Abstract.
We previously described the thyroid volume, which was calculated by measuring the thyroid width, thickness, and longitudinal length using ultrasonography, in children and adolescents. We have proposed a simplified method for quantitatively assessing the thyroid size, to overcome the inaccuracy and challenges in measuring the longitudinal length of the thyroid. Based on measurements of 317,847 (girls: 156,913, boys: 160,934) children and adolescents, we calculated sex-specific means and standard deviations of thyroid width and thickness, and of the cross-sectional area computed by multiplying them, for every age and 0.1 m2 of body surface area, after ensuring normal distribution with Box–Cox transformation. Multivariate regression analysis revealed that female sex, age, and body surface area were independently associated with areas of each thyroid lobe. Our novel method may be useful in quantitatively assessing the thyroid size, and appropriately diagnosing pathological conditions, such as hypoplasia, atrophy, and enlargement of the thyroid gland, in children and adolescents.
Keywords: thyroid volume, area, child, ultrasonography, health survey
Highlights
● We propose a novel method for assessing thyroid size using ultrasonography in children.
● Sex-specific reference values of thyroid width, thickness, and width multiplied by the thickness of each lobe, for every age, and 0.1 m2 of body surface area were determined.
● This method, namely “The Fukushima Method”, could be useful for the quantitative assessment of thyroid size, by providing supportive information on hypoplasia, atrophy, and enlargement of the thyroid gland in children and adolescents.
Introduction
After the Fukushima Daiichi Nuclear Power Plant accident of 2011, the Fukushima prefectural government initiated the Fukushima Health Management Survey, involving a mass ultrasonographic thyroid examination. We previously demonstrated that the thyroid volume estimated using ultrasonography in children and adolescents (1, 2), corresponded to the body surface area (BSA). While the volume of the right lobe was greater than that of the left lobe, that of the entire gland was greater among girls, than that among boys, after correcting for BSA. However, three major concerns are encountered when calculating the thyroid volume, as described in the previous study (1). First, measuring the longitudinal length is challenging in study participants, whose neck lengths are short or whose thyroid lengths are greater than that of the ultrasound probe. Second, the shape of the thyroid may vary, and not match with the fusiform simulation. Finally, the isthmus volume is not assessed. Therefore, the earlier method may be insufficient to elicit the exact volume.
The shape-simulation concern is resolved by using a digital scanner, that measures multiple cross-sectional areas by tracing the thyroid, and produces serial longitudinal sections, to accurately calculate the thyroid volume (3). Although the thyroid gland is composed of bilateral lobes, an isthmus, and a pyramidal lobe, the volume of the isthmus could not be determined in previous studies (1, 2); an additional scanning method could address this issue, along with overcoming the shape-simulation challenge. However, assessing the thyroid volume in those with neck and probe length discrepancies may be possible if the normal reference width and thickness can be defined without requiring the longitudinal length measurement.
Thus, addressing these concerns is important for accurately assessing the thyroid volume, which is occasionally crucial for the diagnosis of autoimmune, infectious, and hereditary thyroid diseases, especially in children, owing to the non-invasiveness of ultrasonography.
Considering the clinical significance and associated challenge of measuring the thyroid volume, we provided the means and standard deviations (SDs) for every age between 0 and 20 yr, and for every 0.1 m2 of BSA in each sex, after correction to the optimal distribution with Box–Cox transformation (4). We further proposed the practical application of assessing the thyroid volume using ultrasound. The transverse area of the thyroid was calculated by multiplying the width by thickness of each lobe to provisionally determine the thyroid size, instead of the practical tracing area, which was unavailable in the previous study.
Materials and Methods
Participants
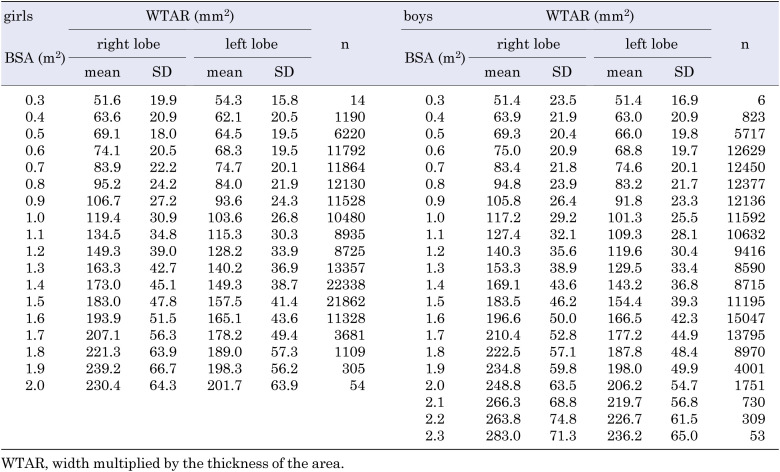
The thyroid ultrasound examination (TUE) program of the Fukushima Health Management Survey began in October 2011, with an intended repetition every 2 or 5 yr (5). An ultrasound volumetric examination was performed using a commercial ultrasound machine equipped with a linear array probe (10–12 MHz, 4 cm in length), to measure the width, thickness, and longitudinal length of the thyroid, in addition to detecting thyroid nodules and cysts (6). Currently, a fifth-round examination is in progress. Data were obtained from 300,472 and 270,552 participants, who underwent their first examination in the first and second rounds of TUE, and 299,927 and 269,660 of them were analyzed, respectively, following the confirmation by a written informed consent. We excluded 7,203 participants because of invalid measurements of height, weight, and thyroid size. Among the study population, 240,337 (42.7%) participants underwent the examinations twice. Participants who underwent a second-round examination, and were > 20 yr of age were excluded. Finally, we analyzed 317,847 (girls: 156,913, boys: 160,934) participants. With only a single girl participant at 2.1 m2 of BSA (0.00064%), the corresponding results are not shown in Table 2, nor in Supplementary Tables 2 and 4.
Table 2. Mean WTAR in the right and left thyroid lobes, for every 0.1 m2 of BSA in girls and boys.
Methods
BSA was calculated based on height and weight using the Du Bois method (7), and rounded off to the first decimal place, to ease grouping. The transverse area of the thyroid was calculated by multiplying the width by thickness (WTAR; width multiplied by thickness of the area). The distribution of each measurement was normalized by Box–Cox transformation (4).
Statistics
The WTAR at different ages was compared between the right and left thyroid lobes, using paired student’s t-test. The participants were divided into two groups (1 and 2), depending on the thyroid longitudinal length: ≤ 40 mm and > 40 mm, respectively. The Spearman’s correlation matrix coefficient was estimated between WTAR and thyroid volume, which was calculated using the width, thickness, and longitudinal length (WTLV), for each lobe. All probability values for statistical tests were two-tailed, and p values < 0.05 were considered statistically significant. Multivariate regression analysis was used to determine whether sex, age, and BSA, independently affected the WTAR.
Ethics
This study was approved by the Ethics Committee of Fukushima Medical University (No. 1318). Written informed consent was obtained from the surveyed participants or their parents or guardians, if < 16 yr of age. The raw data used to create all tables in this study are unavailable because of restrictions outlined in the informed consent form.
Results
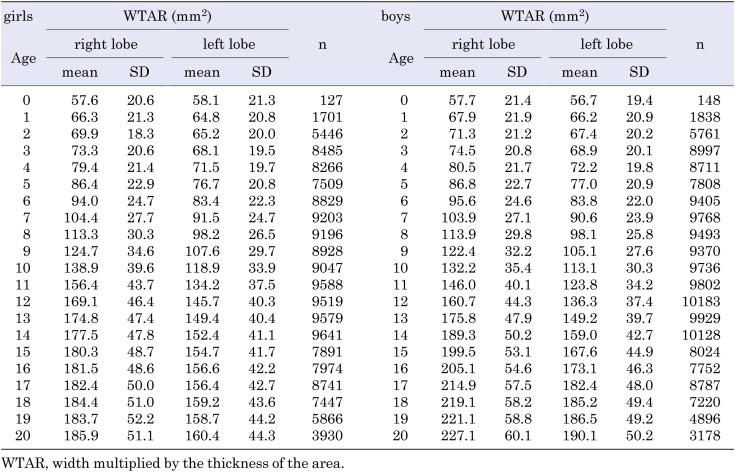
The number of participants and the mean (± SD) WTAR at every age (0–20 yr) for both sexes, are shown in Table 1, and those for every 0.1 m2 BSA are shown in Table 2. The mean (± SD) thyroid width and thickness in girls and boys, based on age (Supplementary Table 1), and BSA (Supplementary Table 2) are shown for reference. In addition, the WTAR of the bilateral lobes for both sexes, based on age and BSA, are shown in Supplementary Tables 3 and 4, respectively. The mean (± SDs) body height, weight, and BSA are shown in Supplementary Table 5; these were not corrected by the Box–Cox transformation.
Table 1. Mean WTAR in the bilateral thyroid lobes, at the ages of 0 to 20 yr, in girls and boys.
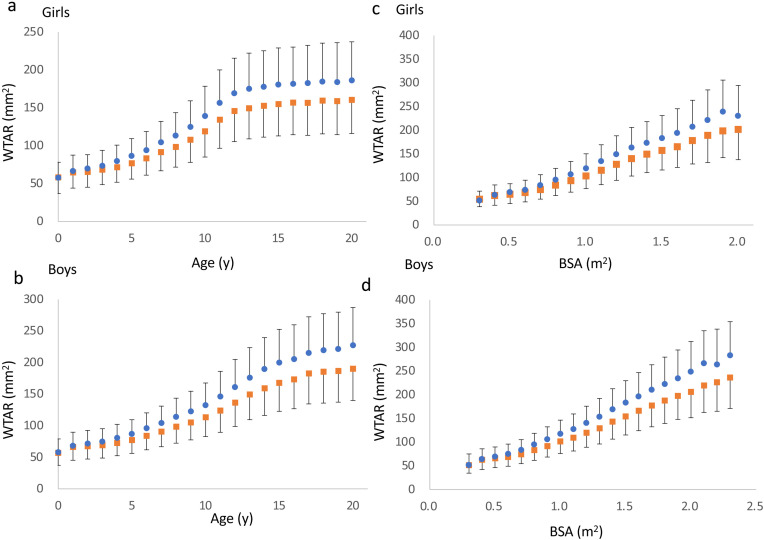
The mean WTAR of each lobe based on sex, age, and BSA, are shown in Fig. 1. The WTAR of the right lobe was significantly larger than that of the left lobe at all measures, except for boys at 0.3 m2 of BSA (P = 0.081). WTAR in girls and boys, rapidly increased with age until approximately 12 and 14 yr, respectively, with a decline in this rate, henceforth (Fig. 1a, b). The relationship between BSA and WTAR increased linearly (Fig. 1c, d).
Fig. 1.
Graphs representing the relationships between WTAR, and age (a, b) and BSA (c, d) in girls and boys, respectively. Orange- square and blue-circle symbols represent the left and right lobes, respectively. Mean ± standard deviations are shown.
A longitudinal length of > 40 mm may be inaccurate because the length of the probe is 40 mm. To estimate the accuracy of WTAR, we assessed the relationship between WTAR and WTLV. The participants were divided into two groups depending on their longitudinal length. Spearman’s coefficients between WTAR and WTLV were calculated groupwise, for each lobe; the coefficient values in group 1 were higher than those in group 2 (right lobe in group 1: r = 0.648, p < 0.001; right lobe in group 2: r = 0.379, p < 0.001; left lobe in group 1: r = 0.657, p < 0.001; left lobe in group 2: r = 0.338, p < 0.001).
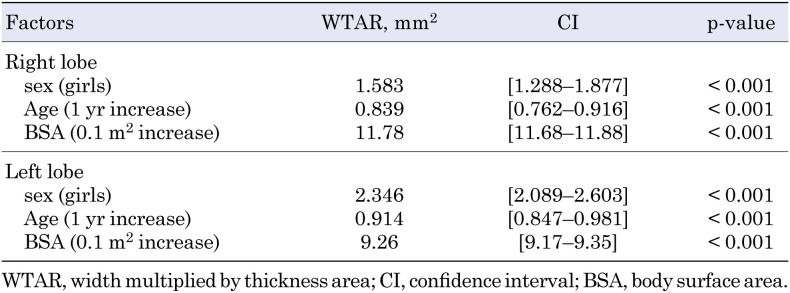
To clarify the factors affecting WTAR in each thyroid lobe, we used a multivariate regression analysis (Table 3); sex, age, and BSA were found to independently affect the thyroid volume. The WTAR in girls was significantly larger than that in boys, after correcting for age and BSA (p < 0.001).
Table 3. Association of right (upper panel) and left (lower panel) WTAR with sex, age, and BSA.
Discussion
The systematic measurement of thyroid size using ultrasonographic devices was first reported in 1974 (8). Several studies on reference ranges for thyroid volume estimated by ultrasound in childhood included more than 5,000 participants (1, 2, 9,10,11,12).
In this study, we estimated the means ± SDs of the thyroid width, thickness, and WTAR based on age and BSA in girls and boys, using > 600,000 determinants of thyroid size measurements. To achieve an optimal distribution of each measurement, we applied Box–Cox transformation, that provides a coefficient value such as lambda to correct for the skewness of the distribution. The lambda-mu-sigma (LMS) method is commonly used for creating growth curve standards in children using lambda, mean, and sigma as the SD (4). The rapid growth in infants, especially at 0 yr of age, does not permit the availability of few measurable values in few of them.
Although several thyroid volume measurements in children and adolescents have been published, comparisons between the reported values are challenging (1), owing to the difficulty in obtaining longitudinal measurements. Yasumoto et al. assessed the thyroid volume in children by measuring the width and thickness of thyroid gland, and the diameter of trachea (13). Although this method is easily available for the assessment of thyroid volume without measuring the longitudinal length, the number of participants was small (n = 30), and they were predominantly hyper- or hypothyroidism (7), and thus, would be insufficient for evaluating the normal population.
In this study, the right thyroid lobe was larger than the left lobe in girls and boys (Fig. 1 and Table 3), except for boys at 0.3 m2 of BSA, possibly because of an insufficient number of boys in this category. As previously speculated, laterality may be affected by genetic factors or the surrounding organs, such as the esophagus, which is almost always present on the left side (1). The relationship between BSA and WTAR was linear, in contrast to that between age and WTAR; a difference of approximately 2 yr was observed, when the WTAR plateaued in girls and boys. However, the precise reason for this finding remains unknown. As shown in Supplementary Table 5, the BSA plateaued after 15 and 17 yr of age in girls and boys, respectively. Because thyroid volume is related to BSA, this 2-yr difference may be attributed to the sex difference in BSA. Alternatively, thyrotropin may affect thyroid size as regulation of the pituitary-thyroid axis may develop in a sex-dependent manner, similar to puberty (14). WTAR in the right and left lobes were independently associated with sex, age, and BSA, and were larger in girls than in boys. This difference may be accredited to sex hormones and sex-specific regulation of thyroid hormones as previously described (1, 14). These features are consistent with previous results obtained using three determinants: longitudinal length, width, and thickness (1).
When we grouped the participants based on the longitudinal length, the relationship between the WTAR and thyroid volume was stronger in the group with longitudinal length ≤ 40 mm, suggestive of inappropriate measurement in the other group.
In conclusion, we have proposed a simple method for assessing thyroid volume. This study provides the mean (± SD) width and thickness of the thyroid, at each year of age, and for every 0.1 m2 of BSA in children and adolescents. Our method facilitates the quantitative assessment of thyroid size and expectantly aids in the accurate diagnosis of hypoplasia, atrophy, and enlargement of the thyroid gland in children and adolescents.
The findings and conclusions of this study are solely the responsibility of the authors, and do not represent the official views of the Fukushima Prefecture government.
Conflict of interests
The authors declare no conflict of interest, in relation to this study. HO is a member of the “Department of Mesenchymal Stem Cell Research”, endowed by Alfresa Corporation.
Supplementary Tables
Acknowledgments
We express our gratitude to all the participants of the Fukushima Health Management Survey. We appreciate Satoshi Narumi, MD, PhD for providing useful suggestions for the manuscript. We are grateful to Tatsuya Okada, PhD, for general advice on mathematical transformations. We thank Ms. Miyuki Konno for her excellent secretarial assistance.
This survey was conducted as part of Fukushima Prefecture’s post-disaster recovery plans, and was supported by the national “Health Fund for Children and Adults Affected by the Nuclear Incident”.
Appendix
Other participating expert committee members, advisors, and staff members in the Fukushima Health Management Survey: Hiroyuki Yaginuma, Kenneth E. Nollet, Kumiko Tsuboi, Masaharu Maeda, Keiya Fujimori, Tetsuo Ishikawa, Shigehira Saji, Michio Shimabukuro, Mitsuaki Hosoya, Masaharu Maeda, Masaharu Tsubokura, Hiroshi, Zaima, Maho Momoi, Shinji Meguro, Mizuki Sekino, Toshie Sakagami, Norikazu Abe, Masahiko Henmi, Sakiko Meguro, Rina Tasaki, Yuko Namekata, Tomoko Ito, Kunio Shibayama, Yuka Hamaya, Ryouko Hata, Takako Takahashi, and Noriko Seto.
References
- 1.Suzuki S, Midorikawa S, Fukushima T, Shimura H, Ohira T, Ohtsuru A, et al. Thyroid Examination Unit of the Radiation Medical Science Center for the Fukushima Health Management Survey. Systematic determination of thyroid volume by ultrasound examination from infancy to adolescence in Japan: the Fukushima Health Management Survey. Endocr J 2015;62: 261–8. doi: 10.1507/endocrj.EJ14-0478 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Midorikawa S, Matsuzuka T, Fukushima T, Ito Y, Shimura H, et al. Prevalence and Characterization of Thyroid Hemiagenesis in Japan: The Fukushima Health Management Survey. Thyroid 2017;27: 1011–6. doi: 10.1089/thy.2016.0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoyama N, Nagayama Y, Kakezono F, Kiriyama T, Morita S, Ohtakara S, et al. Determination of the volume of the thyroid gland by a high resolutional ultrasonic scanner. J Nucl Med 1986;27: 1475–9. [PubMed] [Google Scholar]
- 4.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44: 45–60. [PubMed] [Google Scholar]
- 5.Yasumura S, Hosoya M, Yamashita S, Kamiya K, Abe M, Akashi M, et al. Fukushima Health Management Survey Group. Study protocol for the Fukushima Health Management Survey. J Epidemiol 2012;22: 375–83. doi: 10.2188/jea.JE20120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki S, Yamashita S, Fukushima T, Nakano K, Midorikawa S, Ohtsuru A, et al. The protocol and preliminary baseline survey results of the thyroid ultrasound examination in Fukushima [Rapid Communication]. Endocr J 2016;63: 315–21. doi: 10.1507/endocrj.EJ15-0726 [DOI] [PubMed] [Google Scholar]
- 7.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5: 303–11, discussion 312–3. [PubMed] [Google Scholar]
- 8.Rasmussen SN, Hjorth L. Determination of thyroid volume by ultrasonic scanning. J Clin Ultrasound 1974;2: 143–7. doi: 10.1002/jcu.1870020211 [DOI] [PubMed] [Google Scholar]
- 9.Ashizawa K, Shibata Y, Yamashita S, Namba H, Hoshi M, Yokoyama N, et al. Prevalence of goiter and urinary iodine excretion levels in children around Chernobyl. J Clin Endocrinol Metab 1997;82: 3430–3. [DOI] [PubMed] [Google Scholar]
- 10.Foo LC, Zulfiqar A, Nafikudin M, Fadzil MT, Asmah AS. Local versus WHO/International Council for Control of Iodine Deficiency Disorders-recommended thyroid volume reference in the assessment of iodine deficiency disorders. Eur J Endocrinol 1999;140: 491–7. doi: 10.1530/eje.0.1400491 [DOI] [PubMed] [Google Scholar]
- 11.Delange F, Benker G, Caron P, Eber O, Ott W, Peter F, et al. Thyroid volume and urinary iodine in European schoolchildren: standardization of values for assessment of iodine deficiency. Eur J Endocrinol 1997;136: 180–7. doi: 10.1530/eje.0.1360180 [DOI] [PubMed] [Google Scholar]
- 12.Johnson A, Edwards C, Reddan T. A review of sonographic thyroid volume and iodine sufficiency in children: An Australian perspective. Australas J Ultrasound Med 2019;23: 33–8. doi: 10.1002/ajum.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasumoto M, Inoue H, Ohashi I, Shibuya H, Onishi T. Simple new technique for sonographic measurement of the thyroid in neonates and small children. J Clin Ultrasound 2004;32: 82–5. doi: 10.1002/jcu.10234 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Suzuki S, Iwadate M, Matsuzuka T, Shimura H, Ohira T, et al. Possible association between thyroid nodule formation and developmental alterations in the pituitary-thyroid hormone axis in children and adolescents: The Fukushima Health Management Survey. Thyroid 2022; (in press). doi: 10.1089/thy.2022.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.