Abstract
Longitudinal data on the immune response from the first dose to several months after the third dose of COVID-19 vaccine are limited. We analyzed the immune response in 406 Japanese healthcare workers who received at least three doses of vaccine. The geometric mean anti-receptor binding domain IgG antibody titers and antigen-stimulated T-cell interferon-gamma levels after 6 months after receiving a third dose were similar to those 8 weeks after receiving a second dose. Humoral and cellular immunity induced by the third dose was more durable than that induced by the second dose.
UMIN Clinical Trials Registry ID: UMIN000043340.
Keywords: COVID-19 vaccine, BNT162b2, Third dose, Antibody, Cellular immunity
1. Background
Administration of a third dose of COVID-19 vaccine is currently recommended for all adults, whereas a fourth dose is only recommended for immunocompromised people in most countries [1]. Therefore, most people have received only three doses of vaccine. Data on the long-term immunity dynamics after the third dose are important for estimating vaccine-acquired immunity at the population level. However, limited longitudinal data are available from large studies describing the immune response of healthy individuals from the period before the first dose to several months after the third dose. Reports on long-term cellular immunity after vaccination are particularly important because of its role in protecting again the Omicron variant [2].
We conducted a prospective cohort study of the BNT162b2 vaccine administered to Japanese healthcare workers, and previously reported the relationship between adverse reactions following vaccination and immunogenicity [[3], [4]] and described waning immunity after administration of a second dose of this vaccine [5]. In this paper, we describe the humoral and cellular immune response up to 6 months after the third dose of vaccine.
2. Participants and methods
2.1. Participants
From February 16 to September 3, 2021, unvaccinated healthcare workers and university staff of Keio University Shinanomachi Campus (Tokyo, Japan) were recruited before mass vaccination with the BNT162b2 monovalent vaccine (COMIRNATY intramuscular injection, Pfizer, New York, USA). The study was approved by the ethics committee of Keio University School of Medicine (20200330), and written informed consent was obtained from all participants. Three doses of the vaccine were administered by mass vaccination of the employees. Blood sample collections of all participants were performed before the first dose; 3 and 8 weeks after the second dose; 3 and 6 months after the second dose; 3 and 8 weeks after the third dose; and 6 months after the third dose (Table S1).
The aim of this study was to observe the dynamics of acquired immunity after each of the three doses of BNT162b2 vaccine; therefore, we obtained information about breakthrough infections and receipt of a fourth dose of vaccine, because these might have affected the immune response.
According to the campus labor regulations, all participants had to undergo SARS-CoV-2 testing (PCR or antigen detection test) and report the results if they had fever, respiratory symptoms, or close contact with COVID-19 patients. The employees’ health records were reviewed, and data were extracted on laboratory-confirmed breakthrough infections in the participants from enrollment up to 6 months after the third dose.
In Japan, fourth dose administration for people over 60 years old and those with underlying illness started on May 25, 2022, and some study participants received a fourth dose of COVID-19 vaccine. All participants were questioned about whether they had received a fourth dose at the time of sample collection 6 months after the third dose.
2.2. Measurement of anti-RBD-IgG antibody titer for the spike protein and neutralizing antibody titer for the variants
IgG antibody titers against the SARS-CoV-2 receptor-binding domain spike protein (RBD-IgG) were measured in all serum samples, using SARS-CoV-2 IgG II Quant reagents and an Alinity Analyzer (Abbott Laboratories, Abbott Park, IL, USA), according to the manufacturer’s instructions.
To estimate the relationships between neutralizing antibody titers against the Omicron variant (NA-Omicron) and for RBD-IgG, neutralizing antibody titers in the serum samples collected 3 weeks after the third dose were measured using Omicron (B.1.1.529) variant strains clinically isolated at Keio University Hospital. The detailed methods are described in a previous report [6].
2.3. Interferon gamma releasing assay
To evaluate T-cell immunity, QuantiFERON SARS-CoV-2 tests (QFN) were performed on the first 600 participants selected at first sample collection. Whole blood samples were collected into lithium heparin tubes before vaccination, 8 weeks and 6 months after the second dose, and 8 weeks and 6 months after the third dose. The collected samples were transferred to four QuantiFERON SARS-CoV-2 tubes (Qiagen, Hilden, Germany) coated with antigen 1, antigen 2, phytohemagglutinin (positive control), and no peptide (negative control). Antigen 1 is an epitope of CD4 + T-cells derived from the S1 subunit and antigen 2 is an epitope of CD4 + and CD8 + T-cells derived from the S1 and S2 subunits. The tubes were incubated at 37 °C for 16–24 h, and then the samples were tested using the QuantiFERON SARS-CoV-2 ELISA kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and a DS2 fully automated microplate enzyme immunoassay reader (Dynex Technologies, Chantilly, VA, USA). Interferon-gamma levels for antigen 1 (IFN-γ for Ag1) and antigen 2 (IFN-γ for Ag2) were calculated correcting for the negative control value.
2.4. Statistical analysis
The participants who were not administered all three doses of the BNT162b2 vaccine and those whose blood samples were not collected were excluded from the analysis. Additionally, the antibody and QFN data of samples after a breakthrough infection event or fourth dose administration were excluded.
Antibody titers and interferon gamma levels were expressed as geometric means and 95 % confidence intervals (95 % CI), and a parametric test of the logarithm was used to compare antibody titers and interferon gamma levels of each sample collection point.
The linear mixed-effects model was fitted to the log10-transformed antibody titers, which included the fixed effects of sex, age, and time, as well as the random intercept and slope. Model parameters were estimated using the restricted maximum likelihood (REML) approach, and the variance–covariance structure was assumed to be compound symmetry. The degree of freedom estimates were determined using the Kenward–Roger method. To account for multiple testing, the Benjamini–Hochberg procedure with a 5 % false discovery rate was used to determine statistical significance for the mixed-effects model analysis.
All statistical analyses were performed using SAS version 9.4 and JMP version 16 (SAS Institute, Cary, NC). Statistical significance was set at p < 0.05.
3. Results
3.1. Participants
From the 673 participants included in the present study, 406 participants remained after the exclusions (Fig. S1). Their demographic characteristics are shown in Table S2. The median age was 47 years, the cut-off age was rounded up to 50 years, and the participants were divided into two groups: ≥ 50 years and < 50 years old.
During the study period, 28 participants had an episode of breakthrough infection. Among them, 24 had elevated RBD-IgG titers afterwards (Table S3). No significant difference was observed in RBD-IgG titers and QFN results at 8 weeks after the third dose administration between the participants who had an episode of breakthrough infection from 8 weeks to 6 months after the third dose administration and those who had no episodes of breakthrough infections during the study period (Fig S2). Nineteen participants received a fourth dose of vaccine within 6 months after receiving the third dose (Table S4). The antibody data and QFN results of these participants were not included in the dynamic analysis.
3.2. Dynamics of the RBD-IgG titer and correlation with NA-Omicron
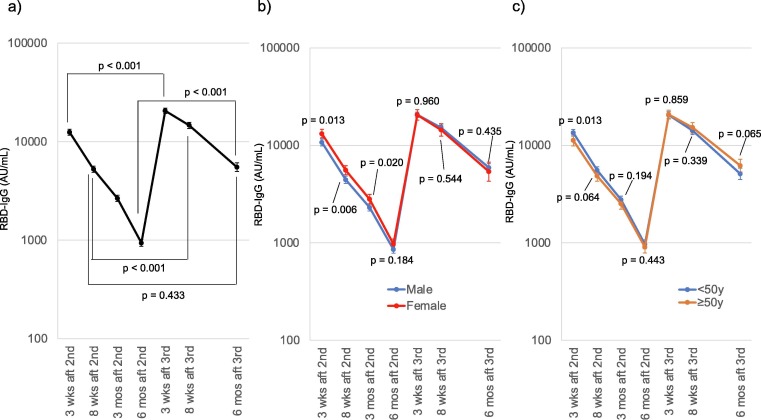
The geometric mean RBD-IgG titer was elevated to 20,558.9 AU/mL 3 weeks after the third dose and decreased to 6,622.2 AU/mL 6 months after the third dose, which was close to the titer 8 weeks after the second dose (5,248.1 AU/mL) (Fig. 1 a).
Fig. 1.
Receptor-binding domain (RBD)-IgG dynamics in study participants (a) RBD-IgG dynamics in all the participants analyzed are shown. Geometric means and 95 % confidential intervals of RBD-IgG of each blood collection point are shown. The paired t-tests of logarithms of RBD-IgG titers revealed higher geometric mean RBD-IgG titer at 3 weeks, 8 weeks, and 6 months after the third dose comparing with the corresponding after the second dose, while the geometric mean RBD-IgG titer was the highest at 8 weeks after the second dose and at 6 months after the third dose. b) Difference in the geometric mean RBD-IgG between male (blue) and female (red) participants. Female participants demonstrated higher geometric mean RBD-IgG titer until 3 months after the second dose, while the geometric mean RBD-IgG titers were not different 6 months after the second dose (t-test of logarithms of the RBD-IgG titer). c) Difference in the geometric mean RBD-IgG between participants aged 50 years and older (orange) and participants younger than 50 years (blue). Younger participants demonstrated higher geometric mean RBD-IgG titer 3 weeks after the second dose, while the geometric mean RBD-IgG titers were not different 8 weeks after the second dose (t-test of logarithms of the RBD-IgG titer). Abbrrrreviations: RBD-IgG, anti-receptor binding domain IgG titer; wks, weeks; mos, months; aft, after; 2nd, administration of the second dose; 3rd, administration of the third dose.
The titer was lower in male participants than female participants after the second dose; however, no difference was observed 6 months after the second dose, or after the third dose (Fig. 1b). In the participants younger than 50 years, a higher titer was observed 3 weeks after the second dose, but no significant difference was observed subsequently (Fig. 1c).
The RBD-IgG and NA-Omicron titers were significantly correlated 3 weeks after the third dose (r = 0.747) (Fig. S3).
3.3. Dynamics of the QuantiFERON results
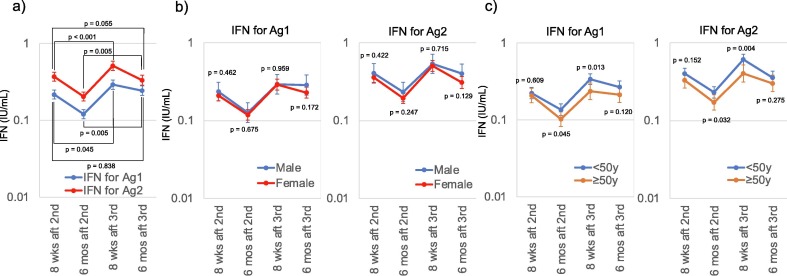
The geometric mean of IFN-γ for Ag2 was higher than that for Ag1 at each sample collection point; however, 6 months after the third dose, it was similar to that 8 weeks after the second dose (Fig. 2 a).
Fig. 2.
Interferon gamma release assay dynamics in the participants. (a) Dynamics of QuantiFERON (QFN) SARS-CoV-2 of all the participants. Geometric means and 95 % confidential interval of the interferon gamma concentration released by CD4 + T-cells after antigen 1 stimulation (IFN-γ for Ag1) are shown in blue, and geometric means and 95 % confidential interval of interferon gamma concentration released by CD4 + T-cells and CD8 + T-cells after antigen 2 stimulation (IFN-γ for Ag2) are shown in red. Paired t-tests of logarithms of the geometric means of IFN-γ for Ag1 and Ag2 8 weeks after the second dose were equivalent to those 6 months after the third dose. (b) Difference in the geometric means of QFN results between male (blue) and female (red) participants. No difference between male and female was observed at any time point. (c) Difference in the geometric mean of QFN results between participants aged 50 years and older (orange) and participants younger than 50 years (blue). Younger participants tended to have higher geometric mean QFN results than those of the older participants, and statistically significant differences were observed 6 months after the second dose and 8 weeks after the third dose (t-test of logarithms). Abbrrrreviations: QFN, QuantiFERON SARS-CoV-2 assay; IFN-γ, interferon gamma; Ag1, antigen 1; Ag2, antigen 2; wks, weeks; mos, months; aft, after; 2nd, administration of the second dose; 3rd, administration of the third dose.
No difference in the mean value was observed according to sex (Fig. 2b). Younger participants had a higher geometric mean of IFN-γ for Ag1 and Ag2 than the older group, but the differences were statistically significant only at 6 months after the second dose and 8 weeks after the third dose (Fig. 2c).
3.4. Mixed-effects model analysis
The mixed-effects model analysis of RBD-IgG titers revealed statistically significant differences between the adjusted mean RBD-IgG levels at each blood collection point, except 8 weeks after the second dose and 6 months after the third dose (Fig. S4a). Additionally, no sex- or age-related differences were observed in the RBD-IgG titers.
The mixed-effects model analysis of the QFN results confirmed these statistically significant differences, which were the smallest 8 weeks after the second dose (IFN-γ for Ag1: 0.207 IU/mL, IFN-γ for Ag2: 0.362 IU/mL) and 6 months after the third dose (IFN-γ for Ag1:0.236 IU/mL, IFN-γ for Ag2:0.319 IU/mL) (Fig. S4b). No sex-related differences were observed in IFN-γ for Ag1 and Ag2, whereas age-related differences were observed in IFN-γ for Ag2, but not in IFN-γ for Ag1.
4. Discussion
This prospective cohort study revealed that RBD-IgG titers and IFN-γ levels 6 months after the third dose were similar to those 8 weeks after the second dose, suggesting that the immunogenicity after three doses was longer lasting than that after two doses. Although the SARS-CoV-2 vaccine effectiveness varies depending on the variant, T-cell responses contribute to protection against hospitalization and death, regardless of the variant [7]. Therefore, our data suggested that third dose administration might prolong vaccine effectiveness and prevent severe outcomes, which is in accordance with the mass surveillance data on vaccine effectiveness after the third dose [8].
A sex-related difference in RBD-IgG titers observed in the weeks after the second dose [3], also found in other studies [[9], [10]], was no longer apparent in the months after the second and third dose. Although the exact reason for this decrease is unclear, our results were similar to those of two reports describing sex-related differences in RBD-IgG titers after SARS-CoV-2 infection in Wuhan, China. In the acute phase of severe COVID-19, the SARS-CoV-2 IgG antibody titer was higher in female patients than in male patients [11], but no sex-related differences were observed among convalescent COVID-19 patients [12]. Further investigations are essential to understand the trends of RBD-IgG titers in the acute phase after antigen exposure and in the late phase, in order to improve the understanding of sex-related differences in short-and long-term humoral immunity against SARS-CoV-2.
In Japan, the Omicron variant has been dominant since the beginning of 2022; thus, most breakthrough infections were caused by the Omicron variant. However, the RBD-IgG titer, the epitope of which was the wild-type strain, was markedly elevated after the breakthrough episode in most cases. This was in accordance with previous studies that demonstrated a broad neutralizing antibody response after a breakthrough [13].
Our study had three limitations. First, the study participants were limited to healthy individuals aged 20–60 years. Second, the epitope of RBD-IgG reagents was the RBD of the wild-type strain, and the QFN antigen used for stimulating T-cells originated from the wild-type strain. However, our analysis of the samples obtained 3 weeks after the third dose showed that the RBD-IgG titers were correlated with NA-Omicron, and T-cell immunity was reported to have cross-reactivity against the Omicron variant [[2], [14]]. Third, monovalent vaccine was used for the third dose. The use of a bivalent vaccine, which induces stronger humoral immunity against the Omicron variant, is now recommended for the third dose.
In conclusion, the humoral and cellular immunity 6 months after third dose BNT162b2 administration were equivalent to those at 8 weeks after the second dose, suggesting that the humoral and cellular immunity induced after the third dose were more long-lasting than those induced after the second dose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Funding
This work was supported by the Japan Agency for Medical Research and Development [grant number: JP21fk0108469], the Keio University School of Medicine, and the Public Foundation of the Vaccination Research Center, Japan.
Conflict of interest
The QuantiFERON SARS-CoV-2 ELISA kit was partially provided free of charge by Qiagen, and the DS2 fully automated microplate enzyme immunoassay reader and its consumables were supplied by Qiagen.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.01.049.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Mbaeyi S., Oliver S.E., Collins J.P., Godfrey M., Goswami N.D., Hadler S.C., et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines — United States, 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70(44):1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uwamino Y., Kurafuji T., Sato Y., Tomita Y., Shibata A., Tanabe A., et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022;40(7):1019–1025. doi: 10.1016/j.vaccine.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakui M, Uwamino Y, Yatabe Y, Nakagawa T, Sakai A, Kurafuji T, et al. Assessing anti-SARS-CoV-2 cellular immunity in 571 vaccinees by using an interferon-γ release assay. Eur J Immunol 2022. 10.1101/2021.12.14.21267039. Published online ahead of print. [DOI] [PMC free article] [PubMed]
- 5.Uwamino Y., Kurafuji T., Takato K., Sakai A., Tanabe A., Noguchi M., et al. Dynamics of antibody responses, cellular immunity, and breakthrough infections among Japanese healthcare workers during the 6 months after receiving two doses of BNT162b2 mRNA vaccine. medRxiv. 2022 doi: 10.1101/2022.01.29.22270052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uwamino Y., Yokoyama T., Shimura T., Nishimura T., Sato Y., Wakui M., et al. The effect of the E484K mutation of SARS-CoV-2 on the neutralizing activity of antibodies from BNT162b2 vaccinated individuals. Vaccine. 2022;40(13):1928–1931. doi: 10.1016/j.vaccine.2022.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss P. The T cell immune response against SARS-CoV-2. Nature Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 8.UK Health Security Agency. COVID-19 vaccine surveillance report Week 16. 21 April 2022. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1070356/Vaccine-surveillance-report-week-16.pdf [accessed 24 April 2022].
- 9.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. doi: 10.1016/j.eclinm.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect 2021;27:1861.e1. 10.1016/j.cmi.2021.07.042. [DOI] [PMC free article] [PubMed]
- 11.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng F., Wu M., Wang J., Li J., Hu G., Wang L. Over 1-year duration and age difference of SARS-CoV-2 antibodies in convalescent COVID-19 patients. J Med Virol. 2021;93:6506–6511. doi: 10.1002/jmv.27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walls A.C., Sprouse K.R., Bowen J.E., Joshi A., Franko N., Navarro M.J., et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185(5):872–880.e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marco L., D’Orso S., Pirronello M., Verdiani A., Termine A., Fabrizio C., et al. Assessment of T-cell reactivity to the SARS-CoV-2 Omicron variant by immunized individuals. JAMA Network Open. 2022;5(4):e2210871. doi: 10.1001/jamanetworkopen.2022.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.