Carpenter TO, Jue NK, et al. Genetic pathways disrupted by ENPP1 deficiency provide insight into mechanisms of osteoporosis, osteomalacia, and paradoxical mineralization. Bone 2021;142: 115656. doi: 10.1016/j.bone.2020.115656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet 2007;81: 906–12. doi: 10.1086/522240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, et al. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res 2013;28: 1378–85. doi: 10.1002/jbmr.1850 [DOI] [PubMed] [Google Scholar]
- 80.Takeyari S, Yamamoto T, Kinoshita Y, Fukumoto S, Glorieux FH, Michigami T, et al. Hypophosphatemic osteomalacia and bone sclerosis caused by a novel homozygous mutation of the FAM20C gene in an elderly man with a mild variant of Raine syndrome. Bone 2014;67: 56–62. doi: 10.1016/j.bone.2014.06.026 [DOI] [PubMed] [Google Scholar]
- 81.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 2012;336: 1150–3. doi: 10.1126/science.1217817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamazaki M, Kawai M, Miyagawa K, Ohata Y, Tachikawa K, Kinoshita S, et al. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J Bone Miner Metab 2015;33: 342–54. doi: 10.1007/s00774-014-0598-2 [DOI] [PubMed] [Google Scholar]
- 83.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet 2005;76: 361–7. doi: 10.1086/427956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rausch S, Föller M. The regulation of FGF23 under physiological and pathophysiological conditions. Pflugers Arch 2022;474: 281–92. doi: 10.1007/s00424-022-02668-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simic P, Babitt JL. Regulation of FGF23: Beyond Bone. Curr Osteoporos Rep 2021;19: 563–73. doi: 10.1007/s11914-021-00703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006;17: 1305–15. doi: 10.1681/ASN.2005111185 [DOI] [PubMed] [Google Scholar]
- 87.Haussler MR, Livingston S, Sabir ZL, Haussler CA, Jurutka PW. Vitamin D receptor mediates a myriad of biological actions dependent on its 1,25-dihydroxyvitamin D ligand: distinct regulatory themes revealed by induction of Klotho and fibroblast growth factor-23. JBMR Plus 2020;5: e10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone 2008;42: 1235–9. doi: 10.1016/j.bone.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 89.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 2005;90: 1519–24. doi: 10.1210/jc.2004-1039 [DOI] [PubMed] [Google Scholar]
- 90.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 2005;146: 5358–64. doi: 10.1210/en.2005-0777 [DOI] [PubMed] [Google Scholar]
- 91.Brown WW, Jüppner H, Langman CB, Price H, Farrow EG, White KE, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab 2009;94: 17–20. doi: 10.1210/jc.2008-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011;49: 636–43. doi: 10.1016/j.bone.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bär L, Feger M, Fajol A, Klotz LO, Zeng S, Lang F, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci USA 2018;115: 5804–9. doi: 10.1073/pnas.1800160115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawai M, Kinoshita S, Ozono K, Michigami T. Lack of PTEN in osteocytes increases circulating phosphate concentrations by decreasing intact fibroblast growth factor 23 levels. Sci Rep 2020;10: 21501. doi: 10.1038/s41598-020-78692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell 2008;134: 728–42. doi: 10.1016/j.cell.2008.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 2010;330: 1349–54. doi: 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawai M, Kinoshita S, Shimba S, Ozono K, Michigami T. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J Biol Chem 2014;289: 1457–66. doi: 10.1074/jbc.M113.500850 [DOI] [PMC free article] [PubMed] [Google Scholar]