Abstract
Introduction
In Africa almost half of healthcare services are delivered through private sector providers. These are often underused in national public health responses. To support and accelerate the public sector's COVID-19 response, we facilitated recruitment of additional private sector capacity by initiating a public-private partnership (PPP) in Kisumu County, Kenya. In this manuscript we demonstrate this PPP's performance.
Methods
COVID-19 diagnostic testing formed the basis for a PPP between Kenyan Medical Research Institute (KEMRI), Department of Health Kisumu County, PharmAccess Foundation, and local faith-based and private healthcare facilities: COVID-Dx. First phase COVID-Dx was implemented from June 01, 2020, to March 31, 2021 in Kisumu County, Kenya. Trained laboratory technologists in participating healthcare facilities collected nasopharyngeal and oropharyngeal samples from patients meeting the Kenyan MoH COVID-19 case definition. Healthcare workers in participating facilities collected patient clinical data using a digitized MoH COVID-19 Case Identification Form. We shared aggregated results from these data via (semi-) live dashboards with all relevant stakeholders through their mobile phones and tablets. Statistical analyses were performed using Stata 16 to inform project processes.
Results
Nine private facilities participated in the project. A patient trajectory was developed from case identification to result reporting, all steps supported by a semi-real time digital dashboard. A total of 4,324 PCR tests for SARS-CoV-2 were added to the public response, identifying 425 positives, accounting for 16% of all COVID-19 tests performed in the County over the given time-period. Geo-mapped and time-tagged information on incident cases was depicted on Google maps through PowerBI-dashboards and fed back to policymakers for informed rapid decision making. Preferential COVID-19 testing was performed on health workers at risk, with 1,009 tests performed (up to 43% of all County health workforce).
Conclusion
We demonstrate feasibility of rapidly increasing the public health sector COVID-19 response through coordinated private sector efforts in an African setting. Our PPP intervention in Kisumu, Kenya was based on a joint testing strategy and demonstrated that semi-real time digitalization of patient trajectories can gain significant efficiencies, linking public and private healthcare efforts, increasing transparency, support better quality health services and informing policy makers to target interventions.
Keywords: public-private partnership, COVID-19, digital dashboard, epidemic preparedness, developing country
Introduction
Most health systems in low and middle income countries (LMIC) are underfunded and understaffed, with limited isolation and intensive care infrastructure (1, 2). In sub-Saharan Africa, health systems are facing disproportional challenges and are ill-equipped and under-res [o]ourced to deal with additional burdens, such as those caused by the recent COVID-19 pandemic. There is an urgent need for innovative approaches to accelerate strengthening African health systems to work toward Universal Health Coverage (UHC).
Kenya confirmed the first COVID-19 case on March 13, 2020. By mid-March 2021, Kenya recorded the beginning of its third wave with a notable steep increase in daily COVID-19 cases and deaths (3, 4). As of November 28, 2021, 254,951 confirmed cases with 5,333 fatalities had been reported (5).
On March 4 2021 Kenya received the first batch of COVAX COVID-19 AstraZeneca vaccines, prioritizing vaccination of its high-risk population, including frontline healthcare workers, adults above 58 years, teachers, police officers and persons with pre-existing conditions (6). However, the 1st weeks of roll-out were met with considerable vaccine hesitancy amongst various target groups, which lasts until today (7). Kenya aimed to vaccinate 30% (15 Million) of its total population of 50 M by the end of June 2023 (8). This vaccination target falls significantly below the 70% goals set for the world by WHO and for Africa by CDC-Africa (9, 10).
In the absence of vaccine-induced immunity, the options to combat COVID-19 in Kenya were relatively limited and mostly included so called non-pharmaceutical interventions, including lockdowns, curfews, social distancing, personal hygiene and protective clothing, particularly at healthcare facilities. When diagnostic testing became available, those who appeared SARS-CoV-2 infected were (advised to) quarantine (d). At the start of the pandemic, diagnostic testing for SARS-CoV-2 was led by the Kenyan MoH at the national and county levels through centralized PCR testing in dedicated high-throughput laboratories, with KEMRI playing a central role. However, centralized testing in Kenya faced multiple challenges, including lack of funding, stockouts of reagents and testing kits, manpower challenges, PCR equipment breakdowns due to heavy workload; this all resulting in test access limitations and prolonged test turnaround time. During most of 2020, RDTs for SARS-CoV-2 according to WHO standards were unavailable in Kenya. In December 2020 the first Kenyan interim guide for RDTs was launched (11). MoH supported COVID-19 testing remained confined to designated public hospitals with limited tapping of the private sector's potential (12).
The private sector (for-profit, not-for-profit foundations and faith-based organizations) is a significant player in health service delivery in sub-Saharan Africa (13) and particularly in Kenya (14). The private healthcare sector can add substantial capacity to the public health infrastructure, which often faces challenges in terms of quality of care, drug stockouts, health worker shortages, industrial action, and lack of diagnostic equipment (15). PPPs can play an essential role in LMICs health system strengthening (9), particularly during outbreaks and epidemics, where a coordinated, rapidly scalable approach is required. A health PPP is a (long-term) partnership between the public sector and the private sector with the aim to coordinate delivery of a health(care) service. Strengthening and coordination of public and private health systems can accelerate ensure progress toward UHC and global health security (16). The challenge is to combine private and public efforts in healthcare delivery in a mutually supportive and collaborative manner. Achieving a supportive PPP is complex, fraught with challenges and evidence of their effectiveness is limited (17, 18). There are ample examples of (inter)national responses where private healthcare sector initiatives were crowded out by parallel public sector efforts (19). Crowding out implies that private investments in healthcare are replaced instead of supplemented by public funds, and the total amount of funds in the healthcare system remains unchanged. If supported well, PPP models can enhance capacity, increase quality of services offered, promote access, and offer innovative and sustainable solutions to healthcare challenges in developing countries (20).
The Dutch NGO PharmAccess gained extensive experience supporting innovative PPP models for healthcare, “crowding in” private funding. Notable PPP models established by PharmAccess include the first risk equalization fund for HIV in Africa (21), the first Medical Credit Fund for Africa that provides loans to private-sector health entrepreneurs through public-private funding (22) and the first digital healthcare exchange platform M-TIBA, a.o. supporting Kisumu County's UHC (23). Due to PharmAccess' experience with timely interventions and through rapid donor contributions and necessary regulatory support by the local Department of Health, a unique joint PPP named “COVID-Dx” was started in early May 2020 in Kisumu County, Kenya. COVID-Dx was designed to enhance Kisumu's capacity for COVID-19 sample collection and testing, rapid digital clinical and socio-demographic data collection and timely reporting to inform policy decisions. The COVID-Dx intervention connected private- and faith-based healthcare facilities in Kisumu County to the existing MoH network, it implemented COVID-19 clinical guidelines, it enabled COVID-19 testing at the KEMRI central laboratories and it created semi-real time dashboards to improve reporting efficiency and decision-making by both healthcare workers and policymakers.
The aim of this study is to describe the performance of COVID-Dx, this rapidly-formed PPP in responding to the COVID-19 pandemic, in a sub-Saharan African setting and to underscore the importance of digitalization of the health system in order to markedly accelerate its responsiveness to sudden capacity challenges.
Kisumu County, located in the western part of Kenya, was selected for this PPP because of its unique track record as a county pioneering UHC using the M-TIBA health platform (23). M-TIBA digitally connects and is used by patients, healthcare providers and healthcare payers (insurers and donors) to create real-time transparency of healthcare transactions. M-TIBA is the vehicle for compulsory health insurance in Kisumu and thus creates the potential of reaching out to the entire 1.2 M population and work on a replicable model of digital infrastructure that can serve future epidemic preparedness for other settings in Kenya.
Materials and methods
Context
After a preparation phase, COVID-Dx officially started in Kisumu, Kenya on June 1, 2020, and its first phase, that this paper reports about, ended March 31, 2021. The intervention was well-timed, starting exactly at the time that Kisumu reported the first two COVID-19 cases on May 27, 2020. Healthcare facilities in Kisumu were selected for participation based on a set of criteria, including: possession of a valid license, a MoH COVID-19 certificate, being within reasonable geographic distance from KEMRI testing laboratories, serving minimally 100 patients per week, participating in the PharmAccess SafeCare quality improvement program, being connected to M-TIBA (24), having an average staff of at least 25, possessing an operational and regularly serviced fridge and generator for sample storage and proving higher management willingness to participate in COVID-Dx. Nine healthcare facilities were eligible, labeled as A-I throughout this manuscript. Table 1 provides an overview of the key characteristics of these healthcare facilities. Facility A was a small NGO facility used a pilot to test steps of the COVID-Dx intervention before scaling to the other eight facilities. Each participating facility had trained staff collecting nasopharyngeal and oropharyngeal swabs from patients who fulfilled the COVID-19 case definition as per the Kenyan Ministry of Health COVID-19 Clinical Case Definition Guidelines. The main eligibility criteria to be tested for COVID-19 were: (1) people presenting with signs and symptoms of COVID-19, fulfilling the criteria of the Kenyan COVID Clinical Identification Form (CCIF) and (2) risk groups as defined in the Kenyan MoH guidelines: healthcare workers, contacts of confirmed COVID-19 cases, travelers from high-risk areas (26). The CCIF is included as Supplementary material 1. Participation of patients was completely voluntary.
Table 1.
Overview of key characteristics participating COVID-Dx facilities.
| Facility | Kenyan healthcare category | Estimated patients/day | Beds | SafeCare level (25) | Staff | Setting | Start project | First case identified |
|---|---|---|---|---|---|---|---|---|
| A | Level 3 Health Center | 40 | 0 | 3 | 12 | Urban | Jun 2020 | Aug 2020 |
| B | Level 4 Hospital | 100 | 100 | 2 | 80 | Urban | Jul 2020 | Sep 2020 |
| C | Level 4 Hospital | 100 | 60 | 3 | 60 | Rural | Jul 2020 | Sep 2020 |
| D | Level 5 Hospital | 180 | 86 | NA | 200 | Urban | Aug 2020 | Aug 2020 |
| E | Level 4 Hospital | 70 | 62 | 3 | 50 | Urban | Aug 2020 | Sep 2020 |
| F | Level 5 Hospital | 50 | 50 | NA | 80 | Urban | Sep 2020 | Sep 2020 |
| G | Level 5 Hospital | 300 | 180 | NA | 400 | Urban | Dec 2020 | Dec 2020 |
| H | Level 5 Hospital | 100 | 70 | 5 | 400 | Urban | Dec 2020 | Jan 2020 |
| I | Level 5 Hospital | 1,000 | 550 | NA | 600 | Urban | Feb 2021 | Feb 2021 |
Laboratory methods for SARS-CoV-2
Patient samples were collected in viral transport medium according to manufacturer's instructions [F&S scientific (27)] and transported by motorbike in cool boxes to the KEMRI central laboratory for SARS-CoV-2 RT-PCR testing within 24 h. Results were reported through KEMRI and the MoH system to participating providers who reported to patients usually within 24–48-h. Complementary (telephone and personal) counseling services were provided involving two counseling session per client (pre and posttest counseling). The duration of the phone calls could range from < 5 min to more than 10 min per client, depending on the situation at hand. KEMRI central laboratory trained staff carried out the PCR test procedures according to standard manufacturer prescribed testing protocols. Laboratory staff used MagMAX™ Viral RNA Isolation Kit (28) to manually extract SARS-CoV-2 viral RNA from the paired nasopharyngeal and oropharyngeal samples. Post-RNA extraction, the TaqPath™ 19 kit (28) was used to carry out real-time SARS-CoV-2 PCR. Laboratory staff employed the following thermocycling conditions; 2 min at 25°C incubation, 10 min at 53°C for reverse transcription, 2 min at 95 °C for enzyme activation and 40 cycles of 3 s at 95°C and 30's at 60°C. Samples having exponential growth curve and Ct <40 in at least two SARS-CoV-2 targets were considered positive. Between December 28, 2020, and March 31, 2021, an additional prospective diagnostic evaluation of a rapid antigen kit was carried out and the results of this evaluation are reported in a separate paper (29). All Ag-RDTs were followed by a confirmatory PCR test, which is the basis for the quantitative analyses reported in the current manuscript.
Use of digital tools
The official Kenyan CCIF used by the MoH to screen and report all COVID-19 cases was digitalized to run on simple tablets that were distributed to participating providers. The digital CCIF tool additionally collected important logistical information including full tracking and tracing of samples and data flows: sample collection, courier receipt, road transport, KEMRI laboratory receipt and triaging into various sub-laboratories, specifics of PCR tests performed, result verification, transmission of final diagnostic result to MoH and finally to healthcare provider for release to patients reporting. The CCIF-Tool data was stored in a dedicated database (CommCare; Dimagi), a robust mobile data collection and service delivery platform hosted in a highly secured ISO27001 environment that is HIPAA and GDPR compliant. KEMRI provided oversight of scientific accuracy and quality of SARS-CoV-2 testing data. Aggregated results from the CommCare database were shared via a PowerBI dashboard with pertinent policymakers and relevant stakeholders through password-protected personal tablets and mobile phones. Microsoft PowerBI is a collection of software services, apps and connectors that visualize interactive insights of data sources (30). The dashboard presents an overview of the clinical and socio-demographic characteristics of patients tested, positivity rates, participating facilities, and maps with the patients per place of residence. Supplementary material 2 includes a screenshot of the dashboard (Supplementary Figure 1).
Data analyses
We conducted data analyses for operational purposes to inform and improve project processes, monitor general progress of the project, inform participating providers, and generate aggregate data to inform policy makers. We performed descriptive statistical analyses using Stata 16, tested relationships between categorical variables using chi-square tests, and used an independent sample t-test to explore relationships between categorical and continuous variables. We considered a p-value of <0.05 to be statistically significant during the analyses.
Ethical clearance
Ethical clearance for this project was obtained from Jaramogi Oginga Odinga Teaching & Referral Hospital (JOOTRH) on June 16, 2020, with approval number IERC/JOOTRH/230/2020. KEMRI also provided ethical clearance on September 30, 2020, with approval number KEMRI/RES/7/3/1. Research License was obtained from the National Commission for Science, Technology, and Innovation (NACOSTI) on July 6, 2020 (NACOSTI/P/20/5616).
Patient and public involvement
The Kisumu DoH, KEMRI and participating healthcare facilities were actively involved in co-creating the design, conduct, reporting, and dissemination plans of the research. Feedbacks from patients and public was collected through ongoing counseling services and fed back into the COVID-Dx operations to further improve.
Building the PPP model
In Table 2, we present the main tasks that needed to be executed during the PPP and which of the PPP stakeholders (gradually) took the lead in executing that task. Choices regarding task division were based on the partners' different levels of authority, partner's capabilities, and capacities, such as time, knowledge about regulatory or laboratory processes, and staff available. In the case of this PPP, the stakeholders were all operational on different levels, and authority levels of each partner were clear for the other partners. If unclarities emerged, these were resolved by weekly stakeholder meetings coordinated by PharmAccess. Generally, the DoH was responsible for approving selected participating facilities, providing guidelines, performing trainings, carrying out contact tracing, and application of epidemic control guidelines. PharmAccess provided kickstart funding, managed day-to-day operations and supported psychosocial counseling to clients and providers staff, procurement of reagents and personal protective equipment (PPE), database hosting, cleaning, quality control and translation into the COVID-Dx data dashboard. KEMRI provided supportive supervision, complementary training, conducted social research and was responsible for the full laboratory component (SARS-CoV-2 testing and RDT evaluation). Healthcare facilities executed patient management, sample taking, data entry into the CCIF-tool, assisted with contact tracing of COVID-19 patients, disseminated test results to patients and provided counseling.
Table 2.
Roles and tasks of public and private partners in COVID-Dx project*.
| PPP stakeholder: PPP tasks | KEMRI | Department of health | Pharmaccess foundation | Healthcare providers |
|---|---|---|---|---|
| Management and monitoring | ||||
| Overall responsibility | X | |||
| Day to day management of the project | X | |||
| COVID-19 Case management | X | |||
| Provide, revise and monitor adherence to COVID-19 guidelines | X | |||
| Overseeing lab operations | X | |||
| Preparation | ||||
| Process ethical clearance of the project | X | |||
| Contract healthcare providers | X | |||
| Set up data collection tools and dashboards | X | |||
| Get a SafeCare (4COVID) assessment | X | |||
| Supplies and resources | ||||
| Procure PPEs and tablets for providers | X | |||
| Sample Courier, logistics for sample transport and storage and distribution of Ag. RDT | X | |||
| Trainings | ||||
| Laboratory practice, patient management training | X | |||
| Train HCPs for COVID-19 sample taking | X | |||
| CCIF-tool training for healthcare providers | X | |||
| Sample taking, testing and support | ||||
| Triage and fast track patients for COVID-19 testing | X | |||
| COVID-19 Sample collection | X | |||
| Run PCR tests on the COVID-19 samples | X | |||
| Contact tracing and support to the field teams | X | |||
| COVID-19 counseling support to the testing teams | X | |||
| Reporting of results | ||||
| Data capture for patients receiving a COVID-19 test | X | |||
| Data reporting to providers | X | |||
| Receive and disseminate results to the patients | X | |||
| Process and disseminate PCR results to stakeholders | X | |||
| Information dissemination through SITREPs | X | |||
| Dashboard updates with CCIF data | X | |||
| Data cleaning and analyses of CCIF data | X | |||
| Studies | ||||
| Antigen RDT evaluation | X | |||
| Conduct the feasibility and acceptability studies | X | |||
| Scientific publication on COVID-Dx | X | |||
for each task the leading institution is indicated.
Results
Key events
Supplementary material 2 include a Gantt Chart (Supplementary Figure 2), outlining our key experiences during this PPP. Preparations for COVID-19 started well before Kisumu County reported the first COVID-19 cases on May 27, 2020. At the onset of COVID-Dx, patient sample collection and central testing via MoH-linked KEMRI laboratories was restricted to designated public hospitals. MoH supported a limited set of public hospitals collecting samples and central laboratories testing for COVID-19 with providing PPE, sample collection materials, sample transportation materials such as viral transport medium (VTM), cool boxes, and sample testing reagents.
For private facilities to be added to this initial COVID-19 response and have access to public sector COVID-19 testing services through the KEMRI central laboratories, it was initially indicated that COVID-Dx had to be positioned as a research project. This implied a phase of comprehensive protocol writing and ethical clearance procedures. Later it was clarified that any private facility intending to provide COVID-19 testing through MoH-supported central laboratories were required to do so via the MoH COVID-19 public sector response at the County level. This meant selected private healthcare facilities just required DoH approval as official sample collection sites supporting Kisumu County to scale up COVID-19 testing.
In the PPP preparation phase, the PPP partners held multiple meetings to align project objectives culminating in signed contracts with expected deliverables clearly outlined. Subsequently, PharmAccess contracted selected private providers as approved by the DoH. These private providers gradually became known to the public as additional COVID-19 service sites featuring free diagnostic tests. In the 1st months of implementation the DOH arranged trainers for multiple trainings for providers (COVID-19 Guidelines, safety measures, sample collection, sample transport, data entry using tablets). During COVID-Dx roll-out, PharmAccess organizedregular complementary refresher trainings.
Several procurement rounds of PPEs occurred during the project (May, August, October, December 2020). At project initiation, PharmAccess developed the PowerBI digital dashboard to share the live operational results of the project. PharmAccess continuously improved and extended the dashboard throughout the project, based on continuous feedbacks of the users. In addition to the healthcare providers, by November 2020, the first external stakeholders (policy makers) got access to the dashboard.
Some months into the project, several healthcare facilities noticed fewer patients coming in to get tested. We hypothesized this reflectedincreasing COVID-19 stigma (due to a.o. “fake news” in (social) media as well-implementation of quarantine measures, which were feared since these deprived people from daily income generation) could explain this, which the counselor of the project later confirmed. Additionally, Kisumu County DoH made patient contact tracing mandatory. As the positive cases increased, COVID-Dx assisted by providing complementary contact tracing services. PharmAccess contributed a senior counselor to COVID-Dx, who provided mental support to clients and in addition trained public sector counselors to address COVID-19 stigma. Most facility-based counselors were already HIV counselors, with previous experience, which facilitated the COVID-19 counseling training.
At times the DoH or KEMRI requested some private facilities to stop testing or the federal MoH restricted the criteria that selected patients for testing. For instance, around October 2020, KEMRI and DoH temporarily paused sample collection from one of the COVID-Dx facilities when samples spilt during transportation, creating a health and safety hazard. This incident triggered an audit and mandatory refresher training of the affected site. Also, the MoH updated guidelines for targeted testing, where only those who met the CCIF case definition and had symptoms were eligible for testing; this continued for several months, restricting testing to fewer patients. Progress of the project was disseminated regularly through stakeholder involvement meetings, supplemented with the semi-real-time mobile phone dashboard.
Quantitative data
Project impact
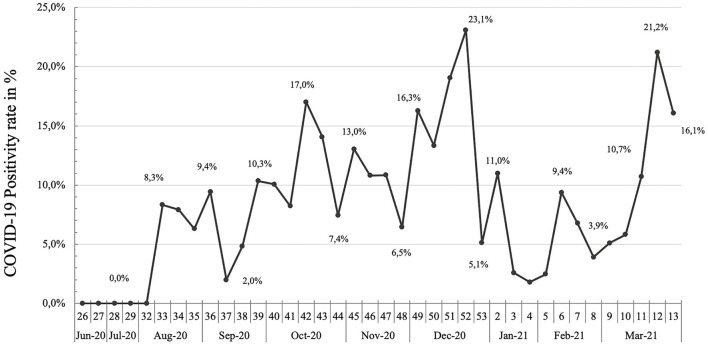
Through nine participating facilities, the COVID-Dx project supported a total of 4,324 PCR tests for SARS-CoV-2, of which 425 tested positive (49.4% female and 50.5% male). There was no significant association between gender and test result, Chi square p = 0.11. The overall COVID-19 positivity rate during the project was 9.8%. Figure 1 presents weekly positivity rate based on PCR tests during project implementation. As shown, there were no COVID-19 cases found during the 1st months of the project (June 2020 and July 2020). We noted a peak in COVID-19 cases in December 2020, which mirrored the Kenyan “second wave,” with a positivity rate of 23.1% at its highest point.
Figure 1.
PCR positivity rate over time in COVID-Dx project. June 2020–March 2021.
Table 3 provides an overview of everyone tested the percentage positive tested for COVID-19, by several groups.
Table 3.
Overview tested patients and % positive, by sub-groups.
| Number of tested patients (% of the total tested) | Positive tested within group, Number and (%) | |||
|---|---|---|---|---|
| Total | 4,324 | (100%) | 425 | (9.8%) |
| Female | 2,138 | (49.4%) | 194 | (9.1%) |
| Male | 2,186 | (50.5%) | 231 | (10.6%) |
| HCW | 1,009 | (23.3%) | 77 | (7.6%) |
| Non-HCW | 3,315 | (76.6%) | 348 | (10.5%) |
| Age < 15 | 295 | (6.8%) | 31 | (10.5%) |
| Age 15–24 | 534 | (12.3%) | 34 | (6.4%) |
| Age 25–34 | 1,348 | (31.2%) | 112 | (8.3%) |
| Age 35–44 | 998 | (23%) | 101 | (10.1%) |
| Age 45–54 | 581 | (13.4%) | 56 | (9.6%) |
| Age 55–64 | 349 | (8.1%) | 52 | (14.9%) |
| Age >65 | 199 | (4.6%) | 37 | (18.6%) |
Clinical presentation
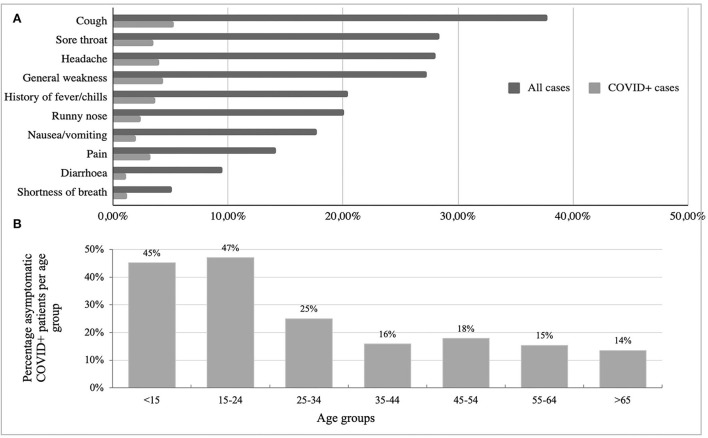
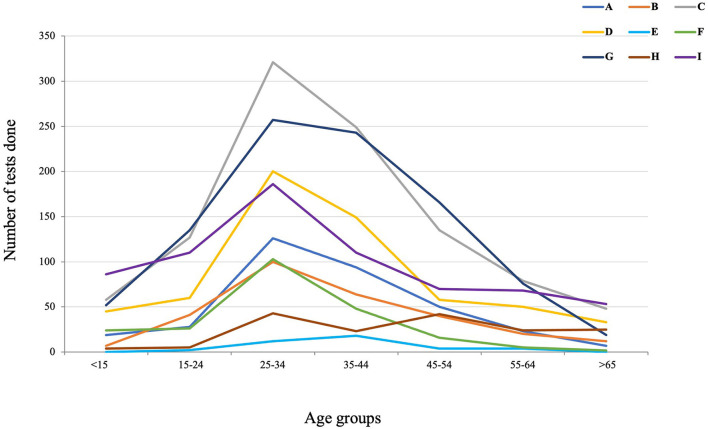
Patient reported clinical symptoms were captured using the CCIF-Tool. Reported symptoms from COVID-19 positive patients in order of frequency included: cough, headache, general weakness, and history of fever/chills. Figure 2A presents an overview of the most common symptoms reported for all cases and COVID-19 positive cases. Of note, 22.8% of all positively tested patients were asymptomatic at the time of testing. Twenty-two percent of all positive cases (both symptomatic and asymptomatic) reported pre-existing conditions, with cardiovascular disease as the most common; 9.9% of all positive cases had cardiovascular diseases, and 5.6% had diabetes. Figure 2B presents the percentage of COVID-19 positive cases who were asymptomatic per age group. This figure demonstrates a significant inverse correlation between age group and COVID-19 positive cases that were asymptomatic (Chi-square p = 0.00). Figure 3 shows the number of tests performed per age group during the project for all participating facilities. No facility differences in age distributions of tested individuals were found. The average age of negative tested patients was 36.4 years, and the average age of positive tested patients was significantly higher at 40.2 years (two-sample t-test p = 0.00).
Figure 2.
(A) Overview of the most common symptoms reported during COVID-Dx project roll-out (all cases vs. COVID+ cases in percentage). (B) Percentage asymptomatic COVID-19 patients per age group.
Figure 3.
Number of SARS-CoV-2 test done per age group per facility.
Healthcare workers
At these early stages of the COVID-19 epidemic, our PPP particularly prioritized healthcare workers who were at increased risk at the frontline of healthcare delivery. We performed 1,009 SARS-CoV-2 tests on healthcare workers (23.3% of total), representing 43% of total Kisumu County healthcare workforce. We noted a positivity rate of 7.6% among healthcare workers, which was slightly but significantly lower than the overall (9.8%) positivity rate (Chi-square p = 0.01). Moreover, 36% of tested healthcare workers who tested positive were asymptomatic. Other regularly tested groups were the self-employed (12.5%) and students (11.0%).
Scalability
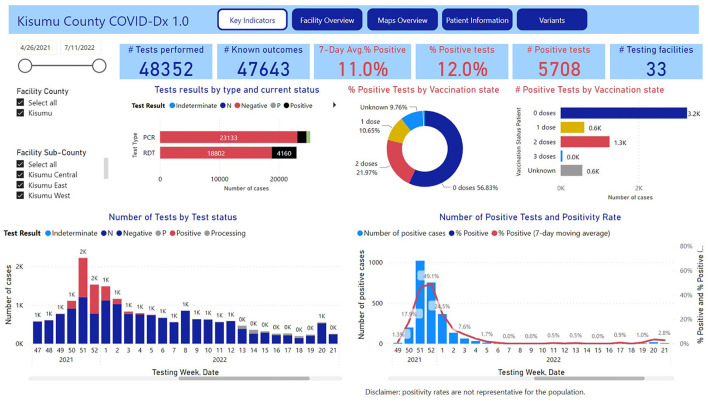
In early Q2 2021 when this PPP was virtually coming to an end, a sudden outbreak of COVID-19 Delta variant took place at a sugar estate in Kisumu (31). The DoH requested immediate support from COVID-Dx, which was provided in the form of procurement of RDTs to identify and possibly ring-fence hotspots of infection. More importantly, the COVID-Dx PPP team developed within 48 h a new dashboard that could accommodate data from 33 public and private health facilities in Kisumu. Up to date this dashboard has accumulated almost 50 K test results, 10-fold the original number realized by the 9 private facilities (Figure 4). Later in 2021 (Q3), the Lake Region Economic Bloc, a consortium of 14 Counties in West Kenya covering a population of 15 million Kenyans expressed its interest to adopt COVID-Dx. This subsequently was realized, with 84 participating COVID-19 service points and ~60 K tests performed up to today. The latest development is the transition of COVID-Dx into Epi-Dx, a digital platform that not only covers COVID, but also 19 additional epidemical diseases corresponding to the official Kenyan IDSR tool (32). Again, rapid scaling was quickly realized due to the digital framework of COVID-Dx and the trust already established working with PPP stakeholders.
Figure 4.
Screenshot dashboard COBID-Dx as per July, 2022.
Discussion
Qualitative insights
This paper describes experiences and findings during a rapid PPP intervention responding to the COVID-19 pandemic in Kisumu, Kenya. Lessons learnt could serve epidemic preparedness, in comparable sub-Saharan African settings. Typically, COVID-19 responses in Africa are addressed through “vertical” programs rolled out mainly through the public sector (33). This was done for HIV in the past, leading to ‘HIV exceptionalism’ (34) [parallel healthcare delivery and financing infrastructure in Africa and other LMICs, that is crucially dependent on international funding mechanisms through institutions like the GFATM and PEPFAR (35)].
This paper presents a model: integrating private healthcare facilities into the public COVID-19 response, embed this into the existing health system infrastructure: providers, staff, policy makers as well as research (KEMRI) institutions, and supporting this with a full digital trajectory tool. In Kenya, almost half of healthcare is delivered through private healthcare facilities. Therefore, at the onset of COVID-19, with then-unknown consequences, we opted for an immediate scaling of healthcare capacity to deliver COVID-19 services by creating a PPP model. This was informed by our experience with HIV service delivery starting in the private sector in Africa and expanding to the public sector (36). In addition, we wished to make use of important digital developments that are taking place in Africa and particularly Kenya that include the mobile phone revolution, the development of “bankless banking” through M-PESA, the digitalization of health data exchange (M-TIBA), the availability of smart-phone apps to support data dashboards and the advent of new digital diagnostic testing methods.
Challenges of building the PPP
Several challenges were encountered building the PPP. First, providing private healthcare facilities with access to public COVID-19 testing facilities could only be realized by positioning the PPP as a ‘scientific research project’. This implied developing a full research proposal along the KEMRI format and submission to various entities for ethical clearance. The process was accelerated by applying a 2-step procedure: first going for an expedited operational clearance at the Kisumu County level (1 month) to get COVID-Dx started and next to go in parallel for more extensive clearance at the national level (6 months). Our general recommendation from this experience is to install Ethical Review processes at the national (MoH) level in Kenya that can act within weeks and are allowed to be used when a health emergency is declared by the government.
Second, operational and material challenges for the PPP were frequently encountered, including high costs and lack of adequate laboratory supplies, laboratory equipment repair delays, and procuring PPE supplies for healthcare facilities. The essential PPE supplies were not readily available in Kenya when the project started and when available their prices were grossly overrated. Given the urgency to respond to the pandemic, we started with paying approximately double the price of PPEs. Waiting a few months into the project for prices to fall was not tenable at the time and would have resulted in significant delays. Throughout the project, the supply chain of PPEs was restored, and prices went gradually down, although laboratory equipment breakdown and supply chain disruptions remained a regular challenge. In general, the emergency required continuous adaptations and frequent consultations, which were facilitated by PharmAccess NGO. This helped building trust between the stakeholders and facilitated developing task divisions rapidly and naturally.
Third, during COVID-Dx, decisions by public entities sometimes had consequences for the set research protocols and processes in the project. For instance, the MoH revised the national guidelines on COVID-19 testing several times throughout the project, and some sample taking sites closed unilaterally without consulting other partners. The COVID-Dx project team continuously tried to manage all parties and adhere to National Guidelines. Additionally, the team requested the highest decision-makers in Kisumu County to have some decisions reviewed, based on practical experiences in the field.
The challenge of stigma during COVID-Dx
COVID-19 related stigma appeared much more important than expected. Some private facilities were afraid of being labeled COVID-19 testing sites. This fear was partially the result of (lockdown) measures implemented by government authorities early in the pandemic. When a public facility had staff who tested positive for COVID-19, the authorities closed the facility. Loss of income due to such measures was reportedly an important reason for private facilities to participate in COVID-Dx. To mitigate this, our project concentrated on testing healthcare staff at the participating facilities (and elsewhere), to keep them optimally informed, and in case of positivity, provide mental support services during the quarantines. Additionally, the facilities hesitated to join COVID-Dx because they were afraid patients would avoid visiting them out of fear of being infected, which would also reduce the number of patients with chronic conditions. To mitigate these fears, a letter from the Kisumu DoH was secured in which it was reassured that private facilities would not be closed if any of their patients tested positive for COVID-19. At the onset of COVID-Dx some reference to moral responsibilities of private facilities to pick up their role appeared important. When the first facilities eventually joined, this created confidence for other private facilities to follow suit.
To effectively combat COVID-19 stigma the COVID-Dx project recruited a senior Psychosocial Counselor to provide direct services to clients. During the roll-out these services appeared very much appreciated and needed scaling. Thus, the senior counselor trained the existing HIV counselors of participating providers to also address the mental challenges of COVID-19 (testing). All counselors underwent a training from the County's Mental Health and Psychosocial Support department as well as refreshers from the Senior Counselor. The counselors remained active throughout the project and were a great help to the facilities, the outreach team, and patients in contact tracing, encouraging contacts to test, reporting results to participants as part of post-test counseling and addressing patient fears around COVID-19 stigma. This intervention helped reducing fear, stigma, depression and other mental health related cases among the patients and health care workers.
Challenges identified by other studies
Challenges identified by other studies of PPPs in the healthcare sector in LMICs include providing diagnostics, the capacity to train and supervise private providers, disruptions in funding, slow implementation of the public sector, lack of information sharing, and mismatched organizational styles and differing priorities (37–40). Findings from Ghana implied that NGOs could be valuable to government for their ability to increase reach and to offer technical expertise (38). A different Kenyan study suggested consistency and flexibility are crucial to make PPPs successful (37). Our PPP experience confirms these findings. We learned, and literature also confirms, that to prevent, control and manage future outbreaks before these become epidemics, sub-Saharan African countries will need investments and political will so public health resources, PPPs and scientific expertise can be aligned (41). Additionally, we learned integrating digital technologies markedly improve and supports the responses to a pandemic. Digitalization increased transparency of funding channels, improved quality and efficiency of health service delivery and empowered policy makers to make data-based decisions in semi-real time.
Data challenges
Developing the CommCare App was a continuous process, with constant improvements to adapt to the needs of the healthcare facilities, needs in sample tracking and transportation, and reporting needs from the laboratories. These changes sometimes affected how we presented the data in the final database. Regular extensive data cleaning was required, which was done manually regularly, the database issues were resolved in time for the final data analyses. It is also vital to highlight that digitization of CCIF was a unique aspect of this project- which promoted quick data transmission compared to paper forms employed by MOH during that time. Health care facilities were motivated to use the COVID-Dx tools, because they could directly see their inputs uploaded into the dashboards visible on their mobile phones.
While first focusing on building a robust PPP model, during COVID-Dx we explored other opportunities to scale up. Early in the project, we experienced the substantial limitations of large-scale PCR testing, such as high costs, requirement of trained staff, sophisticated equipment, and good logistics. Often it was not possible to test all symptomatic patients. Moreover, even if testing was available, the time to report back testing results to patients took long. The original goal of 24–48 h was often not achieved and certainly not realized during the Christmas holiday season. Prolonged turnaround time for COVID-19 resulted in increased patient anxiety, impaired tracking of cases/contacts and hampered public health efforts against the pandemic. To exacerbate these diagnostic challenges and in the midst of stockouts of COVID-19 PCR test kits, VTMs and PPEs, limited molecular testing capacity seriously hampered efforts to tame to pandemic.
All these challenges led us to explore the possibilities of rapid testing for COVID-19 using antigen testing. The costs of RDTs are lower, results can be available within 20 min, no equipment is necessary, and less training is required. A sub-study within COVID-Dx was implemented to validate RDTs and compare them to PCR tests. The favorable results of this sub-study are published in a different paper (29).
Trust gained through COVID-Dx during the first part of the pandemic, led to a closer working relationship with Kisumu County to fight the next phase of the epidemic: the outbreak of the Delta variant in sub-Saharan Africa (4). As described in this paper, in May 2021, Kisumu County health officials decided to deploy the existing COVID-Dx network as a County-wide approach. PharmAccess became technical assistance partner to realize a COVID-Dx dashboard for Kisumu. This dashboard became accessible to key County policymakers and decision-makers, showing COVID-19 hotspots, positive cases, vaccination status, etc. Moreover, based on direct requests of DoH and participating health care providers a dashboard was developed that indicated availability of oxygen, numbers of hospital beds, ambulances, diagnostic tests, etc. This largely helped facilities encountering shortages to link up to neighboring facilities that according to the dashboard still had surpluses. Rapid scalability of COVID-Dx was thus proven on several occasions, with its digitalized trajectory monitoring tools and dashboard playing a crucial role.
Quantitative insights
COVID-Dx encountered an overall operational COVID-19 positivity rate of 9.8%. This figure should not be considered as representative for the Kisumu general population, since the number of weekly tests fluctuated due to operational issues, political decisions, or social challenges. Nevertheless, the positivity rate graph served as guidance for the project team to manage the project in terms of operational challenges, like staff fluctuations, procurement of supplies and utensils, transport arrangements. The project proved to be timely at the very onset of the COVID-19 epidemic in Kisumu, starting with a 0% positivity rate in June 2020 and moving upwards after the first positive cases were reported in Kisumu (42). The highest operational positivity rate was by December 2020 (23.1%), which is probably related to increased traveling and human contacts due to the festive season, creating the Kenyan “second wave,” which was more severe than the first wave (43, 44).
The average age of COVID-19 positive patients (40.2 years) appeared significantly higher than negative tested patients (36.4 years). This confirms the increased likeliness of older individuals to report for testing, possibly due to higher symptomatology in this age group. The data also showed older positive adults (34 and above) would be more likely to be symptomatic than younger patients. These observations are in line with other COVID-19 epidemiological data in Kenya (45) and elsewhere (46, 47). Importantly, the socio-demographic profiles of tested patients in COVID-Dx are not representative for Kisumu County or Kenya, since patients were selected according to regularly changing MoH national testing guidelines and priorities, due to lack of diagnostic supplies.
We considered healthcare workers a priority in the project. Goal was to keep them informed, working, and supported, in line with the MoH priorities. Healthcare workers were tested regularly during the project as per national protocol, on top of that we tested healthcare workers whenever they were showing symptoms or per personal request. Word of mouth through the staff of the 9 participating providers resulted in additional healthcare workers from other providers reporting for a COVD-19 test. The actual number of 1,009 tests performed would reflect a maximum 43% of the Kisumu health work force. The actual figure will be lower, since healthcare staff often went for repeat testing, which was not documented for using the CCIF. Testing healthcare workers regularly contributed to them feeling more safe while executing their work, motivating them to continue sample-taking and assisting patients.
Conclusion
Sudden health emergencies such as pandemics are a serious challenge to already strained health systems in resource-poor settings, particularly in sub-Saharan Africa. In those circumstances, public health sector effort benefit from complementary private efforts. This paper describes a successful PPP, involving both public and private sector health facilities in Western Kenya. We demonstrated feasibility by taking a “can do” approach and addressing operational challenges step-by-step as they unfolded, facilitated by a coordinating NGO. The model was proven scalable in practice, expanding to Kisumu (and recently even to 13 other Counties) and can serve as an example of PPPs for epidemic preparedness in SSA. Specific additional strength of this approach was the investment in digitalization and digital dashboards for project monitoring, patient service delivery, health facility improvement and rapid data processing to inform policy makers and health managers. This accelerated operational decision-making, such as timely identification of COVID-19 hotspots so DoH outreach teams could be deployed efficiently. Building epidemic preparedness benefits from African health systems going digital.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon written request without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical clearance for this project was obtained from Jaramogi Oginga Odinga Teaching & Referral Hospital (JOOTRH) on June 16, 2020, with approval number IERC/JOOTRH/230/2020. KEMRI also provided ethical clearance on September 30, 2020, with approval number KEMRI KEMRI/SERU/CGHR/05-05/4038. Research License was obtained from the National Commission for Science, Technology, and Innovation (NACOSTI) on July 6, 2020 (NACOSTI/P/20/5616). The roll-out of this project was in complete coordination with the Kisumu Department of Health. Sample collection in patients in this project was voluntary. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
TR designed the project and the research study, and additionally scientifically supervised the project. NH assisted with the design and implementation of the study and managed the operations in Kenya of this project. SK and AO oversaw research activities and were involved at decision-making level. HB contributed to the project design and implementation and managed KEMRI's role. EM was responsible for onsite day-to-day operations during the project and managed relationships between all partners. MO assisted in the field and was responsible for the social sciences. RA managed our psychosocial support on the ground. AK'O regularly visited facilities for data management and managed the database. TS developed and updated the dashboard. MJ supported with clinical supervision. KO managed the laboratory aspects of this study. SO contributed research, such as the RDT evaluation. CW managed relations with our donors and reported back results to them. SD conducted data analyses, wrote the manuscript, and submitted this manuscript.
Acknowledgments
The authors would like to express gratitude to all clinicians, nurses, lab technicians, motorbike riders, data entry personnel and all other personnel who were active in the field during this study. The CommCare mobile data collection software was provided by Dimagi free of charge. We would also like to thank the Kisumu Department of Health for its guidance and cooperation to execute this project. We would like to thank our funders: Achmea Foundation, Pfizer Foundation, and the Netherlands Ministry of Foreign Affairs.
Funding Statement
This project was financially supported by Achmea Foundation (Donation Agreement Initiatief 2020.002), Pfizer Foundation, and the Netherlands Ministry of Foreign Affairs (Institutional Grant to Pharm Access Foundation 2016-2022). The funders did not have any role in study design, data collection, analysis, interpretation, summarizing the data or decision to submit the manuscript for publication.
Abbreviations
CCIF, COVID Case Identification Form; COVAX, COVID-19 Global Vaccine Access; COVID-19, coronavirus disease of 2019; DoH, Department of Health; GDPR, General Data Protection Regulation; GFATM, The Global Fund to Fight AIDS, Tuberculosis and Malaria; HIPAA, Health Insurance Portability and Accountability Act of 1996; KEMRI, Kenya Medical Research Institute; LMIC, low-and middle-income countries; MoH, Ministry of Health; NGO, non-governmental organization; PCR, polymerase chain reaction; PEPFAR, President's Emergency Plan for AIDS Relief; PPE, personal protective equipment; PPP, public-private partnership; RDT, rapid diagnostic test; RNA, Ribonucleic acid; ToT, trainers of trainers; UHC, universal health coverage; VTM, viral transport medium; WHO, World Health Organization.
Conflict of interest
SD, TS, CW, TR, EM, RA, and NH are employed by PharmAccess Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.837215/full#supplementary-material
References
- 1.Shamasunder S, Holmes SM, Goronga T, Carrasco H, Katz E, Frankfurter R, et al. COVID-19 reveals weak health systems by design why we must re-make global health in this historic moment. Glob Public Health. (2020) 15:1083–9. 10.1080/17441692.2020.1760915 [DOI] [PubMed] [Google Scholar]
- 2.Dzinamarira T, Dzobo M, Chitungo I. COVID-19 A perspective on Africa's capacity and response. J Med Virol. (2020) 92:2465–72. 10.1002/jmv.26159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Q, Dong XP. Rapid Global Spread of the SARS-CoV-2 Delta (B. 1617 2) variant spatiotemporal variation and public health impact. Zoonoses. (2021). 10.15212/ZOONOSES-2021-0005 [DOI] [Google Scholar]
- 4.WHO . Rife COVID-19 variants fuel Africa's surging wave. (2021). Available online at: https://www.afro.who.int/news/rife-covid-19-variants-fuel-africas-surging-wave (accessed August 5, 2021).
- 5.MoH . COVID-19 (2021). Available online at: https://www.health.go.ke/ (accessed August 30, (2021).
- 6.WHO . Kenya receives COVID-19 vaccines and launches landmark national campaign. (2021). Available online at: https://www.afro.who.int/news/kenya-receives-covid-19-vaccines-and-launches-landmark-national-campaign (accessed March 17, 2021).
- 7.Orangi S, Pinchoff J, Mwanga D, Abuya T, Hamaluba M, Warimwe G, et al. Assessing the level and determinants of COVID-19 vaccine Confidence in Kenya. Vaccines. (2021) 9:2021.06.11.21258775. 10.3390/vaccines9080936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muleshe S. COVID-19 Vaccine Deployment-Overview & Rollout Plan. Planning and Coordination Covid-19 Vaccines Deployment and Vaccination Taskforce. Nairobi: Ministry of Health; (2021). [Google Scholar]
- 9.CDC . COVID-19 Vaccine. (2021). Available online at: https://africacdc.org/document-tag/covid-19-vaccine/ (accessed July 12, 2021).
- 10.WHO . Achieving 70% COVID-19 Immunization Coverage by Mid-2022. (2021). Available online at: https://www.who.int/news/item/23-12-2021-achieving-70-covid-19-immunization-coverage-by-mid-2022 (accessed July 12 2022).
- 11.Kenya M. COVID-19 Antigen Rapid Diagnostic Testing Interim Guide KENYA Kenya Ministry of Health. Nairobi: Ministry of Health; (2020). [Google Scholar]
- 12.WHO . Maintaining Essential Health Services Operational Guidance for the COVID-19 Context. Geneva: World Health Organization; (2020). [Google Scholar]
- 13.WHO . State of Health Financing in the African Region World Health Organization. Brazzaville: WHO Regional office for Africa; (2013). [Google Scholar]
- 14.Chakraborty NM, Montagu D, Wanderi J, Oduor C. Who serves the poor? An equity analysis of public and private providers of family planning and child health services in Kenya. Front Public Health. (2018) 6:374. 10.3389/fpubh.2018.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu S, Andrews J, Kishore S, Panjabi R, Stuckler D. Comparative performance of private and public healthcare systems in low- and middle-income countries a systematic review. PLoS Med. (2012) 9:e1001244. 10.1371/journal.pmed.1001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elebesunu EE, Oke GI, Adebisi YA, Nsofor IM. COVID-19 calls for health systems strengthening in Africa A case of Nigeria. Int J Health Plann Manage. (2021) 36:2035–43. 10.1002/hpm.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanzo J, Shawar YR, Shyam T, Das S, Shiffman J. Challenges to establish effective public-private partnerships to address malnutrition in all its forms. Int J Health Policy Manag. (2021). 10.34172/ijhpm.2020.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker LA, Zaragoza GA, Hernández-Aguado I. Promoting population health with public-private partnerships where's the evidence? BMC Public Health. (2019) 19:1438. 10.1186/s12889-019-7765-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellekens O, Lindner ME, Lange JMA, van der Graaf J. A New Paradigm for Increased Access to Healthcare in Africa. Amsterdam: PharmAccess Foundation; (2007). [Google Scholar]
- 20.Healthcare in Africa Public – Private Partnerships are key. (2019). Available online at: https://african.business/2019/02/economy/healthcare-in-africa-public-private-partnerships-are-key/ (accessed April 7, 2021).
- 21.Schellekens OP, de Beer I, Lindner ME, van Vugt M, Schellekens P, Rinke de Wit TF. Innovation in Namibia preserving private health insurance and HIV/AIDS treatment. Health Aff. (2009) 28:1799–806. 10.1377/hlthaff.28.6.1799 [DOI] [PubMed] [Google Scholar]
- 22.Convergence . Case study Medical Credit Fund (MCF). Amsterdam: Medical Credit Fund; (2019). [Google Scholar]
- 23.PharmAccess . County Government of Kisumu signs a new partnership to establish a scheme for those who cannot afford health insurance. (2020). Available online at: https://www.pharmaccess.org/update/county-government-of-kisumu-signs-a-new-partnership-to-establish-a-scheme-for-those-who-cannot-afford-health-insurance/ (accessed April 7, 2021).
- 24.Mtiba . M-TIBA Homepage. (2021). Available online at: https://mtiba.com/ (accessed July 12, 2022).
- 25.Johnson MC, Schellekens O, Stewart J, van Ostenberg P, de Wit TR, Spieker N. SafeCare an innovative approach for improving quality through standards, benchmarking, and improvement in low- and middle- income countries. Jt Comm J Qual Patient Saf. (2016) 42:350–71. 10.1016/S1553-7250(16)42049-0 [DOI] [PubMed] [Google Scholar]
- 26.Kenya M. Interim Guidelines on Management of COVID-19 in Kenya. Nairobi: Ministry of Health Kenya; (2020). [Google Scholar]
- 27.Scientific FS. F&S scientific - for all your laboratory requirements. (2021). Available online at: https://fnscientific.com/ (accessed November 23, 2021).
- 28.Thermo Fisher Scientific, MA, USA . (2021). Available online at: https://www.thermofisher.com/nl/en/home/global/forms/life-science/sars-cov-2-mutations-variants.html?icid=WB312750 (accessed December 2, 2021).
- 29.Onsongo SN, Otieno K, van Duijn S, Adams E, Omollo M, Odero IA, et al. Performance of a rapid antigen test for SARS-CoV-2 in Kenya. Diagn Microbiol Infect Dis. (2022) 102:115591. 10.1016/j.diagmicrobio.2021.115591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Microsoft . Microsoft PowerBI. (2021). Available online at: https://powerbi.microsoft.com/en-us/ (accessed November 22, 2021).
- 31.Yusuf M. Kenya's Kisumu Emerges as New COVID-19 Hotspot. Voanews.com (2021). [Google Scholar]
- 32.Health Mo WHO . Standard Case Definition for Priority Diseases in Kenya. In (IDSR) IDSaR (editor). Nairobi: Ministry of Health; (2015). [Google Scholar]
- 33.Murewanhema G, Dzinamarira T. The COVID-19 Pandemic Public Health Responses in Sub-Saharan Africa. Int J Environ Res Public Health. (2022) 19:84448. 10.3390/ijerph19084448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sands P, HIV. from exceptionalism to endgame. Lancet. (2018) 392:261–2. 10.1016/S0140-6736(18)31434-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessema GA, Kinfu Y, Dachew BA, Tesema AG, Assefa Y, Alene KA, et al. The COVID-19 pandemic and healthcare systems in Africa a scoping review of preparedness, impact and response. BMJ Glob Health. (2021) 6:7179. 10.1136/bmjgh-2021-007179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Borght S, Rinke de. Wit TF, Janssens V, Schim van der Loeff MF, Rijckborst H, Lange JMA. HAART for the HIV-infected employees of large companies in Africa. The Lancet. (2006) 368:547–50. 10.1016/S0140-6736(06)69165-4 [DOI] [PubMed] [Google Scholar]
- 37.Hill J, Kimani K, White A. Daisy's eye cancer fund and The Kenyan national retinoblastoma strategy group. Achieving optimal cancer outcomes in East Africa through multidisciplinary partnership a case study of the Kenyan National retinoblastoma strategy group. Global Health. (2016) 12:23. 10.1186/s12992-016-0160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hushie M. Public-non-governmental organisation partnerships for health an exploratory study with case studies from recent Ghanaian experience. BMC Public Health. (2016) 16:1–13. 10.1186/s12889-016-3636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakaya J, Uplekar M, Mansoer J, Kutwa A, Karanja G, Ombeka V, et al. Public-private mix for control of tuberculosis and TB-HIV in Nairobi, Kenya outcomes, opportunities, and obstacles. The Int J Tubercul Lung Dis. (2008) 12:1274–8. [PubMed] [Google Scholar]
- 40.Ravishankar N, O'Hanlon B, Dunworth A, Nakyanzi A, Wawire, S. Private Capacity, Public Payment: Private Business Participation in Government Initiatives to Improve Access to Critical Health Services. London: The BEAM Exchange; (2016). Available online at: https://www.beamexchange.org [Google Scholar]
- 41.Osseni IA. COVID-19 pandemic in sub-Saharan Africa preparedness, response, and hidden potentials. Trop Med Health. (2020) 48:1–3. 10.1186/s41182-020-00240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MOH K. COVID-19 Outbreak in Kenya Daily Situation Report – 128. Nairobi: Ministry of Health Kenya; (2020). [Google Scholar]
- 43.Edward-Ekpu U. Scientists are worried a second wave of Covid-19 infections is starting in Kenya and South Africa. (2020). https://qz.com/africa/1942066/scientists-worry-second-wave-of-covid-19-in-kenya-south-africa/ (accessed August 5, 2021).
- 44.Salyer SJ, Maeda J, Sembuche S. The first and second waves of the COVID-19 pandemic in Africa a cross-sectional study. Lancet. (2021) 397:1265–75. 10.1016/S0140-6736(21)00632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ombajo LA, Mutono N, Sudi P, Mutua M, Sood M, Loo AM, et al. Epidemiological and clinical characteristics of patients hospitalised with COVID-19 in Kenya a multicentre cohort study. BMJ Open. (2022) 12:e049949. 10.1136/bmjopen-2021-049949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arima Y, Kanou K, Arashiro T, Ko YK, Otani K, Tsuchihashi Y, et al. Epidemiology of coronavirus disease. 2019 in japan descriptive findings and lessons learned through surveillance during the first three waves. JMA J. (2021) 4:198–206. 10.31662/jmaj.2021-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehmer TK, DeVies J, Caruso E, van Santen KL, Tang S, Black CL, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August. 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1404–9. 10.15585/mmwr.mm6939e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon written request without undue reservation.