Abstract
Objectives
Our aim was to compare patterns of musculoskeletal-related healthcare utilisation between male and female patients before and after the diagnosis of inflammatory arthritis (IA).
Methods
We used Ontario administrative health data to create three inception cohorts of adult patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) diagnosed between April 2010 and March 2017. Healthcare utilisation indicators including visits to physicians, and use of musculoskeletal imaging and laboratory tests were assessed in each year for 3 years before and after diagnosis and compared between male and female patients using regression models adjusting for sociodemographic factors and comorbidities. Results were reported as ORs with 95% CIs for female patients compared with male patients.
Results
A total of 41 277 patients with RA (69% female), 8150 patients with AS (51% female) and 6446 patients with PsA (54% female) were analysed.
Similar trends of sex-related differences were observed in all three cohorts. Before diagnosis, female patients were more likely to visit rheumatologists (OR 1.32–2.28) and family physicians (OR 1.03–1.15) for musculoskeletal reasons, whereas male patients were more likely to visit the emergency for musculoskeletal reasons (OR 0.76–0.87). A similar female predominance was observed regarding musculoskeletal imaging and laboratory tests before diagnosis. After diagnosis, female patients were more likely to remain in rheumatology care (OR 1.12–1.24).
Conclusion
Female patients with IA have higher healthcare utilisation than male patients which may indicate biological differences in disease course or sociocultural differences in healthcare-seeking behaviour.
Keywords: rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, health services research, epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Sex of the patient influences disease course and treatment outcomes in inflammatory arthritis (IA).
Delayed access to healthcare and suboptimal utilisation of healthcare resources delay diagnosis and management of IA. Thus, pattern of healthcare utilisation may affect disease outcomes.
Limited information exists on sex differences in patterns of healthcare utilisation in patients with IA.
WHAT THIS STUDY ADDS
Female patients with IA have more visits to physicians for musculoskeletal reasons, imaging and laboratory investigations before diagnosis compared with male patients.
These differences were more pronounced in older patients compared with their younger counterparts.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Addressing the impact of sex and gender in accessing and using healthcare could help to devise more efficient sex-specific strategies for early diagnosis and treatment of IA, which could reduce the difference in disease outcomes between male and female patients with IA.
Introduction
The term inflammatory arthritis (IA) denotes a group of immune-mediated conditions, including rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA), which are marked by chronic musculoskeletal inflammation.1 Sex and gender are important determinants of disease course and treatment response in IA.2 Timely diagnosis and initiation of therapy are critical for favourable disease outcomes and are influenced by healthcare-appropriate access and utilisation. Little is known about differences in healthcare access and utilisation between men and women with IA. Better understanding of such differences could potentially explain sex-related and gender-related differences in disease course and help to develop gender-sensitive approaches for better management of patients with IA.
The terms ‘sex’ and ‘gender’ have distinct meanings even though they are used synonymously in the medical literature. Sex is a biological characteristic which classifies individuals into ‘male’ or ‘female’. In regard to IA, sex-related mechanisms may influence immune dysregulation, pain processing and pharmacokinetics. On the other hand, gender is a sociocultural construct that denotes the level of ‘masculinity’ or ‘femininity’ experienced by individuals in a society. The impact of gender on IA can be described in terms of perception of illness, healthcare-seeking behaviour, interaction with healthcare providers and coping mechanisms.3
Sex-related disease characteristics (eg, acute vs insidious onset, pain perception) and gender-related factors (eg, health-seeking behaviour) are associated with healthcare utilisation in IA. For example, sex-related differences in access and utilisation of primary and specialty care prior to IA diagnosis can influence time from symptom onset to diagnosis of IA. Recent studies reported no sex differences in time to diagnosis of RA and time to treatment with systemic therapy.4 5 On the other hand, female patients with AS have a significantly delayed diagnosis compared with male patients,6 and are frequently misdiagnosed with fibromyalgia.7 Following IA diagnosis, regular assessments by a rheumatologist and timely initiation of systemic therapy to control disease activity are the cornerstone of best practice management of IA. In RA, female sex has been associated with more ambulatory physician visits including family physicians, rheumatologists and other physicians, and male sex has been associated with higher rates of hospitalisation and emergency visits.8 Male patients with AS were more likely to have outpatient physician visits compared with female patients.9
Overall, limited data exist regarding sex-related differences in healthcare utilisation in IA and existing studies show conflicting results. Furthermore, this topic has not been studied in PsA and no study so far compared these differences across IA types. Exploration of intersectionality of sex with other important sociodemographic health determinants has also been limited to date. Finally, development of new classification criteria for IA, utilisation of sensitive imaging modalities for diagnosis and updated management strategies render results from previous studies less relevant in the modern context.
To address these gaps in knowledge, we assessed sex-related differences in patterns of musculoskeletal-related healthcare utilisation at the population level between male and female patients with IA. Specifically, we used population-based administrative health data from Ontario, Canada to compare a wide range of indicators of musculoskeletal-related healthcare between male and female patients with three types of IA immediately before and after the diagnosis. These indicators of healthcare utilisation included visits to physicians, musculoskeletal imaging and laboratory testing. Additionally, we assessed the intersection of sex and selected health determinants with regard to healthcare utilisation.
Identifying sex-related differences in healthcare utilisation could uncover important sex-related barriers in healthcare utilisation in IA. Effective and timely management of these barriers could result in better short-term and long-term disease outcomes and improved health-related quality of life.
Methods
Study settings and data sources
We conducted a population-based retrospective cohort study using health administrative data from Ontario, Canada. Ontario residents receive health insurance coverage through a publicly funded single-payer system, the Ontario Health Insurance Plan (OHIP),10 which covers all necessary physician and hospital services and procedures. In Ontario, family physicians are often the point of first contact with the healthcare system for patients with musculoskeletal symptoms and initiate referrals to specialists, whereas rheumatologists typically diagnose IA, initiate rheumatic therapy and monitor patients following the diagnosis.
We used the OHIP claims database to create cohorts of RA, AS and PsA as well as to assess indicators of healthcare utilisation. Information on physician specialty and demographics was obtained from ICES Physicians Database, and information on sociodemographic characteristics of the patients from the OHIP Registered Persons Database. Several sources of data were used to assess pre-existing comorbidities including the OHIP claims database, ICES-derived cohorts, Discharge Abstract Database and the National Ambulatory Care Reporting System Database, the latter of which was also used to assess visits to the emergency department. These data sets are held securely in a linked, coded form and are analysed at ICES (www.ices.on.ca).
IA case ascertainment
We created three mutually exclusive inception cohorts of adult patients with RA, AS and PsA. We used validated case definitions for RA11 12 and PsA.13 For AS, we used a similar approach to the RA case definition based on a combination of physician visits for AS, including at least one by a rheumatologist or internist (online supplemental table 1). The date of diagnosis (index date) was the first date a patient received the diagnosis code of the relevant IA by a rheumatologist or internist.
ard-2022-222779supp001.pdf (169.1KB, pdf)
We included patients residing in Ontario, who were 20 years or older on index date, with a valid health insurance number for at least 5 years before the index date and diagnosed between 1 April 2010 and 31 March 2017. We excluded patients with missing information of key characteristics (age, sex, residence) and those fulfilling criteria for more than one IA.
Study outcomes and covariates
Study outcomes included indicators of musculoskeletal-related healthcare utilisation during the 3-year period before and after diagnosis. We evaluated a combination of physician encounters and emergency department visits for a wide range of inflammatory and non-inflammatory musculoskeletal conditions (see online supplemental tables 2 and 3). The following outcomes were evaluated: (1) visits to family physicians for musculoskeletal reasons (before diagnosis); (2) visits to rheumatologists (before and after diagnosis); (3) visits to non-rheumatologist musculoskeletal specialists (before diagnosis); (4) visits to an emergency department for musculoskeletal reasons (before diagnosis); (5) visits to dermatologists, only in the PsA cohort (before and after diagnosis); (6) musculoskeletal imaging including radiographs, ultrasound, CT and MRI (before and after diagnosis); (7) diagnostic laboratory tests including rheumatoid factor (before diagnosis) and acute inflammatory markers (before and 1 year after diagnosis); (8) general laboratory tests including complete blood count, liver and kidney function tests (1 year after diagnosis) and lipid profile (in any of the 3 years after diagnosis). For each outcome, the proportion of patients with utilisation of at least one outcome category was assessed for each year before and after diagnosis (index date) as relevant.
Sex of the patient was considered the primary predictor of the study. Baseline patient characteristics included patient age, area of residence using rural index of Ontario 2008 to define rural residence,14 socioeconomic status (SES) by census neighbourhood income quintiles and marginalisation index,15 comorbidities and extra-articular disease manifestations, both individual and as Aggregated Diagnosis Group (ADG) categories using the Johns Hopkins ACG System V.11. The ADG system assigns International Classification of Diseases (ICD) codes to 1 of 32 diagnosis clusters known as ADG. ICD codes within the same ADG are similar in both clinical criteria and expected need for healthcare resource. In addition, access to rheumatologists in terms of regional density of rheumatologists and remote distance to rheumatologist was assessed. The Canadian Rheumatology Association recommends 1 rheumatologist per 75 000 population,16 which was considered ‘optimal’ in our study and less than one rheumatologist to be ‘suboptimal’. Remote distance to rheumatologist indicated a distance of 100 km or more between patient and the nearest rheumatologist.
Subgroup analyses were conducted to investigate the effect of prespecified variables on sex differences in healthcare utilisation in IA. The following variables were assessed as effect modifiers: age (20–65 years vs >65 years), area of residence (urban vs rural), SES (neighbourhood income quintiles 1 and 2 were collapsed to form low, quintiles 3 and 4 collapsed to form middle and quintile 5 alone to form high SES categories) and sex of diagnosing specialist (male vs female).
Statistical analysis
Analyses were performed on each of the three cohorts separately. Patient characteristics (at diagnosis date) were compared between male and female patients using Student’s t-tests for continuous variables and Χ2 tests for binary variables. Since formal statistical tests may show statistical difference in the absence of clinically meaningful difference in large samples, we examined the differences in characteristics between male and female patients by using standardised differences. We considered a standardised difference of greater than 0.1 to be significant.
The association between sex of the patients and probability of healthcare utilisation (study outcomes) was assessed using multivariable logistic regression models with binomial distributions separately for each IA cohort. Sex was the primary predictor in these models which were adjusted for age, residence, SES, comorbidities by ADG categories and regional density of rheumatologists. Similar multivariable regression models were constructed for the subgroup analysis. Only patients with complete information on all model covariates were included in the regression analysis. Each model was run separately for each indicator as an outcome and results were reported as ORs with 95% CIs for female patients compared with male patients.
Results
A total of 41 277 patients with RA (69% female), 8150 patients with AS (51% female) and 6446 patients with PsA (54% female) were included in the study and their sociodemographic characteristics at the time of diagnosis are summarised in table 1. Male patients were significantly older than female patients with RA (mean age 60.4 (14.2) years in men vs 57.1 (15.2) years in women). Multimorbidity (ADG category ≥10), depression and osteoporosis were more common in female patients and cardiovascular disease was more common in male patients across the cohorts. Male patients with PsA had a significantly higher prevalence of psoriasis than female patients with PsA (46.8% in men vs 40.2% in women).
Table 1.
Patient characteristics at the time of diagnosis, by sex
| Patient characteristics | Rheumatoid arthritis | Ankylosing spondylitis | Psoriatic arthritis | |||
| Male N=12 702 N (%) |
Female N=28 575 N (%) |
Male N=3990 N (%) |
Female N=4160 N (%) |
Male N=2963 N (%) |
Female N=3483 N (%) |
|
| Age, mean (SD) | 60.4 (14.2) | 57.1 (15.2) | 46.8 (15.5) | 48.2 (14.9) | 51.7 (13.8) | 52.7 (13.8) |
| Residence | ||||||
| Rural | 1263 (9.9) | 2400 (8.4) | 307 (7.7) | 288 (6.9) | 203 (6.9) | 217 (6.2) |
| Urban | 11 242 (88.5) | 25 799 (90.3) | 3649 (91.5) | 3828 (92.0) | 2743 (92.6) | 3240 (93.0) |
| Socioeconomic status (SES) | ||||||
| Low SES | 4724 (37.2) | 11 250 (39.4) | 1415 (35.5) | 1465 (35.2) | 1008 (34.0) | 1244 (35.7) |
| Middle SES | 5338 (42.0) | 11 692 (40.9) | 1624 (40.7) | 1717 (41.3) | 1249 (42.2) | 1438 (41.3) |
| High SES | 2603 (20.5) | 5534 (19.4) | 942 (23.6) | 967 (23.2) | 701 (23.7) | 790 (22.7) |
| Marginalisation | ||||||
| Least deprived | 5282 (41.6) | 11 216 (39.3) | 1762 (44.2) | 1878 (45.1) | 1383 (46.7) | 1576 (45.2) |
| Moderately deprived | 2567 (20.2) | 5671 (19.8) | 823 (20.6) | 781 (18.8) | 579 (19.5) | 682 (19.6) |
| Most deprived | 4730 (37.2) | 11 414 (39.9) | 1379 (34.6) | 1479 (35.6) | 991 (33.4) | 1206 (34.6) |
| Comorbidities/extra-articular manifestations | ||||||
| Psoriasis | 332 (2.6) | 470 (1.6) | 229 (5.7) | 232 (5.6) | 1388 (46.8) | 1399 (40.2) |
| Uveitis | 54 (0.4) | 123 (0.4) | 165 (4.1) | 199 (4.8) | 25 (0.8) | 37 (1.1) |
| IBD | 261 (2.1) | 492 (1.7) | 310 (7.8) | 317 (7.6) | 79 (2.7) | 175 (5.0) |
| Hypertension | 6307 (49.7) | 12 320 (43.1) | 1185 (29.7) | 1157 (27.8) | 1161 (39.2) | 1307 (37.5) |
| Diabetes mellitus | 2844 (22.4) | 4619 (16.2) | 491 (12.3) | 491 (11.8) | 472 (15.9) | 588 (16.9) |
| CVD | 1935 (15.2) | 2070 (7.2) | 272 (6.8) | 155 (3.7) | 248 (8.4) | 171 (4.9) |
| Cerebrovascular disease | 103 (0.8) | 144 (0.5) | 23 (0.6) | 11 (0.3) | 15 (0.5) | 13 (0.4) |
| Cancer | 1314 (10.3) | 2210 (7.7) | 211 (5.3) | 231 (5.6) | 188 (6.3) | 228 (6.5) |
| Depression | 467 (3.7) | 1870 (6.5) | 162 (4.1) | 371 (8.9) | 116 (3.9) | 298 (8.6) |
| Lung disease | 3442 (27.1) | 8357 (29.2) | 863 (21.6) | 1132 (27.2) | 624 (21.1) | 1042 (29.9) |
| Liver disease | 283 (2.2) | 437 (1.5) | 72 (1.8) | 65 (1.6) | 51 (1.7) | 70 (2.0) |
| Kidney disease | 650 (5.1) | 892 (3.1) | 138 (3.5) | 98 (2.4) | 89 (3.0) | 77 (2.2) |
| Osteoporosis | 168 (1.3) | 1618 (5.7) | 50 (1.3) | 217 (5.2) | 21 (0.7) | 135 (3.9) |
| ADG categories | ||||||
| 0–5 | 1005 (7.9) | 1393 (4.9) | 411 (10.3) | 116 (2.8) | 284 (9.6) | 113 (3.2) |
| 6–9 | 3287 (25.9) | 5722 (20.0) | 1269 (31.8) | 666 (16.0) | 909 (30.7) | 614 (17.6) |
| 10+ | 8410 (66.2) | 21 460 (75.1) | 2310 (57.9) | 3378 (81.2) | 1770 (59.7) | 2756 (79.1) |
| Regional density of rheumatologists | ||||||
| Optimal | 1798 (60.7) | 2211 (63.5) | 2648 (66.4) | 2684 (64.5) | 1798 (60.7) | 2211 (63.5) |
| Suboptimal | 1165 (39.3) | 1272 (36.5) | 1342 (33.6) | 1476 (35.5) | 1165 (39.3) | 1272 (36.5) |
| Remote distance to rheumatologist | 100 (3.4) | 122 (3.5) | 183 (4.6) | 274 (6.6) | 100 (3.4) | 122 (3.5) |
Statistically significant (standardised difference >0.1) results are bolded.
Regional density of rheumatologists: 1 rheumatologist per 75 000 population—optimal; <1 rheumatologist per 75 000 population—suboptimal.
Remote distance to rheumatologist: ≥100 km between patient’s residence and nearest rheumatologist.
ADG, Aggregated Diagnosis Group; CVD, cardiovascular disease; IBD, inflammatory bowel disease.
Visits to physicians and emergency department
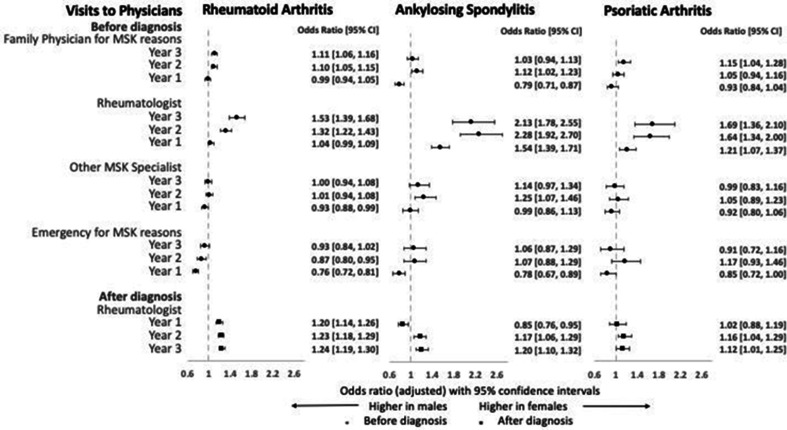
Overall pattern of healthcare utilisation both before and after diagnosis showed similar trends across the three cohorts. Analyses of physician visits revealed most prominent sex-related differences in visits to family physicians and rheumatologists before diagnosis. Visits to family physicians for musculoskeletal issues gradually increased from around 35%–47% to about 66%–80%, and visits to rheumatologists increased abruptly from around 4%–11% to about 20%–31% over the 3 years before diagnosis (see table 2 and figure 1). Multivariable logistic regression models showed that female patients across the three cohorts were more likely to visit family physicians for musculoskeletal reasons (adjusted OR 1.03–1.15) and rheumatologists (adjusted OR 1.32–2.28) 2-3 years before diagnosis (figure 1). Sex differences were greater in the earlier pre-diagnosis periods and tended to diminish with time. Three years after diagnosis, female patients were more likely to remain in rheumatology care with adjusted OR ranging from 1.12 to 1.24 across the cohorts (figure 1). In contrast, male patients with RA and AS were more likely to visit the emergency department for a musculoskeletal reason immediately before diagnosis with adjusted OR ranging from 0.76 to 0.78. In the PsA cohort, male patients had higher odds of visiting dermatologists both before and after diagnosis.
Table 2.
Visits to physicians before and after diagnosis in patients with inflammatory arthritis, by sex of the patients
| Visits to physicians | Rheumatoid arthritis (RA) | Ankylosing spondylitis (AS) | Psoriatic arthritis (PsA) | |||
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| Before diagnosis | ||||||
| Visits to family physicians for MSK reasons | ||||||
| Year 3 | 5016 (39.5) | 12 467 (43.6) | 1657 (41.5) | 1968 (47.3) | 1053 (35.5) | 1500 (43.1) |
| Year 2 | 5611 (44.2) | 13 719 (48.0) | 1736 (43.5) | 2121 (51.0) | 1199 (40.5) | 1599 (45.9) |
| Year 1 | 10 144 (79.9) | 22 867 (80.0) | 2980 (74.7) | 2982 (71.7) | 1966 (66.4) | 2360 (67.8) |
| Visits to rheumatologists for non-specific MSK causes | ||||||
| Year 3 | 640 (5.0) | 2195 (7.7) | 198 (5.0) | 470 (11.3) | 129 (4.4) | 284 (8.2) |
| Year 2 | 904 (7.1) | 2682 (9.4) | 215 (5.4) | 535 (12.9) | 160 (5.4) | 336 (9.6) |
| Year 1 | 3627 (28.6) | 8493 (29.7) | 842 (21.1) | 1300 (31.3) | 584 (19.7) | 873 (25.1) |
| Visits to other MSK specialists | ||||||
| Year 3 | 1370 (10.8) | 3092 (10.8) | 293 (7.3) | 418 (10.0) | 283 (9.6) | 389 (11.2) |
| Year 2 | 1514 (11.9) | 3450 (12.1) | 310 (7.8) | 471 (11.3) | 300 (10.1) | 431 (12.4) |
| Year 1 | 2162 (17.0) | 4654 (16.3) | 498 (12.5) | 595 (14.3) | 435 (14.7) | 539 (15.5) |
| Visits to the emergency department for MSK reasons | ||||||
| Year 3 | 708 (5.6) | 1629 (5.7) | 198 (5.0) | 264 (6.3) | 131 (4.4) | 170 (4.9) |
| Year 2 | 921 (7.3) | 1966 (6.9) | 206 (5.2) | 281 (6.8) | 135 (4.6) | 219 (6.3) |
| Year 1 | 2327 (18.3) | 4433 (15.5) | 484 (12.1) | 480 (11.5) | 312 (10.5) | 362 (10.4) |
| Visits to dermatologists for psoriasis | ||||||
| Year 3 | Not assessed | Not assessed | 506 (17.1) | 540 (15.5) | ||
| Year 2 | 568 (19.2) | 544 (15.6) | ||||
| Year 1 | 845 (28.5) | 913 (26.2) | ||||
| After diagnosis | ||||||
| Visits to rheumatologists | ||||||
| Year 1 | 9781 (78.3) | 22 982 (81.1) | 3122 (78.5) | 3114 (75.1) | 2544 (86.0) | 3001 (86.3) |
| Year 2 | 8203 (67.0) | 19 902 (71.0) | 2522 (63.9) | 2762 (66.7) | 1984 (67.5) | 2447 (70.7) |
| Year 3 | 7373 (61.6) | 18 284 (66.2) | 2154 (55.0) | 2434 (59.1) | 1868 (64.0) | 2299 (66.8) |
| Visits to dermatologists for psoriasis | ||||||
| Year 1 | N/A | N/A | 884 (29.9) | 959 (27.6) | ||
| Year 2 | 665 (22.6) | 739 (21.3) | ||||
| Year 3 | 651 (22.3) | 689 (20.0) | ||||
Denominators for RA cohort:
Year 1 after index date: male patients—12 488, female patients—28 339.
Year 2 after index date: male patients—12 250, female patients—28 014.
Year 3 after index date: male patients—11 971, female patients—27 628.
Denominators for AS cohort:
Year 1 after index date: male patients—3975, female patients—4149.
Year 2 after index date: male patients—3948, female patients—4141.
Year 3 after index date: male patients—3913, female patients—4118.
Denominators for PsA cohort:
Year 1 after index date: male patients—2957, female patients—3478.
Year 2 after index date: male patients—2940, female patients—3462.
Year 3 after index date: male patients—2919, female patients—3440.
Bolded results are statistically significant (standardised difference >0.1).
MSK, musculoskeletal.
Figure 1.
Adjusted ORs for visits to physicians for female patients compared with male patients by IA group. Error bars represent 95% CIs. ORs >1 indicate higher odds in women and ORs <1 indicate higher odds in men. IA, inflammatory arthritis; MSK, musculoskeletal.
Musculoskeletal imaging
A female predominance was found in receiving musculoskeletal radiographs before diagnosis and ultrasounds after diagnosis of IA (see table 3 and figure 2). The proportion of patients who underwent musculoskeletal radiographs nearly doubled in the year before diagnosis in all three cohorts (table 3). Only in the AS cohort, approximately 30% of patients underwent MRI immediately before and after diagnosis without significant sex differences. Female patients across all three cohorts were more likely to receive musculoskeletal radiographs (adjusted OR 1.02–1.18) in the earlier pre-diagnosis periods and musculoskeletal ultrasounds (adjusted OR 1.07–1.36) following diagnosis (figure 2).
Table 3.
Musculoskeletal (MSK) imaging before and after diagnosis in patients with inflammatory arthritis, by sex of the patients
| MSK imaging | Rheumatoid arthritis (RA) | Ankylosing spondylitis (AS) | Psoriatic arthritis (PsA) | |||
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| Before diagnosis | ||||||
| X-ray | ||||||
| Year 3 | 3817 (30.1) | 9674 (33.9) | 1093 (27.4) | 1424 (34.2) | 835 (28.2) | 1180 (33.9) |
| Year 2 | 4409 (34.7) | 10 943 (38.3) | 1211 (30.4) | 1564 (37.6) | 1013 (34.2) | 1349 (38.7) |
| Year 1 | 9313 (73.3) | 20 906 (73.2) | 2754 (69.0) | 2726 (65.5) | 1976 (66.7) | 2375 (68.2) |
| Ultrasound | ||||||
| Year 3 | 557 (4.4) | 1413 (4.9) | 127 (3.2) | 201 (4.8) | 120 (4.0) | 195 (5.6) |
| Year 2 | 727 (5.7) | 1772 (6.2) | 129 (3.2) | 234 (5.6) | 149 (5.0) | 221 (6.3) |
| Year 1 | 1997 (15.7) | 4689 (16.4) | 330 (8.3) | 472 (11.3) | 330 (8.3) | 472 (11.3) |
| CT scan | ||||||
| Year 3 | 227 (1.8) | 462 (1.6) | 66 (1.7) | 80 (1.9) | 35 (1.2) | 63 (1.8) |
| Year 2 | 258 (2.0) | 488 (1.7) | 105 (2.6) | 79 (1.9) | 45 (1.5) | 56 (1.6) |
| Year 1 | 372 (2.9) | 671 (2.3) | 193 (4.8) | 180 (4.3) | 68 (2.3) | 68 (2.0) |
| MRI | ||||||
| Year 3 | 779 (6.1) | 1792 (6.3) | 324 (8.1) | 423 (10.2) | 163 (5.5) | 250 (7.2) |
| Year 2 | 851 (6.7) | 2022 (7.1) | 354 (8.9) | 477 (11.5) | 212 (7.2) | 271 (7.8) |
| Year 1 | 1608 (12.7) | 3421 (12.0) | 1037 (26.0) | 1215 (29.2) | 378 (12.8) | 500 (14.4) |
| After diagnosis (year 1) | ||||||
| X-ray | 7046 (56.4) | 17 107 (60.4) | 2150 (54.1) | 2231 (53.8) | 1881 (63.6) | 2299 (66.1) |
| Ultrasound | 1885 (15.1) | 4785 (16.9) | 312 (7.8) | 461 (11.1) | 402 (13.6) | 598 (17.2) |
| CT scan | 279 (2.2) | 608 (2.1) | 99 (2.5) | 123 (3.0) | 72 (2.4) | 85 (2.4) |
| MRI | 1447 (11.6) | 3430 (12.1) | 1193 (30.0) | 1340 (32.3) | 499 (16.9) | 627 (18.0) |
Denominator for RA cohort in year 1 after index date: male patients—12 488, female patients—28 339.
Denominator for AS cohort in year 1 after index date: male patients—3975, female patients—4149.
Denominator for PsA cohort in year 1 after index date: male patients—2957, female patients—3478.
Bolded results are statistically significant (standardised difference >0.1).
Figure 2.
Adjusted ORs for musculoskeletal (MSK) imaging for female patients compared with male patients by inflammatory arthritis group. Error bars represent 95% CIs. ORs >1 indicate higher odds in women and ORs <1 indicate higher odds in men.
Laboratory testing
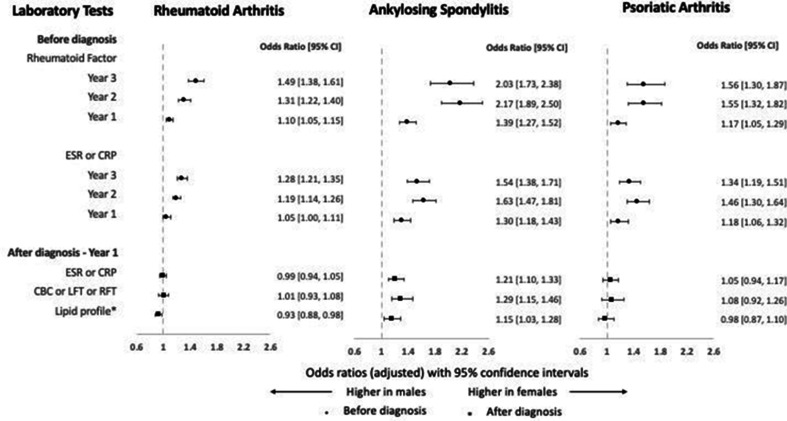
A similar trend of an abrupt rise in testing was seen for laboratory tests in the year before diagnosis (table 4). Female patients had higher odds of these tests before diagnosis across the three cohorts (figure 3), which tended to diminish with time (approaching diagnosis). No significant sex-related difference was observed in laboratory tests after diagnosis.
Table 4.
Laboratory tests before and after diagnosis in patients with inflammatory arthritis, by sex of the patients
| Laboratory tests | Rheumatoid arthritis (RA) | Ankylosing spondylitis (AS) | Psoriatic arthritis (PsA) | |||
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| Before diagnosis | ||||||
| Rheumatoid factor | ||||||
| Year 3 | 916 (7.2) | 3169 (11.1) | 260 (6.5) | 586 (14.1) | 211 (7.1) | 405 (11.6) |
| Year 2 | 1383 (10.9) | 4160 (14.6) | 352 (8.8) | 782 (18.8) | 271 (9.1) | 498 (14.3) |
| Year 1 | 8484 (66.8) | 19 667 (68.8) | 1610 (40.4) | 1986 (47.7) | 1364 (46.0) | 1719 (49.4) |
| ESR or CRP | ||||||
| Year 3 | 2224 (17.5) | 6306 (22.1) | 744 (18.6) | 1202 (28.9) | 602 (20.3) | 958 (27.5) |
| Year 2 | 2848 (22.4) | 7576 (26.5) | 886 (22.2) | 1433 (34.4) | 671 (22.6) | 1106 (31.8) |
| Year 1 | 9888 (77.8) | 22 520 (78.8) | 2501 (62.7) | 2843 (68.3) | 1889 (63.8) | 2372 (68.1) |
| After diagnosis | ||||||
| ESR or CRP—year 1 | 9853 (78.9) | 22 269 (78.6) | 2360 (59.4) | 2693 (64.9) | 2146 (72.6) | 2559 (73.6) |
| CBC or LFT or RFT—year 1 | 11 202 (89.7) | 25 420 (89.7) | 3002 (75.5) | 3415 (82.3) | 2585 (87.4) | 3095 (89.0) |
| Lipid profile—year 1 | 5708 (45.7) | 11 926 (42.1) | 1392 (35.0) | 1690 (40.7) | 1314 (44.4) | 1558 (44.8) |
| Lipid profile—year 2 | 6052 (49.4) | 12 988 (46.4) | 1533 (38.8) | 1876 (45.3) | 1368 (46.5) | 1644 (47.5) |
| Lipid profile—year 3 | 5946 (49.7) | 12 741 (46.1) | 1577 (40.3) | 1866 (45.3) | 1389 (47.6) | 1617 (47.0) |
Denominators for RA cohort:
Year 1 after index date: male patients—12 488, female patients—28 339.
Year 2 after index date: male patients—12 250, female patients—28 014.
Year 3 after index date: male patients—11 971, female patients—27 628.
Denominators for AS cohort:
Year 1 after index date: male patients—3975, female patients—4149.
Year 2 after index date: male patients—3948, female patients—4141.
Year 3 after index date: male patients—3913, female patients—4118.
Denominators for PsA cohort:
Year 1 after index date: male patients—2957, female patients—3478.
Year 2 after index date: male patients—2940, female patients—3462.
Year 3 after index date: male patients—2919, female patients—3440.
Bolded results are statistically significant (standardised difference >0.1).
CBC, complete blood count; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; LFT, liver function test; RFT, renal function test.
Figure 3.
Adjusted ORs for laboratory tests for female patients compared with male patients by inflammatory arthritis group. Error bars represent 95% CIs. ORs >1 indicate higher odds in women and ORs <1 indicate higher odds in men. *OR for lipid profile was calculated for patients who survived the 3 years after diagnosis. CBC, complete blood count; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; LFT, liver function test; RFT, renal function test.
Subgroup analysis
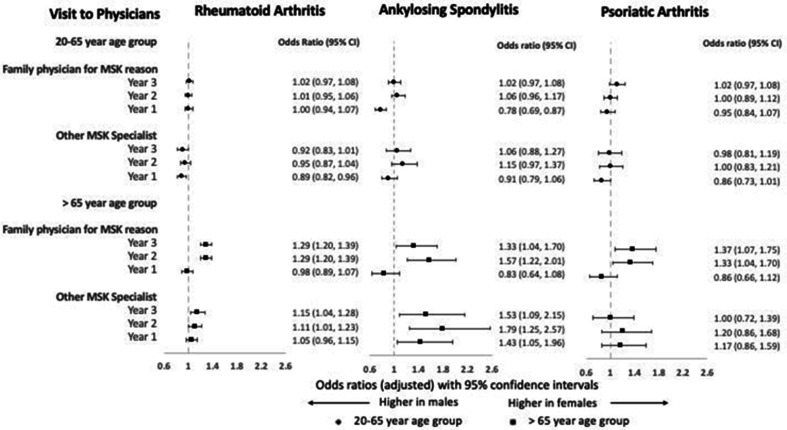
Subgroup analysis revealed striking effect modification of sex by age, especially in physician visits before diagnosis (online supplemental tables 4–6). In earlier pre-diagnosis periods, older female patients were more likely to visit a family physician for musculoskeletal reasons with adjusted OR ranging from 1.29 to 1.57 across all three cohorts (figure 4). Additionally, odds were also higher for older female patients for visits to other musculoskeletal specialists 2-3 years before diagnosis (adjusted OR 1.00–1.79) (figure 4) and radiograph testing (adjusted OR 1.31–1.65) before and after diagnosis (online supplemental figure 1). In contrast, no such sex differences were found among younger patients. The remaining subgroup analyses did not suggest substantial modification of the effect of sex on healthcare utilisation.
Figure 4.
Adjusted ORs for visits to physicians for female patients compared with male patients before diagnosis by inflammatory arthritis and age group. Error bars represent 95% CIs. ORs >1 indicate higher odds in women and ORs <1 indicate higher odds in men. MSK, musculoskeletal.
Discussion
Our study explored sex-related differences in musculoskeletal-related healthcare utilisation in three large cohorts of patients with IA at the population level. Besides the magnitude, the study also detects patterns and trends of sex-related differences in healthcare access and utilisation in IA. Thus, the results help to capture and speculate about the sex-specific barriers to accessing healthcare in IA. Overall, the study identified higher access to outpatient primary and specialty care, and increased utilisation of imaging and laboratory investigations in female patients before and after diagnosis of IA. On the other hand, use of emergency care for musculoskeletal reasons was more common in male patients. These differences were more pronounced in older patients compared with their younger counterparts.
Both sex-related and gender-related factors could explain the observed differences in pattern of healthcare utilisation between men and women. Early prodromal phase of disease, lower threshold for pain reporting or higher healthcare-seeking behaviour common in female patients17 could have triggered the increased healthcare encounters seen in this study. Men, on the other hand, might be reluctant to seek care, ignore symptoms, have a high threshold for reporting pain, self-medicate, present to healthcare providers only when disease is severe or symptoms intolerable or lack a usual source of care.18 Additionally, several factors may hinder the diagnosis of IA in women thus contributing to suboptimal healthcare utilisation and delayed diagnosis, for example, low suspicion of male-predominant disease (such as AS) in women and unique disease presentation in women, such as peripheral arthritis in AS, a disease that typically manifests with spinal inflammation. Moreover, the presence of female-predominant musculoskeletal comorbidities, such as osteoarthritis (OA)19 and fibromyalgia,20 and misinterpretation of subjective complaints of pain and fatigue that are common in women21 further add to the diagnostic dilemma of IA in women. Such bias could cause underutilisation of more sensitive imaging modalities in women. Heavy reliance on the test results, especially when they comply with physicians’ inherent biases, could further delay referrals and diagnosis of IA.
Our subgroup analyses showed substantial effect modification of sex differences in healthcare utilisation in IA by age. Older female patients were more likely to visit family physicians and other musculoskeletal specialists before diagnosis, and receive radiographs before and after diagnosis. Musculoskeletal pain may be considered an effect of age-associated ‘wear and tear’,22 making both patients and physicians reluctant in seeking a diagnosis. High prevalence of OA in older women23 could lower the suspicion of IA in this patient group.
Our study had several limitations. First, lack of clinical information on patient symptoms and physician findings prevented determining a definite linkage between physician visits and IA during the pre-diagnosis period. In addition, the lack of information on IA disease activity limited our ability to determine the causes for sex-related differences in visits to rheumatologists after the diagnosis of IA. Lack of imaging data prevented us from distinguishing radiographic from non-radiographic spondyloarthritis (SpA) and it is likely that patients with non-radiographic SpA were included in the AS group. However, best practice guidelines require at least one annual rheumatology visit, including for patient with stable IA, thus the higher persistence of female patients in rheumatology care is likely multifactorial and not solely explained by lower disease activity in male patients. Medication information is only available in Ontario in those above 65 years of age; therefore, we could not consider use of disease-modifying medications and biologics, either as outcome or confounder. The prevalence of extra-articular manifestations of SpA (eg, psoriasis, uveitis) may be underestimated due to use of more stringent case definition (favouring specificity over sensitivity). Finally, lack of data on gender-related variables limited our analysis and findings to sex of the patient, even though many of the sex differences are in reality gender related.
Our study has several major strengths. First, this is one of the largest studies to date that describes sex differences in healthcare access and utilisation in three types of IA at the population level. Additional strengths of the study include use of validated case definitions, use of data from a publicly funded healthcare system removing sex biases in coverage of healthcare services and the large study population with diverse sociodemographic characteristics which makes our findings generalisable to similar populations.
In conclusion, our study found overall higher healthcare utilisation in female patients with IA, which could be due to sex-related biological differences in disease course or gender-related differences in patient behaviour, access to care and interaction with healthcare providers. Further research could be carried out to evaluate economic impact of such excess healthcare utilisation before diagnosis of IA and to determine more effective sex-specific and gender-specific strategies to overcome barriers to efficient and timely healthcare use in IA. External validation of our study findings could also be done by linking health administrative databases with disease registries of IA. Patient satisfaction with the healthcare system from a gender focus could be explored to identify barriers to care from the patient perspective.
Acknowledgments
This study was supported by ICES which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by the MOH, MLTC, Ontario Health and the Canadian Institute for Health Information. ST was supported by the Enid Walker Graduate Student Award for Research in Women’s Health for this study. JW receives support from the Arthritis Society Stars Career Development Award (STAR-19-0610). SRJ has been awarded a Canadian Institutes of Health Research New Investigator Award. PR holds the RTOERO Chair in Geriatric Medicine at the University of Toronto. LE has been awarded Early Researcher Award from the Ontario Ministry of Research, Innovation and Science and Canada Research Chair (Tier 2) in Inflammatory Rheumatic Diseases.
Footnotes
Handling editor: Josef S Smolen
Twitter: @lihi_eder
Presented at: The abstract of this study was presented as posters at the annual scientific meeting of the Canadian Rheumatology Association in January 2022 and at the 2022 SPARTAN Annual meeting and Trainee Symposium.
Contributors: LE, ST, JW, PR and SRJ conceptualised the study. LE, ST, JW, PR, SRJ and CFW were involved in study design. CFW did the statistical analysis. LE, ST, JW, PR, SRJ and CFW interpreted the study findings. ST and LE wrote the manuscript. JW, PR, SRJ and CFW reviewed and provided comments on the manuscript. All authors approved the final version of the manuscript. LE is responsible for the overall content as the guarantor.
Funding: This project was partially funded by the Enid Walker Graduate Student Award for Research in Women’s Health (recipient–ST).
Disclaimer: The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Competing interests: ST, JW, CFW, SRJ and PR (none for LE) received educational and research grants and consultation fees from Abbvie, UCB, Eli Lilly, Novartis, Sandoz and Pfizer.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data were obtained through ICES.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The data in this study were authorised for use under section 45 of Ontario’s Personal Health Information Protection Act and do not require review by a research ethics board.
References
- 1. Brent LH. Inflammatory arthritis: an overview for primary care physicians. Postgrad Med 2009;121:148–62. 10.3810/pgm.2009.03.1987 [DOI] [PubMed] [Google Scholar]
- 2. Oertelt-Prigione S. Sex and Gender in Medical Literature. In: Sex and gender aspects in clinical medicine. Springer-Verlag London Limited, 2012. [Google Scholar]
- 3. Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr 2007;25:47–61. [PMC free article] [PubMed] [Google Scholar]
- 4. Barnabe C, Xiong J, Pope JE, et al. Factors associated with time to diagnosis in early rheumatoid arthritis. Rheumatol Int 2014;34:85–92. 10.1007/s00296-013-2846-5 [DOI] [PubMed] [Google Scholar]
- 5. Jamal S, Alibhai SMH, Badley EM, et al. Time to treatment for new patients with rheumatoid arthritis in a major metropolitan City. J Rheumatol 2011;38:1282–8. 10.3899/jrheum.101315 [DOI] [PubMed] [Google Scholar]
- 6. Jovaní V, Blasco-Blasco M, Ruiz-Cantero MT, et al. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and Metaanalysis. J Rheumatol 2017;44:174–83. 10.3899/jrheum.160825 [DOI] [PubMed] [Google Scholar]
- 7. Ogdie A, Benjamin Nowell W, Reynolds R, et al. Real-World patient experience on the path to diagnosis of ankylosing spondylitis. Rheumatol Ther 2019;6:255–67. 10.1007/s40744-019-0153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanly JG, Thompson K, Skedgel C. A longitudinal study of ambulatory physician encounters, emergency room visits, and hospitalizations by patients with rheumatoid arthritis: a 13-year population health study. J Rheumatol 2017;44:1421–8. 10.3899/jrheum.170056 [DOI] [PubMed] [Google Scholar]
- 9. Chen H-H, Chen T-J, Chen Y-M, et al. Gender differences in ankylosing spondylitis-associated cumulative healthcare utilization: a population-based cohort study. Clinics 2011;66:251–4. 10.1590/S1807-59322011000200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ontario ca . Health care in Ontario, 2022. Available: http://www.ontario.ca/page/health-care-ontario
- 11. Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res 2013;65:1582–91. 10.1002/acr.22031 [DOI] [PubMed] [Google Scholar]
- 12. Widdifield J, Bombardier C, Bernatsky S, et al. An administrative data validation study of the accuracy of algorithms for identifying rheumatoid arthritis: the influence of the reference standard on algorithm performance. BMC Musculoskelet Disord 2014;15:216. 10.1186/1471-2474-15-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eder L, Widdifield J, Rosen CF, et al. Identifying and characterizing psoriasis and psoriatic arthritis patients in Ontario administrative data: a population-based study from 1991 to 2015. J Rheumatol 2020;47:1644–51. 10.3899/jrheum.190659 [DOI] [PubMed] [Google Scholar]
- 14. Kralj B. Measuring rurality - RIO 2008_BASIC: methodology and results. Toronto, Ontario: Ontario Medical Association Economics Department; 2013. https://www.deslibris.ca/ID/237943 [Google Scholar]
- 15. Public Health Ontario . Ontario marginalization index (ON-Marg), 2022. Available: https://www.publichealthontario.ca/en/Data-and-Analysis/Health-Equity/Ontario-Marginalization-Index
- 16. Barber CEH, Jewett L, Badley EM, et al. Stand up and be counted: measuring and mapping the rheumatology workforce in Canada. J Rheumatol 2017;44:248–57. 10.3899/jrheum.160621 [DOI] [PubMed] [Google Scholar]
- 17. Pinkhasov RM, Wong J, Kashanian J, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract 2010;64:475–87. 10.1111/j.1742-1241.2009.02290.x [DOI] [PubMed] [Google Scholar]
- 18. Samulowitz A, Gremyr I, Eriksson E, et al. "Brave Men" and "Emotional Women": A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Res Manag 2018;2018:1–14. 10.1155/2018/6358624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355–69. 10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017;29:304–10. 10.1097/BOR.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Losin EAR, Ashar YK, et al. Gender biases in estimation of others' pain. J Pain 2021;22:1048–59. 10.1016/j.jpain.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grime J, Richardson JC, Ong BN. Perceptions of joint pain and feeling well in older people who reported being healthy: a qualitative study. Br J Gen Pract 2010;60:597–603. 10.3399/bjgp10X515106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Litwic A, Edwards MH, Dennison EM, et al. Epidemiology and burden of osteoarthritis. Br Med Bull 2013;105:185–99. 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-222779supp001.pdf (169.1KB, pdf)
Data Availability Statement
Data were obtained through ICES.