Abstract
Aims
To predict the vault and the EVO-implantable collamer lens (ICL) size by artificial intelligence (AI) and big data analytics.
Methods
Six thousand two hundred and ninety-seven eyes implanted with an ICL from 3536 patients were included. The vault values were measured by the anterior segment analyzer (Pentacam HR). Permutation importance and Impurity-based feature importance are used to investigate the importance between the vault and input parameters. Regression models and classification models are applied to predict the vault. The ICL size is set as the target of the prediction, and the vault and the other input features are set as the new inputs for the ICL size prediction. Data were collected from 2015 to 2020. Random Forest, Gradient Boosting and XGBoost were demonstrated satisfying accuracy and mean area under the curve (AUC) scores in vault predicting and ICL sizing.
Results
In the prediction of the vault, the Random Forest has the best results in the regression model (R2=0.315), then follows the Gradient Boosting (R2=0.291) and XGBoost (R2=0.285). The maximum classification accuracy is 0.828 in Random Forest, and the mean AUC is 0.765. The Random Forest predicts the ICL size with an accuracy of 82.2% and the Gradient Boosting and XGBoost, which are also compatible with 81.5% and 81.8% accuracy, respectively.
Conclusions
Random Forest, Gradient Boosting and XGBoost models are applicable for vault predicting and ICL sizing. AI may assist ophthalmologists in improving ICL surgery safety, designing surgical strategies, and predicting clinical outcomes.
Keywords: treatment surgery
INTRODUCTION
Uncorrected or undercorrected myopia is a major cause of visual impairment, which has caused billions of US$ in lost productivity worldwide.1 It is a common occurrence that high myopia, particularly extremely high myopia, is likely to be undercorrected as many patients with extremely high myopia cannot tolerate full correction with spectacles because of spectacle magnification problems or aniseikonia.2 Corneal refractive surgeries are efficient for myopia correction, while the amount of correction is limited by corneal thickness (CT) and keratometry. Contact lenses are able to correct refractive errors ranging from −20 dioptres (D) to +20 D. However, improper daily cleaning and maintenance may lead to keratitis.
Posterior chamber phakic intraocular lens (mode V4C, including non-toric implantable collamer lens (ICL) V4C and toric ICL V4C) implantation has gained popularity among myopes and has become one of the mainstream surgeries for myopia correction, especially for extremely high myopia. With a soft, biocompatible lens been implanted into the posterior chamber, myopia up to −18 D and astigmatism up to 6 D can be fully corrected with satisfying visual outcome and life quality.3 4 Nevertheless, the majority of reported complications after ICL implantation are related to improper ICL size. An undersized ICL will lead to an insufficient vault, less than 90 μm, which may increase the risk of cataracts due to the contact between the ICL and the crystalline lens. In contrast, an oversized ICL may result in an excessive vault (>1000 µm), which pushes the iris forward, leading to acute angle closure. The idea vault is recommended ranging from 250 µm to 750 μm.5
Accurate sizing is key to maintaining a safe vault and achieving a successful ICL implantation procedure. The manufacturer has provided an online calculator for ICL sizing, mainly accords to horizontal corneal diameter (white to white (WTW)) and anterior chamber depth (ACD) values measurements. However, literature has reported that the present ICL calculator may cause excessively low or high vault in some cases5 6 as there are only four sizes (diameter of 12.1 mm, 12.6 mm, 13.2 mm or 13.7 mm) of ICL-V4C (non-toric or toric ICL-V4C) available for selection, which do not fit all patients. To optimise the method of ICL sizing and improve the accuracy and precision of vault prediction, more detailed anatomic dimensional parameters, including angle-to-angle, anterior chamber area or ciliary sulcus diameter (sulcus to sulcus (STS)),7–9 are obtained using various ophthalmic devices, for instance, the scheimpflug technique based anterior segment analyzer (Pentacam HR), optical coherence tomography, and ultrasound biomicroscopy (UBM). Meanwhile, many formulas have been developed9–11 using regression models.
However, rather than a single parameter, the vault is affected by multiple factors.5 9–13 The accuracy and precision of those formulas are suboptimal.6–10 Up to the present, the prediction of vault and ICL sizing remains technically problematic.
Artificial intelligence (AI) technology has been employed to diagnose eye diseases, such as age-related macular degeneration,14 glaucoma15 and diabetic retinopathy16 for several years. Nevertheless, so far, the application of big data-based AI in ICL implantation has not been reported yet. Machine learning (ML), a subset of AI, combined with big data, is a powerful tool that identifies relationships among various variables and analyses multidimensional data. This study explores ML and big data analytics applications for predicting vault and sizing ICL.
Methods
Patients
Patients who underwent ICL-V4C implantation from 2015 to 2020 at the Eye and ENT Hospital of Fudan University in Shanghai, China, were enrolled in this cross-sectional study. Patients were excluded from the study if they had a history of other ocular diseases or surgery that could affect any measurement. Finally, 6942 eyes were collected, after eliminating the missing data, 6297 eyes (3181 right eyes and 3116 left eyes) of 3536 patients (800 male (1397 eyes) and 2736 female (4900 eyes), mean age: 26.1±5.9 years)were included in this study.
All study procedures adhered to the tenets of the Declaration of Helsinki. Each participant was provided with informed consent after a detailed explanation of the procedure prior to treatment. In this study, all patients’ names were hidden.
Measurements
All patients underwent an ophthalmic examination before the operation, including uncorrected distance visual acuity, corrected distance visual acuity, autorefraction, manifest refraction, intraocular pressure assessment (Canon Full Auto Tonometer TX-F; Canon, Tokyo, Japan), slit lamp examination and funduscopic examinations. Anterior segment parameters, including steepest meridian keratometry (K1), flattest meridian keratometry (K2), ACD, anterior chamber angle (ACA), pupil size, CT and WTW, were obtained by using an anterior segment analyzer (Pentacam HR, OCULUS; Wetzlar, Germany). Axial length (AL) was measured using laser-assisted optical biometers (IOL-master 1322–734, Carl Zeiss Meditec AG, Jena, Germany). STS was measured by using a UBM (Quantel Medical, France). All the ICLs were prescribed by an experienced technician according to all the parameters above. After the operation, all the above anterior segment parameters and the vault values were measured using the anterior segment analyzer. The follow-up periods were 30.5 weeks (ranging from 1 to 270 weeks).
Machine learning
Selection of the input parameters
Permutation importance and Impurity-based feature importance are used to investigate the importance between the vault and input parameters (including ACA, pupil size, AL, K1, K2, K1 axis, K2 axis, sphere, cylinder, ACD, CT, WTW, spherical equivalent (SE), ICL size, type of ICL, the sphere of ICL, the cylinder of ICL, the time after surgery). The data are randomly divided into the training set and testing set according to the ratio of 9:1 (we use the random package in the Python programming language to generate a random number sequence).
Prediction of the vault
Regression models and classification models are applied to predict the vault. The accurate value of the vault is set as the output of the regression model. The regression models include Lasso, Ridge, Decision Tree, Random Forest, AdaBoost, Gradient Boosting, XGboost and SVR (Support Vector Regression). In the classification model, patients are divided into three groups based on their vault: (0, 250), (250, 750) and (750, +∞), respectively. The classification models include Decision Tree, Random Forest, AdaBoost, Gradient Boosting, XGBoost and Support Vector Classification.
These models' inputs are 18-dimensional features, including preoperative parameters, information of ICL and the time after surgery. Fivefold cross-validation is employed to reduce the uncertainty caused by data partition.
The data are randomly divided into the training set and test set according to the ratio of 8:2. R2-score and root mean square error (RMSE) are applied as the regression models’ evaluation metrics, and the accuracy and mean area under the curve (AUC) for the classification models.
Prediction of the ICL size
The ICL size is set as the target of the prediction, and the vault and the other input features are set as the new inputs for the ICL size prediction. The Random Forest, Gradient Boosting and the XGBoost are performed to build the model for predicting the ICL size.
Results
Selection of the input parameters
The importance of the ICL size, ACD and pupil size are 1.00, 0.64 and 0.51 in Permutation importance. The importance of the ICL size and ACD are 1.00 and 0.52 in Impurity-based feature importance. The cylinder and cylinder of ICL are not as crucial as the others (importance=0.08 and 0.08). Table 1 lists the importance between input features and the vault.
Table 1.
The importance of 18 features in two methods
| Permutation importance | Impurity-based feature importance | ||
| Feature | Importance | Feature | Importance |
| ICL size | 1.00 | ICL size | 1.00 |
| ACD | 0.64 | ACD | 0.52 |
| Pupil size | 0.51 | Type of ICL | 0.29 |
| ACA | 0.47 | The time after surgery | 0.19 |
| CT | 0.45 | Pupil size | 0.16 |
| AL | 0.44 | WTW | 0.14 |
| The time after surgery | 0.40 | AL | 0.11 |
| K2 value | 0.31 | CT | 0.09 |
| K2 axis | 0.31 | ACA | 0.08 |
| K1 value | 0.31 | K2 value | 0.07 |
| K1 axis | 0.28 | Sphere | 0.07 |
| WTW | 0.27 | K1 value | 0.06 |
| Sphere | 0.26 | Sphere of ICL | 0.04 |
| Sphere of ICL | 0.18 | K2 axis | 0.04 |
| SE of ICL | 0.17 | SE of ICL | 0.02 |
| Type of ICL | 0.13 | K1 axis | 0.02 |
| Cylinder | 0.08 | Cylinder | 0.01 |
| Cylinder of ICL | 0.08 | Cylinder of ICL | 0.0 |
The highest importance aligned to 1.0 and scale the left values accordingly.
ACA, anterior chamber angle; ACD, anterior chamber depth; AL, axial length; CT, corneal thickness; ICL, implantable collamer lens; K1, steepest meridian keratometry; K2, flattest meridian keratometry; SE, spherical equivalent; WTW, white to white.
Prediction of the vault
In all patients, 305 eyes’ vaults are under 250, 5131 eyes are with normal vault and 861 eyes’ vaults are greater than 750. The Random Forest has the best results in the regression model (R2=0.315, RMSE=159.026), then follows the Gradient Boosting (R2=0.291, RMSE=161.862) and XGBoost (R2=0.285, RMSE=162.527). The performance of the regression models for vault prediction is listed in table 2.
Table 2.
Performance of the regression models for postoperative vault prediction
| Model | R2-Score (95% CI) | RMSE (95% CI) |
| Lasso | 0.237 (0.216 to 0.257) | 167.924 (165.551 to 170.297) |
| Ridge | 0.237 (0.216 to 0.258) | 167.869 (165.566 to 170.173) |
| Decision Tree | 0.170 (0.145 to 0.195) | 175.098 (172.201 to 177.996) |
| Random Forest | 0.315 (0.295 to 0.336) | 159.026 (155.988 to 162.065) |
| Adaboost | 0.157 (0.136 to 0.178) | 176.519 (172.779 to 180.259) |
| Gradient Boosting | 0.291 (0.267 to 0.315) | 161.862 (158.963 to 164.761) |
| XGBoost | 0.285 (0.260 to 0.310) | 162.527 (159.163 to 165.890) |
| SVR | 0.154 (0.142 to 0.165) | 176.900 (173.008 to 180.791) |
RMSE, root mean square error; SVR, Support Vector Regression.
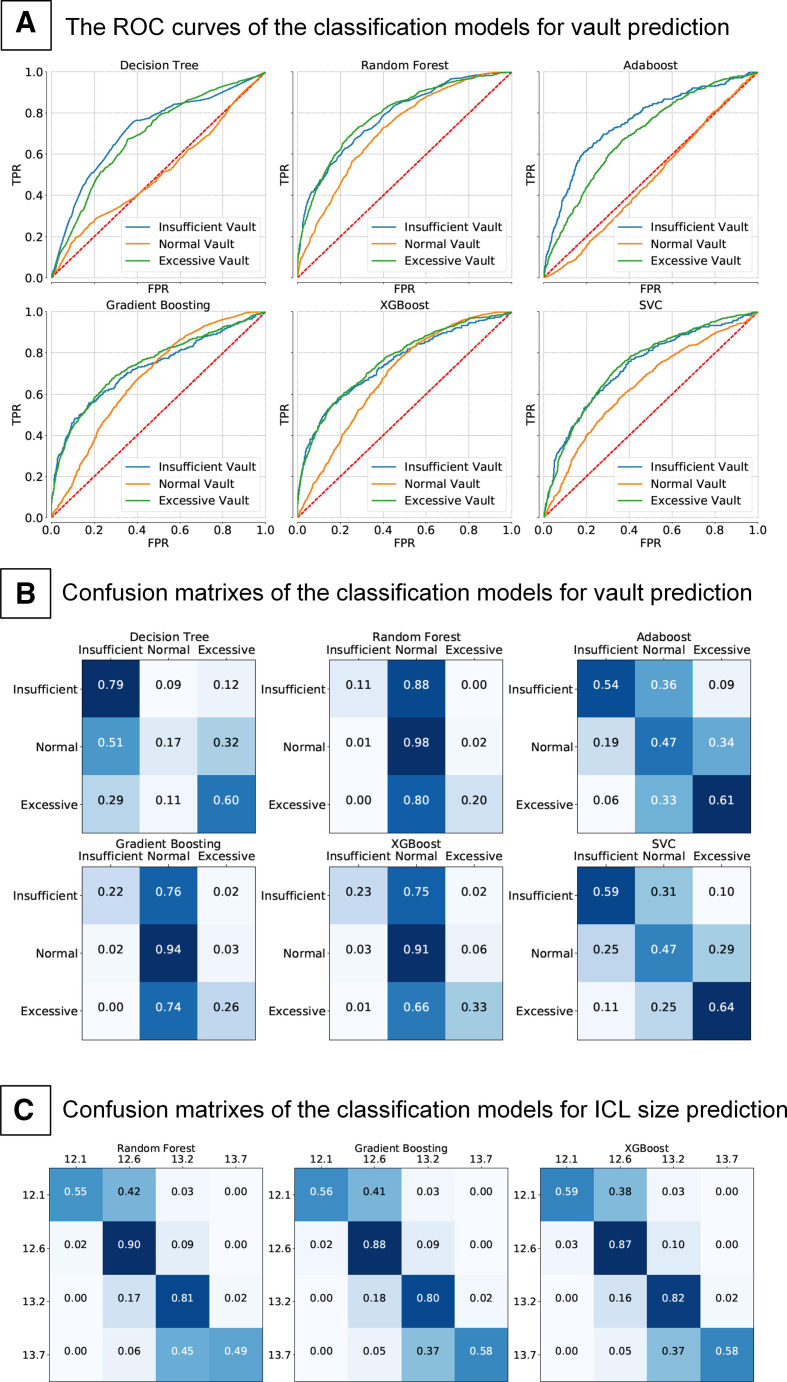
The results show that the Random Forest, Gradient Boosting and XGBoost outperform other classification methods. As shown in table 3 and figure 1A, the maximum classification accuracy (0.828) is achieved by Random Forest, and the mean AUC is 0.765. The confusion matrixes of the classification models' results are shown in figure 1B.
Table 3.
Performance of the classification models for postoperative vault prediction
| Model | Accuracy (95% CI) | Mean AUC (95% CI) |
| Decision Tree | 0.261 (0.232 to 0.290) | 0.637 (0.630 to 0.643) |
| Random Forest | 0.828 (0.819 to 0.836) | 0.765 (0.744 to 0.785) |
| Adaboost | 0.492 (0.486 to 0.499) | 0.645 (0.629 to 0.661) |
| Gradient Boosting | 0.815 (0.809 to 0.821) | 0.718 (0.690 to 0.746) |
| XGBoost | 0.802 (0.791 to 0.813) | 0.733 (0.707 to 0.759) |
| SVC | 0.497 (0.494 to 0.499) | 0.705 (0.690 to 0.721) |
AUC, area under the curve; SVC, Support Vector Classification.
Figure 1.
The ROC curves of the classification models for vault prediction (A). Confusion matrixes of the classification models for vault prediction (B). Confusion matrixes of the classification models for ICL size prediction (C). ICL, implantable collamer lens.
Prediction of ICL size
As listed in table 4, the Random Forest predicts the ICL size with an accuracy of 82.2% and the Gradient Boosting and XGBoost, which are also compatible with 81.5% and 81.8% accuracy, respectively. The confusion matrixes of the classification models for ICL size prediction are shown in figure 1C.
Table 4.
Performance of the ICL size prediction
| Model | Accuracy (95% CI) |
| Random Forest | 0.822 (0.813 to 0.831) |
| Gradient Boosting | 0.815 (0.805 to 0.825) |
| XGBoost | 0.818 (0.808 to 0.828) |
ICL, implantable collamer lens.
Discussion
The global demand for refractive surgery is booming. ICL implantation demonstrates outstanding efficacy5 17 18 and has been reaching a great prospect in refractive error correction in recent years. However, the safety of the procedure is tightly related to an appropriate ICL size and vault height.
The present study screened 18 characteristic parameters, trained and tested by 8 classic ML models to predict vault and ICL size. As expected, ICL size is the most important feature among the 18 parameters for vault prediction, consistent with Akihito Igarashi’s study.19 Meanwhile, the preoperative values of ACD, pupil size, ACA, AL, CT and the time after surgery weighted heavily with ICL vault prediction, indicating all the above parameters should not be ignored when predicting the vault or guide ICL sizing. Alfonso et al 20 reported that the vaults had a statistically significant correlation with preoperative ACD and WTW, but the R2 values were only around 0.1. It is no wonder that widely distributed vaults (0 to over 1000 µm) were reported in the majority of the previous studies.5 Trancón et al identified the ICL size and ICL SE as predictors of the vault height. However, their multivariate model only explained 34% of vault variability.13 Lee et al 12 reported that ICL size, age and K-reading significantly associated with vault, but ICL size—WTW did not, which is similar to our findings.
The present study revealed that pupil size significantly affects the vault, thus affecting ICL sizing. It is known that pupil diameters and pupil constriction amplitude may decrease after operation.21 Moreover, the decrease in pupil diameter is always associated with the decrease in the vault. Mechanical contact between the ICL and the posterior iris surface may be the potential mechanisms.8 22 Chen23 evaluated the vault changes after ICL implantation and assessed its correlation with pupil size changes. They concluded that pupil movement was demonstrated to be a critical factor in vault change. We hypothesise that preoperative pupil diameter may associate with iris tension, which pushes ICL back towards the crystalline lens after ICL implantation, resulting in a lower vault.
Long-term studies have detected that the vault keeps changing over time after operation.4 24 ICL rotation25 and age-related lens thickening24 are the most probable reasons. Our results demonstrated that the time after surgery contributes similarly to pupil size and AL in the feature importance, which ranks the seventh among the 18 feature parameters, indicating the vault changes dynamically over time after the operation and may affect the ICL sizing accuracy.
As the vault is the key parameter to the procedure’s safety, we assessed the performance of eight ML models for vault value prediction. Although the Random Forest model demonstrated the best fitting precision among the eight models, the R2 value is still too low (R2=0.316) to meet the clinical requirement. One probable explanation can be that the ocular dimensional parameters are continuously and normally distributed variables. In contrast, the ICL size is non-continuous (only four sizes are available), which cannot fit all the patients, leading to a wide deviation in vault values among the population after ICL implantation. Moreover, the vault values change over time. The exact value of the vault value may be undeterminable. In practice, it is not necessary to predict the exact value of the vault. A reliable prediction on the possible range of vault may be more applicable and meaningful to guide ICL sizing, thus achieving a safe vault height after the operation.
In order to improve the performance of the models, the vault data are divided into three categories, including insufficient vault (<250 µm), normal vault (250–750 μm) and excessive-high vault (>750 µm). Random Forest, Gradient Boosting, XGBoost, Adaboost, Decision Tree, and SVC were performed to train the vault category prediction models. The results demonstrated that Random Forest yielded the highest accuracy of 0.828 and an average AUC of 0.764 in predicting the vault category. These tree-based ensemble methods are powerful and stable algorithms, which performed competently with continuous and categorical variables.26 Precisely, ICL vault prediction also involves continuous variables (such as ocular biometric parameters) and categorical variables. That may be why Random Forests, Gradient Boost and so on showed relatively higher AUC and accuracy than the models such as Lasso, Ridge, Decision tree and SVR. Furthermore, methods like Random Forests are more applicable in classification tasks than regression as they cannot give precise continuous nature prediction.27 That may explain why the tree-based models showed a poor performance in predicting vault values but demonstrated outstanding accuracy in vault categories classification.
The present study also trained ML models to determine ICL size.28 Gradient Boosting and XGBoost showed similar global accuracy and average AUC to Random Forests when predicting ICL size, indicating Random Forests, XGBoost, and Gradient Boosting are all valuable for ICL size prediction. The further analysis detected Random Forests, XGBoost, and Gradient Boosting demonstrated similar accuracy when predicting ICL sizes of 12.6 mm and 13.2 mm. XGBoost showed higher accuracy in predicting the ICL size of 12.1 mm than Random Forests. Besides, XGBoost showed the highest accuracy in predicting the ICL size of 12.1 mm among the three models. Hence, in order to obtain the highest accuracy of prediction, all the above three models were recommended to be applied.
There are some limitations in the present study. As the present study is a cross-sectional study rather than a longitudinal study, the vault changes over time are not assessed and remaining unknown. A long-term follow-up is essential to predict the changes in vault height over time. The second limitation is the relatively small sample size of the patients implanted with extremely small (12.1 mm) or large (13.7 mm) ICLs. As the majority of the patients were implanted with ICLs of 12.6 mm and 13.2 mm, the accuracy of ICL sizing models performs much better in ICLs of 12.6 mm and 13.2 mm when compared with the sizes of 12.1 mm and 13.7 mm. To enrol, more patients who had been implanted ICLs of extreme sizes would improve the models’ performance for ICL sizing. Third, only a single source of data is deployed in the present study. It will be beneficial for the accuracy of the models to involve multicentre data and out-of-group validation data. In addition, the present study predicted ICL vaults mainly by using WTW values, and the prediction may be more accurate if ciliary sulcus diameter (obtained by UBM) could be involved. In future research, we will try to introduce some external data through cooperation and combine different modal data (such as ultrasound bio-microscopic or anterior segment optical coherence tomographic data),hoping to predict the postoperative vault more accurately.
In summary, AI is applicable for vault prediction and ICL sizing. Random Forests, Gradient Boosting and XGBoost are the most preferred ML models that assist ophthalmologists to fit ICLs to achieve appropriate vaults, hence improving the safety of the ICL implantation technique.
Footnotes
Contributors: YS, LW and WJ were responsible for the initial plan, study design, data collection, data extraction, data interpretation, manuscript drafting, statistical analysis and conducting the study. JS, XW, LJ, ML, JZ, XC and ZG were responsible for data collection, extraction and critical revisions of the manuscript. YS, LW, WJ, XYW and XZ were responsible for data interpretation, manuscript drafting, supervision and critical revisions of the manuscript for important intellectual content. YS, LW and WJ should be considered as equal first authors.
Funding: This study was supported by National Natural Science Foundation of China (Grant No. 81770955); Joint research project of new frontier technology in municipal hospitals (SHDC12018103); Project of Shanghai Science and Technology (Grant No.20410710100); Major clinical research project of Shanghai Shenkang Hospital Development Center (SHDC2020CR1043B) and Project of Shanghai Xuhui District Science and Technology (2020-015).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the Ethics Committee of the Eye and ENT Hospital of Fudan University.
References
- 1. Naidoo KS, Fricke TR, Frick KD, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology 2019;126:338–46. 10.1016/j.ophtha.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 2. Freeman CE, Evans BJW. Investigation of the causes of non-tolerance to optometric prescriptions for spectacles. Ophthalmic Physiol Opt 2010;30:1–11. 10.1111/j.1475-1313.2009.00682.x [DOI] [PubMed] [Google Scholar]
- 3. Ieong A, Hau SCH, Rubin GS, et al. Quality of life in high myopia before and after implantable collamer lens implantation. Ophthalmology 2010;117:2295–300. 10.1016/j.ophtha.2010.03.055 [DOI] [PubMed] [Google Scholar]
- 4. Choi JH, Lim DH, Nam SW, et al. Ten-Year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J Cataract Refract Surg 2019;45:1555–61. 10.1016/j.jcrs.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 5. Packer M. Meta-Analysis and review: effectiveness, safety, and central Port design of the intraocular collamer lens. Clin Ophthalmol 2016;10:1059–77. 10.2147/OPTH.S111620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alfonso JF, Lisa C, Palacios A, et al. Objective vs subjective vault measurement after myopic implantable collamer lens implantation. Am J Ophthalmol 2009;147:e971:978–83. 10.1016/j.ajo.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 7. Salouti R, Nowroozzadeh MH, Zamani M, et al. Comparison of horizontal corneal diameter measurements using the Orbscan IIz and Pentacam HR systems. Cornea 2013;32:1460–4. 10.1097/ICO.0b013e3182a40786 [DOI] [PubMed] [Google Scholar]
- 8. Lee H, Kang SY, Seo KY, et al. Dynamic vaulting changes in V4c versus V4 posterior chamber phakic lenses under differing lighting conditions. Am J Ophthalmol 2014;158:e1191:1199–204. 10.1016/j.ajo.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 9. Kojima T, Yokoyama S, Ito M, et al. Optimization of an implantable collamer lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol 2012;153:632–7. 10.1016/j.ajo.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 10. Reinstein DZ, Lovisolo CF, Archer TJ, et al. Comparison of postoperative vault height predictability using white-to-white or sulcus diameter-based sizing for the visian implantable collamer lens. J Refract Surg 2013;29:30–5. 10.3928/1081597X-20121210-02 [DOI] [PubMed] [Google Scholar]
- 11. Zheng Q-Y, Xu W, Liang G-L, et al. Preoperative biometric parameters predict the vault after ICl implantation: a retrospective clinical study. Ophthalmic Res 2016;56:215–21. 10.1159/000446185 [DOI] [PubMed] [Google Scholar]
- 12. Lee D-H, Choi S-H, Chung E-S, et al. Correlation between preoperative biometry and posterior chamber phakic Visian implantable collamer lens vaulting. Ophthalmology 2012;119:272–7. 10.1016/j.ophtha.2011.07.047 [DOI] [PubMed] [Google Scholar]
- 13. Trancón AS, Manito SC, Sierra OT, et al. Determining vault size in implantable collamer lenses: preoperative anatomy and lens parameters. J Cataract Refract Surg 2020;46:728–36. 10.1097/j.jcrs.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 14. Russakoff DB, Lamin A, Oakley JD, et al. Deep learning for prediction of AMD progression: a pilot study. Invest Ophthalmol Vis Sci 2019;60:712–22. 10.1167/iovs.18-25325 [DOI] [PubMed] [Google Scholar]
- 15. Phene S, Dunn RC, Hammel N, et al. Deep learning and glaucoma specialists: the relative importance of optic disc features to predict glaucoma referral in fundus Photographs. Ophthalmology 2019;126:1627–39. 10.1016/j.ophtha.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 16. Abràmoff MD, Lou Y, Erginay A, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 2016;57:5200–6. 10.1167/iovs.16-19964 [DOI] [PubMed] [Google Scholar]
- 17. Yang W, Zhao J, Sun L, et al. Four-Year observation of the changes in corneal endothelium cell density and correlated factors after implantable collamer lens V4c implantation. Br J Ophthalmol 2021;105:625–30. 10.1136/bjophthalmol-2020-316144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei R, Li M, Niu L, et al. Comparison of visual outcomes after non-toric and toric implantable collamer lens V4c for myopia and astigmatism. Acta Ophthalmol 2021;99:511–8. 10.1111/aos.14652 [DOI] [PubMed] [Google Scholar]
- 19. Igarashi A, Shimizu K, Kato S, et al. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg 2019;45:1099–104. 10.1016/j.jcrs.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 20. Alfonso JF, Fernández-Vega L, Lisa C, et al. Central vault after phakic intraocular lens implantation: correlation with anterior chamber depth, white-to-white distance, spherical equivalent, and patient age. J Cataract Refract Surg 2012;38:46–53. 10.1016/j.jcrs.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 21. Li D, Yang Y, Su C, et al. Pupil diameter changes in high myopes after collamer lens implantation. Optom Vis Sci 2015;92:1161–9. 10.1097/OPX.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kato S, Shimizu K, Igarashi A. Vault changes caused by light-induced pupil constriction and accommodation in eyes with an implantable collamer lens. Cornea 2019;38:217–20. 10.1097/ICO.0000000000001785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Miao H, Naidu RK, et al. Comparison of early changes in and factors affecting vault following posterior chamber phakic implantable collamer lens implantation without and with a central hole (ICl V4 and ICl V4c). BMC Ophthalmol 2016;16:161. 10.1186/s12886-016-0336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidinger G, Lackner B, Pieh S, et al. Long-Term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology 2010;117:1506–11. 10.1016/j.ophtha.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 25. Matarazzo F, Day AC, Fernandez-Vega Cueto L, et al. Vertical implantable collamer lens (ICl) rotation for the management of high vault due to lens oversizing. Int Ophthalmol 2018;38:2689–92. 10.1007/s10792-017-0757-2 [DOI] [PubMed] [Google Scholar]
- 26. Breiman L. Random forests. Mach Learn 2001;45:5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 27. Hengl T, Nussbaum M, Wright MN, et al. Random forest as a generic framework for predictive modeling of spatial and spatio-temporal variables. PeerJ 2018;6:e5518. 10.7717/peerj.5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen T, Guestrin C. Xgboost: a scalable tree boosting system. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 13-17 Aug 2016, San Francisco, CA, USA, 2016:785–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Data are available upon reasonable request.