Abstract
The conclusions of the European Food Safety Authority (EFSA) following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Czech Republic, and co‐rapporteur Member State, France, for the pesticide active substance fat distillation residues are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative uses of fat distillation residues as a repellent on seedlings of coniferous and deciduous trees. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns were not identified.
Keywords: fat distillation residues, peer review, risk assessment, pesticide, repellent
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012 as amended by Commission Implementing Regulation (EU) No 2016/183. Fat distillation residues is one of the active substances listed in that Regulation.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Czech Republic, and co‐rapporteur Member State (co‐RMS), France, received an application from NeraAgro, spol. s r.o. for the renewal of approval of the active substance fat distillation residues.
An initial evaluation of the dossier on fat distillation residues was provided by the RMS in the renewal assessment report (RAR), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The uses of fat distillation residues according to the representative field uses as a ruminant game repellent on seedlings of conifer and deciduous trees, by coating individual plants with a brush or gloves, resulted in a sufficient game repellent efficacy.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to identity, physical and chemical properties and analytical methods.
No critical area of concern or issues that could not be finalised were identified in the area of mammalian toxicity.
In the residues area, for the representative uses, a consumer exposure via dietary intake is not expected.
The information available and its evaluation regarding the environmental fate and behaviour of the active substance were considered sufficient to complete the assessments necessary regarding the environmental exposure assessment for the representative uses assessed. Considering the nature of the substance mixture and the representative uses assessed, a definition of residue in the environment for risk assessment is considered unnecessary for fat distillation residues.
No concerns were identified in the area of ecotoxicology.
It is unlikely that fat distillation residues meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
Background
Commission Implementing Regulation (EU) No 844/20121, as amended by Commission Implementing Regulation (EU) No 2018/16592 (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20093. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS, Czech Republic, and co‐RMS, France, received an application from NeraAgro, spol. s r.o. for the renewal of approval of the active substance fat distillation residues. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (France), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on fat distillation residues in the RAR, which was received by EFSA on 25 February 2021 (Czech Republic, 2021).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, NeraAgro, spol. s r.o., for consultation and comments on 7 December 2021. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 9 February 2022. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of reporting table. In addition, the applicant was invited to respond to the comments received. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 30 March 2022. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in November–December 2022.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the formulation for representative uses, evaluated on the basis of the representative uses of fat distillation residues as a repellent on seedlings of coniferous and deciduous trees, as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion.
A list of the relevant end points for the active substance and the formulation is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for fat distillation residues according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2022), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (30 March 2022);
the evaluation table (16 December 2022);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Czech Republic, 2022), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulation for representative uses
The name of this active substance is fat distillation residues, for which there is no ISO common name.
The formulation for representative uses for the evaluation is ‘Morsuvin’, a paste (PA) containing 40 g/kg fat distillation residues and 255 g/kg quartz sand.4
The representative field uses evaluated are as a ruminant game repellent on seedlings of conifer and deciduous trees by application with a brush or gloves. Full details of the good agricultural practice (GAP) can be found in the list of end points in Appendix B.
Information was submitted to conclude that the use of fat distillation residues according to the representative uses proposed at EU level results in a sufficient repellent efficacy against the target organisms, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance document was followed in the production of this conclusion: European Commission, 2000.
The proposed specification for fat distillation residues is based on batch data from industrial plant production. The proposed minimum purity is 400 g/kg cleaved fatty acids (free/ester bonded) and minimum acid value 70 mg KOH/g. Palmitic, stearic and oleic acids are proposed as reference substances of the active substance with a content of 19%, 18% and 37% of the total content of cleaved fatty acids, respectively. Nickel is considered as a relevant impurity with a maximum content of 0.1 g/kg (see Section 2). It is noted that the measured levels of nickel in all five representative batches are above this limit. In addition, toxicological relevance of some impurities potentially present in the technical material is open (see Section 2). Consequently, new data such as quantification of the impurities in the recent five batches, spectral data, content of the impurities before and after the storage of the formulation and methods for analysis of the relevant impurities in the formulation might be required. Based on the data for the renewal, the reference specification should be amended (i.e. lower maximum levels for the relevant impurity and consideration of palmitic, stearic and oleic acids as reference substances of the active substance). An assessment of the compliance of the material tested in (eco)toxicological studies with the specification(s) was not required (see Sections 2 and 5). An FAO specification is not available for fat distillation residues.
The main data regarding the identity of fat distillation residues and its physical and chemical properties are given in Appendix B. A data gap was identified for shelf‐life study of the formulation for representative uses at ambient temperature and self‐heating data (data gap, see Section 10).
Methods of analysis are available for the determination of the active substance and the relevant impurity in the technical material. It should be noted that the method used for the determination of nickel in the technical material is a standard method (CSN ISO 8070) for which full validation is not needed; however, it is required to demonstrate the applicability of the method for determination of nickel in fat distillation residues (data gap, see Section 10). Method for determination of the active substance in the formulation for representative uses was provided; however, a data gap for demonstration of the accuracy of the method was identified (data gap, see Section 10). Method for determination of the relevant impurity in the formulation is missing (data gap, see Section 10).
Methods for the analysis of residues in food and feed of plant origin, animal products, body fluids and tissues and environmental compartments are not required as residue definitions were not set.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: European Commission, 2012; ECHA, 2017.
The active substance ‘fat distillation residues’ is a mixture of natural compounds, including fatty acids (i.e. palmitic, stearic, oleic acids), which are approved as food additives (E 570) under Regulation (EC) No 1333/20085. EFSA re‐evaluated the safety of E 570 in 2017 (EFSA ANS Panel, 2017) and concluded it to be of no safety concern. Palmitic acid, stearic acid and oleic acid are also included in the Union list of authorised substances that may be intentionally used in the manufacture of plastic layers in plastic materials and articles (Annex I to Commission Regulation (EU) No 10/20116) and they are permitted in cosmetic products (European Commission database‐CosIng7).
Reliable toxicity data with the active substance are limited to acute toxicity studies, and therefore, an assessment of the compliance of the material tested with the specifications was not required (see Section 1).
In the proposed reference specification, nickel is identified as relevant impurity. Nickel is classified as Carcinogenic Cat. 2, Skin Sens. 1 and STOT RE1 according to Regulation 1272/20088 with a generic concentration limit of 0.01% (0.1 g/kg) which is exceeded in the case of fat distillation residues technical material (see Section 1). EFSA noted that the toxicological profile of some impurities potentially present in the technical material has not been addressed (data gap, see Section 10).
No ADME (Absorption, Distribution, Metabolism, Excretion) studies with fat distillation residues have been submitted by the applicant. Fatty acids, the reference substances of the fat distillation residues, are readily and extensively absorbed from the gastrointestinal tract and are further metabolised to carbon dioxide, which is finally excreted via exhalation. Based on the available data, fat distillation residues have no acute toxicity via the oral and dermal routes of exposure. It is not an eye or skin irritant and does not cause skin sensitisation. Testing for phototoxicity/photogenotoxicity is not required considering the nature of the active substance. No concern on the genotoxicity potential of fatty acids, including stearic‐, palmitic‐, oleic acid, has been raised under other regulatory framework (EFSA ANS Panel, 2017). This is also supported by a supplementary Ames Test on fat distillation residues submitted in the context of the current peer review. Fat distillation residues are unlikely to be genotoxic.
No data on medical surveillance on manufacturing plant personnel and monitoring studies have been provided (data gap, see Section 10).
Based on its chemical composition (i.e. fatty acids), all toxicological studies can be waived, and toxicological reference values are not required for fat distillation residues. Thus, a low risk to operators, workers, bystanders and residents from the representative uses was concluded. It is noted that a similar approach has been used and accepted for similar active substances such as sheep fat and fish oil.
In the formulation for representative uses ‘Morsuvin®’, the presence of a second active substance, i.e. quartz sand, is noted. Quartz sand has been subject of a recent peer review re‐evaluation (EFSA et al., 2022).
‘Morsuvin®’ also contains two co‐formulants of potential toxicological concern, i.e. titanium dioxide (TiO2) and a co‐polymer of acrylic esters and styrene. 9
Titanium dioxide (TiO 2 ) of unknown particle size is present at a final concentration higher than 1%. Titanium dioxide is classified as a suspected carcinogen (Category 2) by inhalation according to Regulation (EC) No 1272/200810. This classification specifically applies to TiO2 in powder form containing 1% or more particles with aerodynamic diameter ≤10 μm. The presence of TiO2 at a level >1% might trigger the classification of the product as carcinogen category 2, pending further considerations of the aerodynamic diameter of particles in the product. Additionally, EFSA has recently revised its safety assessment of TiO2 as a food additive (EFSA FAF Panel, 2021) and has concluded that a genotoxic concern for TiO2 particles (with unknown relationship to particle size) cannot be ruled out.
With regard to the co‐polymer of acrylic ester and styrene, EFSA noted that the constituent monomers, styrene and butyl acrylate are classified11 according to Regulation (EC) No 1272/2008. EFSA has recently re‐assessed styrene safety for use as a food contact material (EFSA CEP Panel, 2020) and concluded that a concern for genotoxicity associated with oral exposure to styrene cannot be excluded. No information on the presence of free‐styrene, either as unreacted monomer or as released from the co‐polymer, is reported in the current renewal. In addition, 1,2‐benzisothiazolin‐3‐one (BIT) and 2‐methyl‐2H‐isothiazoline‐3‐one (MIT), also included in the co‐polymer formulation, are classified12 according to Regulation (EC) No 1272/2008.
The lack of additional toxicological information on the above co‐formulants is not considered relevant for the representative uses and the formulation (paste), given that exposure by inhalation and/or ingestion is not expected (see also Section 3). However, this might be an issue for different types of formulations and/or other potential uses triggering inhalation exposure, to which consideration should be given by Member States.
3. Residues
Metabolism studies were not submitted, and they are not needed since the representative uses are on coniferous and deciduous trees thus residues in food and edible crops are not expected. Consequently, exposure to the consumer via dietary intake following the representative uses is not expected and a consumer dietary risk assessment can be waived.
4. Environmental fate and behaviour
The environmental fate and behaviour of fat distillation residues is expected to follow the normal pathways of dissipation and degradation common to naturally occurring cleaved fatty acid residues of biological origin.
After application (by brush or glove), the formulation dries and forms a protective coating. The dried preparation is not water soluble. Soil and surface water exposure is expected to be limited consequent to the correct application in accordance with the representative uses. Based on the nature of the ingredients and the formulation, it is unlikely that residues of the preparation would be detected in air.
Considering the nature of the substance mixture and the limited environmental exposure resulting from the representative uses being assessed, further consideration of its fate and behaviour in the environment was concluded to be unnecessary for fat distillation residues.
5. Ecotoxicology
Reliable toxicity data with the active substance or the formulation for representative uses were not available for any group of non‐target organisms. Therefore, an assessment of the compliance of the material tested with the specifications was not required. Only supportive studies (aquatic organisms, honeybees, earthworms, soil microorganisms) were available. Although none of the studies were considered reliable, the results indicated low toxicity. The representative uses of fat distillation residues are a repellent by a localised application to individual tree seedlings, with a special brush or with a glove. The uses are anticipated to result in a low exposure to all groups of non‐target organisms (see Section 4). On the basis of the low exposure and considering the method of application, the repellent mode‐of‐action and the supportive studies, a low risk to all groups of non‐target organisms from the representative uses was concluded.
As acknowledged in Section 2, in relation to the co‐formulants of ‘Morsuvin®’, i.e. titanium dioxide (TiO2) and co‐polymer of acrylic esters and styrene, EFSA noted that hazard classifications13 according to Regulation (EC) No 1272/2008 for the aquatic environment are reported for the constituent monomers, styrene and butyl acrylate, for 1,2‐benzisothiazolin‐3‐one (BIT) and 2‐methyl‐2H‐isothiazoline‐3‐one (MIT), also included in the co‐polymer formulation, and for TiO2. However, the exposure to surface water was considered limited (see Section 4) for the representative uses and formulation (a paste). It is unlikely that undegraded constituents of the copolymer solution would reach the aquatic environment.
6. Endocrine disruption properties
With regard to the assessment of the endocrine disruption potential of fat distillation residues for humans and non‐target organisms according to the ECHA/EFSA guidance (2018), no (eco) toxicological data are available to assess the endocrine‐disrupting properties. This was discussed at the Pesticide Peer Review Experts' Meeting Teleconference 89 (September 2022),14 where it was agreed that additional data do not appear to be scientifically necessary based on the following considerations:
Fat distillation residues are a mixture of natural compounds, including fatty acids (i.e. palmitic, stearic, oleic acids), which are approved as food additives.
The natural occurrence of the fatty acids, and the soil and surface water exposure which is expected to be limited consequent to the correct application in accordance with the representative uses.
The pesticide mode of action, repellent (paste) applied on terminal sprouts or top whirls of seedlings, is considered of no/low concern for potential endocrine disruption.
Negative outcome on endocrine activity based on supporting information from ToxCast (i.e. E (oestrogen), A (androgen) models negative for palmitic, stearic and oleic acids; no evidence for S (steroidogenesis) and T (thyroid) activity in ToxCast assays). Oleic acid, palmitic acid and stearic acid were not tested for steroidogenesis but were tested for aromatase activity.
EFSA (EFSA NDA Panel, 2010) opinion concluding that setting of an acceptable daily intake (ADI) was considered not scientifically necessary and that fatty acids are included in Annex 4 of REACH regulation15 indicating low concern.
Based on the available information, it is unlikely that fat distillation residues meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3–4)16
Table 1.
Soil
| Compound (name and/or code) | Ecotoxicology |
|---|---|
|
Not applicable. Considering the nature of the substance mixture and the limited exposure from the representative uses, a definition of residue in the environment for risk assessment triggering assessment of effects data is considered unnecessary for fat distillation residues. |
Not triggered. |
Table 2.
Groundwater
| Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses (a) Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
|
Not applicable. Considering the nature of the substance mixture and the limited exposure from the representative uses, a definition of residue in the environment for risk assessment triggering assessment of effects data is considered unnecessary for fat distillation residues. |
Not relevant (b) | Yes | – | – |
Yes |
FOCUS scenarios or relevant lysimeter. Ranges indicated for FOCUS scenarios include the result from the model giving the highest concentration at each scenario, as needed to comply with European Commission (2014a) guidance.
Attractants and repellents are not defined as pesticides in Council Directive 98/83/EC.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
|
Not applicable. Considering the nature of the substance mixture and the limited exposure from the representative uses, a definition of residue in the environment for risk assessment triggering assessment of effects data is considered unnecessary for fat distillation residues. |
Not triggered. |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
|
Not applicable. Considering the nature of the substance mixture and the limited exposure from the representative uses, a definition of residue in the environment for risk assessment triggering assessment of effects data is considered unnecessary for fat distillation residues |
Not triggered. |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant's proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
No particular conditions are proposed for the representative uses evaluated.
9. Concerns and related data gaps
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/201117 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
Issues or assessments that could not be finalised were not identified.
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical areas of concerns were not identified.
9.3. Overview of the concerns identified for each representative use considered (Table 5)
Table 5.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use | Seedlings of coniferous and deciduous trees | Seedlings of coniferous and deciduous trees | |
|---|---|---|---|
| Coating of individual plants with special brush or with rubber or plastic glove | Coating of individual plants with special brush or with rubber or plastic glove | ||
| Max 0.200 kg a.i./1,000 seedlings | Max 0.240 kg a.i./1,000 seedlings | ||
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | ||
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | ||
| Assessment not finalised | |||
| Consumer risk | Risk identified | ||
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to aquatic organisms | Risk identified | ||
| Assessment not finalised | |||
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breached | ||
| Parametric value of 10 μg/L (a) breached | |||
| Assessment not finalised | |||
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5).
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections:
Shelf‐life study at ambient temperature and self‐heating data (relevant for all representative uses evaluated; see Section 1).
Data demonstrating applicability of the CSN ISO 8070 method for determination of nickel in fat distillation residues (relevant for all representative uses evaluated; see Section 1).
Data on accuracy of the method for determination of the active substance in the formulation for representative uses (relevant for all representative uses evaluated; see Section 1).
Validated analytical method for analysis of nickel in the formulation for representative uses (relevant for all representative uses evaluated; see Section 1).
Consideration on the toxicological profile of some impurities potentially present in fat distillation residues technical material (relevant for all representative uses evaluated; see Section 2).
Data on medical surveillance on manufacturing plant personnel and monitoring studies (relevant for all representative uses evaluated; see Section 2).
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
Wavelength
- ε
decadic molar extinction coefficient
- AMA
Amphibian Metamorphosis Assay
- a.s.
active substance
- ADI
acceptable daily intake
- bw
body weight
- ECHA
European Chemicals Agency
- ErC50
effective concentration (growth rate)
- FAO
Food and Agriculture Organisation of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GCPF
Global Crop Protection Federation (formerly known as International Group of National Associations of Manufacturers of Agrochemical Products; GIFAP)
- GGT
gamma glutamyl transferase
- GM
geometric mean
- GS
growth stage
- GSH
glutathione
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
intravenous
- mm
millimetre (also used for mean measured concentrations)
- MS
mass spectrometry
- Pa
pascal
- PIE
potential inhalation exposure
- pKa
negative logarithm (to the base 10) of the dissociation constant
- PPE
personal protective equipment
- ppm
parts per million (10−6)
- PT
proportion of diet obtained in the treated area
- PTT
partial thromboplastin time
- QSAR
quantitative structure–activity relationship
- RAR
Renewal Assessment Report
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for fat distillation residues according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
| Properties | Conclusion (a) | |
|---|---|---|
| CMR | Carcinogenicity (C) | The active substance fat distillation residues is not considered to be mutagenic, carcinogenic or toxic for reproduction according to points 3.6.2, 3.6.3 and 3.6.4 of Annex II of Regulation (EC) 1107/2009. |
| Mutagenicity (M) | ||
| Toxic for Reproduction (R) | ||
| Endocrine‐disrupting properties | It is unlikely that fat distillation residues meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605. | |
| POP | Persistence | The active substance fat distillation residues is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Long‐range transport | ||
| PBT | Persistence | The active substance fat distillation residues is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Toxicity | ||
| vPvB | Persistence | The active substance fat distillation residues is not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the formulation for representative uses
Appendix B can be found in the online version of this output (‘Supporting Information’ section): https://doi.org/10.2903/j.efsa.2023.7811
Appendix C – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
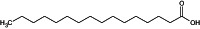
| Palmitic acid |
hexadecanoic acid O=C(O)CCCCCCCCCCCCCCC IPCSVZSSVZVIGE‐UHFFFAOYSA‐N |
|
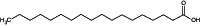
| Stearic acid |
octadecanoic acid O=C(O)CCCCCCCCCCCCCCCCC QIQXTHQIDYTFRH‐UHFFFAOYSA‐N |
|
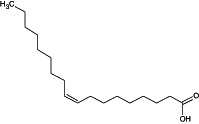
| Oleic acid |
(9Z)‐octadec‐9‐enoic acid O=C(O)CCCCCCC/C=C\CCCCCCCC ZQPPMHVWECSIRJ‐KTKRTIGZSA‐N |
|
The compound name in bold is the name used in the conclusion.
ACD/Name 2021.1.3 ACD/Labs 2021.1.3 (File Version N15E41, Build 123232, 07 July 2021).
ACD/ChemSketch 2021.1.3 ACD/Labs 2021.1.3 (File Version C25H41, Build 123835, 28 August 2021).
Supporting information
List of end points for the active substance and the formulation for representative uses
Suggested citation EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, De Magistris I, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Nogareda LH, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Panzarea M, Parra Morte JM, Rizzuto S, Serafimova R, Sharp R, Szentes C, Szoradi A, Terron A, Theobald A, Tiramani M, Vianello G and Villamar‐Bouza L, 2023. Conclusion on the peer review of the pesticide risk assessment of the active substance fat distillation residues. EFSA Journal 2023;21(1):7811, 18 pp. 10.2903/j.efsa.2023.7811
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00474
Note/Update: This scientific output, approved on 20 December 2022, supersedes the previous output published on 10 February 2012 (EFSA, 2012).
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: EFSA wishes to thank the rapporteur Member State, Czech Republic, for the preparatory work on this scientific output.
Approved: 20 December 2022
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, pp. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine‐disrupting properties introduced by Regulation (EU) 2018/605.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, pp. 1–50.
For the latter active substance (quartz sand), the peer review of the renewal process was completed and an EFSA Conclusion was issued on 10 August 2022 (https://doi.org/10.2903/j.efsa.2022.7552) (EFSA et al., 2022).
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, pp. 16–33.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 354, 31.12.2008, pp. 16–33.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, pp. 1–1355.
It has to be noted that co‐polymer of styrene has not been flagged during the peer review on active substance Quartz sand, and therefore, it was not reported in the EFSA conclusion (EFSA, 2022). However, EFSA noted that the formulation for representative uses ‘Morsuvin’ is the same as per Quartz sand a.s. and as such it does contain the same co‐formulants.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, pp. 1–1355.
Styrene (CAS No. 100‐42‐5) is classified as Skin Irrit. 2, Eye Irrit. 2, Acute tox 4, STOT RE 1 and Repr. 2 according to Regulation (EC) No 1272/2008. Butyl acrylate (or n‐butyl acrylate, CAS No. 141‐32‐2) is classified as Skin Irrit. 2, Eye Irrit. 2, Skin Sens. 1 and STOT SE 3 according to Regulation (EC) No 1272/2008.
1,2‐benzisothiazolin‐3‐one (BIT) (CAS No. 2634‐33‐5) is classified as Acute Tox. 4, Skin Irrit. 2, Eye Dam. 1 and Skin Sens. 1 according to Regulation (EC) No 1272/2008. 2‐methyl‐2H‐isothiazoline‐3‐one (MIT) (CAS No. 2682‐20‐4) is classified as Acute Tox. 2 (H330), Acute Tox. 3 (H301 and H311), Skin Corr. 1B, Eye Dam. 1 and Skin Sens. 1A according to Regulation (EC) No 1272/2008.
- Monomer of styrene has a notified classification as aquatic chronic 3 (ECHA website).
- Butyl acrylate (or n‐butyl acrylate) has a notified classification as aquatic chronic 3 (ECHA website)
- 1,2‐benzisothiazolin‐3‐one (BIT) is classified as aquatic Acute 1 (ECHA website).
- 2‐methyl‐2H‐isothiazoline‐3‐one (MIT) is classified as aquatic acute 1 and aquatic chronic 1 (ECHA website)
- Titanium dioxide (TiO2) has a notified classification as aquatic chronic 4 (ECHA website)
See experts' consultation points 2.1 and 5.1 at the Pesticide Peer Review Teleconference 89 (EFSA, 2022).
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1988, pp. 32–54.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, pp. 127–175.
References
- Czech Republic , 2021. Renewal Assessment Report (RAR) on the active substance fat distillation residues prepared by the rapporteur Member State Czech Republic in the framework of Commission Implementing Regulation (EU) No 844/2012, February 2021. Available online: www.efsa.europa.eu
- Czech Republic , 2022. Revised Renewal Assessment Report (RAR) on fat distillation residues prepared by the rapporteur Member State Czech Republic in the framework of Commission Implementing Regulation (EU) No 844/2012, November 2022. Available online: www.efsa.europa.eu
- ECHA (European Chemicals Agency) , 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0 July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5. Available online: https://echa.europa.eu/guidance‐documents/guidance‐on‐clp
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance fat distillation residues. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, BinagliaM, Castoldi AF , Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Nogareda LH, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Rizzuto S, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance quartz sand. EFSA Journal 2022;20(9):7552, 21 pp. 10.2903/j.efsa.2022.7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen, A , Aguilar, F , Crebelli, R , Di Domenico, A , Dusemund, B , Frutos, MJ , Galtier, P , Gott, D , Gundert‐Remy, U , Leblanc, J‐C , Lindtner, O , Moldeus, P , Mosesso, P , Parent‐Massin, D , Oskarsson, A , Stankovic, I , Waalkens‐Berendsen, I , Woutersen, RA , Wright, M , Younes, M , Boon, P , Chrysafidis, D , Gürtler, R , Tobback, P , Gergelova, P , Rincon, AM and Lambré, C , 2017. Scientific Opinion on the re‐evaluation of fatty acids (E 570) as a food additive. EFSA Journal 2017;15(5):4785, 48 pp. 10.2903/j.efsa.2017.4785 [DOI] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , 2020. Scientific Opinion on the assessment of the impact of the IARC Monograph Vol. 121on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials. EFSA Journal 2020;18(10):6247, 23 pp. 10.2903/j.efsa.2020.6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , 2021. Scientific Opinion on the safety assessment of titanium dioxide (E171) as a food additive. EFSA Journal 2021;19(5):6585, 130 pp. 10.2903/j.efsa.2021.6585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies) ; 2010. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461, 107 pp. 10.2903/j.efsa.2010.1461. [DOI] [Google Scholar]
- European Commission , 2000. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2003. Guidance document on assessment of the relevance of metabolites in groundwater of substances regulated under council directive 91/414/EEC. SANCO/221/2000-rev. 10 final, 25 February 2003.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2 May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the formulation for representative uses


