Summary
Objectives
Proline/arginine-rich end leucine-rich repeat protein (PRELP) has been reported to contribute to the remodelling of cardiovascular tissues in the ischaemia–reperfusion injury model. However, research is lacking on the role of PRELP in myocardial fibrosis and ventricular remodelling, and the mechanism through which PRELP brings about these changes is not clear. This study aimed to evaluate the role of PRELP in ventricular remodelling and myocardial fibrosis following acute myocardial infarction (AMI) and to explore the underlying mechanism.
Methods
In this study, we established AMI mouse and cellculture models in an oxygen–glucose deprivation environment.
Results
We found that over-expression of PRELP increased the infarct size and interstitial fibrotic area. Expression of the wnt/β–catenin pathway molecules, which are downstream of PRELP, increased more in the PRELP over-expression group than in the AMI group.
Conclusions
Our results showed that PRELP, through the wnt/β–catenin signalling pathway, led to myocardial fibrosis and ventricular remodelling following AMI.
Keywords: PRELP, myocardial fibrosis, acute myocardial infarction, wnt, β-catenin
Acute myocardial infarction (AMI), a serious and common cardiovascular disorder, is the leading cause of heart failure and sudden death,1-3 with increasing incidence worldwide.4 Myocardial fibrosis is an important pathological characteristic linked to AMI.5,6 It can lead to elevated rigidity, induce myocardial sclerosis, trigger ventricular remodelling, affect ventricular compliance and eventually induce heart failure.7 Therefore, more effort should be made to identify the molecular mechanisms of myocardial fibrosis. This will help provide novel insights into therapeutic targets and uncover effective strategies to alleviate myocardial fibrosis and ventricular remodelling following AMI.
The small leucine-rich repeat protein family (SLRR), located in the extracellular matrix of connective tissue, has been found to play an important role in myocardial fibrosis and ventricular remodelling.8,9 There are several members in the SLRRs, such as biglycan, decorin, lumican and glypican-6. Recent studies showed that inhibition of biglycan or lumican expression can reduce myocardial fibrosis.10,11 It was also shown that glypican-6 is involved in cardiac remodelling by the extracellular signalrelated kinase (ERK) signalling pathway.12
Proline/arginine-rich end leucine-rich repeat protein (PRELP), which is another member of the SLRRs, can bind to the basement membranes of connective tissues more easily compared to other members of the SLRRs, as it contains a positively charged N-terminus rich in proline and arginine residue.13 Javier et al., for the first time, reported that several members of the small leucinerich proteoglycan family, including asporin and PRELP, were shown to contribute to cardiac remodelling.14 However, there is little research about PRELP’s role in myocardial fibrosis and ventricular remodelling post AMI.
The mechanism by which PRELP brings about these changes is also not clear. The wnt signalling pathway is a relatively silent pathway that regulates important cellular activity such as cell proliferation, differentiation and apoptosis. β-catenin has been found to be one of the most important members in the wnt signalling pathway. The other downstream members of this pathway are glycogen synthase kinase 3 beta (GSK3β), matrix metallopeptidase 9 (MMP9), c-myc and tissue inhibitor of metalloproteinases-1 (TIMP-1).
Previous studies have shown that wnt signalling gets activated following AMI.15 Sufficient activation of this signalling pathway can decrease the infarct size of the heart and alleviate heart failure, while excess activation can increase myocardial fibrosis and infarct sizes.16,17 A recent research study suggested that PRELP can regulate the differentiation of osteoblasts by the β-catenin pathway.18-20 Whether PRELP increases myocardial fibrosis post AMI by the wnt/β–catenin signalling pathway remains undetermined. In our study, we determined PRELP’s role in myocardial fibrosis post AMI and the underlying mechanisms involved in the process.
Methods
Male SD mice, aged eight weeks, were subjected to MI by ligation of the left anterior descending coronary artery, as previously described.21 After the mice were anaesthetised, the left anterior descending coronary artery was ligated. Mice in the sham group were also anaesthetised and their hearts were exposed. However, ligation of the coronary artery was not performed in this group. PRELP over-expressing lentiviral vector and PRELP interference-expressed lentiviral vector were generated by Genechem Corporation (Shanghai, China).
All mice (n = 60) were randomly divided into four groups: SHAM, MI, MI + PRELP shRNA, and MI + PRELP over group. In the MI + PRELP over group, mice were injected with 1 × 106 PRELP over-expressing lentiviral vector in the myocardium around the ligation point. Additionally, 1 × 106 PRELP interference-expressed lentiviral vector was injected into the myocardium around the point of ligation of the mice in the MI + PRELP shRNA group.
All experimental protocols were carried out in accordance with guidelines. All procedures were under approval from the animal care and use committee of Shandong University Qilu Hospital.
The cardiac fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% foetal bovine serum, 100 units/ml penicillin and 100 mg/ml streptomycin at 37°C with 95% air and 5% CO2. To identify the fibroblasts, microscopy and immunofluorescence analysis were used. The cells were also randomly divided into four groups: SHAM, MI, MI + PRELP shRNA, and MI + PRELP over group.
To mimic the ischaemic conditions, the cells were cultured in DMEM without foetal bovine serum (FBS), and with 0.1% O2 for 24 hours. PRELP over-expressing lentiviral vector was transfected into the cells in the MI + PRELP over group. PRELP interference-expressed lentiviral vector was transfected into the cells in the MI + PRELP shRNA group. Transfection efficiency was assessed by Western blotting, immunohistochemical staining and real-time polymerase chain reaction (PCR).
The left ventricular internal diameter in diastole and the left ventricular internal diameter in systole of all the mice were measured with motion-mode (M-mode) echocardiography. Left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) were automatically calculated according to the formula.
The heart tissues and the cells were lysed in RIPA buffer. The concentration of total proteins for each sample was quantified using a BCA kit, and 30 μg of each sample was electrophoresed in SDS-PAGE gel and transferred onto polyvinylidene difluoride membranes. After blocking with 5% fat-free milk at room temperature for one hour, the membranes were incubated with primary antibodies overnight at 4°C on a refrigerator shaker.
All primary antibodies were dissolved in a 5% solution with a dilution of 1:1 000. Anti-wnt1 antibody (ab15251), anti-PRELP antibody (ab229719), anti-GSK3β antibody (ab93926), anti- MM9P antibody (ab228402), anti-β-catenin antibody (ab6302), anti-c-myc antibody (ab32072) and anti-TIMP-1 antibody (ab81282) were purchased from Abcam Biotechnology. Anti- GAPDH antibody (200306-7E4) was purchased from Zen Bioscience.
Next, the membranes were washed three times at room temperature. Following the addition of the anti-rabbit (or antimouse, anti-goat) secondary antibodies (1:5 000, A0208, A0216, A0181, Beyotime), the membranes were incubated for two hours, followed by three washes at room temperature. Finally, protein expressions were visualised by an enhanced chemiluminescence system.
The myocardial tissues were paraffin-embedded, 100 for 10 minutes. Next, they were incubated with primary antibodies overnight at 4°C. After three phosphatebuffered saline (PBS) washes, the slices were incubated with secondary antibodies for 50 minutes at 4°C. This was followed by three PBS washes. Finally, the sections were counterstained with haematoxylin to observe the cellular nuclei and were viewed under a microscope.
The heart was sectioned into 1-mm-thick transverse slices and stored at –20°C for 20 minutes. The slices were then incubated with 1% triphenyltetrazolium chloride solution (TTC) at 37°C. The viable tissues stained red, while the infarcted tissues appeared pale. The percentage of infarcted area was quantified using image analysis software (Image-Pro Plus).
The heart tissues were fixed, dehydrated and then sectioned into 4-μm sections. After they were dewaxed, hydrated and stained with haematoxylin and eosin (HE), and Sirius red (SR), the slices were dehydrated by gradient ethanol and finally viewed under a microscope.
The total RNA content was extracted from cells using Trizol reagent. The RNA was quantified by a Nanodrop One, and the obtained RNA (0.6 μl) was subjected to reversetranscription reaction using the RT reagent kit. Real-time PCR of cDNA was done using a SYBR Premix Ex Taq kit, with GAPDH as the internal control. The primer sequences of PRELP were CTTCTGGTTCCTTCCACTTCTC (forward) and GGCCTTGGCTTGGGTTTA (reverse), and the primer sequences of GAPDH were GGGAAACCCATCACCATCTT (forward) and CCAGTAGACTCCACGACATACT (reverse). Results were calculated based on the 2Ct −ΔΔ method.
Statistical analysis
The experimental data for the three separate experiments are represented as mean ± standard deviation (SD). SPSS 23.0 was used for statistical analyses; p-values < 0.05 were considered statistically significant. The t-test was used to compare the data between the two groups.
Results
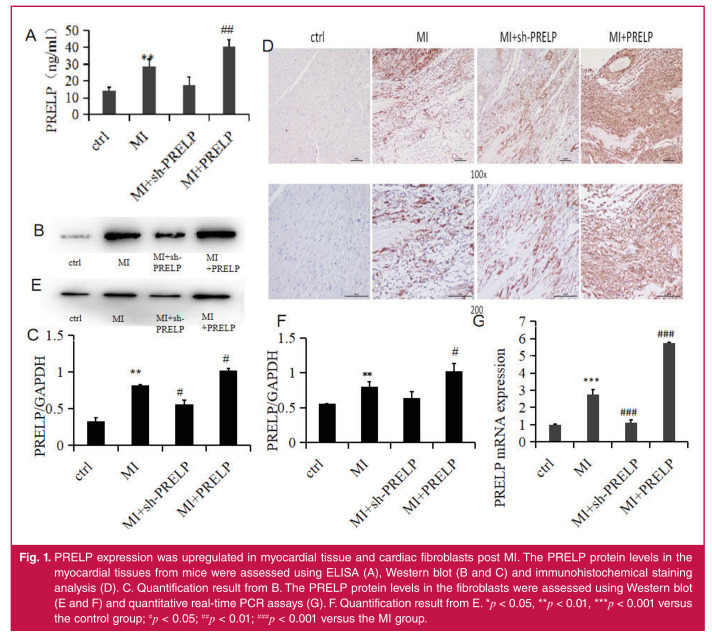
As shown in Fig. 1, the ELISA assay, Western blotting and immunohistochemical staining analysis showed that the PRELP expression in the myocardial tissues was upregulated following AMI compared to that in the control mice. The PRELP expression in the myocardial tissues was upregulated in PRELP over-expression groups compared to that in the MI mice, and that of the sh-PRELP group was down-regulated compared to that in the MI mice.
Fig. 1.
PRELP expression was upregulated in myocardial tissue and cardiac fibroblasts post MI. The PRELP protein levels in the myocardial tissues from mice were assessed using ELISA (A), Western blot (B and C) and immunohistochemical staining analysis (D). C. Quantification result from B. The PRELP protein levels in the fibroblasts were assessed using Western blot (E and F) and quantitative real-time PCR assays (G). F. Quantification result from E. *p < 0.05, **p < 0.01, ***p < 0.001 versus the control group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the MI group.
The Western blot and quantitative real-time PCR analysis also showed that the PRELP expression in the cardiac fibroblasts was increased post MI compared to the control cells. The PRELP expression in the cardiac fibroblasts was upregulated in the PRELP over-expression groups compared to that in the MI groups, and that of the sh-PRELP group was down-regulated compared to that in the MI groups.
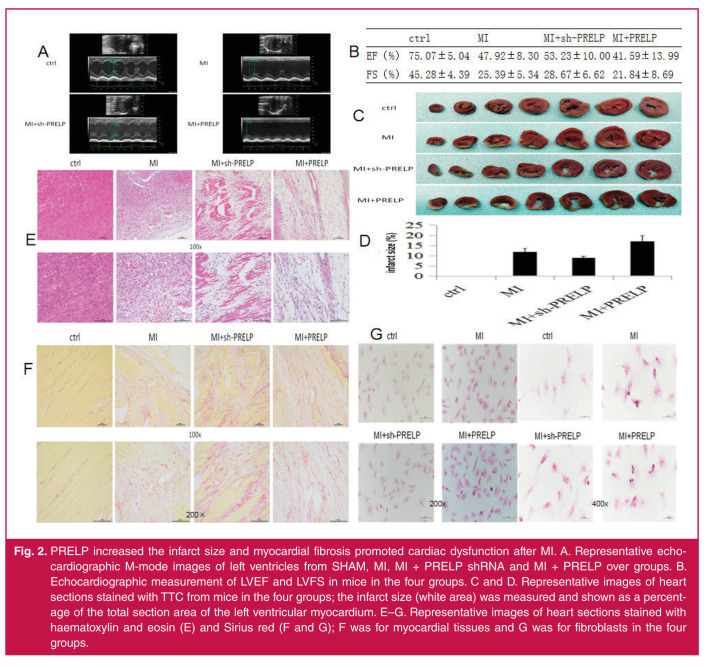
Echocardiography results showed that over-expression of PRELP reduced cardiac function following AMI, as assessed by the significantly decreased percentages of LVFS and LVEF, compared to the MI mice group. On the contrary, in the sh-PRELP groups, there was a significant increase in both LVFS and LVEF of the heart following AMI, compared to the MI mice group (Fig. 2A ,B ). The results indicate that PRELP can promote cardiac dysfunction following AMI. Over-expression of PRELP significantly increased the infarct size of the heart compared to the MI groups, and in the sh-PRELP groups, there was a significant decrease in infarct size of the heart following AMI, as assessed by TTC staining ( Fig. 2C, 2D ).
Fig. 2.
PRELP increased the infarct size and myocardial fibrosis promoted cardiac dysfunction after MI. A. Representative echocardiographic M-mode images of left ventricles from SHAM, MI, MI + PRELP shRNA and MI + PRELP over groups. B. Echocardiographic measurement of LVEF and LVFS in mice in the four groups. C and D. Representative images of heart sections stained with TTC from mice in the four groups; the infarct size (white area) was measured and shown as a percentage of the total section area of the left ventricular myocardium. E–G. Representative images of heart sections stained with haematoxylin and eosin (E) and Sirius red (F and G); F was for myocardial tissues and G was for fibroblasts in the four groups.
These findings showed that PRELP increased adverse myocardial fibrosis and collagen deposition after AMI. HE and SR staining revealed a clear increase in infarct size, interstitial fibrotic area, and collagen accumulation in PRELP overexpression groups following MI. In contrast, in the sh-PRELP groups, there was a significant decrease in the infarct size and interstitial fibrotic area compared to the MI group and fibroblasts (Fig. 2E–G), indicating that PRELP can increase collagen deposition, promote adverse myocardial fibrosis and lead to ventricular re-modelling, which can cause heart failure post MI.
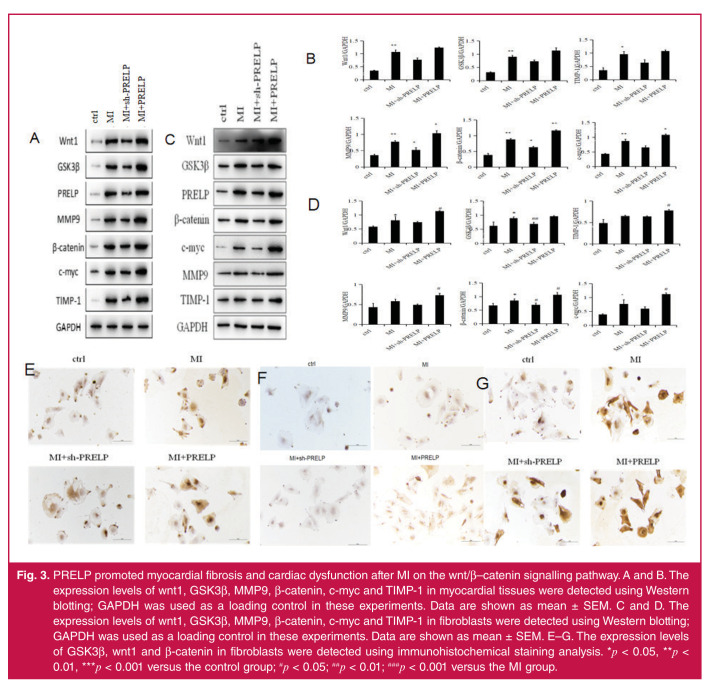
Next, we investigated the effect of PRELP on activation of the wnt/β–catenin signalling pathway. As is already known, the downstream members of this pathway are β-catenin, GSK3β, MMP9, c-myc and TIMP-1. In our experiments, we used Western blotting and immunohistochemical staining analysis to detect the expression levels of the above proteins of the wnt/β– catenin signalling pathway.
Western blotting and immunohistochemical staining analysis showed that the expression levels of wnt1, GSK3β, MMP9, β-catenin, c-myc and TIMP-1 in the myocardial tissues and fibroblasts were more increased in PRELP over-expression groups than in the MI groups (Fig. 3). The expression levels of the above proteins in the sh-PRELP groups were decreased compared to the MI groups, indicating that PRELP increased the infarct size and promoted myocardial fibrosis and ventricular re-modelling following MI through the wnt/β–catenin signalling pathway in vivo and in vitro.
Fig. 3.
PRELP promoted myocardial fibrosis and cardiac dysfunction after MI on the wnt/β–catenin signalling pathway. A and B. The expression levels of wnt1, GSK3β, MMP9, β-catenin, c-myc and TIMP-1 in myocardial tissues were detected using Western blotting; GAPDH was used as a loading control in these experiments. Data are shown as mean ± SEM. C and D. The expression levels of wnt1, GSK3β, MMP9, β-catenin, c-myc and TIMP-1 in fibroblasts were detected using Western blotting; GAPDH was used as a loading control in these experiments. Data are shown as mean ± SEM. E–G. The expression levels of GSK3β, wnt1 and β-catenin in fibroblasts were detected using immunohistochemical staining analysis. *p < 0.05, **p < 0.01, ***p < 0.001 versus the control group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the MI group.
Discussion
In our study, we investigated the role of PRELP in ventricular re-modelling and myocardial fibrosis post AMI and explored the underlying mechanism through which PRELP brings about these changes. In our experiments, over-expression of PRELP increased the infarct size and myocardial fibrosis, and decreased the LVFS and LVEF of the heart, compared to the MI groups. By contrast, in the sh-PRELP group, there was a decrease in the infarct size and myocardial fibrosis, and an increase in LVFS and LVEF of the heart, compared to the MI groups.
The results indicate that PRELP increased the infarct area and myocardial fibrosis and reduced the cardiac function post MI. The expression of wnt1, GSK3β, MMP9, β-catenin, c-myc and TIMP-1 was greater in the group with over-expression of PRELP than in the MI groups, both in vivo and in vitro. The expression of these proteins in sh-PRELP groups was lower than that in the MI groups. The results indicate that PRELP takes part in myocardial fibrosis and ventricular re-modelling post AMI through the wnt/β–catenin signalling pathway.
Several previous studies have shown that other members of the SLRR family such as biglycan, glypican-6 and lumican take part in myocardial fibrosis and ventricular re-modelling.10-12,22,23 Javier et al. reported, for the first time, that several members of the small leucine-rich proteoglycan family, including asporin and PRELP, contribute to cardiac re-modelling.24 Bengtsson et al. showed that PRELP was an important regulator of cell adhesion, due to the positively charged N-terminal region in its chemical structure.24,25 Fibroblasts can adhere to PRELP and that can be inhibited by heparin.26 However, little research on PRELP’s role in myocardial fibrosis and ventricular re-modelling following AMI has been done.
Our study determined the role of PRELP in myocardial fibrosis and re-modelling post AMI. Li et al. reported PRELP promotes osteoblastic differentiation via the β-catenin/connexin 43 pathway, and β-catenin acts as a hub gene in the PRELP gene network.5 Some studies showed that expression of the wnt pathway increased following AMI, and appropriate activation of the wnt pathway can decrease the infarct size of the heart and alleviate heart failure, while excess activation can lead to myocardial fibrosis.16,17 Other studies also noted that the wnt/β– catenin signalling pathway is involved in myocardial fibrosis post MI.27-31 However, there was no research until now that looked at PRELP’s role in myocardial fibrosis through the wnt/β–catenin signalling pathway post AMI.
Conclusion
Our study has shown for the first time that PRELP takes part in cardiac myofibrosis and ventricular re-modelling following AMI through the wnt/β–catenin signalling pathway. It further identified the cardiac fibrotic molecular mechanisms, provided novel insights into therapeutic targets and uncovered effective strategies to alleviate myocardial fibrosis after AMI.
Acknowledgments
This study was supported by Natural Science Foundation of Shandong Province (ZR2017MH122).
Contributor Information
Yu Zhang, Email: zhangy19851208@126.com.
Xiangju Liu, Email: xiangjuliu@163.com.
Honglei Jiang, Department of Cardiology, Shandong Provincial Western Hospital, Jinan, Shandong, People’s Republic of China.
Wei Li, Department of Anesthesia, Shandong Provincial Hospital affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China.
References
- 1.Cai Y, Xie KL, Wu HL. et al. Functional suppression of Epiregulin impairs angiogenesis and aggravates left ventricular re-modelling by disrupting the extracellular‐signal‐regulated kinase1/2 signaling pathway in rats after acute myocardial infarction. J Cell Physiol. 2019;234:18653–18665. doi: 10.1002/jcp.28503. [DOI] [PubMed] [Google Scholar]
- 2.Yuan X, Pan J, Wen L. et al. MiR-590-3p regulates proliferation, migration and collagen synthesis of cardiac fibroblast by targeting ZEB1. J Cell Mol Med. 2020;24:227–237. doi: 10.1111/jcmm.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu L, Zhang W, Huang C. et al. LncRNA ANRIL protects H9c2 cells against hypoxia-induced injury through targeting the miR-7-5p/SIRT1 axis. J Cell Physiol. 2020;235:1175–1183. doi: 10.1002/jcp.29031. [DOI] [PubMed] [Google Scholar]
- 4.Gong X, Zhu Y, Chang H. et al. Long noncoding RNA MALAT1 promotes cardiomyocyte apoptosis after myocardial infarction via targeting miR-144-3p . Biosci Rep. 2019;39:BSR20191103. doi: 10.1042/BSR20191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Jiang P, He Y. et al. A novel mechanism of Smads/miR-675/ TGFβR1 axis modulating the proliferation and re-modelling of mouse cardiac fibroblasts. J Cell Physiol. 2019;234:20275–20285. doi: 10.1002/jcp.28628. [DOI] [PubMed] [Google Scholar]
- 6.Van Rooij E, Olson EN. Searching for miracles in cardiac fibrosis. Circ Res. 2009;104:138–140. doi: 10.1161/CIRCRESAHA.108.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furtado MB, Nim HT, Boyd SE. et al. View from the heart: cardiac fibroblasts in development, scarring and regeneration. Development. 2016;143:387–397. doi: 10.1242/dev.120576. [DOI] [PubMed] [Google Scholar]
- 8.Itoh A, Nonaka Y, Ogawa T. et al. Small leucine-rich repeat proteoglycans associated with mature insoluble elastin serve as binding sites for galectins. Biosci Biotechnol Biochem. 2017;8:2098–2104. doi: 10.1080/09168451.2017.1374828. [DOI] [PubMed] [Google Scholar]
- 9.Matsushima N, Takatsuka S, Miyashita H. et al. Leucine rich repeat proteins: sequences, mutations, structures and diseases. Protein Pept Lett. 2019;26:108–131. doi: 10.2174/0929866526666181208170027. [DOI] [PubMed] [Google Scholar]
- 10.Beetz N, Rommel C, Schnick T. et al. Ablation of biglycan attenuates cardiac hypertrophy and fibrosis after left ventricular pressure overload. J Mol Cell Cardiol. 2016;101:145–155. doi: 10.1016/j.yjmcc.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Chen SW, Tung YC, Jung SM. et al. Lumican-null mice are susceptible to aging and isoproterenol-induced myocardial fibrosis. Biochem Biophys Res Commun. 2017;482:1304–1311. doi: 10.1016/j.bbrc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Melleby AO, Strand ME, Romaine A. et al. The heparan sulfate proteoglycan glypican-6 is upregulated in the failing heart, and regulates cardiomyocyte growth through ERK1/2 signaling. PLos One. 2016;11:e0165079. doi: 10.1371/journal.pone.0165079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengtsson E, Neame PJ, Heinegard D. et al. The primary structure of a basic leucine-rich repeat protein, PRELP, foundin connective tissues. J Biol Chem. 1995;270:25639–25644. doi: 10.1074/jbc.270.43.25639. [DOI] [PubMed] [Google Scholar]
- 14.Barallobre-Barreiro J, Didangelos A, Schoendube FA. et al. Proteomics analysis of cardiac extracellular matrix re modelling in a porcine model of ischemia/reperfusion injury. Circulation. 2012;125:789–802. doi: 10.1161/CIRCULATIONAHA.111.056952. [DOI] [PubMed] [Google Scholar]
- 15.Duan J, Xu H, Ma S. et al. Cre-mediated targeted gene activation in the middle silk glands of transgenic silkworms (Bombyx mori). Transgenic Res. 2013;3:607–619. doi: 10.1007/s11248-012-9677-0. [DOI] [PubMed] [Google Scholar]
- 16.Hahn JY, Cho HJ, Bae JW. et al. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem. 2006;41:30979–30989. doi: 10.1074/jbc.M603916200. [DOI] [PubMed] [Google Scholar]
- 17.Jones SE, Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;9:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Cui Y, Luan J. et al. PRELP (proline/arginine-rich end leucine-rich repeat protein) promotes osteoblastic differentiation of preosteoblastic MC3T3-E1 cells by regulating the β-catenin pathway. Biochem Biophys Res Commun. 2016;470:558–562. doi: 10.1016/j.bbrc.2016.01.106. [DOI] [PubMed] [Google Scholar]
- 19.Pillai VS, Kundargi RR, Edathadathil F. et al. Identfication of prolargin expression in articular cartilage and its significance in rheumatoid arthritis pathology. Int J Biol Macromol. 2018;110:558–566. doi: 10.1016/j.ijbiomac.2018.01.141. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Puente P, Gonzalez-Rodriguez L, Calamia V. et al. Analysis of endogenous peptides released from osteoarthritic cartilage unravels novel pathogenic markers. Mol Cell Proteomics. 2019;18:2018–2028. doi: 10.1074/mcp.RA119.001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lal H, Ahmad F, Zhou J. et al. Cardiac fibroblast glycogen synthase kinase-3b regulates ventricular re-modelling and dysfunction in ischemic heart. Circulation. 2014;130:419–430. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara T, Yoshida E, Shinkai Y. et al. Biglycan intensifies ALK5-Smad2/3 signaling by TGF-β1 and downregulates syndecan-4 in cultured vascular endothelial cells. J Cell Biochem. 2017;118:1087–1096. doi: 10.1002/jcb.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jazi MF, Biglari A, Mazloomzadeh S. et al. Recombinant fibromodulin has therapeutic effects on diabetic nephropathy by down-regulating transforming growth factor-β1 in streptozotocin-induced diabetic rat model. Iran J Basic Med Sci. 2016;19:265–271. [PMC free article] [PubMed] [Google Scholar]
- 24.Bengtsson E, Lindblom K, Tillgren V. et al. The leucine-rich repeat protein PRELP binds fibroblast cell-surface proteoglycans and enhances focal adhesion formation. Biochem J. 2016;473:1153–1164. doi: 10.1042/BCJ20160095. [DOI] [PubMed] [Google Scholar]
- 25.Liu GH, David E, Martin E. et al. PRELP enhances host innate immunity against the respiratory tract pathogen. Moraxella catarrhalis. J Immunol. 2017;198:2330–2340. doi: 10.4049/jimmunol.1601319. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson E, Aspberg A, Heinegard D. et al. The amino-terminal part of PRELP binds to heparin and heparan sulfate . J Biol Chem. 2000;275:40695–40702. doi: 10.1074/jbc.M007917200. [DOI] [PubMed] [Google Scholar]
- 27.Sumida T, Naito AT. Complement C1q-induced activation of β-catenin signaling causes hypertensive arterial re-modelling. Nat Commun. 2005;26:6241. doi: 10.1038/ncomms7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Wu Q, Guo F. et al. Expression of dishevelled-1 in wound healing after acute myocardial infarction: possible involvement in myofibroblast proliferation and migration. J Cell Mol Med. 2004;8:257–264. doi: 10.1111/j.1582-4934.2004.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popova AP, Bentley JK, Anyanwu AC. et al. Glycogen synthase kinase- 3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L439–L448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong MH, Kim HJ, Pyun JH. et al. Cdon deficiency causes cardiac remodeling through hyperactivation of wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2017;114:E1345–E1354. doi: 10.1073/pnas.1615105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Angeli M, Chung KJ. et al. sFRP2 activates wnt/β-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am J Physiol Cell Physiol. 2016;311:C710–C719. doi: 10.1152/ajpcell.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]