Abstract
Rationale
Patients with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are at higher risk of ventilator-associated pneumonia (VAP) and may have an increased attributable mortality (increased or decreased risk of death if VAP occurs in a patient) and attributable fraction (proportion of deaths that are attributable to an exposure) of VAP-related mortality compared with subjects without coronavirus disease (COVID-19).
Objectives
Estimation of the attributable mortality of the VAP among patients with COVID-19.
Methods
Using the REA-REZO surveillance network, three groups of adult medical ICU patients were computed: control group (patients admitted between 2016 and 2019; prepandemic patients), pandemic COVID-19 group (PandeCOV+), and pandemic non–COVID-19 group (PandeCOV−) admitted during 2020. The primary outcome was the estimation of attributable mortality and attributable fraction related to VAP in these patients. Using multistate modeling with causal inference, the outcomes related to VAP were also evaluated.
Measurements and Main Results
A total of 64,816 patients were included in the control group, 7,442 in the PandeCOV− group, and 1,687 in the PandeCOV+ group. The incidence of VAP was 14.2 (95% confidence interval [CI], 13.9 to 14.6), 18.3 (95% CI, 17.3 to 19.4), and 31.9 (95% CI, 29.8 to 34.2) per 1,000 ventilation-days in each group, respectively. Attributable mortality at 90 days was 3.15% (95%, CI, 2.04% to 3.43%), 2.91% (95% CI, −0.21% to 5.02%), and 8.13% (95% CI, 3.54% to 12.24%), and attributable fraction of mortality at 90 days was 1.22% (95% CI, 0.83 to 1.63), 1.42% (95% CI, −0.11% to 2.61%), and 9.17% (95% CI, 3.54% to 12.24%) for the control, PandeCOV−, and PandeCOV+ groups, respectively. Except for the higher risk of developing VAP, the PandeCOV− group shared similar VAP characteristics with the control group. PandeCOV+ patients were at lower risk of death without VAP (hazard ratio, 0.62; 95% CI, 0.52 to 0.74) than the control group.
Conclusions
VAP-attributable mortality was higher for patients with COVID-19, with more than 9% of the overall mortality related to VAP.
Keywords: COVID-19, ventilator-associated pneumonia, attributable mortality, population attributable fraction
At a Glance Commentary
Scientific Knowledge on the Subject
Patients with coronavirus disease (COVID-19) are at higher risk of ventilator-associated pneumonia (VAP). Little is known regarding VAP in patients without COVID-19 admitted in ICUs during the pandemic.
What This Study Adds to the Field
In patients with COVID-19, VAP accounts for 9% of ICU deaths at 90 days. Patients without COVID-19 admitted to an ICU during the pandemic have a higher risk of VAP, but VAP is not responsible for a higher risk of death in this population than in ICU patients admitted before the pandemic.
After the outbreak of coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan in early 2020, the World Health Organization (WHO) declared the COVID-19 global pandemic on March 11, 2020 (1). This resulted in massive waves of hospitalizations of patients potentially requiring intensive care resources (dialysis or hemodynamic support) and specific acute respiratory distress syndrome management, including high-flow nasal oxygen and protective ventilation (2–4).
Compared with regular ICU patients and regardless of mechanical ventilation and ICU stay duration, patients with COVID-19 are at higher risk of ventilator-associated pneumonia (VAP), with approximately 40% of patients presenting at least one episode of VAP (5, 6). The overall incidence of VAP in this specific population is around 25 per 1000 ventilation-days (5, 7). The time from mechanical ventilation to VAP onset as well as the microbiological ecology are similar between patients with COVID-19 and regular ICU patients (5). However, the burden related to developing VAP in this specific population has not yet been examined thoroughly.
Several measurements are used to estimate the burden of healthcare-associated infections. It has been pointed out that while accounting for the competing risk associated with healthcare-associated infections, epidemiological methods suffer from heterogeneity and require proper definitions (8). For instance, attributable mortality is sometimes used as a synonym of attributable fraction (or population attributable fraction), whereas some authors propose different definitions for each measurement (8). In this study, the definition by von Cube and colleagues will be used to define attributable mortality as the increase in risk at a given time if one individual is exposed to an event (9). The attributable fraction indicates, herein, the increase or decrease in overall mortality due to an exposure (Table 1). For illustration purposes, the increased risk of death related to VAP might be 50% (i.e., attributable mortality), and the impact in the studied population on the global mortality will vary depending on the prevalence of VAP in the population (i.e., attributable fraction of death). The attributable fraction of mortality related to VAP in the overall ICU population varies among studies and, when using appropriate statistical methods, ranges from 1.5% to 5% at 60 days (10, 11).
Table 1.
Definition of Attributable Mortality and Attributable Fraction
| Definition | Formula | |
|---|---|---|
| Attributable mortality (AM) | Increase or decrease in death (D) if a patient presents an exposure (E) at the time (t). | AM(t) = P[D(t) = 1|E(t) = 1] − P[D(t) = 1|E(t) = 0] |
| Attributable fraction (AF) | Proportion of deaths (D) that are attributable to an exposure (E) at the time (t). | AF(t) = {(P[D(t) = 1] − P[D(t) = 1|E(t) = 0]}/P[D(t) = 1] |
| Proportion of deaths (D) that would not have occurred if no patient presented the exposure (E) at the time (t). |
We therefore aimed to estimate the attributable mortality and attributable fraction of mortality related to VAP in patients with COVID-19. We compared patients with COVID-19 to regular ICU patients (admitted before the pandemic) and to patients without COVID-19 who were admitted during the pandemic. This was performed using the French REA-REZO surveillance network database, which is dedicated to the surveillance of ICU-acquired infections related to invasive devices (12).
Methods
Study Setting
This observational study was conducted using a prospective cohort resulting from a continuous multicenter surveillance network of ICU-acquired infections (REA-REZO) (12).
The detailed protocol for continuous data collection and monitoring in the REA-REZO surveillance network is available at http://rearezo.chu-lyon.fr/. All patients received information about the use of their personal data for research purposes and were given the opportunity to refuse this. According to French law, written informed consent was not required. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The database was approved by the national data protection agency (Commission Nationale de l’Informatique et des Libertés, number 919149) and by the regional institutional review board (CPP SUD EST—IRB 00009118).
Participants and Variables
The studied population consisted of patients admitted for at least 2 days to an adult French ICU, between January 1, 2016, and December 31, 2020. Patients under the age of 18 years old, undergoing extracorporeal membrane oxygenation, with immunodepression according to the Acute Physiology and Chronic Health Evaluation II definition (13), transferred from another ICU, or with a traumatic or nonmedical type of admission were excluded, as were those who did not receive mechanical ventilation.
The following data were analyzed: demographic characteristics (age and sex), presence of an antimicrobial treatment (excluding prophylaxis) 2 days before or after admission date, simplified acute physiology score II (SAPS II) at admission, type of admission (direct admission or secondary to conventional ward admission), time between ICU admission and mechanical ventilation, total duration of mechanical ventilation, length of ICU stay, diagnosis of VAP episode, and date of discharge from the ICU or death.
Definition of VAP
VAP was defined according to the clinical, radiological, and bacteriological criteria proposed by the European Center for Disease Control (14). The microbiological diagnosis was based on either a positive semiquantitative culture from a minimally contaminated lower respiratory tract specimen such as distal protected aspirate, a positive semiquantitative culture from a possibly contaminated lower respiratory tract specimen such as endotracheal aspiration, or alternative microbiology methods such as positive blood cultures not related to another source of infection. VAP was defined as a pneumonia with bacteriological identification, occurring after 48 hours of mechanical ventilation.
Classification of the Studied Population
Among the studied population, three groups were defined. The control group consisted of the prepandemic patients admitted between January 1, 2016, and December 31, 2019. The pandemic COVID-19 group (PandeCOV+) was composed of patients with a COVID-19 diagnosis during ICU stay, admitted between January 1, 2020, and December 31, 2020. The pandemic non–COVID-19 group (PandeCOV−) consisted of patients admitted during the pandemic, between January 1, 2020, and December 31, 2020, with no diagnosis of COVID-19.
Outcome
This study aimed to estimate the attributable mortality and the attributable fraction of mortality associated with VAP among the control, PandeCOV+, and PandeCOV− groups.
As a secondary exploratory analysis, the occurrence of VAP, the occurrence of death after or without VAP, and the occurrence of extubation after or without VAP were estimated in each group.
Statistical Methods
Descriptive statistics were expressed by the median and interquartile range for quantitative variables and by the number and percentage for qualitative variables. Differences between groups were estimated using the Wilcoxon rank-sum test for quantitative variables, and the chi-square test for qualitative variables or Fisher exact test when applicable. If heterogeneity between groups was detected, a two-by-two comparison was performed in order to detect the group differences. The statistical threshold for between-group comparisons was set at 0.01.
The overall incidence rate was calculated using the number of VAP episodes (all VAP episodes during the ICU stay) and the sum of ventilation exposure days among all patients. The 95% confidence intervals (95% CIs) were estimated using the Ulm method (15).
To estimate the impact of the group on the mortality attributable to VAP, the method previously reported by von Cube and colleagues and Coeurjolly and colleagues was used (9, 16).
Briefly, a multistate modeling strategy, using an extended illness death model (Figure E1A), was used to estimate the attributable mortality and the attributable fraction at the time t. The attributable mortality of VAP was defined as AM(t) = P(D|VAP,t) − P(D|no VAP,t). The attributable fraction of VAP was defined as AF(t) = (P(D|,t) − P(D|no VAP,t))/P(D|,t). A previously described parametric approach was used (9). The time-specific attributable mortality and attributable fraction were estimated at 60 and 90 days.
Concerning the interpretation of the differences in these metrics, the attributable mortality and attributable fraction were finally interpreted for the 90th day value. A first comparison was performed between the PandeCOV− group and the control group, then between the PandeCOV+ and control groups. In case of discrepancies between the PandeCOV− and control groups, a comparison between PandeCOV+ and PandeCOV− was performed. A difference between groups was considered if there was no overlap between the estimated 95% CI.
Then, using a disability model (Figure E1B), we studied the impact of the group of the different transition of the model: the risk of developing VAP, the risk of death or extubation without presenting VAP, and the risk of death or extubation after the occurrence of VAP. The specific transition time-fixed covariate candidates were selected as potential predictors of VAP, extubation, and death in the ICU: age, sex, presence of an antimicrobial treatment, SAPS II, type of admission, time between admission and mechanical ventilation, and presence of immunosuppression. Except for the time to mechanical ventilation, continuous variables were categorized according to the quartile of distribution.
Then, the effect of the different groups on each transition was estimated using a proportional intensity model (Markov model), by estimating adjusted hazard ratios (HRs) associated with their 95% CI on the covariates selected through backward regression. During this backward regression, the group was systematically kept into the final model.
As a sensitivity analysis, and to assess the causal effect of each group regarding each transition in the multistate model, we used inverse probability of treatment weighting–regression adjusted using a multinomial regression model including all confounders (age, sex, presence of an antimicrobial treatment, SAPS II, type of admission, time between admission and mechanical ventilation, and presence of immunosuppression). The propensity score was computed using the probability for each patient to belong in his own group and using all confounders. The stabilized weights were estimated by taking the ratio between the marginal probability of belonging to a group and the propensity score. Then, the HRs were reestimated using these weights and covariate adjustment.
Analyses were performed using the mstate package from the R software version 3.4.3 (17).
Results
Among the 267,730 patients admitted to an ICU between 2016 and 2020, 73,945 were included and categorized into the control group (n = 64,816), the PandeCOV− group (n = 7,442), and the PandeCOV+ group (n = 1,687) (Figure E2).
Description of the Population
Compared with the control group, the patients included in the PandeCOV− group were of younger age and were more likely to be male, to be admitted directly from home, and to have received antibiotics at ICU admission. The severity was significantly lower in the PandeCOV− group, although this decrease was not clinically relevant (SAPS II, 54 [41–67] vs. 55 [42–68]). Both groups were comparable in terms of time from admission to mechanical ventilation, duration of mechanical ventilation, and ICU length of stay. Compared with the control group, the PandeCOV+ patients were older, mostly male, and more likely to have been admitted after a conventional hospitalization. They had more frequently received antibiotics at admission and were significantly less severe (SAPS II, 42 [33–53] vs. 55 [42–68]). Compared to both the control and PandeCOV− groups, the time from admission to mechanical ventilation was longer, as were the duration of mechanical ventilation and the ICU length of stay. The ICU case fatality was comparable between groups, with a total of 21,379 patients (28.9%) who died in the ICU (Table 2).
Table 2.
Patient Characteristics
| Pandemic COVID-19 Group (n = 1,687) | Pandemic Non–COVID-19 Group (n = 7,442) | Control Group (n = 64,816) | P Value* | |
|---|---|---|---|---|
| Age, y | 68.3 (59.7–74.2) | 66.1 (55.2–74.4) | 66.6 (55.5–76.2) | <0.001†§ |
| <55 | 280 (16.6) | 1,843 (24.8) | 15,712 (24.2) | <0.001†‡§ |
| 55–66 | 432 (25.6) | 1,864 (25.0) | 15,701 (24.2) | — |
| 67–76 | 663 (39.3) | 2,203 (29.6) | 17,028 (26.3) | — |
| >76 | 312 (18.5) | 1,532 (20.6) | 16,375 (25.3) | — |
| Sex, male | 1,231 (73.0) | 4,872 (65.5) | 40,702 (62.8) | <0.001†‡§ |
| Type of admission | <0.001†‡§ | |||
| Home | 869 (51.5) | 5,020 (67.5) | 42,225 (65.1) | — |
| Hospital | 818 (48.5) | 2,422 (32.5) | 22,591 (34.9) | — |
| Antibiotics at admission | 1,255 (74.4) | 4,812 (64.7) | 40,854 (63.0) | <0.001†‡§ |
| SAPS II | 42 (33–53) | 54 (41–67) | 55 (42–68) | <0.001†‡§ |
| <42 | 869 (51.5) | 2,031 (27.3) | 16,661 (25.7) | <0.001†‡§ |
| 42–54 | 416 (24.7) | 1,814 (24.4) | 15,541 (24.0) | — |
| 55–67 | 248 (14.7) | 1,819 (24.4) | 16,222 (25.0) | — |
| >67 | 154 (9.1) | 1,778 (23.9) | 16,392 (25.3) | — |
| Time from ICU admission to mechanical ventilation | <0.001†§ | |||
| < 24 hours | 891 (52.8) | 6,296 (84.6) | 54,573 (84.2) | — |
| 24 – 72 hours | 499 (29.6) | 771 (10.4) | 6,882 (10.6) | — |
| > 72 hours | 297 (17.6) | 375 (5.0) | 3,361 (5.2) | — |
| Duration of mechanical ventilation, days | 11 (5–21) | 5 (2–11) | 5 (2–10) | <0.001†§ |
| Length of ICU stay, days | 15 (9–26) | 7 (4–15) | 8 (4–15) | <0.001†§ |
| Number of VAP episodes | <0.001†‡§ | |||
| None | 1,064 (63.1) | 6,447 (86.6) | 57,976 (89.4) | — |
| 1 | 463 (27.4) | 816 (11.0) | 5,844 (9.0) | — |
| 2 | 113 (6.7) | 144 (1.9) | 813 (1.3) | — |
| >2 | 47 (2.8) | 35 (0.5) | 183 (0.3) | — |
| Time between mechanical ventilation and first VAP, days | 8 (5–12) | 8 (5–12) | 9 (5–14) | 0.024 |
| ICU case fatality | 513 (30.4) | 2,127 (28.6) | 18,739 (28.9) | 0.332 |
Definition of abbreviations: COVID-19 = coronavirus disease; SAPS II = simplified acute physiology score II; VAP = ventilator-associated pneumonia.
Results are expressed as the number of patients (n) and percentage (%) or median (interquartile range). P values for the comparison between groups, except for the matching parameters.
P value of the heterogeneity test between groups.
Significant difference between pandemic COVID-19 and pandemic non–COVID-19 group.
Significant difference between pandemic non–COVID-19 and control group.
Significant difference between pandemic COVID-19 and control group.
Overall Incidence of VAP
There was a significantly higher proportion of patients who presented with VAP in the PandeCOV+ group (623 [36.9%]) than in the PandeCOV− group (995 [13.4%]) and the control group (6840 [10.6%]). The time from mechanical ventilation to the first VAP episode was comparable between groups, with an overall median (interquartile range) delay of 8 (5–13) days (Table 2).
The overall incidence of VAP was estimated at 14.2 (95%CI, 13.9–14.6) per 1000 ventilation-days in the control group, 18.3 (95% CI, 17.3–19.4) in the PandeCOV− group, and 31.9 (95% CI, 29.8–34.2) in the PandeCOV+ group.
Attributable Mortality and Attributable Fraction of Mortality
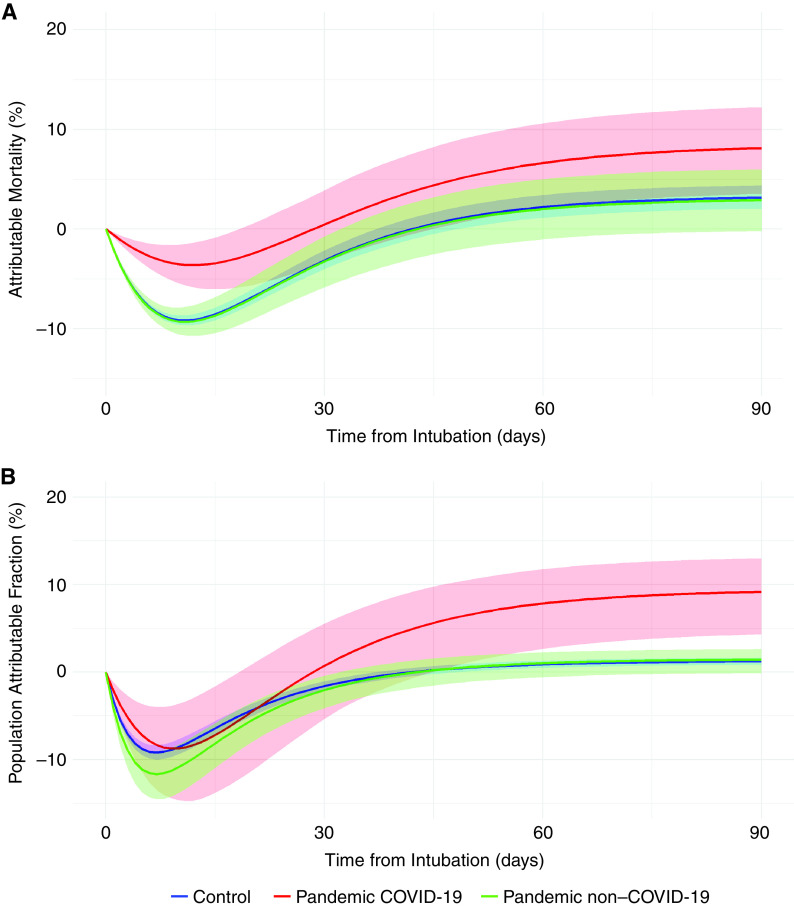
The attributable mortality and the attributable fraction of mortality of VAP were comparable between the control group and the PandeCOV− group. The attributable mortality was 2.21% (95% CI, 1.14% to 3.43%) and 3.15% (95% CI, 2.04% to 3.43%) in the control group, and 2.02% (95% CI, −1.03% to 5.02%) and 2.91% (95% CI, −0.21% to 5.02%) in the PandeCOV− group, for the 60th and 90th days, respectively. The attributable fraction of mortality was 0.89% (95% CI, 0.48% to 1.32%) and 1.22% (95% CI, 0.83% to 1.63%) in the control group, and 1.02% (95% CI, −0.58% to 2.26%) and 1.42% (95% CI, –0.11% to 2.61%) in the PandeCOV− group, for the 60th and 90th days, respectively. The attributable mortality and attributable fraction were not significantly different between the PandeCOV− group and the control group.
The PandeCOV+ patients presented a higher attributable mortality (6.64% [95% CI, 2.14–10.61%] and 8.13% [95% CI, 3.54–12.24%] for the 60th and 90th days, respectively) and a higher attributable fraction of mortality (7.85% [95% CI, 2.14–10.61%] and 9.17% [95% CI, 3.54–12.24%] for the 60th and 90th days, respectively) than the control group (Figure 1).
Figure 1.
Attributable mortality and attributable fraction of mortality related to ventilator-associated pneumonia over time among the control, pandemic coronavirus disease (COVID-19), and pandemic non–COVID-19 groups.
Difference between Groups for the Different Transitions of the Multistate Model
No significant difference was observed between the control group and the PandeCOV− group regarding the risk of death or extubation, with or without VAP. However, the PandeCOV− group had a higher risk of VAP (HR, 1.28; 95% CI, 1.20–1.37) than the control group.
The patients from the PandeCOV+ group presented a higher risk of VAP (HR, 2.35; 95% CI, 2.17–2.56). These patients were more likely to die with VAP (HR, 1.17; 95% CI, 1.01–1.36), and less likely to be extubated after VAP (HR, 0.90; 95% CI, 0.81–1.00) than those in the control group. However, if they did not acquire VAP, they were less likely to die than the control group (HR, 0.66; 95% CI, 0.58–0.75) (Table 3 and Table E2). These HRs were also reestimated by considering the PandeCOV− group as the reference group (Table E3).
Table 3.
Adjusted Hazard Ratios of the Proportional Intensity Model for the Different Transition Intensities
| VAP | Death without VAP | Extubation without VAP | Death with VAP | Extubation with VAP | |
|---|---|---|---|---|---|
| Group | |||||
| Control | 1 | 1 | 1 | 1 | 1 |
| Pandemic non–COVID-19 | 1.28 (1.20–1.37) | 1.05 (1.00–1.11) | 0.97 (0.94–1.00) | 1.04 (0.91–1.18) | 0.99 (0.91–1.07) |
| Pandemic COVID-19 | 2.35 (2.17–2.56) | 0.66 (0.58–0.75) | 0.56 (0.52–0.60) | 1.17 (1.01–1.36) | 0.90 (0.81–1.00) |
| Sex | |||||
| Female | 1 | 1 | 1 | 1 | 1 |
| Male | 1.30 (1.24–1.37) | —* | 0.86 (0.85–0.88) | 1.11 (1.01–1.21) | —* |
| Age | |||||
| ⩽55 | 1* | 1* | 1* | 1* | 1* |
| 55–66 | 0.94 (0.89–1.00) | 1.21 (1.15–1.28) | 0.81 (0.79–0.83) | 1.32 (1.15–1.52) | 0.92 (0.85–0.98) |
| 67–76 | 0.94 (0.89–1.00) | 1.31 (1.24–1.38) | 0.77 (0.75–0.79) | 1.80 (1.58–2.05) | 0.83 (0.77–0.89) |
| >76 | 0.85 (0.79–0.91) | 1.68 (1.60–1.77) | 0.82 (0.80–0.84) | 2.33 (2.03–2.67) | 0.91 (0.84–0.98) |
| Type of admission | |||||
| Home | 1* | 1* | 1* | 1* | 1* |
| Hospital | 0.92 (0.88–0.96) | 0.91 (88–0.94) | 0.86 (0.84–0.87) | —* | 0.91 (0.86–0.95) |
| Antibiotics at admission | |||||
| No | 1* | 1* | 1* | 1* | 1* |
| Yes | 0.82 (0.78–0.86) | 0.68 (0.65–0.70) | 0.76 (0.75–0.77) | —* | 0.86 (0.82–0.91) |
| Time to mechanical ventilation | |||||
| <24 hours | 1* | 1* | 1* | 1* | 1* |
| 24–72 hours | 0.99 (0.98–1.05) | 1.15 (1.09–1.21) | 0.75 (0.73–0.77) | 0.87 (0.77–0.99) | 0.91 (0.85–0.98) |
| >72 hours | 0.92 (0.84–1.00) | 1.72 (1.62–1.83) | 0.62 (0.59–0.65) | 1.19 (1.02–1.38) | 0.80 (0.72–0.88) |
| SAPS II | |||||
| ⩽42 | 1* | 1* | 1* | 1* | 1* |
| 42–54 | —* | 1.68 (1.58–1.78) | 0.92 (0.89–0.94) | 1.28 (1.13–1.45) | —* |
| 55–67 | —* | 2.53 (2.39–2.68) | 0.76 (0.75–0.78) | 1.49 (1.32–1.69) | —* |
| >67 | —* | 3.71 (3.51–3.92) | 0.58 (0.56–0.59) | 1.67 (1.48–1.89) | —* |
For definition of abbreviations, see Table 2.
Results expressed as hazard ratio associated with their 95% confidence intervals.
The corresponding covariate is not used for this transition.
Using a causal inference analysis, the higher risk of VAP among PandeCOV− and PandeCOV+ patients was confirmed. Similarly, the reduced risk of death without VAP in the PandeCOV+ group was also confirmed. The causal inference analysis also found that when PandeCOV+ patients had VAP, their risk of death was not significantly different (Table 4).
Table 4.
Hazard Ratios of the Proportional Intensity Model for the Different Transition Intensities Estimated by Inverse Probability of Treatment Weighting–Regression Adjusted
| VAP | Death without VAP | Extubation without VAP | Death with VAP | Extubation with VAP | |
|---|---|---|---|---|---|
| Group | |||||
| Control | 1 | 1 | 1 | 1 | 1 |
| Pandemic non–COVID-19 | 1.28 (1.20–1.37) | 1.05 (1.00–1.11) | 0.97 (0.95–1.00) | 1.02 (0.90–1.16) | 0.99 (0.92–1.06) |
| Pandemic COVID-19 | 2.12 (1.88–2.40) | 0.62 (0.52–0.74) | 0.53 (0.49–0.59) | 1.07 (0.86–1.33) | 0.90 (0.75–0.96) |
Definition of abbreviations: COVID-19 = coronavirus disease; VAP = ventilator-associated pneumonia.
Results expressed as hazard ratio associated with their 95% confidence intervals.
Microbial Ecology of VAP
Of the 8,458 cases of VAP documented, 2,259 (27%) were polymicrobial with no significant difference between groups (1,797 [26%], 275 [28%], and 187 [30%] for the control, PandeCOV−, and PandeCOV+ groups, respectively; P = 0.101). The distribution of the types of microbiological pathogens involved in VAP was homogeneous between groups (P = 0.319). The main microbial species involved in VAP were Enterobacterales (4680, 44%), nonfermenting gram-negative bacteria (2664, 25%), and gram-positive bacteria (2411, 22%) (Table 5). Specific resistance for Enterobacterales, Pseudomonas spp., and Staphylococcus aureus are available in Table E4.
Table 5.
Microbial Ecology of Ventilator-associated Pneumonia
| Pandemic COVID-19 Group (n = 807) | Pandemic Non–COVID-19 Group (n = 1,257) | Control Group (n = 8,589) | |
|---|---|---|---|
| Gram-negative bacteria | |||
| Enterobacterales | 371 (46) | 577 (45) | 3,732 (43) |
| Escherichia coli | 62 (17) | 109 (19) | 816 (22) |
| Citrobacter spp. | 30 (8) | 52 (9) | 251 (7) |
| Enterobacter spp. | 102 (27) | 148 (26) | 958 (26) |
| Hafnia spp. | 25 (7) | 24 (4) | 143 (4) |
| Klebsiella spp. | 83 (22) | 131 (23) | 809 (22) |
| Morganella spp. | 14 (4) | 15 (3) | 161 (4) |
| Proteus spp. | 21 (6) | 41 (7) | 229 (6) |
| Serratia | 32 (9) | 50 (9) | 344 (9) |
| Other | 2 (1) | 7 (1) | 21 (1) |
| Nonfermenting | 179 (22) | 296 (23) | 2,189 (25) |
| Pseudomonas aeruginosa | 143 (80) | 224 (76) | 1,683 (77) |
| Stenotrophomonas maltophilia | 24 (13) | 48 (16) | 338 (15) |
| Acinetobacter baumannii | 10 (6) | 18 (6) | 153 (7) |
| Other | 2 (1) | 6 (2) | 15 (1) |
| Other | 38 (5) | 57 (4) | 447 (5) |
| Haemophilus spp. | 32 (84) | 44 (77) | 335 (75) |
| Other | 6 (16) | 13 (23) | 112 (25) |
| Gram-positive bacteria | 185 (23) | 293 (23) | 1,933 (22) |
| Enterococcus spp. | 36 (19) | 30 (10) | 151 (8) |
| Staphylococcus aureus | 109 (59) | 191 (65) | 1,305 (67) |
| Staphylococcus spp. | 10 (5) | 13 (4) | 136 (7) |
| Streptococcus spp. | 22 (12) | 47 (16) | 302 (16) |
| Other | 8 (4) | 12 (4) | 39 (2) |
| Other | 34 (5) | 34 (3) | 336 (3) |
| Other microorganism | 4 (11) | 8 (45) | 93 (28) |
| Candida spp. | 20 (55) | 19 (40) | 189 (56) |
| Aspergillus spp. | 8 (22) | 6 (13) | 32 (9) |
| Virus (CMV, HSV, etc.) | 5 (13) | 1 (2) | 22 (6) |
Definition of abbreviation: CMV = cytomegalovirus; COVID-19 = coronavirus disease; HSV = herpes simplex virus.
Group name of microbial species are in bold character.
Discussion
The occurrence of VAP is a major factor of poor prognosis in patients with COVID-19. The causal inference analysis performed herein showed that when patients with COVID-19 are exposed to VAP, their risk of death tends to increase compared with patients without COVID-19, and that they are at a lower risk of death if they do not acquire VAP. The findings of the present study also demonstrated that during the pandemic, even the patients without COVID-19 presented a higher risk of VAP, whereas their attributable mortality and attributable fraction of mortality associated with VAP was comparable to that of prepandemic regular ICU patients.
The moderate but significant increase in the risk of VAP among patients admitted to ICUs without COVID-19 during the pandemic is noteworthy. The higher incidence of VAP among this specific population is likely to be an indirect consequence of the pandemic-related burden of care and the surge of patients admitted to ICUs. During the pandemic, healthcare workers faced time-consuming protocols, which included the need to wear personal protective equipment as well as dealing with the management of a high number of patients requiring invasive ventilation, hemodynamic, and renal support (18, 19). The pandemic also negatively impacted the mental health of the healthcare professionals mainly owing to work overload (20, 21). It is therefore possible that the prevention of VAP, which relies mainly on a bundle of care performed by the ICU nurses, was suboptimal during the pandemic owing to the work overload and understaffing (22, 23).
Beyond the COVID-19 context, the estimation of the attributable mortality and attributable fraction of mortality associated with VAP varies greatly in the literature, ranging from 0% to 50% (24). This is partly owing to the heterogeneity in the methods used for these estimations that might over- or underestimate the burden of VAP. One of the best methodological studies, from Bekaert and colleagues in 2011, used the OUTCOMEREA database with 4,479 patients and took into account the competing risk and time-dependent variables (11). At 60 days, the attributable fraction of mortality was higher (5.9% [95% CI, 2.5% to 9.1%]) than that estimated in the present study. These discrepancies could reflect the differences between the two cohorts, as the present study included only medical patients with higher SAPSII who were older. Recently, Steen and colleagues tested four different approaches to estimate the attributable fraction of mortality of VAP in a cohort of 2,720 patients, using a competitive risk analysis and multistate modeling. They reported that a method using multistate modeling associated with propensity score weighting was the best method to estimate the attributable fraction of mortality (at 60 days, 3.7 [95% CI, 0.8 to 6.6]), and they found a similar result to that estimated in the present study in the control and the PandeCOV− groups (10). A meta-analysis of the individual data from a randomized controlled trial of VAP prevention was performed by Melsen and colleagues in 2013 and estimated the attributable fraction at 13% (95% CI, −14% to 38%]) (25). Despite a well-performed meta-analysis, these results suffer from a high heterogeneity but pointed out that in some specific subpopulations, such as surgical patients, the attributable fraction of mortality is higher.
To fully acknowledge the subtle difference between attributable mortality and attributable fraction of mortality, it is essential to note that the attributable mortality of VAP, as defined herein, does not depend on the prevalence of VAP (9). It thus simply represents the excess of mortality that a patient might suffer when acquiring VAP. However, the attributable fraction depends on the prevalence of the event: If no VAP occurs in any of the patients, the attributable fraction of mortality attributable to VAP will be 0. It would be maximal if every patient in a population acquired VAP. If the attributable mortality is interesting at the individual level, the attributable fraction represents the percentage of deaths that could be avoided by preventing every VAP in the observed cohort.
Several studies have pointed out that certain characteristics of VAP are similar whether they occur in patients with COVID-19 or regular ICU patients. For instance, the overall delay for VAP occurrence, between 8 and 10 days, is similar between these two populations. The main difference concerning VAP among patients with COVID-19 is its high incidence rate, between 25 and 30 VAP per 1,000 ventilation-days, compared with that of regular ICU patients outside of the pandemic context for whom the incidence ranges between 10 and 15 VAP per 1,000 ventilation-days. This higher incidence in patients with COVID-19 is also observed in case of a second episode of VAP (5). The microbial ecology is also similar with a large predominance of Gram-negative bacteria and Enterobacterales, nonfermenting bacteria (Pseudomonas aeruginosa), and Staphylococcus aureus. The resistance pattern of these bacteria is also similar to the microbiological ecology found in regular ICU patients and cannot explain this higher incidence or related mortality (5).
To date, no study has thoroughly studied the influence of VAP on mortality within the COVID-19 context. However, this increased mortality risk related to VAP was suggested: In an ancillary analysis of the coVAPip cohort, a relationship was found between VAP and COVID-19 mortality (26). The present study found that the differences in VAP between patients with COVID-19 and regular ICU patients are not only related to an increased incidence in VAP, but also a higher attributable mortality and attributable fraction of mortality of VAP in patients with COVID-19. The VAP occurring in a patient with COVID-19 drastically changes the prognosis of this patient: Before presenting with VAP, the mortality risk of a patient with COVID-19 is 1.5 times lower than that of a regular ICU patient with no VAP. However, after developing VAP, the ICU mortality of a patient with COVID-19 tends to increase. At 90 days, nearly 10% of the ICU deaths among patients with COVID-19 are attributable to VAP solely. The reason for this increased mortality caused by VAP is not clear. COVID-19 is associated with a major inflammatory reaction occurring in the lungs (27, 28), which is followed by an immunosuppressive state, that can favor the occurrence of VAP and lead to an increased risk of death (29). In a recent study, it has been shown that the immunosuppressive state, affecting both the innate and adaptative immune systems, frequently occurs during COVID-19 and was associated with mortality at 28 days (30).
On the basis of the present results, it appears that patients with COVID-19 represent a specific population in terms of VAP prevention, and that reducing VAP among these patients could be a point of care, leading to a decrease in mortality. For example, in their study, Luque-Paz and colleagues compared two independent cohorts of ICU patients with COVID-19 from two different centers, one applying selective digestive decontamination (SDD) (n = 77), and the other without SDD (n = 101) (31). They found a large decrease of VAP incidence in the SDD cohort compared with the non-SDD cohort (9 vs. 23 VAP events per 1,000 ventilation-days, respectively). This decrease was also associated with a decrease in mortality (6% vs. 21%), even after adjustment for confounding variables (HR, 0.36; 95% CI, 0.20–0.63). An observational single-center noncomparative study from van der Meer and colleagues also reported similar results (32). SDD and other preventive measures such as subglottic suction endotracheal tubes, probiotics, or implementation of prevention bundle to avoid VAP could lead to a decrease in overall mortality among patients with COVID-19, associated with cost savings (the cost of a VAP episode has been estimated between $7,000 and $35,000) (33–35).
Owing to the nature of the surveillance network, some variables such as antibiotic treatment during ICU stay and the COVID-19 treatments used (including corticosteroids) could not be documented. Other important confounders such as the presence of coVAPid, hemodynamic failure, and comorbidities were also not available. Moreover, the follow-up was limited to the time of ICU stay. The wide use of corticosteroids in the ICU for the treatment of COVID-19 could be linked to the incidence of VAP. Corticosteroids have been used as a standard of care since the Randomised Evaluation of COVID-19 Therapy trial, and although their efficacy is currently being discussed, they do not seem to be associated with an increased risk of VAP (36, 37). Moreover, a heterogeneity between groups was noticed herein, with a longer delay between admission and mechanical ventilation and a higher rate of antibiotics at admission in the COVID-19 group.
Conclusions
In patients with COVID-19, acquiring VAP is a factor of poorer prognosis as it increases mortality in this specific population. A potential benefit of the prevention of VAP in patients with COVID-19 hospitalized in the ICU would be a reduction of overall mortality of nearly 10% at 90 days.
Acknowledgments
Acknowledgment
The authors thank all intensivists, ICU nurses, and infection control teams of the REA-REZO network.
Footnotes
Author Contributions: C.-H.V. helped in writing the first draft of the manuscript, reviewing the manuscript, and performing the statistical analysis. A.L., A.F., A.S., and A.M. helped in data collection, conception, interpretation of the data, and revising the manuscript substantially. F.B. is supported by the French National Research Agency in the artificial intelligence Chair of excellence MyWayTOHealth MIAI @ Grenoble Alpes (ANR-19-P3IA-0003). D.M.-B., S.B., F.B., J.F.T., and P.V. helped in statistical analysis, conception, interpretation of the data, and revising the manuscript substantially. S.C., T.L., G.C., and V.L. helped in the interpretation of the data, data collection, and revising the manuscript substantially.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202202-0357OC on May 10, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Coronavirus disease (COVID-19) situation reports https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2. Abrams D, Agerstrand C, Beitler JR, Karagiannidis C, Madahar P, Yip NH, et al. Risks and benefits of ultra-lung-protective invasive mechanical ventilation strategies with a focus on extracorporeal support. Am J Respir Crit Care Med . 2022;205:873–882. doi: 10.1164/rccm.202110-2252CP. [DOI] [PubMed] [Google Scholar]
- 3. Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med . 2021;204:303–311. doi: 10.1164/rccm.202009-3467OC. [DOI] [PubMed] [Google Scholar]
- 4. Mellado-Artigas R, Ferreyro BL, Angriman F, Hernández-Sanz M, Arruti E, Torres A, et al. COVID-19 Spanish ICU Network High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care . 2021;25:58. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vacheron CH, Lepape A, Savey A, Machut A, Timsit JF, Vanhems P, et al. REA-REZO Study Group Increased incidence of ventilator-acquired pneumonia in coronavirus disease 2019 patients: a multicentric cohort study. Crit Care Med . 2022;50:449–459. doi: 10.1097/CCM.0000000000005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pickens CO, Gao CA, Cuttica MJ, Smith SB, Pesce LL, Grant RA, et al. NU COVID Investigators Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med . 2021;204:921–932. doi: 10.1164/rccm.202106-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest . 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Cube M, Timsit J-F, Schumacher M, Motschall E, Schumacher M. Quantification and interpretation of attributable mortality in core clinical infectious disease journals. Lancet Infect Dis . 2020;20:e299–e306. doi: 10.1016/S1473-3099(20)30485-0. [DOI] [PubMed] [Google Scholar]
- 9. von Cube M, Schumacher M, Wolkewitz M. Basic parametric analysis for a multi-state model in hospital epidemiology. BMC Med Res Methodol . 2017;17:111. doi: 10.1186/s12874-017-0379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steen J, Vansteelandt S, De Bus L, Depuydt P, Gadeyne B, Benoit DD, et al. Attributable mortality of ventilator-associated pneumonia. replicating findings, revisiting methods. Ann Am Thorac Soc . 2021;18:830–837. doi: 10.1513/AnnalsATS.202004-385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekaert M, Timsit J-F, Vansteelandt S, Depuydt P, Vésin A, Garrouste-Orgeas M, et al. Outcomerea Study Group Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med . 2011;184:1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 12.Infections et Antibiorésistance en Réanimation http://rearezo.chu-lyon.fr/.
- 13. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med . 1985;13:818–829. [PubMed] [Google Scholar]
- 14. Plachouras D, Lepape A, Suetens C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med . 2018;44:2216–2218. doi: 10.1007/s00134-018-5113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol . 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 16. Coeurjolly J-F, Nguile-Makao M, Timsit J-F, Liquet B. Attributable risk estimation for adjusted disability multistate models: application to nosocomial infections. Biom J . 2012;54:600–616. doi: 10.1002/bimj.201100222. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team 2017https://www.R-project.org/.
- 18. Shelton C, El-Boghdadly K, Appleby JB. The “haves” and “have-nots” of personal protective equipment during the COVID-19 pandemic: the ethics of emerging inequalities amongst healthcare workers. J Med Ethics . 2021 doi: 10.1136/medethics-2021-107501. [DOI] [PubMed] [Google Scholar]
- 19. Bruyneel A, Gallani M-C, Tack J, d’Hondt A, Canipel S, Franck S, et al. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intensive Crit Care Nurs . 2021;62:102967. doi: 10.1016/j.iccn.2020.102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehta S, Yarnell C, Shah S, Dodek P, Parsons-Leigh J, Maunder R, et al. Canadian Critical Care Trials Group The impact of the COVID-19 pandemic on intensive care unit workers: a nationwide survey. Can J Anaesth . 2022;69:472–484. doi: 10.1007/s12630-021-02175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caillet A, Conejero I, Allaouchiche B. Job strain and psychological impact of COVID-19 in ICU caregivers during pandemic period. Anaesth Crit Care Pain Med . 2021;40:100850. doi: 10.1016/j.accpm.2021.100850. [DOI] [PubMed] [Google Scholar]
- 22. Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol . 2014;35:S133–S154. doi: 10.1017/s0899823x00193894. [DOI] [PubMed] [Google Scholar]
- 23. Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S, et al. ADARPEF; GFRUP Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann Intensive Care . 2018;8:104. doi: 10.1186/s13613-018-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timsit J-F, Zahar J-R, Chevret S. Attributable mortality of ventilator-associated pneumonia. Curr Opin Crit Care . 2011;17:464–471. doi: 10.1097/MCC.0b013e32834a5ae9. [DOI] [PubMed] [Google Scholar]
- 25. Melsen WG, Rovers MM, Groenwold RHH, Bergmans DCJJ, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis . 2013;13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 26. Nseir S, Martin-Loeches I, Povoa P, Metzelard M, Du Cheyron D, Lambiotte F, et al. coVAPid study group Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: a planned ancillary analysis of the coVAPid cohort. Crit Care . 2021;25:177. doi: 10.1186/s13054-021-03588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA . 2020;324:1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Agnillo F, Walters K-A, Xiao Y, Sheng Z-M, Scherler K, Park J, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med . 2021;13:eabj7790. doi: 10.1126/scitranslmed.abj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science . 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 30. Hue S, Beldi-Ferchiou A, Bendib I, Surenaud M, Fourati S, Frapard T, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med . 2020;202:1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luque-Paz D, Tattevin P, Jaubert P, Reizine F, Kouatchet A, Camus C. Selective digestive decontamination to reduce the high rate of ventilator-associated pneumonia in critical COVID-19. Anaesth Crit Care Pain Med . 2022;41:100987. doi: 10.1016/j.accpm.2021.100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Meer SB, Figaroa G, van der Voort PHJ, Nijsten MW, Pillay J. Ventilator-associated pneumonia in critically-ill patients with COVID-19 in a setting of selective decontamination of the digestive tract. Crit Care . 2021;25:445. doi: 10.1186/s13054-021-03869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorente L, Blot S, Rello J. New issues and controversies in the prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med . 2010;182:870–876. doi: 10.1164/rccm.201001-0081CI. [DOI] [PubMed] [Google Scholar]
- 34. Branch-Elliman W, Wright SB, Howell MD. Determining the ideal strategy for ventilator-associated pneumonia prevention. Cost-benefit analysis. Am J Respir Crit Care Med . 2015;192:57–63. doi: 10.1164/rccm.201412-2316OC. [DOI] [PubMed] [Google Scholar]
- 35. Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med . 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dupuis C, de Montmollin E, Buetti N, Goldgran-Toledano D, Reignier J, Schwebel C, et al. OutcomeReaTM research network Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS One . 2021;16:e0255644. doi: 10.1371/journal.pone.0255644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med . 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]