This secondary analysis of a randomized clinical trial examines the association of SER-109 with health-related quality of life in 182 patients with recurrent Clostridioides difficile infection in the US and Canada.
Key Points
Question
Is treatment with an investigational microbiome therapeutic, SER-109, associated with disease-specific health-related quality-of-life (HRQOL) in adults with recurrent Clostridioides difficile infection compared with placebo after standard-of-care antibiotics?
Findings
In this secondary analysis of a randomized clinical trial in 182 adults, patients treated with SER-109 had significantly greater improvements in disease-specific HRQOL scores compared with patients treated with placebo as early as week 1, with continued steady and durable improvements by week 8 after dosing.
Meaning
These findings suggest that an investigational microbiome therapeutic may improve disease-related HRQOL, an important patient-related outcome.
Abstract
Importance
Recurrent Clostridioides difficile infection (CDI) is a debilitating disease leading to poor health-related quality of life (HRQOL), loss of productivity, anxiety, and depression. The potential association of treatment with HRQOL has not been well evaluated.
Objectives
To explore the association of SER-109 compared with placebo on HRQOL in patients with recurrent CDI up to week 8.
Design, Setting, and Participants
This study was a secondary analysis of a randomized, double-blind, placebo-controlled trial that took place at 56 sites in the US and Canada from July 2017 to April 2020 and included 182 patients randomized to SER-109 or placebo groups.
Interventions
SER-109 or placebo (4 capsules once daily for 3 days) following antibiotics for CDI.
Main Outcomes and Measures
Exploratory analysis of HRQOL using the disease specific Clostridioides difficile Quality of Life Survey (Cdiff32) assessed at baseline, week 1, and week 8.
Results
In this study, 182 patients (109 [59.9%] female; mean age, 65.5 [16.5] years) were randomized to SER-109 (89 [48.9%]) or placebo (93 [51.1%]) groups and were included in the primary and exploratory analyses. Baseline Cdiff32 scores were similar between patients in the SER-109 and placebo groups (52.0 [18.3] vs 52.8 [18.7], respectively). The proportion of patients with overall improvement from baseline in the Cdiff32 total score was higher in the SER-109 arm than placebo at week 1 (49.4% vs 26.9%; P = .012) and week 8 (66.3% vs 48.4%; P = .001).Greater improvements in total and physical domain and subdomain scores were observed in patients in the SER-109 group compared with placebo as early as week 1, with continued improvements observed at week 8. Among patients in the placebo group, improvements in HRQOL were primarily observed in patients with nonrecurrent CDI while patients in the SER-109 group reported improvements in HRQOL, regardless of clinical outcome.
Conclusions and Relevance
In this secondary analysis of a phase 3 clinical trial, SER-109, an investigational microbiome therapeutic was associated with rapid and steady improvement in HRQOL compared with placebo through 8 weeks, an important patient-reported outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT03183128
Introduction
Clostridioides difficile (C difficile) is the most common health care-associated infectious agent in the US and is estimated to cause more than 460 000 infections and 20 000 deaths annually.1 C difficile infection (CDI) is a debilitating disease with up to 10 to 20 watery bowel movements daily leading to poor health-related quality of life (HRQOL), loss of productivity, anxiety, and depression.2,3,4,5,6 HRQOL changes related to CDI are long lasting, deeply affect patients’ lives, including mental, physical, and relational aspects.2,7,8 A multicenter study in the UK demonstrated a 46% lower HRQOL measured by the general instrument EuroQol (EQ-5D) index9 in adults who are hospitalized with CDI as compared with age- and gender-matched population norms.10 In addition, general measures have shown that recurrent CDI is even more devastating on quality of life than primary infection.8
Unfortunately, 20% to 36% of patients with primary CDI experience recurrence, and a history of recurrence is associated with 40% or greater risk of future episodes.11,12,13,14 Currently approved antibiotics generally lead to symptom resolution through reduction of toxin-producing bacteria. However, sustained efficacy rates remain modest since antibiotics do not kill dormant C difficile spores nor address the disrupted microbiome, the underlying cause of recurrent disease.3 While fidaxomicin and vancomycin are effective at killing the C difficile bacteria in its vegetative form, they do not restore the disrupted microbiome.15,16,17
SER-109 is an oral investigational microbiome therapeutic comprising live purified Firmicutes bacterial spores designed to compete metabolically with C difficile and restore colonization resistance to C difficile. In a recent Phase 3, double-blind, randomized clinical trial (ECOSPOR III), SER-109 was observed to be superior to placebo in reducing the risk of CDI recurrence up to 8 weeks after dosing, the primary endpoint, and was well-tolerated.18
The Clostridiodes difficile Quality of Life Survey (Cdiff32) is a disease-specific measure designed to assess HRQOL changes related to recurrent CDI.19 Initially validated against the 36-Item Short Form Health Survey (SF-36), an instrument considered to be a general measure of HRQOL, subsequent studies have confirmed and extended these findings by also demonstrating HRQOL differences based on CDI recurrence and severity.20 Prior studies of the Cdiff32 have shown HRQOL scores to be 10 or more points lower in patients with recurrent CDI than those with primary CDI.19,20 In addition, the Cdiff32 was also validated against the general instrument EQ-5D in a planned analysis of matching data sets from ECOSPOR III and found to be more sensitive in assessing disease-specific changes in HRQOL.21 Herein, we present an exploratory analysis of quality of life in adults with a history of recurrent CDI between patients randomized to SER-109 vs placebo groups through 8 weeks after dosing in ECOSPOR III.
Methods
Trial Design, Study Population, Intervention, and Follow-up
This was an exploratory analysis of a Phase 3, double-blind, randomized, placebo-controlled trial conducted at 56 sites in the US and Canada from July 2017 to April 2020.18 The reporting of this study follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline (eFigure 1 in Supplement 1). The trial protocol is available in Supplement 2. Ethical approval for the study was obtained by the institutional review boards at all participating sites and written informed consent was obtained from all patients prior to screening.18
Participant’s race and ethnicity data were entered into the database by clinical staff based on patient self-report or from electronic health records. The categories used to describe racial and ethnic groups were aligned with the US National Institutes of Health and US Office of Management and Budget minimum requirements as well as with US Food and Drug Administration guidance. These variables were assessed to determine if our study demographics were consistent with other epidemiologic data and clinical trials, which also showed that a majority of patients with CDI are White non-Hispanic.
Eligible participants were persons 18 years of age or older with 3 or more episodes of CDI within 12 months, inclusive of their qualifying episode. A previous episode of CDI was defined as (1) the presence of diarrhea (3 or more unformed bowel movements over 2 consecutive days); (2) a positive C difficile toxin test; and (3) enzyme immunoassay or cell cytotoxicity neutralization assay, and resolution of symptoms following 10 to 21 days of standard of care therapy with vancomycin or fidaxomicin. Participants were stratified by age (younger than 65 years or 65 years or older) and antibiotic treatment (vancomycin or fidaxomicin) prior to randomization. After completing standard of care antibiotics with resolution of CDI symptoms, patients were randomized 1 to 1 to receive 4 oral capsules of SER-109 (approximately 3 × 107 spore colony-forming units) or matching placebo daily for 3 days. Patients were monitored for 8 weeks for CDI recurrence defined as onset of 3 of more unformed bowel movements per day over 2 consecutive days, a positive C difficile stool toxin assay, assessment by the investigator that antibiotic treatment was warranted, and diarrheal persistence until initiation of antibiotic therapy.
Health-Related Quality of Life Instruments
The Cdiff32 includes 32 items on 3 major domains (physical, mental, social) with 4 subdomains (general physical complaints, specific physical complaints, anxiety future, and anxiety current) and a total score19 (eTable in Supplement 1). Questions are answered on a 5-point Likert scale and ordinal items were transformed to 0, 25, 50, 75, and 100 points. Total, domain, and subdomain scores were calculated as the mean of included items resulting in a 0 to 100 score. A minimally important difference of 10-points in total, domain, and subdomain scores was supported.21 Patients completed the Cdiff32 after resolution of symptoms on standard-of-care antibiotics for CDI, but prior to initiation of treatment (baseline), and at the week 1 and week 8 visit after treatment initiation. To assess total HRQOL between groups, patients also completed the EQ-5D-5L at baseline, a general HRQOL instrument.
Outcome Measures
The primary efficacy outcome measure in the ECOSPOR III study was the rate of CDI recurrence up to 8 weeks after treatment initiation in patients who received SER-109 compared with patients who received placebo.18 For this exploratory analysis, Cdiff32 measures were collected at day 1 (baseline), week 1, and week 8, or at the recurrence visit or early termination visit prior to week 8.
Statistical Analysis
Participants who completed baseline and week 8 Cdiff32 results were included. For patients with a CDI recurrence visit or early termination visit prior to week 1 or week 8, last observation carried forward (LOCF) was applied for any missing week 1 or week 8 data. Total and individual domain (physical, mental, and social) Cdiff32 scores were calculated for each patient at day 1 (baseline) and at weeks 1 and 8. Change from baseline at weeks 1 and 8 was calculated. Comparison between treatment groups were performed and further adjusted for baseline covariates of age, gender, the number of prior CDI episodes, and prior antibiotic use using an analysis of covariance (ANCOVA) model. Least square (LS) means, 95% CIs for LS means, and P values are presented.
A 10-point change in Cdiff32 total or domain scores has been suggested as the minimally important difference (MID) for the Cdiff3221 in line with thresholds commonly used for other HRQOL measures22,23,24 and indicate a 10 percent change in score. Disease-specific HRQOL outcome status was characterized as (1) improved, defined by an increase from baseline in Cdiff32 score of at least 10 points; (2) unchanged, defined as a change from baseline in Cdiff32 score of less than 10 points in either direction; or (3) worsened, defined by a decrease from baseline in Cdiff32 score of at least 10 points. The Mantel-Haenszel χ2 test was used to test differences in treatment arms in ranked ordinal outcomes (improved, unchanged, and worsened) in Cdiff32 scores (total, mental, physical, and social and relationship domains) at week 1 and week 8. All statistical tests were performed using a 2-sided α level of .05 to determine statistical significance. Additionally, a post hoc analysis compared Cdiff32 scores from patients with and without recurrences in the overall population and within each treatment group to determine if on-study recurrence negatively impacted HRQOL. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study Population
Of the 281 patients screened, 182 were randomized (intent to treat population) to receive SER-109 (89 [51.1%]) or placebo (93 [48.9%]) and were included in the primary analysis as well as this exploratory analysis (eFigure 1 in Supplement 1). All 182 patients completed the Cdiff32 at baseline and week 8, and 160 (87.9%) patients completed the baseline HRQOL measures before dosing, as per protocol and the remaining 22 (12.1%) patients completed it within the 3-day period of drug dosing. However, 8 (4.4%) patients (2 in the SER-109 arm and 6 in the placebo arm) did not complete the Cdiff32 at week 1 and are not included in week 1 data. The mean (SD) age of participants was 65.5 (16.5) years; 109 (59.9%) were female, 170 (93.4%) were White patients, 171 (94.0%) were non-Hispanic patients, and 180 (98.9%) were outpatients. Comorbidities were highly prevalent in the study population with a mean age adjusted Charlson comorbidity index25 of 4.1. Baseline demographics and clinical characteristics were balanced between treatment arms with the exception that there was a greater proportion of females randomized to SER-109 compared with placebo (60 [67%] vs 49 [53%], respectively). Baseline EQ-5D VAS scores were similar in the SER-109 (75.7 [16.9]) and the placebo (69.8 [18.2]) groups. As previously reported, 11 of 89 patients (12%) who received SER-109 experienced an on-study CDI recurrence after treatment initiation compared with 37 of 93 patients (40%) who received placebo (P < .001), a 68% relative risk reduction.18 Notably, the majority of these recurrences (41 of 48; 85%) occurred on or before Week 4. Participants who experienced recurrence after week 1, but prior to week 8 completed the week 8 Cdiff32 questionnaire at the recurrence visit.
Changes From Baseline in CDI-Related HRQOL by Treatment Group
Baseline total and domain Cdiff32 scores were similarly low19 in both study arms (eFigure 2 in Supplement 1). To examine treatment-related differences in HRQOL scores, we evaluated (1) the mean change in HRQOL scores from baseline to weeks 1 and 8; and (2) the proportion of patients with improved, unchanged, or worsened disease-specific HRQOL outcome status.
At week 1 after dosing, all Cdiff32 total and domain scores improved in both treatment groups, although the change from baseline was not significant in the placebo group across all domain and subdomain scores (Figure 1). The magnitude of improvement in the total Cdiff32 and physical domain and subdomain scores was significantly greater for patients in the SER-109 arm compared with the placebo arm (P < .05). Improvements in the mental and social domain scores were also consistently greater for SER-109–treated patients compared with placebo although between group differences were not statistically significant at week 1 (Figure 1). In addition, the overall proportion of patients with improved, unchanged, or worsened HRQOL outcome status (49.4%, 38.2%, and 10.1% in SER-109 vs 26.9%, 54.8%, and 11.8% in placebo, respectively) was significantly different between treatment arms in the total Cdiff32 score at week 1 (P = .01) (eFigure 3 in Supplement 1). In the SER-109 arm, 44 of 89 (49%) of patients had at least a 10-point improvement from baseline in Cdiff32 total score vs 25 of 94 (27%) of patients in the placebo arm.
Figure 1. Change From Baseline at Week 1 and Week 8 in Cdiff32 Score by Treatment Group, ITT Population.
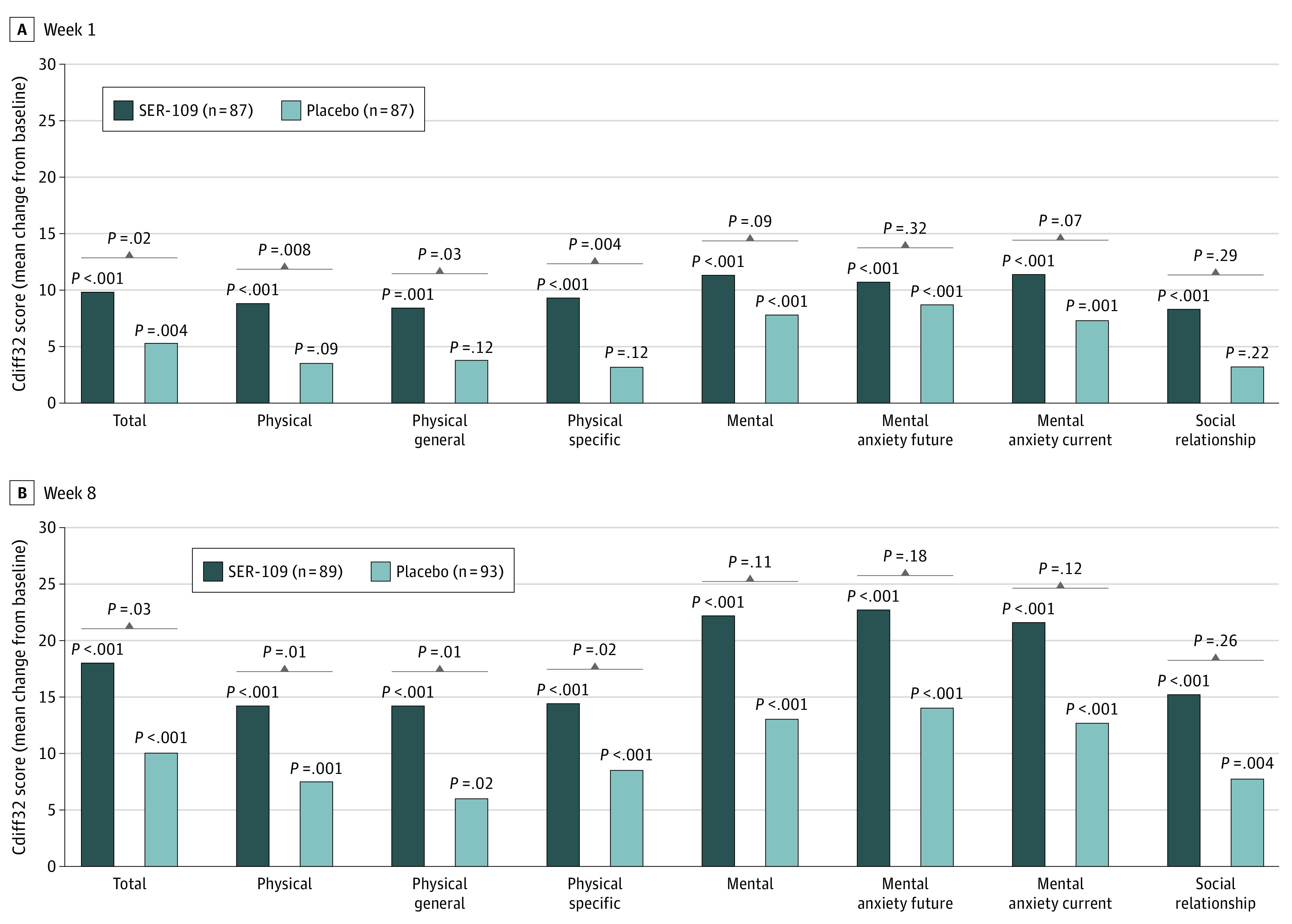
P values above bars represent change from baseline within each treatment group. Brackets represent between treatment group comparisons. Cdiff32 indicates Clostridioides difficile Quality of Life Survey; ITT, intent to treat.
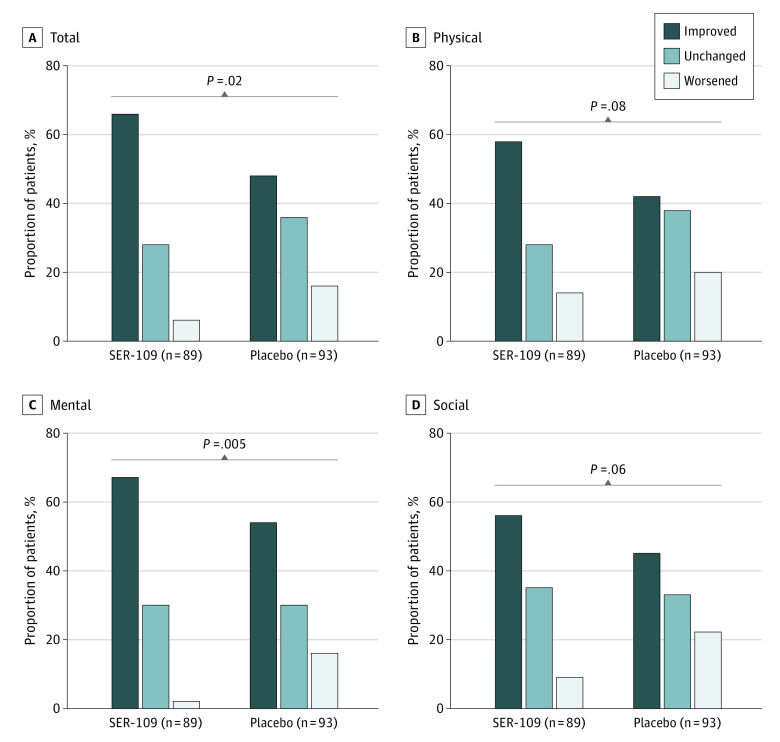
At week 8, patients in both treatment arms continued to show improvement from baseline in Cdiff32 total and domain scores. In the SER-109 arm, improvements in Cdiff32 scores surpassed a greater than 10-point increase from baseline in all domains. In contrast, improvements in Cdiff32 scores in the placebo arm were more limited. The magnitude of improvements in overall and physical domain and subdomain scores in the SER-109 arm were significantly greater at week 8 compared with placebo (least squares mean treatment difference [95% CI] for total: 11.3 [1.4 – 21.2]; P = .03; for physical: 14.1 [3.4 – 24.8]; P = .01; for physical-general: 16.1 [3.6 – 28.7]; P = .01; for physical-specific: 12.6 [2.2 – 23.0]; P = .02) (Figure 1). Similarly, the overall proportion of patients with improved, unchanged, or worsened HRQOL outcome status was significantly different between treatment arms in the total score (66.3%, 28.1%, and 5.6% in SER-109 vs 48.4%, 35.5%, and 16.1% in placebo, respectively; P = .02) and the mental domain score (67.4%, 30.3%, 2.2% in SER-109 vs 53.8%, 30.1%, and 16.1% in placebo, respectively; P = .005) (Figure 2). For the Cdiff32 total score, the proportion of patients with at least a 10-point improvement in the SER-109 arm was 59 (66%) vs 45 (48%) in the placebo arm. Similarly, for the mental domain score, 60 (67%) of patients in the SER-109 arm vs 50 (54%) in the placebo arm had at least a 10-point improvement from baseline.
Figure 2. Week 8 Disease-Specific HRQOL Outcome Status (Defined by 10-Point Change) in Postbaseline Cdiff32 HRQOL Questionnaire by Treatment Arm, ITT Population.
Week 8 disease-specific HRQOL outcome status, characterized as (1) improved, defined by an increase from baseline of at least 10 points; (2) unchanged, defined as a change from baseline of less than 10 points in either direction; or (3) worsened, defined by a decrease from baseline of at least 10 points, in Cdiff32 scores (total, mental, physical, and social/relationship domains) in SER-109 vs placebo treatment arms. Brackets represent between treatment group comparisons. Cdiff32 indicates Clostridioides difficile Quality of Life Survey; HRQOL, health-related quality-of-life; ITT, intent to treat.
Association of On-Study CDI Recurrence With HRQOL in the Overall Population and Within Each Treatment Arm
The association between on-study CDI recurrence at week 8 and HRQOL was evaluated in the overall population. There were significant differences in Cdiff32 total and all domain and subdomain scores between patients who experienced on-study recurrence vs those without recurrence. Patients without on-study recurrence reported significantly greater improvements from baseline in total Cdiff32 and domain scores compared with patients who experienced on-study recurrence through week 8 (least squares mean treatment difference [95% CI] for total: −17.1 [−23.1 to −11.2]; P < .001; for physical: −17.7 [−24.6 to −10.8]; P < 0.001; for mental: −16.0 [−23.1 to −8.9]; P < .001; for social: −18.8 [−26.2 to −11.4]; P < .001) (Figure 3).
Figure 3. Change in Cdiff32 HRQOL Score From Baseline to Week 8 by On-study Recurrence Status, ITT Population.
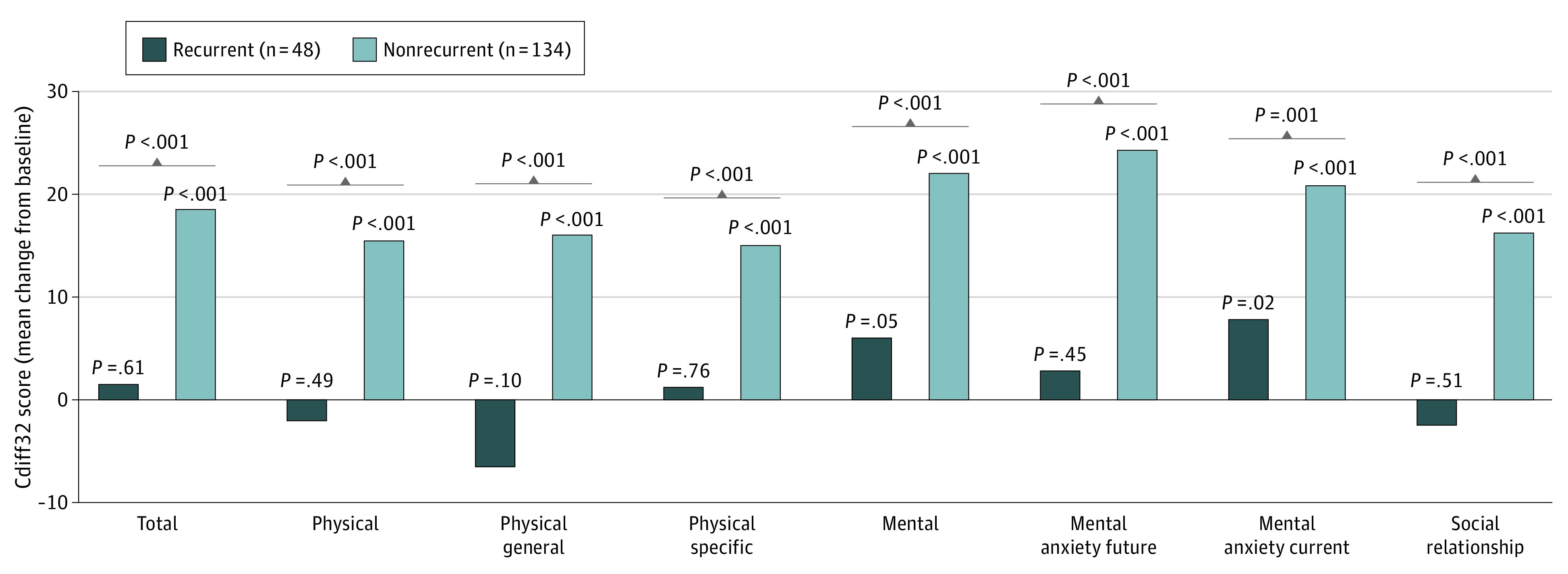
P values above bars represent change from baseline within each treatment group. Brackets represent between treatment group comparisons. Cdiff32 indicates Clostridioides difficile Quality of Life Survey; HRQOL, health-related quality-of-life; ITT, intent to treat.
However, these outcomes differed when examined within each treatment arm. In the placebo arm, only patients without on-study CDI recurrence had significant improvements in Cdiff32 scores at week 8 (Figure 4). Patients in the placebo group who experienced on-study CDI recurrence generally showed decreased Cdiff32 scores and significant differences by CDI recurrence status were observed in total and all domain and subdomain scores at week 8 (Figure 4). In contrast, within the SER-109 arm, total and individual domain Cdiff32 scores improved, regardless of on-study CDI recurrence through week 8 (Figure 4). Notably, patients in the SER-109 arm who experienced on-study CDI recurrence showed significant improvements from baseline in mental domain and subdomain scores at Week 8 (Figure 4).
Figure 4. Change From Baseline at Week 8 in Cdiff32 HRQOL Questionnaire by On-study Recurrence Status in the Placebo and SER-109 Arms, ITT Population.
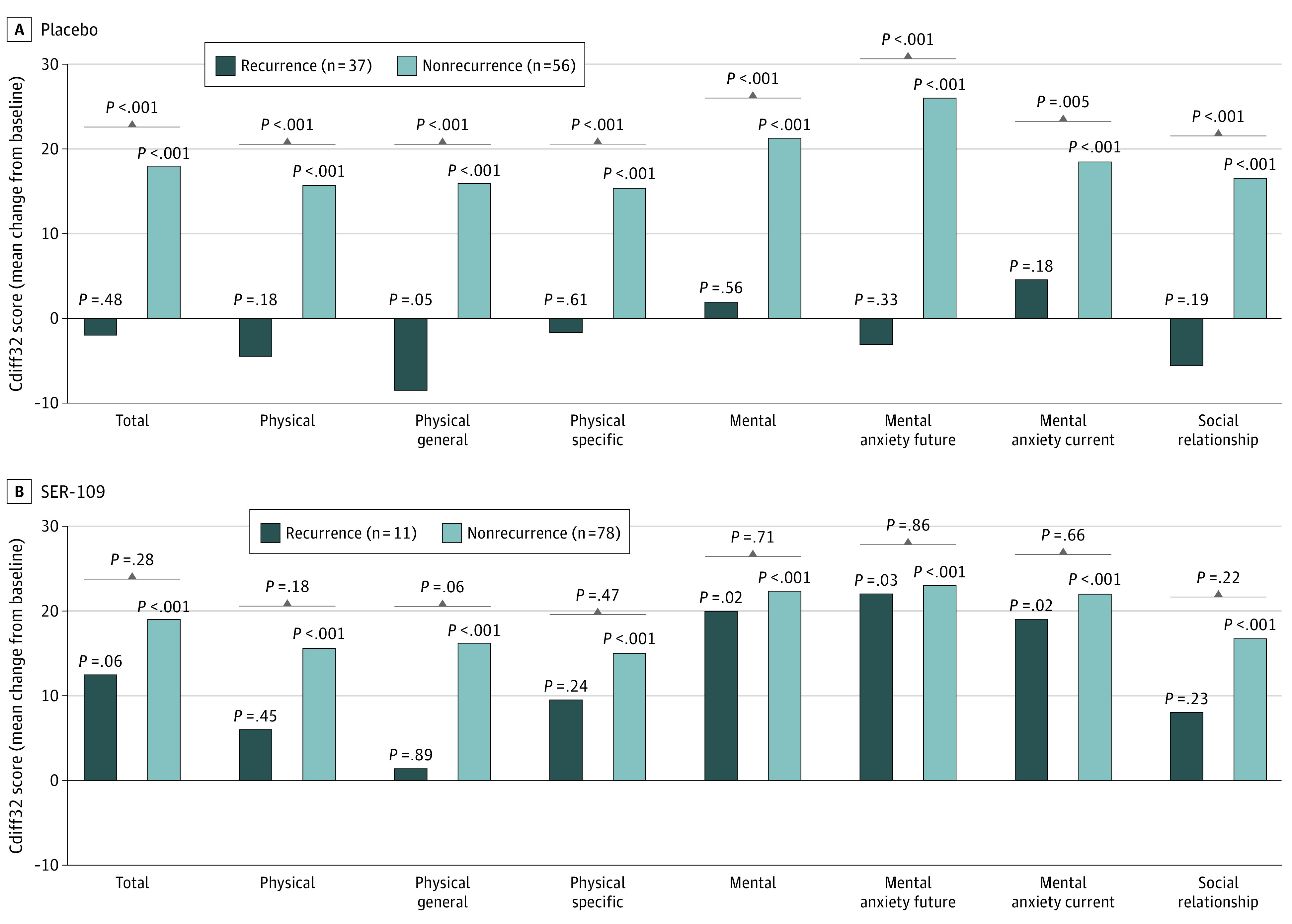
P values above each bar represents change from baseline within each treatment group. Brackets represent between treatment group comparisons. Cdiff32 indicates Clostridioides difficile Quality of Life Survey; HRQOL, health-related quality-of-life; ITT, intent to treat.
Discussion
In this exploratory analysis of the ECOSPOR III trial in patients with a history of recurrent CDI and prevalent comorbidities, patients in the SER-109 treatment group had significantly greater improvements in HRQOL scores compared with patients in the placebo group as early as week 1, with continued steady and durable improvements by week 8, as assessed by the disease-specific Cdiff32 measure. Improvement was noted in the total score and across all 3 Cdiff32 domains (physical, mental, and social) after a single dosing regimen. These data suggest that an investigational microbiome therapeutic not only offers the clinical benefits of reduced CDI recurrence, but may also improve HRQOL, an important patient-reported outcome of great interest to patients, clinicians, payers, and regulators.
Another positive finding from this study was the observed improvements in the mental domain and subdomain scores at week 8 in patients in the SER-109 arm, regardless of clinical outcome. However, given the low number of patients who had a recurrence on the SER-109 arm (n = 11), the benefit of SER-109, regardless of recurrence, will need to be confirmed in future studies of this investigational agent. Several interesting hypotheses arise from this novel observation, which may be related to the potential role of the microbiome in disorders related to the gut-brain axis.26,27,28 CDI is associated with a disrupted microbiome which has been associated with mood disorders, including anxiety and depression.6 In the ECOSPOR III trial, engraftment of SER-109 dose species was associated with metabolic changes, critical to inhibition of C difficile spore germination.18 Whether the consortium of purified Firmicutes spores may also improve the balance of neurotransmitters, which play a role in anxiety or depression (eg, serotonin or gamma aminobutyric acid)29 will require further study and investigation.
Another important finding from this study was the performance of the disease-specific Cdiff32 measure in the context of this placebo-controlled randomized trial. In ECOSPOR III, the average baseline Cdiff32 total scores in patients with recurrent CDI were similar between the 2 arms and consistent with previous studies, with a range of approximately 26 to 50.4,19,20 Importantly, in this Phase 3 trial, the Cdiff32 detected significant change from baseline in HRQOL scores by clinical outcome in the overall population at week 8. Finally, the post hoc analyses of on-study CDI recurrence between study arms clearly differentiate the sensitivity of the Cdiff32 in assessing important patient-related clinical outcomes. Based on the totality of these findings, including the validation of this disease-specific measure against other general instruments,21 we propose that the Cdiff32 is fit for purpose in future clinical trials of CDI-directed therapeutics in both inpatients20 and outpatients.19
In the overall population, Cdiff32 scores were significantly lower in patients who experienced an on-study CDI recurrence compared with patients without recurrence, highlighting the debilitating impact of recurrent infection on HRQOL. These data are consistent with prior epidemiologic measures that have assessed the marked impact of CDI, particularly recurrent infection, on every aspect of daily living.2 Improving HRQOL is an important objective for the overall management of patients with CDI. Multiple epidemiologic studies have demonstrated long-lasting, reduced HRQOL in patients with this common hospital- and community-associated infection.8,30 Patients with CDI also describe significant physical and psychological consequences of the disease.8 In 1 study, despite eradication of infection, large proportions of patients continued to experience reduced HRQOL and 41% of respondents believed they would never attain relief of their post-CDI symptoms. Such findings underscore the lasting and vexing impact of CDI and emphasizes the need to address both the physical and psychological aspects of the disease.8 Furthermore, the severe diarrhea associated with prior episodes coupled with the anxiety of a future recurrence has led some experts to refer to these anticipated events as typical of a CDI-related post-traumatic stress disorder.31
Strengths and Limitations
Our study has many strengths. This was a planned exploratory analysis from a robust randomized clinical trial that recruited an outpatient population of patients with recurrent CDI from 56 North American sites. A free toxin CDI assay was used for study entry and to confirm CDI recurrence per guideline recommendations, assuring a study population with active disease.32 Using the intent-to-treat analysis population, all patients completed the Cdiff32 questionnaire at baseline and week 8. Although patients were not randomized based on baseline HRQOL scores, these scores were evenly matched between treatment arms.
This study also has limitations. Changes in HRQOL have not been mapped yet to changes in microbiome composition, so it is unclear if the HRQOL improvements are due to sustained clinical response or partially mediated by effects on the gut-brain axis, an area of worthy future investigation. In addition, the baseline estimates of HRQOL were performed after clinical resolution on antibiotics, which may have limited our ability to see the full spectrum of improvement after the qualifying CDI episode. Finally, a 10-point change was used as the minimally important difference based on results of a recent study on the validity and responsiveness of the Cdiff32 in patients with a history of CDI.21 However, more studies are needed to formally evaluate a minimally important difference.
Conclusions
In this secondary analysis of a randomized clinical trial (ECOSPOR III) of patients with a history of recurrent CDI and prevalent comorbidities, this exploratory analysis suggested that SER-109 significantly was associated with improved disease-specific HRQOL at week 8 after dosing compared with placebo. In addition to the observed clinical benefit of SER-109, this is the first evidence of improved HRQOL scores following treatment with an investigational microbiome agent. This study also further validated the Cdiff32 for use in future clinical trials and may offer a new patient-reported outcome when evaluating investigational agents for clinical management of patients with recurrent CDI.
eTable. Relationship Between Cdiff32 Questions, Domains, and Subdomains
eFigure 1. CONSORT Diagram
eFigure 2. Baseline Cdiff32 HRQOL Questionnaire Scores by Treatment Arm, ITT Population
eFigure 3. Week 1 Disease-Specific HRQOL Outcome Status (Defined by 10-Point Change) in Postbaseline Cdiff32 HRQOL Questionnaire by Treatment Arm, ITT Population
Trial Protocol
Data Sharing Statement
References
- 1.Guh AY, Mu Y, Winston LG, et al. ; Emerging Infections Program Clostridioides difficile Infection Working Group . Trends in US burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330. doi: 10.1056/NEJMoa1910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrich K, Harnett J, Vietri J, Chambers R, Yu H, Zilberberg M. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci. 2018;63(11):2864-2873. doi: 10.1007/s10620-018-5222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB. Fecal microbiota transplantation for recurrent Clostridium difficile infection: the patient experience. Am J Infect Control. 2016;44(5):554-559. doi: 10.1016/j.ajic.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 4.Hengel RL, Schroeder CP, Jo J, et al. Recurrent Clostridioides difficile infection worsens anxiety-related patient-reported quality of life. J Patient Rep Outcomes. 2022;6(1):49. doi: 10.1186/s41687-022-00456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17(1):6. doi: 10.1186/s12955-019-1081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bear T, Dalziel J, Coad J, Roy N, Butts C, Gopal P. The microbiome-gut-brain axis and resilience to developing anxiety or depression under stress. Microorganisms. 2021;9(4):723. doi: 10.3390/microorganisms9040723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vent-Schmidt J, Attara GP, Lisko D, Steiner TS. Patient experiences with Clostridioides difficile infection: results of a Canada-wide survey. Patient Prefer Adherence. 2020;14:33-43. doi: 10.2147/PPA.S229539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurienne L, Bandinelli PA, Galvain T, Coursel CA, Oneto C, Feuerstadt P. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. 2020;4(1):14. doi: 10.1186/s41687-020-0179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547-1553. doi: 10.1053/j.gastro.2014.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649-656. doi: 10.1093/cid/ciy171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis. 2016;62(5):574-580. doi: 10.1093/cid/civ958 [DOI] [PubMed] [Google Scholar]
- 13.Dubberke ER, Lee CH, Orenstein R, Khanna S, Hecht G, Gerding DN. Results From a randomized, placebo-controlled clinical trial of a RBX2660-A microbiota-based drug for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018;67(8):1198-1204. doi: 10.1093/cid/ciy259 [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154-S161. doi: 10.1093/cid/cis462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and clostridium difficile. Annu Rev Microbiol. 2015;69(1):445-461. doi: 10.1146/annurev-micro-091014-104115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2(1):16020. doi: 10.1038/nrdp.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorpe CM, Kane AV, Chang J, Tai A, Vickers RJ, Snydman DR. Enhanced preservation of the human intestinal microbiota by ridinilazole, a novel Clostridium difficile-targeting antibacterial, compared to vancomycin. PLoS One. 2018;13(8):e0199810. doi: 10.1371/journal.pone.0199810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an Oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220-229. doi: 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- 19.Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol. 2016;50(8):631-637. doi: 10.1097/MCG.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Z, Lapin B, Garey KW, Donskey CJ, Deshpande A. Impact of Clostridioides difficile infection on patient-reported quality of life. Infect Control Hosp Epidemiology. October 7, 2021. doi: 10.1017/ice.2021.413 [DOI] [PubMed] [Google Scholar]
- 21.Lapin B, Garey KW, Wu H, et al. Validation of a health-related quality of life questionnaire in patients with recurrent Clostridioides difficile infection in ECOSPOR III, a Phase 3 randomized trial. Clin Infect Dis. 2022;ciac554. doi: 10.1093/cid/ciac554 [DOI] [PubMed] [Google Scholar]
- 22.Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual Life Res. 2016;25(6):1585-1596. doi: 10.1007/s11136-015-1196-z [DOI] [PubMed] [Google Scholar]
- 23.Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J Clin Epidemiol. 2017;89:188-198. doi: 10.1016/j.jclinepi.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Zanini A, Aiello M, Adamo D, et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care. 2015;60(1):88-95. doi: 10.4187/respcare.03272 [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 26.Morais LH, Schreiber HL IV, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241-255. doi: 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- 27.Koppenol E, Terveer EM, Vendrik KEW, et al. Fecal microbiota transplantation is associated with improved aspects of mental health of patients with recurrent Clostridioides difficile infections effect of FMT on affect in rCDI patients. J Affect Disord Rep. 2022;9:100355. doi: 10.1016/j.jadr.2022.100355 [DOI] [Google Scholar]
- 28.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087-1092. doi: 10.1126/science.abi6087 [DOI] [PubMed] [Google Scholar]
- 29.Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995;34(4):275-281. doi: 10.1016/0165-0327(95)00025-I [DOI] [PubMed] [Google Scholar]
- 30.Wilcox MH, Ahir H, Coia JE, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72(9):2647-2656. doi: 10.1093/jac/dkx174 [DOI] [PubMed] [Google Scholar]
- 31.Hohmann EL. Are microbial politics local? Ann Intern Med. 2016;165(9):667-668. doi: 10.7326/M16-1784 [DOI] [PubMed] [Google Scholar]
- 32.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi: 10.1093/cid/cix1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Relationship Between Cdiff32 Questions, Domains, and Subdomains
eFigure 1. CONSORT Diagram
eFigure 2. Baseline Cdiff32 HRQOL Questionnaire Scores by Treatment Arm, ITT Population
eFigure 3. Week 1 Disease-Specific HRQOL Outcome Status (Defined by 10-Point Change) in Postbaseline Cdiff32 HRQOL Questionnaire by Treatment Arm, ITT Population
Trial Protocol
Data Sharing Statement