Abstract
Objective
To assess and compare the incidence of venous thromboembolism (VTE) in patients with rheumatoid arthritis (RA) treated with Janus kinase inhibitors (JAKi), tumour necrosis factor inhibitors (TNFi) or other biological disease modifying antirheumatic drugs (bDMARDs). For contextualisation, to assess VTE incidences in the Swedish general population and in the RA source population.
Methods
We performed a nationwide register-based, active comparator, new user design cohort study in Sweden from 2010 to 2021. The Swedish Rheumatology Quality Register was linked to national health registers to identify treatment cohorts (exposure) of initiators of a JAKi, a TNFi, or a non-TNFi bDMARD (n=32 737 treatment initiations). We also identified a general population cohort (matched 1:5, n=92 108), and an ‘overall RA’ comparator cohort (n=85 722). Outcome was time to first VTE during the follow-up, overall and by deep vein thrombosis (DVT) and pulmonary embolism (PE). We calculated incidence rates (IR) and multivariable-adjusted HRs using Cox regression.
Results
Based on 559 incident VTE events, the age- and sex-standardised (to TNFi) IR (95% CI) for VTE was 5.15 per 1000 person-years (4.58 to 5.78) for patients treated with TNFi, 11.33 (8.54 to 15.04) for patients treated with JAKi, 5.86 (5.69 to 6.04) in the overall RA cohort and 3.28 (3.14 to 3.43) in the general population. The fully adjusted HR (95% CI) for VTE with JAKi versus TNFi was 1.73 (1.24 to 2.42), the corresponding HR for PE was 3.21 (2.11 to 4.88) and 0.83 (0.47 to 1.45) for DVT.
Conclusions
Patients with RA treated with JAKi in clinical practice are at increased risk of VTE compared with those treated with bDMARDs, an increase numerically confined to PE.
Keywords: Arthritis, Rheumatoid; Cardiovascular Diseases; Antirheumatic Agents; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Meta-analyses of phase III trials of Janus kinase inhibitors (JAKi), and a recent postapproval safety trial of tofacitnib versus tumour necrosis factor inhibitors (TNFi), both indicate a higher risk of venous thromboembolism (VTE) with JAKi.
WHAT THIS STUDY ADDS
Our study (1) confirms a 50%–100% increased risk of VTE for JAKi as used in clinical practice, (2) demonstrates that the VTE rate with JAKi is higher than with TNFi, with other biological disease-modifying antirheumatic drugs, and in the background rheumatoid arthritis population, and (3) shows that the increased VTE rate seems to be explained by an increased rate of pulmonary embolism rather than deep venous thrombosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results add to the concerns regarding VTE risk in patients treated with JAKi, and underscore the need for VTE risk stratification before initiating JAKi treatment.
Introduction
Patients with rheumatoid arthritis (RA) are at 50%–100% increased risk of venous thromboembolism (VTE), that is, pulmonary embolism (PE) and deep vein thrombosis (DVT), compared with individuals without RA.1–6 In part, this increase may be explained by an increased occurrence of established VTE risk factors, such as cancer, immobilisation and joint replacement surgery7 in patients with RA. Importantly, inflammation is known to upregulate procoagulatory factors and cause endothelial damage8 9; we and others recently demonstrated an association between RA disease activity and VTE risk.10–12
Janus kinase inhibitors (JAKi) are the most recently introduced class of disease-modifying antirheumatic drugs (DMARDs), also referred to as targeted synthetic (ts)DMARDs. Initially, a signal for VTE risk was identified by an increased number of VTE events in randomised controlled trials of baricitinib and upadacitinib versus placebo.13 Further, the very recently published ORAL surveillance postauthorisation safety trial of tofacitinib versus etanercept/adalimumab observed a dose-dependent increase in VTE risk in the two tofacitinib arms compared with the tumour necrosis factor inhibitors (TNFi) reference arm.14 Based on interim results from this trial, regulatory agencies (European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA)) issued warnings regarding VTE risk with the class of JAKi.15 16 So far, however, published data on VTE risk with JAKi as used in clinical practice (from the U.S. CorEvitas RA registry and from U.S. insurance claims) have not demonstrated any increased risk.17 18 Importantly, ORAL surveillance used TNFi as reference, thus providing relative estimates of risk where the effect of the comparator on the baseline risk of VTE is not fully understood, and may not be neutral.
The aims of this study were therefore to assess and compare the incidence of VTE, by type, in patients with RA treated with JAKi or biological DMARDs (bDMARDs) as used in clinical practice (using TNFi as reference), and to contextualise these rates by estimation of the corresponding incidences in the overall RA population, irrespective of specific DMARD treatment, and in the general population.
Methods
Study design
We conducted a nationwide register-based, active comparator, new user design cohort study that made use of prospectively recorded and linked data from clinical and other Swedish registers.
Setting
In Sweden, healthcare and drug prescriptions are publicly funded with an annual threshold of payment for the individual patients of €230. Prescription of drugs approved by EMA and the national drug reimbursement agency is at the discretion of individual physicians with guidelines issued by the Swedish Society for Rheumatology. The vast majority of patients with RA are treated by rheumatologists at public rheumatology clinics. VTE events are usually diagnosed and/or treated at public hospitals.
Data sources
The Swedish Rheumatology Quality Register (SRQ) is a longitudinal clinical register capturing information on RA disease activity and treatment, based on data entered by the treating rheumatologist as well as the patients at outpatients visit. The estimated national coverage in SRQ for patients with RA and any DMARD treatment is 85%–90%. The National Patient Register (NPR) contains information on inpatient care since 1969 (nationwide since 1987) and non-primary outpatient care since 2001, with diagnoses coded according to International Classification of Diseases (ICD). The coverage for somatic conditions such as RA and VTE, and typical RA comorbidities, is reported to be over 95%.19 The Prescribed Drug Register (PDR) contains information on all filled prescriptions, coded according to the Anatomic Therapeutic Chemical Classification system (ATC) since 2005. The Cause of Death Register contains information on all deaths and cause(s) of death registered using ICD codes since 1961. The Swedish Population Register contains information on residency, civil status (marriage or civil partnership/union) and migration data for all Swedish residents. The Longitudinal integrated database for health insurance and labour market studies (LISA) includes information on sick leave and disability pension since 1990. For details of the data sources, please see online supplemental table S1. Through personal identification numbers, issued to all Swedish residents, individual-level data from registers and other data sources may be linked together.
ard-2022-223050supp001.pdf (328.6KB, pdf)
Study population
Through SRQ, we identified all b/tsDMARD treatment initiations between 1 January 2010 and 31 December 2020 among all registered patients with RA above 18 years of age.
Using validated algorithms applied to NPR,20 we further identified the entire RA population in Sweden (the ‘overall RA cohort’) defined by at least two separate visits listing RA at a rheumatology or internal medicine clinic before or during the study period.
For each patient with RA who contributed to any of the b/tsDMARD cohorts, we randomly matched five individuals without RA from the general population by age, sex and residential area (at time of RA diagnosis for the index RA patient).
Exposure b/tsDMARDs were categorised into the following treatment initiation cohorts: TNFi (etanercept, infliximab, adalimumab, golimumab, certolizumab pegol), rituximab, interleukin 6 inhibitors (IL6i) (tocilizumab, sarilumab), abatacept and JAKi (tofacitinib, baricitinib, upadacitinib). Dates for introduction of each JAKi on the Swedish market are presented in online supplemental table S2. Treatment initiation was defined as the registered date of treatment start in the SRQ. For each of these treatment cohorts, we used a first initiation per molecule approach, meaning that one individual could contribute to each treatment cohort more than once, but only once with each individual drug. For instance, an individual who, during the study period, initiated treatment with adalimumab, switched to rituximab, and later to etanercept contributed two initiations to the TNFi cohort, and one to the rituximab cohort. Similarly, an individual who initiated treatment with baricitinib followed by tofacitinib contributed two initiations to the JAKi cohort. Switch from an originator to biosimilar was considered the same treatment episode, as was restarting the same treatment within 90 days after discontinuation (180 days for rituximab) if no other bDMARD was initiated in between. In an additional analysis, baricitinib and tofacitinib were assessed as separate cohorts.
Outcome
The outcome was defined as the first registration with an ICD10 code for VTE in primary or secondary position in the NPR, or PE listed as the underlying cause of death in the Cause of Death Register during follow-up, plus the requirement of a filled anticoagulant prescription within 30 days (unless death from any cause within 30 days). See online supplemental table S3 for all ICD and ATC codes. Subjects with a VTE registered during the year prior to start of follow-up were excluded. This outcome definition was recently validated in our RA study population and was found to have a PPV of 98% (95% CI 95% to 100%), with only a minor loss in sensitivity (86%) compared with when not applying anticoagulant requirement and not excluding those with VTE within the previous year.21 We used a time-to-first event approach for each drug. One individual could thus only contribute one VTE event for each individual drug treatment-exposure, but could contribute VTE events to more than one drug or drug class. VTE events that occurred within the 60-day window after discontinuation of one b/tsDMARD but after the start of any new b/tsDMARD (n=9) were attributed to both exposures. We also defined two separate outcomes: first PE and first DVT during the follow-up, with the same requirement of dispensation of anticoagulant as for the main outcome.
Follow-up
Start of follow-up was defined as the registered date of treatment start in SRQ. In the main analysis we used an on-drug approach. Treatment discontinuation was defined as either of (1) registered date for discontinuation in the SRQ, (2) start of alternative b/tsDMARD in SRQ or (3) date of filled prescription for alternative b/tsDMARD from the PDR, whichever came first. For the overall RA cohort, follow-up started at first day of study period (or at the time point of the second ICD10 code registration for RA, if later). For the matched general population cohort, follow-up started at the time point of the first recorded treatment initiation in the corresponding index individual with RA. For all cohorts, follow-up ended at 60 days after discontinuation of the DMARD treatment in question, first VTE event, death, emigration or end of study period (31 December 2021), whichever came first.
Statistical analyses
We calculated crude as well as age- and sex-standardised incidence rates (using the TNFi cohort as standard). HRs comparing the rate of VTE between the treatment cohorts (using TNFi as reference) were estimated using Cox proportional hazards regression, adjusted for age, sex and number of previous b/tsDMARDs (ever) (HR1), additionally adjusted for relevant comorbidities and treatments, healthcare consumption and socioeconomic variables (HR2) and for RA disease-related variables and smoking (HR3). Table 1 (except rows 1–4) includes all variables used in these models, each covariate reflecting status at start of follow-up for each observation in each model). For variables with missing data (HR2: one variable, HR3: five variables), we used the missing indicator method. See table 1 for a full list of variables and their definitions. Since the overall RA cohort was partially comprised of patients from the treatment cohorts, and was followed irrespective of any treatment initiation, HRs were not calculated for this cohort.
Table 1.
Characteristics at each treatment initiation for the RA population (by b/tsDMARD treatment cohort and overall) and general population referents, in Swedish patients ith RA from 2010 to 2020
| b/tsDMARD treatment cohort | Gen pop | |||||
| TNFi | Rituximab | IL6i | Abatacept | JAKi | ||
| Treatment initiations (n) | 19 950 | 4032 | 3019 | 3382 | 2354 | 92 180 |
| Individuals (n) | 15 090 | 4032 | 2956 | 3382 | 2150 | 91 207 |
| Year of treatment start, median | 2015 | 2014 | 2015 | 2015 | 2019 | 2014 |
| Follow-up, median years (IQR) | 2 (1–4) | 3 (1–6) | 2 (1–4) | 2 (1–4) | 2 (1–3) | 7 (4–10) |
| Age at Tx start, median (IQR) | 57 (47–67) | 62 (54–72) | 58 (49–68) | 60 (52–70) | 60 (51–70) | 57 (48–68) |
| Females (%) | 77 | 75 | 80 | 80 | 82 | 76 |
| Ever smoker (%) | 58 | 65 | 59 | 62 | 59 | n/a |
| Clinical RA data | ||||||
| RA duration, median years (IQR) | 7 (3–15) | 12 (6–22) | 11 (5–19) | 12 (5–21) | 13 (7–23) | n/a |
| No previous biologics, median (IQR) | 0 (0–1) | 1 (0–2) | 2 (1–3) | 2 (1–3) | 3 (1–4) | n/a |
| Seropositive (%) | 77 | 90 | 80 | 81 | 79 | n/a |
| DAS28CRP, median (IQR) | 4.3 (3.5–5.1) | 4.6 (3.8–5.3) | 4.8 (4.0–5.5) | 4.5 (3.8–5.2) | 4.4 (3.5–5.1) | n/a |
| CRP, median (IQR) | 6 (3–17) | 9 (4–24) | 10 (4–27) | 7 (3–20) | 5 (2–16) | n/a |
| HAQ, median (IQR) | 1.0 (0.5–1.5) | 1.3 (0.8–1.8) | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | 1.3 (0.8–1.8) | n/a |
| Comorbidities* | ||||||
| Previous VTE, since 2001 (%) | 3 | 5 | 5 | 5 | 5 | 2 |
| ACS (%) | 2 | 3 | 2 | 3 | 2 | 1 |
| Other cardiac disease (%) | 21 | 33 | 25 | 32 | 29 | 15 |
| Cerebrovascular disease (%) | 2 | 3 | 2 | 3 | 2 | 2 |
| Chronic kidney disease (%) | 1 | 2 | 2 | 2 | 2 | 1 |
| Cancer (in past 10 years) (%) | 3 | 11 | 4 | 5 | 4 | 5 |
| Hospitalisation listing infection (%) | 9 | 18 | 13 | 18 | 16 | 4 |
| Coagulopathy (%) | 0 | 1 | 1 | 1 | 1 | 0 |
| Varicose veins (%) | 2 | 2 | 2 | 3 | 2 | 1 |
| Major surgery previous 3 months (%) | 3 | 4 | 4 | 4 | 3 | 2 |
| Diabetes (%) | 9 | 11 | 10 | 12 | 10 | 7 |
| COPD or asthma (%) | 14 | 20 | 16 | 22 | 19 | 11 |
| Treatments† | ||||||
| HRT or oestrogen contraceptive (%) | 13 | 14 | 15 | 17 | 14 | 12 |
| Antipsychotics (%) | 1 | 2 | 1 | 1 | 1 | 2 |
| NSAID/ASA (%) | 63 | 61 | 65 | 62 | 58 | 23 |
| Anticoagulant (%) | 6 | 11 | 8 | 10 | 10 | 5 |
| Oral corticosteroids (%) | 70 | 78 | 75 | 76 | 73 | 6 |
| Healthcare consumption | ||||||
| No of specialist care visits, median (IQR) | 13 (7–22) | 19 (10–32) | 19 (10–30) | 21 (12–33) | 25 (15–39) | 2 (0–6) |
| No of hospitalisations, median (IQR) | 0 (0–1) | 1 (0–2) | 0 (0–2) | 1 (0–2) | 1 (0–2) | 0 (0–0) |
| No of filled prescriptions previous year, median (IQR) | 22 (13–36) | 30 (18–45) | 28 (17–42) | 31 (19–48) | 29 (18–46) | 5 (1–16) |
| Socioeconomics | ||||||
| Married/partnership (%) | 51 | 51 | 52 | 52 | 52 | 50 |
| Sick leave in previous year (%) | 17 | 13 | 17 | 16 | 16 | 7 |
| Disability pension in previous year (%) | 2 | 2 | 2 | 2 | 1 | 1 |
*Registered within the last 5 years before treatment initiation, unless otherwise stated.
†Registered within the last year before treatment initiation.
ACS, acute coronary syndrome; ASA, acetylsalisylic acid; b/tsDMARD, biologic/targeted synthetic disease modifying anti-rheumatic drug; COPD, chronic obstructive pulmonary disease; DAS28CRP, Disease Activity Score 28 C reactive protein; HAQ, Health Assessment Questionnaire; HRT, hormone replacement therapy; IL6i, interleukin 6 inhibitor; JAKi, Janus kinase inhibitor; n/a, not applicable; NSAID, non-steroidal anti-inflammatory drugs; Gen pop, general population; RA, rheumatoid arthritis; TNFi, Tumour necrosis factor inhibitor; tx, treatment; VTE, venous thromboembolism.
We also performed separated analyses by sex, RA serostatus, number of previous b/tsDMARDs and time since start of follow-up (0 to ≤1 year, 1 to ≤5 years and ≥5 years). To test the proportional hazards assumption, we used an interaction term between follow-up time (0 to ≤1 year, 1 to ≤5 years and≥5 years) and exposure.
To test the robustness of our results, we performed several sensitivity analyses in which we investigated alternative outcome and follow-up definitions (see online supplemental table S3 for a full description). To maximise statistical precision, our main analysis used data from 2010. Since JAKis were introduced to the Swedish market in 2016, we performed a separate analysis restricted to data from 2016 to 2021. To investigate the role of missingness, we performed a complete case analysis (ie, excluding patients with missing values for the disease-related variables and smoking), as well as an analysis using multiple imputation for variables with missing data (see the online supplemental file for a full description of the imputation). We further performed a separate analysis of patients fulfilling relevant inclusion and exclusion criteria for the ORAL surveillance study, to assess how such enrichment for cardiovascular risk factors affected the VTE incidence and HRs for each exposure (details in online supplemental file 1). All analyses were performed using SAS Enterprise Guide V.7.1 and Stata V.15.
Results
Table 1 displays baseline characteristics of the b/tsDMARD treatment exposure cohorts and the general population referents. Overall, 27 610 unique patients with RA contributed with 32 737 b/tsDMARDs treatment exposures. The overall RA cohort encompassed 85 722 patients and the general population cohort encompassed 91 207 individuals. Compared with TNFi, patients starting other b/tsDMARDs were generally slightly older, had longer-lasting RA, more comorbidities and a higher level of healthcare consumption. Additional covariates are presented in online supplemental table S4, and proportions of missing data are presented in online supplemental table S5.
VTE occurrence and incidence
Table 2 presents the number of VTE events and age- and sex-standardised incidence rates for the b/tsDMARD exposure cohorts, the overall RA cohort and the general population cohort. In the b/tsDMARD exposure cohorts, a total of 559 incident VTE events were observed, corresponding to an overall standardised VTE incidence of 6.09 (95% CI 5.60 to 6.61) per 1000 person-years. For patients initiating TNFi, the standardised incidence rate per 1000 person-years was 5.15 (95% CI 4.58 to 5.78) compared with 11.33 (95% CI 8.54 to 15.04) for patients initiating JAKi. For patients initiating other bDMARDs, the incidence was generally higher than in the TNFi cohort. Based on 4476 VTE events, the standardised VTE incidence in the overall RA cohort was 5.86 (95% CI 5.69 to 6.04), compared with 3.28 (95% CI 3.14 to 3.43) in the general population.
Table 2.
Number of treatment initiations, person-years at risk, VTE events, age- and sex-standardised incidence rates, and HRs for VTE in Swedish patients with RA (by treatment b/tsDMARD cohort and overall) and matched individuals from the general population between 2010 and 2020
| Obs. | PYs at risk | VTE events | Standardised IR/1000 PYs (95% CI) | Unadjusted HR (95% CI) | HR (95% CI) Model 1* |
HR (95% CI) Model 2† |
HR (95% CI) Model 3‡ |
|
| Cohort | ||||||||
| TNFi | 19 950 | 55 765 | 287 | 5.15 (4.58 to 5.78) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Rituximab | 4032 | 14 871 | 102 | 6.05 (4.98 to 7.34) | 1.35 (1.08 to 1.70) | 1.09 (0.86 to 1.38) | 0.97 (0.76 to 1.23) | 0.94 (0.74 to 1.20) |
| IL6i | 3019 | 8 354 | 66 | 7.54 (5.92 to 9.59) | 1.54 (1.18 to 2.01) | 1.44 (1.09 to 1.92) | 1.30 (0.97 to 1.73) | 1.25 (0.94 to 1.67) |
| Abatacept | 3382 | 8 651 | 56 | 5.69 (4.38 to 7.40) | 1.25 (0.94 to 1.67) | 1.10 (0.81 to 1.49) | 0.89 (0.65 to 1.20) | 0.89 (0.66 to 1.21) |
| JAKi | 2354 | 4 184 | 48 | 11.33 (8.54 to 15.04) | 2.16 (1.59 to 2.93) | 1.94 (1.40 to 2.70) | 1.63 (1.17 to 2.28) | 1.73 (1.24 to 2.42) |
| Baricitinib§ | 1825 | 3 412 | 41 | 11.35 (8.35 to 15.41) | 2.27 (1.64 to 3.15) | 2.00 (1.41 to 2.83) | 1.69 (1.19 to 2.40) | 1.79 (1.25 to 2.55) |
| Tofacitinib§ | 424 | 667 | 7 | 11.30 (5.39 to 23.70) | 1.96 (0.92 to 4.15) | 1.91 (0.89 to 4.11) | 1.56 (0.72 to 3.35) | 1.66 (0.77 to 3.59) |
| Overall RA cohort | 85 722 | 633 871 | 4476 | 5.86 (5.69 to 6.04) | n/a | n/a | n/a | n/a |
| Gen pop | 92 180 | 597 854 | 2001 | 3.28 (3.14 to 3.43) | 0.67 (0.59 to 0.76) | 0.66 (0.57 to 0.76) | n/a | n/a |
*Model 1 adjusted for age, sex and line of therapy. Overall RA cohort excluded from model.
†Model 2 additionally adjusted for comorbidities and socioeconomic variables. Overall RA cohort and general population excluded from model.
‡Model 3 additionally adjusted for RA disease variables, civil status and smoking, using an indicator for missing variables. Overall RA cohort and general population excluded from model.
§Estimates obtained from a separate model where JAKi cohort is split into baricitinib and tofacitinib.
b/tsDMARD, biologic/targeted synthetic disease modifying anti-rheumatic drug; Gen pop, general population; IL6i, interleukin 6 inhibitor; IR, incidence rate; JAKi, Janus kinase inhibitor; n/a, not applicable; PY, person years; RA, rheumatoid arthritis; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.
Relative risks for VTE
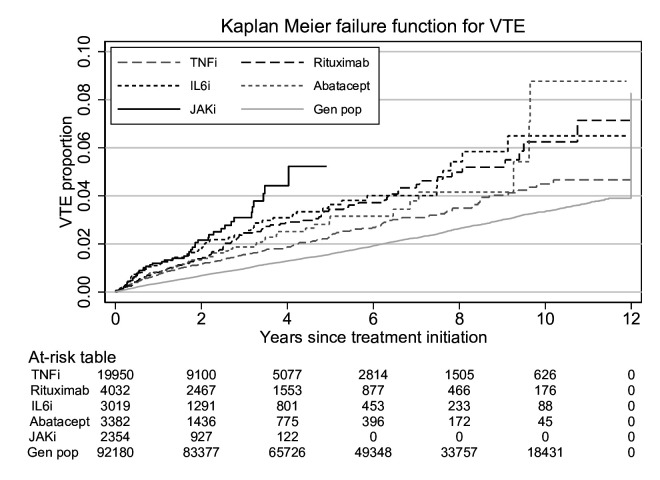
Figure 1 presents unadjusted Kaplan-Meier curves for the treatment and general population cohorts. Table 2 presents crude and adjusted HRs for VTE. Using TNFi as reference, the fully adjusted HR for VTE for patients treated with JAKi was 1.73 (95% CI 1.24 to 2.42). When separating individual JAKis (78% were bariticinib initiations, 18% tofacitinib and 4% upadacitinib), the HR for baricitinib was 1.79 (95% CI 1.25 to 2.55) and 1.66 (95% CI 0.77 to 3.59) for tofacitinib. HRs were not calculated for upadacitinib due to low follow-up time and 0 VTE events. For non-TNFi bDMARDs, HRs were close to 1 except for IL6i for which HRs were generally increased (although not statistically significant) with a fully adjusted HR of 1.25 (95% CI 0.94 to 1.67). For the general population versus TNFi, the age- and sex-adjusted HR was 0.66 (95% CI 0.57 to 0.76). The overall number of observations, events and person-years at risk in each model is presented in online supplemental table S6, and HRs by time since treatment start in online supplemental table S7. When using JAKi as reference, HRs were lower for all bDMARDs, although not statistically significant for IL6i (online supplemental table S8).
Figure 1.
Kaplan-Meier failure function for the incidence of VTE in Swedish patients with RA by b/tsDMARD treatment cohort and matched individuals from the general population between 2010 and 2021. b/tsDMARD, biologic/targeted synthetic disease-modifying antirheumatic drug; JAKi, Janus kinase inhibitor; RA, rheumatoid arthritis; TNFi, Tumour necrosis factor inhibitor; VTE, venous thromboembolism.
Occurrence and relative risks for PE and VTE
When assessing time to first event for PE and DVT separately, the overall incidence rate per 1000 person-years was 2.91 (95% CI 2.59 to 3.28) for PE and 3.51 (95% CI 3.15 to 3.92) for DVT. The fully adjusted HR for PE in patients initiating treatment with JAKi versus TNFi was 3.21 (95% CI 2.11 to 4.88), and the corresponding HR for DVT was 0.83 (95% CI 0.47 to 1.45). The corresponding HRs for DVT and PE with non-TNFi bDMARDs were generally closer to 1 and not statistically significant (figure 2).
Figure 2.
Events, incidence rates per 1000 person-years and HRs for VTE by treatment cohort (TNFi as reference), overall and by VTE subtype, in Swedish patients with RA between 2010 and 2021. b/tsDMARD, biologic/targeted synthetic disease-modifying antirheumatic drug; DVT, deep vein thrombosis; IR, incidence rate; JAKi, Janus kinase inhibitor; PE, pulmonary embolism; RA, rheumatoid arthritis; VTE, venous thromboembolism.
Stratified analyses
When analysed separately by sex, the incidence rates of VTE were approximately 50% higher in males than in females, and the HRs were numerically higher for males compared with females for those treated with IL6i versus TNFi (2.30 (95% CI 1.43 to 3.70) for males and 0.91 (0.63 to 1.32) for females) as well as JAKi versus TNFi (2.66 (95% CI 1.44 to 4.92) for males and 1.53 (1.02–2.30) for females). When stratified by history of VTE, the incidence rate of VTE was almost nine times higher for individuals with a previous VTE versus those without, but all HRs for b/tsDMARDs versus TNFi were close to 1 (table 3). By contrast, among those without a previous VTE, the HRs were increased with IL6i versus TNFi (1.42 (95% CI 1.03 to 1.96)) and with JAKi versus TNFi (1.95 (1.33 to 2.87). All stratified analyses are presented in table 3.
Table 3.
Number of events, time at risk, crude incidence rates and fully adjusted HRs for VTE in Swedish patients with RA by b/tsDMARD cohort between 2010 and 2021. Stratified by sex, age, line of therapy, history of VTE, RA serostatus, any VTE risk factor and RA disease activity.
| Stratification | Obs, N | Events, N | PYs at risk | IR/1000 PYs (95% CI) | HR (95% CI) | ||||
| TNFi | Rituximab | IL6i | Abatacept | JAKi | |||||
| Overall | 32 737 | 559 | 91 824 | 6.09 (5.60 to 6.61) | 1 (ref) | 0.94 (0.74 to 1.20) | 1.25 (0.94 to 1.67) | 0.89 (0.66 to 1.21) | 1.73 (1.24 to 2.42) |
| Sex | |||||||||
| Male | 7 407 | 177 | 22 010 | 8.04 (6.94 to 9.32) | 1 (ref) | 1.09 (0.70 to 1.71) | 2.30 (1.43 to 3.70) | 0.92 (0.49 to 1.70) | 2.66 (1.44 to 4.92) |
| Female | 25 330 | 382 | 69 815 | 5.47 (4.95 to 6.05) | 1 (ref) | 0.90 (0.70 to 1.20) | 0.91 (0.63 to 1.32) | 0.89 (0.62 to 1.26) | 1.53 (1.02 to 2.30) |
| Age | |||||||||
| <60 | 16 820 | 140 | 47 196 | 2.97 (2.51 to 3.50) | 1 (ref) | 1.43 (0.87 to 2.35) | 1.27 (0.69 to 2.34) | 0.69 (0.32 to 1.48) | 2.11 (1.09 to 4.07) |
| 60 or above | 15 917 | 419 | 44 628 | 9.39 (8.53 to 10.33) | 1 (ref) | 0.85 (0.65 to 1.13) | 1.27 (0.92 to 1.77) | 0.97 (0.70 to 1.36) | 1.64 (1.11 to 2.44) |
| No of previous b/tsDMARDs (ever) | |||||||||
| 0 | 15 068 | 259 | 46 795 | 5.53 (4.90 to 6.25) | 1 (ref) | 0.82 (0.56 to 1.19) | 1.36 (0.77 to 2.39) | 0.60 (0.30 to 1.19) | 1.67 (0.81 to 3.44) |
| 1 | 7 981 | 148 | 22 205 | 6.67 (5.67 to 7.83) | 1 (ref) | 1.61 (1.06 to 2.46) | 1.18 (0.69 to 2.02) | 1.25 (0.74 to 2.12) | 1.74 (0.82 to 3.70) |
| two or more | 9 688 | 152 | 22 824 | 6.66 (5.68 to 7.81) | 1 (ref) | 0.65 (0.38 to 1.10) | 1.24 (0.76 to 2.02) | 0.81 (0.49 to 1.36) | 1.60 (0.97 to 2.65) |
| History of VTE | |||||||||
| Yes | 1 249 | 125 | 3 066 | 40.77 (34.22 to 48.59) | 1 (ref) | 0.58 (0.33 to 1.01) | 0.79 (0.42 to 1.49) | 0.78 (0.42 to 1.44) | 1.07 (0.53 to 2.14) |
| No | 31 488 | 434 | 88 759 | 4.89 (4.45 to 5.37) | 1 (ref) | 1.06 (0.81 to 1.39) | 1.42 (1.03 to 1.96) | 0.94 (0.65 to 1.33) | 1.95 (1.33 to 2.87) |
| RA serostatus | |||||||||
| Seropositive | 26 012 | 460 | 74 186 | 6.20 (5.66 to 6.79) | 1 (ref) | 0.91 (0.71 to 1.18) | 1.31 (0.96 to 1.80) | 0.81 (0.57 to 1.16) | 1.71 (1.17 to 2.49) |
| Seronegative | 6 725 | 99 | 17 639 | 5.61 (4.61 to 6.83) | 1 (ref) | 1.20 (0.56 to 2.57) | 1.06 (0.52 to 2.17) | 1.09 (0.57 to 2.08) | 1.76 (0.84 to 3.71) |
| ≥1 VTE risk factor* | |||||||||
| Yes | 18 814 | 401 | 51 553 | 7.78 (7.05 to 8.58) | 1 (ref) | 0.83 (0.63 to 1.11) | 1.16 (0.83 to 1.63) | 0.89 (0.63 to 1.27) | 1.66 (1.13 to 2.43) |
| No | 13 923 | 158 | 40 271 | 3.92 (3.36 to 4.59) | 1 (ref) | 1.36 (0.88 to 2.12) | 1.91 (1.09 to 3.34) | 1.12 (0.50 to 2.11) | 1.72 (0.85 to 3.48) |
| RA disease activity | |||||||||
| DAS28 ≥5.1 (high) | 7 853 | 161 | 22 126 | 7.27 (6.24 to 8.49) | 1 (ref) | 0.52 (0.31 to 0.87) | 1.40 (0.89 to 2.22) | 0.86 (0.50 to 1.50) | 1.33 (0.67 to 2.65) |
| DAS28 <5.1 | 12 232 | 210 | 35 760 | 5.87 (5.13 to 6.72) | 1 (ref) | 0.96 (0.64 to 1.45) | 1.19 (0.71 to 1.99) | 0.75 (0.44 to 1.29) | 1.71 (0.96 to 3.03) |
*Defined as any of age >60 years, cancer, previous VTE, major surgery within 90 days, coagulation disorder, varicose veins, hormone replacement therapy, high DAS28, HAQ score >1 or ever smoker.
b/tsDMARD, biologic/targeted synthetic disease-modifying antirheumatic drug; DAS28, Disease Activity Score 28; HAQ, Health Assessment Questionnaire; IL6i, interleukin 6 inhibitor; IR, incidence rate; JAKi, Janus kinase inhibitor; PY, person years; RA, rheumatoid arthritis; TNFi, Tumour necrosis factor inhibitor; VTE, venous thromboembolism.
Sensitivity analyses
Sensitivity analyses altering the definition of VTE as well as the definition of follow-up showed similar results as our main analyses, although the HRs were generally lower when extending follow-up from 60 days after exposure-drug discontinuation until start of (any) next treatment (online supplemental figure S1). When using multiple imputation for variables with missing data, the fully adjusted HR for JAKi versus TNFi was 1.63 (95% CI 1.16 to 2.30) (see online supplemental Table S9 for additional results). When limiting the study period to 2016–2021, HRs were very similar to those in the main analysis (online supplemental table S10 and figure S2). When performing a complete case analysis, HRs for JAKi and IL6i versus TNFi were close to identical compared with the main analysis (online supplemental table S11).
Oral surveillance trial emulation
When analysing the subgroup of those fulfilling an emulation of the inclusion and exclusion criteria of the ORAL surveillance study, the incidence rates of VTE were around 50% higher, although the HRs for JAKi versus TNFi were similar or lower compared with our main analysis (online supplemental table S12).
Discussion
In this nationwide study including 32 737 b/tsDMARD initiations from clinical practice, we made the following important observations: (1) the incidence of VTE in patients with RA treated with JAKi was 50%–100% higher than the corresponding incidence in patients treated with TNFi, (2) this increase was at least numerically confined to PE rather than DVT, (3) the increase of VTE in the JAKi cohort was on average double that observed in the entire pool of patients with RA, and tripled compared with that in the general population, (4) when restricting the study population to mimic that of ORAL surveillance, the VTE incidence generally increased by some 50% but the HR for JAKi versus TNFi remained largely the same as in our overall analysis.
So far, a few studies have studied the association between VTE risk in patients treated with JAKi using real world data. A comparative safety study for tofacitinib versus a combined bDMARD group from 2021 using the US CorEvitas RA registry (3152 tofacitinib PYs and 12 869 bDMARD PYs) showed no difference in age- and sex-standardised incidence rates of VTE per 100 PYs for JAKi versus bDMARDs (0.29 (95% CI 0.13 to 0.54) versus 0.33 (95% CI 0.24 to 0.45)).17 Another study on tofacitinib versus TNFi from 2021 using three US claims databases (5301 tofacitinib PYs and 75 824 TNFi PYs) resulted in a pooled propensity score-weighted HR of 1.13 (95% CI 0.77 to 1.65).18 For several reasons, the results from these studies are difficult to compare to our current study. First, these previous studies only include tofacitinib initiations, whereas our current study includes both baricitinib and tofacitinib (80% of JAKi PYs were from baricitinib). Second, the CorEvitas study compared standardised incidence rates and used no measure for relative risks due to lack of power. Third, the claims database was restricted to inpatient VTEs. Fourth, use of claims database increases the risk of selection and attrition bias and provides no information on clinical RA variables such as disease activity.
The recently published ORAL surveillance study was a postauthorisation randomised open-label safety study for tofacitinib that enrolled patients with active RA above 50 years of age and with at least one additional cardiovascular risk factor.14 Although not powered to assess VTE, this study found an increased number of VTE events, especially with the 10 mg tofacitinib dose. For tofacitinib 5 mg, the dose currently approved for RA, the HR versus TNFi (etanercept/adalimumab) was 2.93 (95% CI 0.79 to 10.83) for PE and 1.54 (95% CI 0.60 to 3.97) for DVT. The study design raised the question whether these results could partially be explained by the population included in the study, since there is an overlap between risk factors for cardiovascular disease and VTE. In our study, the incidence of VTE was slightly higher for those fulfilling the ORAL surveillance inclusion criteria, but the HRs for the risk of VTE for JAKi versus TNFi were similar or lower compared with our main analysis. The results indicate that the enrichment of cardiovascular risk factors may not be the main explanation for the observed results from the ORAL surveillance trial.
A common denominator of all the above studies is the use of TNFi (or all bDMARDs) as the comparator, which raises the question to what extent, and in what direction, this comparator itself alters the risk of VTE. In order to address this issue, we included an overall RA cohort to assess the incidence in the entire RA population including those not treated with any b/tsDMARD, and a general population cohort. Although comparing age- and sex-standardised incidence rates has its limitations in terms of confounding, the VTE incidence in the TNFi cohort, as well as the other bDMARD cohorts, was similar to the incidence in the overall RA cohort. In addition, when using JAKi as reference, HRs for VTE were very similar and lower (statistically significant) for all bDMARDs except IL6i, where it was only numerically lower than that of JAKi. Thus, it is unlikely that the main results observed in this study are mainly explained by a decreased VTE risk with the active comparator (here: TNFi).
Interestingly, our study verifies the signal of a high number of PE events and thereby a disproportionate PE/DVT ratio, which was observed in JAKi randomised clinical trials and the ORAL surveillance study.14 PE in the absence of DVT has been described to have different risk factors than traditional PE (that arises from a DVT), such as higher age, female sex and cardiovascular disease, which are all enriched in RA.22 It has also been proposed that PE in the absence of DVT (or in situ pulmonary artery embolism) could be a separate entity triggered by local inflammation and ensuing imbalance in the coagulation system.23 On the other hand, since our previous study on RA disease activity and the risk of VTE observed a somewhat stronger association between RA disease activity and PE risk, compared with DVT risk, confounding by indication cannot be ruled out as a partial explanation for our findings (but not for those from ORAL surveillance and other trials).10
Whether, and how much, ongoing anticoagulant therapy alter the risk of VTE for patients with (or without) a history of VTE treated with any b/tsDMARD is an important clinical question; limited statistical precision precluded a detailed assessment of risks by history of VTE by anticoagulant use at treatment start and during the follow-up.
Although a definitive biological explanation for how JAK inhibition could increase VTE risk, PE in particular, is still lacking, our study adds to the body of evidence suggesting this is indeed the case, not only in trial populations but also when and as these drugs are used in clinical practice. Because of the relatively small sample size for tofacitinib, it is difficult to draw any specific conclusions regarding any difference in VTE risk between tofacitinib and baricitinib. In addition to the increased VTE risk for patients treated with JAKis, our study indicated a potential increase in VTE risk for patients treated with IL6i, especially in males. This raises the question whether the similarities between the mechanism of action for JAKis and IL6i may be a clue to a mechanism of the increased VTE risk.
Limitations
This study has some limitations. Defining the main outcome (VTE) from ICD codes in clinical registers might be a source of misclassification, especially since patients with RA might be at increased surveillance as well as having symptoms mimicking those of a DVT. A recent validation study from our group revealed that a rheumatologist was involved in the VTE workup in only 7% of cases of VTE in RA, and there was an initial suspicion of an RA-related symptom (instead of VTE suspicion) in only 1%, indicating a low surveillance and diagnostic bias regarding VTE specifically in the RA population.21
Our study has missing data for some variables (civil status, Disease Activity Score 28 components, Health Assessment Questionnaire and smoking), which is a potential source of residual confounding. On the other hand, results from the complete case analysis as well as multiple imputation were highly similar to the main results. It is therefore unlikely that this limited missingness has affected our results in any major way.
Our emulation of the ORAL surveillance study also had its limitations. First, our study mainly assessed baricitinib rather than tofacitinib, and our TNFi cohort was not limited to etanercept and adalimumab. In addition, some of the inclusion and exclusion criteria were not possible to emulate due to the register-based nature of this study.
Strengths
SRQ includes more than 85% of all Swedish patients with RA treated by rheumatologists, and we linked this population to national and population-based health registers based on prospectively collected data with high internal validity and coverage. Also, data on the outcome (VTE) was collected independently of the exposure (b/tsDMARD, and RA, status). This minimises the risk of selection and misclassification bias, and the external generalisability is likely to be high for many other settings. The inclusion of a general population cohort and an overall RA cohort, not restricted to those treated with b/tsDMARD, allowed for contextualisation of results which has not previously been performed.
Conclusion
In conclusion, as used in routine clinical practice to treat RA, JAKi (here: baricitinib and tofacitinib) are associated with a 50%–100% increased risk of VTE compared with bDMARDs. This increase seems entirely confined to PE. Although our study results add to the concerns regarding cardiovascular safety of JAKi, these risks must be viewed in light of the cardiovascular risks in patients with active RA for whom alternative treatment options may not exist.
Footnotes
Handling editor: Josef S Smolen
Collaborators: The ARTIS study group:Gerd-Marie Alenius (Department of Public Health and Clinical Medicine/Rheumatology, Umeå University), Eva Baecklund (Department of Medical Sciences, Uppsala University), Katerina Chatzidionysiou (Department of Medicine Solna, Karolinska Institutet), Nils Feltelius (Swedish Medical Products Agency, Department of Public Health and Caring Sciences, Uppsala University), Helena Forsblad-d’Elia (Department of Rheumatology and Inflammation Research, Sahlgrenska Academy, University of Gothenburg), Alf Kastbom (Department of Biomedical and Clinical Sciences, Linköping University), Lars Klareskog (Department of Medicine Solna, Karolinska Institutet), Ann Knight (Department of Medical Sciences, Uppsala University), Elisabet Lindqvist (Department of Clinical Sciences, Rheumatology, Lund University, Skåne University Hospital), Ulf Lindström (Department of Rheumatology and Inflammation Research, Sahlgrenska Academy, University of Gothenburg), Lotta Ljung (Department of Medicine Solna, Karolinska Institutet), Carl Turesson (Rheumatology, Department of Clinical Sciences, Malmö, Lund University), Christopher Sjöwall (Department of Biomedical and Clinical Sciences, Linköping University), and Johan Askling (Department of Medicine Solna, Karolinska Institutet).
Contributors: VM is guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish. VM, HB, TF and JA participated in the design of the study. VM and HB conducted the statistical analyses. All authors contributed to interpretation of the results. VM and JA contributed to the drafting of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. The study was supervised by JA.
Funding: This study has received funding from Swedish Research Council, the Swedish Heart Lung Foundation, Nordforsk, Vinnova, and the Karolinska Institutet Region Stockholm funds (ALF).
Competing interests: Karolinska Institutet, with JA as principal investigator, has or has had research agreements with Abbvie, Astra-Zeneca, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB, mainly in the context of safety monitoring of biologics via ARTIS/Swedish Biologics Register.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
The ARTIS study group:
Gerd-Marie Alenius, Eva Baecklund, Katerina Chatzidionysiou, Nils Feltelius, Helena Forsblad-d’Elia, Alf Kastbom, Lars Klareskog, Ann Knight, Elisabet Lindqvist, Ulf Lindström, Lotta Ljung, Carl Turesson, Christopher Sjöwall, and Johan Askling
Data availability statement
Data not available. The study data forms part of a register linkage performed by Karolinska Institutet, and for which further sharing of the data is limited by legal restrictions.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Regional Ethics Committee, Stockholm, Sweden (Dnr 2015/1844-31/2). Informed consent for this study was, in accordance with Swedish law, not required from the Ethics Committee.
References
- 1. Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA 2012;308:1350–6. 10.1001/2012.jama.11741 [DOI] [PubMed] [Google Scholar]
- 2. Kim SC, Schneeweiss S, Liu J, et al. The risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res 2013;65:NA–7. 10.1002/acr.22039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang J-H, Keller JJ, Lin Y-K, et al. A population-based case-control study on the association between rheumatoid arthritis and deep vein thrombosis. J Vasc Surg 2012;56:1642–8. 10.1016/j.jvs.2012.05.087 [DOI] [PubMed] [Google Scholar]
- 4. Choi HK, Rho Y-H, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182–7. 10.1136/annrheumdis-2012-201669 [DOI] [PubMed] [Google Scholar]
- 5. Chung W-S, Peng C-L, Lin C-L, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis 2014;73:1774–80. 10.1136/annrheumdis-2013-203380 [DOI] [PubMed] [Google Scholar]
- 6. Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur Heart J 2018;39:3608–14. 10.1093/eurheartj/ehx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3–14. 10.1007/s11239-015-1311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borensztajn KS, von der Thüsen JH, Spek CA. The role of coagulation in chronic inflammatory disorders: a jack of all trades. Curr Pharm Des 2011;17:9–16. 10.2174/138161211795049813 [DOI] [PubMed] [Google Scholar]
- 9. Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5–9. 10.1055/s-0037-1617146 [DOI] [PubMed] [Google Scholar]
- 10. Molander V, Bower H, Frisell T, et al. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis 2021;80:169–75. 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 11. Liang H, Danwada R, Guo D, et al. Incidence of inpatient venous thromboembolism in treated patients with rheumatoid arthritis and the association with switching biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) in the real-world setting. RMD Open 2019;5:e001013. 10.1136/rmdopen-2019-001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura M, Fujieda Y, Sugawara M, et al. Disease activity as a risk factor for venous thromboembolism in rheumatoid arthritis analysed using time-averaged DAS28CRP: a nested case-control study. Rheumatol Int 2022. 10.1007/s00296-022-05121-4. [Epub ahead of print: 06 Apr 2022]. [DOI] [PubMed] [Google Scholar]
- 13. Xie W, Huang Y, Xiao S, et al. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 2019;78:1048–54. 10.1136/annrheumdis-2018-214846 [DOI] [PubMed] [Google Scholar]
- 14. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 15. EMA . Increased risk of blood clots in lungs and death with higher dose of Xeljanz (tofacitinib) for rheumatoid arthritis, 2019. [Google Scholar]
- 16. FDA . Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate, 2019. [Google Scholar]
- 17. Kremer JM, Bingham CO, Cappelli LC, et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol 2021;3:173–84. 10.1002/acr2.11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai RJ, Pawar A, Khosrow-Khavar F, et al. Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a population-based cohort study. Rheumatology 2021;61:121–30. 10.1093/rheumatology/keab294 [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waldenlind K, Eriksson JK, Grewin B, et al. Validation of the rheumatoid arthritis diagnosis in the Swedish national patient register: a cohort study from Stockholm County. BMC Musculoskelet Disord 2014;15:432. 10.1186/1471-2474-15-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molander V, Bower H, Askling J. Validation and characterization of venous thromboembolism diagnoses in the Swedish national patient register among patients with rheumatoid arthritis. Scand J Rheumatol 2022:1–7. 10.1080/03009742.2021.2001907 [DOI] [PubMed] [Google Scholar]
- 22. Palareti G, Antonucci E, Dentali F, et al. Patients with isolated pulmonary embolism in comparison to those with deep venous thrombosis. Differences in characteristics and clinical evolution. Eur J Intern Med 2019;69:64–70. 10.1016/j.ejim.2019.08.023 [DOI] [PubMed] [Google Scholar]
- 23. Cao Y, Geng C, Li Y, et al. In situ pulmonary artery thrombosis: a previously overlooked disease. Front Pharmacol 2021;12:671589. 10.3389/fphar.2021.671589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223050supp001.pdf (328.6KB, pdf)
Data Availability Statement
Data not available. The study data forms part of a register linkage performed by Karolinska Institutet, and for which further sharing of the data is limited by legal restrictions.